Abstract
There are large interindividual differences in exercise capacity. It is well established that there is a genetic basis for these differences. However, the genetic factors underlying this variation are undefined. Therefore, the purpose of this study was to identify novel putative quantitative trait loci (QTL) for exercise capacity by measuring exercise capacity in inbred mice and performing genome-wide association mapping. Exercise capacity, defined as run time and work, was assessed in male mice (n = 6) from 34 strains of classical and wild-derived inbred mice performing a graded treadmill test. Genome-wide association mapping was performed with an efficient mixed-model association (EMMA) algorithm to identify QTL. Exercise capacity was significantly different across strains. Run time varied by 2.7-fold between the highest running strain (C58/J) and the lowest running strain (A/J). These same strains showed a 16.5-fold difference in work. Significant associations were identified for exercise time on chromosomes 1, 2, 7, 11, and 13. The QTL interval on chromosome 2 (∼168 Mb) contains one gene, Nfatc2, and overlaps with a suggestive QTL for training responsiveness in humans. These results provide phenotype data on the widest range of inbred strains tested thus far and indicate that genetic background significantly influences exercise capacity. Furthermore, the novel QTLs identified in the current study provide new targets for investigating the underlying mechanisms for variation in exercise capacity.
Keywords: treadmill running, single nucleotide polymorphism, quantitative trait loci, wild-derived inbred strains
exercise capacity, as assessed by exercise time during a graded treadmill test, is commonly used to assess cardiorespiratory fitness and is highly correlated to risk of cardiovascular disease (26). In sedentary humans, there are substantial individual differences in exercise capacity, and it is well established that there is a genetic basis for these individual differences (4, 6, 31). Because of the health benefits associated with high levels of fitness, the physiological factors determining exercise capacity have been widely studied. However, little is known about the underlying genetic determinants of exercise capacity (6, 13). To elucidate these genetic factors, Bouchard and colleagues used genome-wide linkage analysis to identify quantitative trait loci (QTL) for exercise capacity, quantified by maximal oxygen consumption (V̇O2max), in the sedentary state and in response to training. Several promising and suggestive QTL for pretraining exercise capacity were reported (4, 31), and these regions differed from those for the V̇O2max response to training. These findings imply that the genes that determine sedentary-state V̇O2 are different from those that determine the response to training (4, 6, 31). Thus, the underlying genes or DNA variants determining the genetic effect on sedentary-state or intrinsic exercise capacity will need to be determined to understand the link between exercise capacity and susceptibility to disease.
Several rodent models have been utilized to investigate the genetic factors contributing to intrinsic endurance exercise capacity assessed by treadmill running (2, 22, 24, 25, 42). Using traditional genome-wide linkage analysis, investigators have identified several QTL for intrinsic endurance exercise capacity in rats (2, 42) and mice (22, 25). However, traditional QTL analyses are limited by the variation present in the genomes of the two mouse strains (3, 16), which can reduce mapping resolution. A relatively new approach for refining large QTL regions and identifying novel QTL for disease and behavior-related traits, including physical activity is genome-wide association mapping (3, 9, 20). Early attempts at genome-wide association mapping in mice were criticized for low statistical power and differences in population structure across inbred strains leading to a high rate of false positive associations due limited genomic information from a small number of mouse strains (12, 41). Recent advances in single nucleotide polymorphism (SNP) discovery and genomic sequencing capabilities have led to the creation of dense SNP maps available for a large number of inbred strains (10, 44). These large SNP databases have facilitated the development of genome-wide association mapping approaches in mice that account for some of the concerns raised with earlier studies (12, 16, 28). In the current study, we employed efficient mixed model association (EMMA) mapping. EMMA relies on a kinship matrix to account for genetic relatedness and population structure in inbred strains of mice and other model organisms, which can reduce the number of false positive associations (16, 17). EMMA has been used successfully to identify QTL for physiological traits such as body weight, bone mineral density, HDL cholesterol, airway responsiveness, and pulmonary adenomas in inbred mice (3, 9, 16). Thus, given the limited information regarding the genetic determinants of intrinsic endurance exercise capacity and the improved methodology for in silico genetic analyses, the purpose of this study was to characterize intrinsic endurance exercise capacity in 34 strains of classical and wild-derived inbred mice and to apply association mapping to identify novel putative QTL for endurance exercise capacity.
METHODS
All procedures in this study were approved by the Institutional Animal Care and Use Committee at Texas A&M University and adhered to the American Physiological Society's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training.
Animals.
Seven-week-old male mice from 34 inbred strains (129S1/SvImJ, 129X1/SvJ, A/J, AKR/J, BALB/cByJ, BUB/BnJ, C3H/HeJ, C57BL/6J, C57BLKS/J, C57L/J, C58/J, CAST/EiJ, CBA/J, CE/J, DBA/2J, FVB/NJ, KK/HlJ, I/LnJ, LP/J, MA/MyJ, MRL/MpJ, NOD/ShiLtJ, NON/ShiLtJ, NZB/BlNJ, NZO/HlLtJ, NZW/LacJ, PL/J, PWD/PhJ, PWK/PhJ, RIIIS/J, SJL/J, SM/J, SWR/J, and WSB/EiJ) (n = 6 per strain) were purchased from Jackson Laboratory (Bar Harbor, ME). These strains were chosen for genetic diversity incorporating inbred and wild-derived strains (27, 44). Animals were housed in group cages and kept on a 12 h light-dark schedule (7:00 AM–7:00 PM) in a temperature-controlled environment (21.0–22.0°C) with food and water provided ad libitum. All mice were allowed 1 wk to become accustomed to the housing facility before completing the exercise protocol. All exercise tests were performed between 9:00 AM and 11:00 AM.
Endurance exercise test.
Intrinsic endurance exercise capacity was defined for each of the 34 strains as time (minutes) and work (kg·m) performed during a graded exercise test. Work performed (kg·m) or vertical work was calculated as a product of body weight (kg) and vertical distance (meters), where vertical distance = (distance run)(sinθ), where θ is equal to the angle of the treadmill from 0° to 15° (24, 25). To identify strain-specific phenotypic differences in endurance exercise capacity, we had all mice perform two graded exercise endurance tests on a six-lane treadmill (Columbus Instruments, Columbus, OH). Prior to completing the exercise testing, mice were familiarized for 2 days running for 10 min up a 10° incline at 9.0 m/min on day 1 and 11.0 m/min on day 2. This protocol introduced the mice to treadmill running but was limited to avoid inducing any training adaptations. Following familiarization, intrinsic exercise capacity was measured in 8 wk old mice by two graded exercise tests separated by 48 h. The test started at a speed of 9.0 m/min for 9 min at 0°. After 9 min the speed was increased to 10 m/min and the grade was increased 5°. Thereafter, speed was increased 2.5 m/min every 3 min and the grade was increased 5° every 9 min to a maximum grade of 15°. The graded exercise test continued until mice maintained contact with the shock grid for more than 15 s continuously or could no longer be motivated to run (24, 25). For each mouse, the average exercise capacity for the two trials was used to calculate strain means and for association mapping. Individual body weights were measured before each exercise test.
Association mapping.
Genome-wide association mapping for exercise phenotypes and body mass were performed with EMMA using the UCLA web-based server (http://mouse.cs.ucla.edu/emmaserver). EMMA uses a linear mixed model algorithm to account for relatedness among inbred strains, which reduces the rate of false positive associations (3, 16, 17). Analysis of individual phenotypic data was performed with SNP panels consisting of 132,285 SNPs (132 K) and 4 million SNPs (4 M) (3, 17). The initial analysis was conducted using the 132 K panel to identify putative loci for endurance exercise capacity. The SNP density of this panel is comparable to previously published mouse genome-wide association mapping studies conducted on a similar number of mouse strains. A second analysis then was conducted using the 4 M SNP panel to compare with the results from the 132 K panel and to identify novel loci not identified with the smaller panel. The 4 M SNP panel includes the SNPs in the 132 K panel. We conducted association mapping on all 34 strains and after omitting phenotype data from the wild-derived inbred strains to assess the affect these strains have on QTL discovery. Genome-wide significance thresholds were calculated based on a false discovery rate of 5% (or q value ≤ 0.05) using the R package q value (34). We determined confidence intervals by expanding the interval around the peak SNP to include all neighboring SNPs surpassing the significance threshold. For single SNP associations, the QTL confidence interval was set at 400 kb (200 kb on either side of the peak SNP), but only the location of the peak SNP is reported. If two QTL overlapped or were separated by <1 Mb they were considered one QTL. All SNP and gene locations were mapped to Build 37.2 of the National Center for Biotechnology Information's mouse genome. SNP-associated P values were transformed with −log10 (P value) for graphing association scores.
Statistical analysis.
All data are represented as means ± SE. Exercise capacity variables and body mass data from the 34 strains was analyzed by JMP 9.0 (SAS Institute, Cary, NC). Analysis of variance (ANOVA) followed by a Tukey's post hoc test was used to identify significant strain-specific differences across all 34 strains. To assess the allele effect for significant peak SNPs, we grouped strains by genotype at each individual SNP and compared exercise time between groups by Mann-Whitney test. We calculated the proportion of variance explained by significant peak SNPs by fitting exercise time against the genotypes using a linear model. Statistical significance was set at P < 0.05.
RESULTS
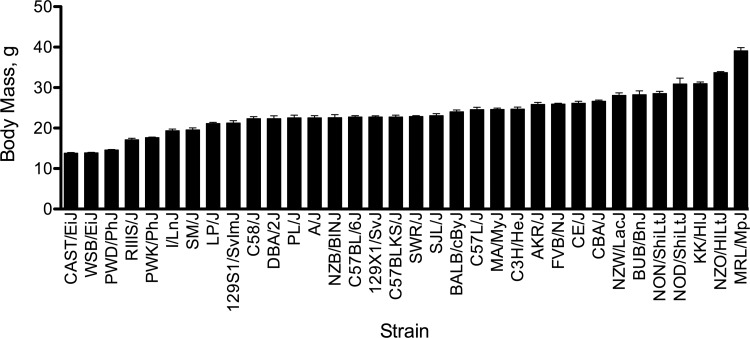
Body mass in 8-wk-old male mice varied significantly among inbred strains (Fig. 1 and Table 1). There was an approximately threefold difference in body mass across strains. The strain with the lowest body mass was the wild-derived CAST/EiJ mice (13.8 ± 0.2 g), whereas the heaviest strain was the MRL/MpJ (39.1 ± 0.8 g). Because there are strain-specific differences in body mass, work performed during the graded exercise test is reported in addition to time.
Fig. 1.
Distribution of body mass in 8-wk-old male mice from 34 inbred strains. Values are expressed as means ± SE. n = 6 mice/strain. Significant differences among strains are indicated in Table 1.
Table 1.
Statistical differences among 34 inbred strains for time, work, and body mass
| Strain | Time (Fig. 2A) | Work (Fig. 2B) | Body Mass (Fig. 1) |
|---|---|---|---|
| C58/J | A | A | GHI |
| AKR/J | B | B | DEF |
| CAST/EiJ | BC | IJKL | M |
| WSB/EiJ | CD | IJKL | M |
| SWR/J | DE | DE | GH |
| PWD/PhJ | DEF | JKLM | LM |
| PWK/PhJ | EFG | HIJK | K |
| MA/MyJ | EFG | CDE | EFG |
| SM/J | EFG | FGHI | IJK |
| NON/ShiLtJ | EFG | C | CD |
| DBA/2J | EFG | EFGH | GHI |
| SJL/J | EFG | EFG | FGH |
| FVB/NJ | FG | DE | DEF |
| C57L/J | G | EF | EFG |
| NOD/ShiLtJ | G | CD | BC |
| LP/J | G | GHIJ | HIJ |
| CBA/J | H | FGHI | DE |
| BALB/cByJ | HI | IJKL | EFGH |
| CE/J | HI | HIJK | DE |
| C57BLKS/J | HI | JKLMN | GH |
| NZB/BlNJ | HI | KLMN | GH |
| 129S1/SvImJ | IJ | MNO | HIJ |
| C3H/HeJ | IJ | KLMN | EFG |
| PL/J | JK | NO | GH |
| C57BL/6J | JK | NO | GH |
| 129X1/SvJ | K | O | GH |
| KK/HlJ | K | LMNO | BC |
| MRL/MpJ | K | IJKL | A |
| I/LnJ | L | PQ | JK |
| NZW/LacJ | L | PQ | CD |
| NZO/HlLtJ | L | P | B |
| RIIIS/J | L | Q | KL |
| BUB/BnJ | L | PQ | CD |
| A/J | M | Q | GH |
Significant differences are based on results from 1-way ANOVA followed by Tukey post hoc analysis. Strains not connected by same letter are significantly different. Strains are organized based on time from the longest time (A) to the shortest time (M). The number of strain groupings varies by phenotype.
Intrinsic endurance exercise capacity.
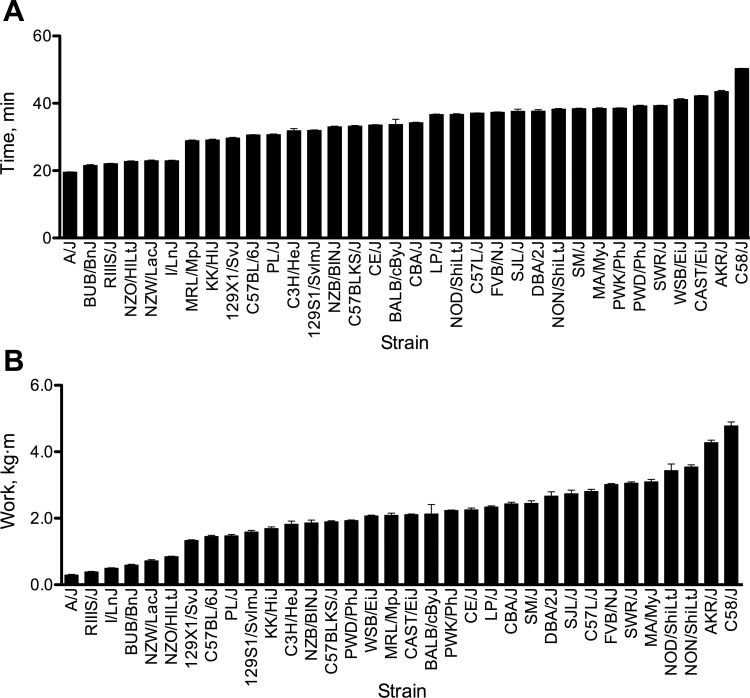
A strain screen was performed to identify strain-specific differences in intrinsic exercise capacity across 34 strains of male mice in a graded exercise test. All mice completed both graded exercise tests. Both tests were conducted by the same individual and were highly reproducible (within-mouse coefficient of variation = 1.56 ± 0.14%). The strain distribution patterns for time and work are shown in Fig. 2, and significant differences are indicated in Table 1. There were significant differences across the strains for each exercise phenotype. For run time, there was a nearly threefold difference (2.73) between the lowest (A/J: 18.4 ± 0.2 min) and highest (C58/J: 50.3 ± 0.1 min) performing strains (Fig. 2A). The variation among strains was greater for work than for time (Fig. 2B). There is a 16.5-fold difference between the lowest performing (A/J: 0.29 ± 0.02 kg·m) and highest performing (C58/J: 4.78 ± 0.12 kg·m) strains. The strain distribution pattern also changed when exercise capacity is expressed as work. This is abundantly clear in the four wild-derived strains (CAST/EiJ, PWD/PhJ, PWK/PhJ, and WSB/EiJ), which had high run times and low body mass, but relatively low work performed (Fig. 2, Table 1). Based on strain means, body mass did not correlate significantly with run time (r = −0.30, P = 0.08).
Fig. 2.
Strain survey of intrinsic endurance exercise capacity in male mice from 34 inbred strains. Exercise capacity is expressed as time (minutes) (A) and work (kg·m) (B). All mice performed 2 graded exercise tests separated by 48 h, and individual means were used to calculate strain means. Values are expressed as means ± SE. n = 6 mice/strain. Significant differences among strains are indicated in Table 1.
Genome-wide association mapping.
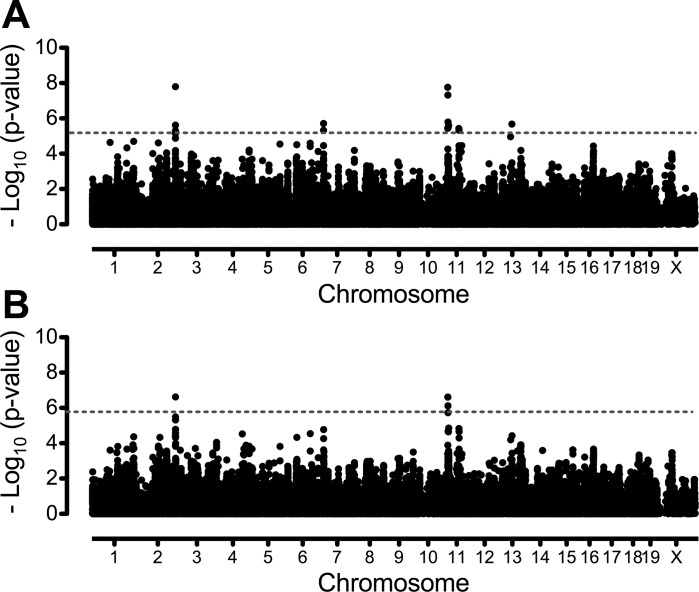
Genome-wide association mapping for exercise phenotypes was performed using 132 K and 4 M SNP panels in EMMA. For each SNP panel, analyses were run two ways, one with all 34 strains including classical and wild-derived inbred mouse strains and a second with only the classical inbred strains. This was done to investigate the influence of the wild-derived strains on the genetic architecture of the exercise phenotypes. For the 132 K panel, significant associations for exercise time using all 34 strains were identified on chromosomes 2, 7, 11, and 13 (Fig. 3A, Table 2). When analyses were repeated on data from the classical inbred strains only, significant associations for exercise time were found on chromosomes 2 (168.40–168.43 Mb) and 11 (21.66–22.56 Mb) (Fig. 3B). Two associations on chromosome 11 (∼25 Mb and ∼70 Mb) and those on chromosomes 7 and 13 were no longer significant. Repeating association mapping for exercise time using the 4 M SNP panel identified two significant associations in the 34-strain cohort and four significant associations in the 30-strain cohort (Table 3). A novel significant association was identified on chromosome 1 in both the 34-strain and 30-strain groups and a suggestive (false discovery rate of 10%) association on chromosome 11 in the 30 classical inbred strains.
Fig. 3.
Genome-wide association mapping for endurance exercise capacity, expressed as time in minutes, in male mice from 34 classical and wild-derived inbred strains (A) and 30 classical inbred strains only (B) using the 132 K SNP panel. Association mapping was conducted with an empirical mixed model algorithm (EMMA) and a 132,285 SNP panel. The x-axis indicates genomic position divided by chromosome. Values on the y-axis are P values transformed using −log10 (P value). Horizontal dashed lines in each plot indicate genome-wide significance thresholds. Significance thresholds were calculated using a 5% false discovery rate and correspond to P values of 7.21 × 10−6 and 1.87 × 10−6 for 34 strain and 30 strain cohorts, respectively.
Table 2.
Genome-wide association mapping for exercise time in male mice from classical and wild-derived inbred strains using a 132 K SNP panel
| Chr. | QTL Location, Mb | Peak SNP | P Value | Known Genes, n |
|---|---|---|---|---|
| Classical and wild-derived inbred strains | ||||
| 2 | 168.27–168.45 | rs27288988 | 1.61 × 10−8 | 1 (Nfatc2) |
| 7 | 16.97–16.99 | rs31142151 | 1.93 × 10−6 | 2 (Sae1) |
| 11 | 21.66–22.59 | rs4135796 | 1.73 × 10−8 | 9 (AV249152) |
| 11 | 24.51–25.56 | rs13480916 | 3.17 × 10−6 | 2 |
| 11 | 70.26–70.81 | rs3707772 | 3.73 × 10−6 | 30 (Med11) |
| 13 | 58.95 | rs29804611 | 2.09 × 10−6 | 2 (Ntrk2) |
| Classical inbred strains only | ||||
| 2 | 168.40–168.43 | rs27288988 | 2.36 × 10−7 | 1 (Nfatc2) |
| 11 | 21.65–22.56 | rs4135796 | 2.41 × 10−7 | 9 (AV249152) |
| rs3708339 | 2.41 × 10−7 | (AV249152) | ||
All results are significant based on genome-wide thresholds corresponding to P values of 7.21 × 10−6 for 34-strain and 1.87 × 10−6 for 30-strain cohorts. Gene symbol in parentheses indicates the gene containing the peak singe nucleotide polymorphism (SNP). Chr., chromosome; QTL, quantitative trait locus; Peak SNP, SNP with highest P value in QTL interval; P value, EMMA-corrected P value; Nfatc2, nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 2; Sea1, SUMO-1 activating enzyme subunit 1; AV249152, WD repeat containing planar cell polarity effector; Med11, mediator of RNA polymerase II transcription, subunit 11 homolog; Auh, AU RNA binding protein/enoyl-coenzyme A hydratase; Ntrk2, neurotrophic tyrosine kinase receptor type 2.
Table 3.
Genome-wide association mapping for exercise time in male mice from classical and wild-derived inbred strains using a 4 million SNP panel
| Chr. | QTL Location, Mb | Peak SNP | P Value | Known Genes, n |
|---|---|---|---|---|
| Classical and wild-derived inbred strains | ||||
| 1 | 180.76 | rs39502136 | 1.01 × 10−7 | 2 (Kif26b) |
| 11 | 21.65–22.58 | rs4135796 | 2.75 × 10−8 | 9 (AV249152) |
| rs26852365 | 2.75 × 10−8 | (Ehbp1) | ||
| Classical inbred strains only | ||||
| 1 | 178.54–180.76 | rs39502136 | 2.43 × 10−8 | 24 (Kif26b) |
| 2 | 168.40–168.43 | rs27288988 | 1.13 × 10−6 | 1 (Nfatc2) |
| 11 | 21.65–22.56 | rs4135796 | 3.29 × 10−7 | 9 (AV249152) |
| rs3708339 | 3.29 × 10−7 | (AV249152) | ||
| rs26852365 | 3.29 × 10−7 | (Ehbp1) | ||
| 11 | 70.92–71.09 | rs282333136 | 3.31 × 10−6* | 3 (Nlrplc) |
P value corresponding to a genome-wide false discovery rate of ≤10% (q value of ≤0.10). All other results are significant based on genome-wide thresholds corresponding to P values of 1.01 × 10−7 for 34-strain and 2.54 × 10−6 for 30-strain cohorts. Gene symbol in parentheses indicates the gene containing the peak SNP. Kif26b, kinesin family member 26B; AV249152, WD repeat containing planar cell polarity effector; Ehbp1, EH domain binding protein 1; Nfatc2, nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 2; Nlrplc, NLR family, pyrin domain containing 1C.
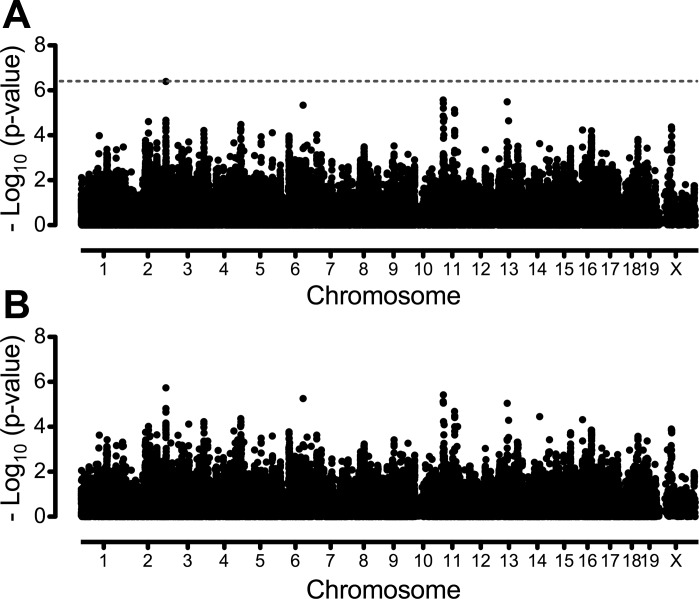
Genome-wide association mapping for work using the 132 K SNP panel is shown in Fig. 4. Only one significant association was identified for work in the 34-strain cohort (Chr. 2 at 168.4 Mb). No significant associations were identified using the 30 classical inbred strains only. Similar analyses using the 4 M SNP panel yielded no significant associations (data not shown).
Fig. 4.
Genome-wide association mapping for endurance exercise capacity, expressed as work in kg·m, in male mice from 34 classical and wild-derived inbred strains (A) and 30 classical inbred strains only (B) using the 132 K SNP panel. Association mapping was conducted with EMMA and a 132,285 SNP panel. The x-axis indicates genomic position divided by chromosome. Values on the y-axis are P values transformed using −log10 (P value). The horizontal dashed line in A indicates genome-wide significance threshold. Significance thresholds were calculated using a 5% false discovery rate and correspond to a P value of 4.01 × 10−7 for 34 strain cohort. P values were too low for the 30-strain cohort to estimate a 5% false discovery rate based on q values.
To assess the contribution of body mass to QTL for exercise capacity, we conducted genome-wide association mapping using body mass from the 34 inbred strains and the 30 classical inbred strains (Table 4). This analysis was performed to identify any body mass QTL that might overlap with QTL for exercise capacity. A significance threshold corresponding to P = 10−5 was used for this analysis to maximize the number of significant associations identified. None of the significant associations for body mass overlapped with significant associations for time or work, suggesting that none of the exercise-related QTL were primarily due to genetic factors controlling body mass. Seven of the putative QTL identified for body mass overlapped with previously identified QTL for body mass or body mass change after an experimental intervention (e.g., high-fat diet) (Table 4), with the exception of the QTL on chromosome 1 located at 54.08 Mb.
Table 4.
Genome-wide association mapping for body mass in male mice from classical and wild-derived inbred strains
| Chr. | QTL Location, Mba | Peak SNP | Known Genes, n | Previous QTL |
|---|---|---|---|---|
| Classical and wild-derived inbred strains | ||||
| 1 | 54.08 | rs6406541 | 1 (Hecw2) | |
| 3 | 109.58 | rs29791710 | 2 | Bwq8 |
| Classical inbred strains only | ||||
| 2 | 59.19 | rs6238344 | 5 | Pbwg1, Bwtq8 |
| 4 | 41.42–41.69 | rs6222957 | 13 | Wtlr3, Bwtq9, Wta1 |
| 9 | 93.76–98.14 | rs6360988 | 28 (Acpl2) | Bwq6 |
| 9 | 99.78 | rs6387071 | 3 | Bwq6 |
| 15 | 29.20 | rs13482501 | 0 | Dob3 |
| 17 | 48.77 | rs29537880 | 3 | Obwq4, W10q12, W6q11, Bodwt2, Wta4 |
All associations with P value <10−5 are reported. Gene symbol in parentheses indicates the gene containing the peak SNP. aFor single SNP associations, the QTL interval for candidate gene identification was estimated to be 400 kb centered around the peak SNP. Hecw2, HECT, C2 and WW domain containing E3 ubiquitin protein ligase 2; Acpl2, acid phosphatase-like 2; Bwq8, body weight QTL 8 (39); Pbwg1, postnatal body weight growth 1 (35); Bwtq8, body weight QTL 8 (36); Wtlr3, weight loss response 3 (32); Bwtq9, body weight QTL 9 (36); Wta1, weight adult 1 (7); Bwq6, body weight, QTL 6 (30); Dob3, dietary obesity 3 (43); Obwq4, obesity and body weight QTL 4 (36); W10q12, weight 10 wk QTL 12 (33); W6q11, weight 6 wk QTL 11 (33); Bodwt2, body weight 2 (38); Wta4, weight adult 4 (7).
Two peak SNPs (rs27288988 and rs4135796) were identified in multiple analyses. To assess the effect of each allele on exercise capacity, we grouped strains according to their genotype and compared them for exercise time. For rs27288988 on chromosome 2, 25 strains carry the G allele and 9 the T allele. Strains carrying the G allele had significantly greater exercise time than strains with the T allele (G = 36.1 ± 1.2 min, T = 26.9 ± 1.9 min; P < 0.05). Similarly, exercise time was significantly greater for strains with the G allele (n = 23 strains) than those with the C allele (n = 8 strains) for rs4135796 on chromosome 11 (G = 36.2 ± 1.2 min, C = 25.6 ± 2.1 min; P < 0.05). Based on provisional estimates using a linear model and considered individually, the locus on chromosome 2 (rs27288988) accounts for 32.6% of the variance in exercise time and the locus on chromosome 11 (rs4135796) accounts for 39.7% of the variance in exercise time. When both SNPs are included in the analysis, the contribution of the peak on chromosome 11 is ∼7.8% (P < 0.001), whereas the variance accounted for by the peak on chromosome 2 is < 1.0% (P = 0.14).
DISCUSSION
In general, efforts to determine the genetic basis for variation in exercise capacity have been limited due to small sample size in human studies (13) or limited genetic diversity in animal studies (2, 22, 25, 42). Advances in sequencing technology and SNP discovery have led to the development of large SNP datasets for inbred mice (10, 44). In turn, these datasets have facilitated the advancement of genome-wide association studies in mice (12, 16, 28). Therefore, the aim of this study was to identify strain-specific differences in intrinsic endurance exercise capacity across 34 strains of inbred mice and to identify significant QTL regulating intrinsic exercise capacity using genome-wide association mapping. Using this large strain set, we found significant variation for intrinsic endurance exercise capacity across strains. This variation allowed for the identification of novel QTL for exercise capacity using association mapping, including one QTL on chromosome 2 that overlaps with a suggestive QTL for the response to exercise training (change in V̇O2max) in a human linkage study. The majority of the peak SNPs for these QTL are located within genes, suggesting that these genes are prospective candidates for influencing variation in intrinsic endurance exercise capacity. Although these QTL require confirmation by traditional approaches, they provide potential new targets for identifying the underlying genetic basis for variation in endurance exercise capacity.
In the current study, phenotype data from 34 classical and wild-derived inbred mouse strains were used to conduct a genome-wide association study for intrinsic endurance exercise capacity. This large phenotype dataset represents a three- to fourfold expansion of exercise capacity measurements over previous reports. Using male mice from seven inbred strains, Lerman et al. (19) reported a 1.7-fold difference in maximal running speed across strains. Lightfoot et al. (21) showed a similar degree of variation in running distance across 10 inbred strains. In the present study, we used 34 strains and included mice from all seven strain groupings from the mouse family tree (27), indicating a wide range of genetic diversity. Many of these strains were chosen based on their priority for inclusion in the Mouse Phenome Database (http://phenome.jax.org/) or their role as a founder strain for the Collaborative Cross recombinant inbred line panel (129S1/SvImJ, A/J, C57BL/6J, CAST/EiJ, NOD/LtJ, PWK/PhJ, WSB/EiJ) (http://compgen.unc.edu). There was a 2.5- to 3-fold difference between the highest and lowest performing strains with respect to time. This range is comparable to previous reports, despite differences in testing protocols and equipment. Only two strains, C57BL/6J and DBA/2J, were common among all three studies with the C57BL/6J strain ranking at or near the bottom in all three studies. High-performing strains FVB/NJ (19) and SWR/J (21) also performed well in our study, suggesting that these strains might be useful for future studies contrasting high (FVB, SWR) and low (C57BL/6J) performing strains.
Interestingly, despite being a low-performing strain in treadmill-based exercise tests (19, 21, 24, 25), C57BL/6J mice are generally considered a high-performing strain for wheel running (19, 20). The lack of correlation between treadmill running performance and voluntary wheel running performance in C57BL/6J and other strains (19) suggests that these models assess different phenotypes related to exercise. Some contributing factors could be differences in protocols for assessing exercise capacity and the exercise intensity. For example, treadmill running as described here is a measure of acute exercise capacity, whereas wheel running is generally measured over >1 day (20). In addition, motivation to run likely has a strong influence on wheel running performance, while workload is controlled in treadmill running (1). Thus, differences in methodology, physiological responses, and contrasting performance within some inbred strains suggest that wheel running and treadmill running represent different phenotypes related to exercise performance.
The large number and genetic diversity of the strains utilized in the current study were amenable to the performance of a genome-wide association study to identify putative QTL for intrinsic endurance exercise capacity. In the current study eight significant or suggestive associations for intrinsic endurance exercise capacity were identified on five different chromosomes (Tables 2 and 3). None of the QTL from the current study overlap with previously identified QTL for intrinsic exercise capacity in mice or rats. Using traditional linkage analysis, Lightfoot et al. (22) identified significant QTL for running distance on chromosomes 8 and X. Significant QTL for pretraining work were identified by us on chromosomes 14 and 19 and suggestive QTL on chromosomes 1 (∼189 Mb), 2 (∼80 Mb), 3, and 8 in F2 mice derived from FVB and B6 strains (25). Ways et al. (42) reported several significant QTL for aerobic running capacity in rats that map to regions of the rat genome that are syntenic with regions on mouse chromosomes 3 and 8. The lack of overlap across studies may be due to differences in the genetic diversity represented in the linkage and association studies. Traditional linkage studies in rodents are dependent on the genetic variation present in the two parental lines, whereas association mapping studies incorporate the genomes of a large number of inbred strains, allowing for greater diversity and enhanced resolution (3, 16). Furthermore, the current study utilized only male mice, whereas previous work included mice from both sexes. Sex differences in exercise performance in rodents have been reported (2, 20, 22) as have sex-specific QTL for various traits, including exercise capacity and physical activity (3, 20, 22, 42). In fact, Lightfoot et al. (20) reported that 67% of physical activity QTL identified in a genome-wide association study were sex-specific. Based on those results, separate studies of exercise capacity for each sex might be warranted.
Two significant associations for time (Chr. 2 and Chr. 11) were present in the 34-strain cohort and the 30 classical inbred strains using the 132 K panel and corroborated using the 4 M SNP panel. The putative QTL interval on chromosome 2 is relatively small (180 Kb) and was further narrowed to ∼27 Kb using the 4 M SNP panel. This interval contains only one gene, nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 2 (Nfatc2). Nfatc2 is a transcription factor belonging to the Nfat family (29). Nfat proteins are regulated by calcineurin, a calcium-calmodulin-dependent phosphatase (29). Nfatc2 is required for calcineurin-mediated cardiac hypertrophy (5) and also contributes to signaling pathways regulating angiogenesis (14) and skeletal muscle growth (15). Furthermore, the marker D20S857 maps to human chromosome 20 (position: 50.1 Mb, q13.2), which is syntenic with the putative QTL on mouse chromosome 2. This marker was linked to the response to exercise training (change in V̇O2max) in African Americans in the HERITAGE Family Study (31). On the basis of the potential links to endurance exercise in humans and mice, this genomic region is of interest and should be considered for more detailed analyses of genetic variants contributing to differences in intrinsic endurance exercise capacity.
The other QTL (Chr. 11, ∼21 Mb) associated with exercise time was identified in all analyses. This interval was relatively large (∼900 Kb) and contains nine known genes with the peak SNPs falling within AV249152 (also identified as Wdpcp) and Ehbp1. No genes within this region have been directly linked to exercise performance. However, several other significant QTL also were identified on chromosome 11, and these regions contain genes with potential links to exercise performance (i.e., Eno3, Zfp3) (8, 18). In addition, a previously reported suggestive QTL for the change in work in response to 4 wk of exercise training in an F2 population derived from FVB and B6 mice was identified on chromosome 11 (72.6 Mb) (25). Although no direct link to exercise capacity was identified on chromosome 11, the collective evidence suggests that this chromosome should be considered for further investigation.
Several of the high-performing strains in this study are classified as wild-derived inbred strains. These strains were captured in the wild and subsequently inbred for 20 or more generations. Inclusion of these wild-derived inbred strains expands the range of strains measured for endurance exercise capacity and provides phenotype information on mouse strains that are extremely different genetically from classical inbred strains (44). Mice from the four wild-derived inbred strains had exercise times that exceeded 38 min with a very narrow range of times (38.5–42.2 min). In general this is in agreement previous reports of exercise capacity in wild mice (11, 40). Therefore, inclusion of wild-derived strains can increase both genetic and phenotypic diversity to strain screens for exercise-related phenotypes. However, including these strains in association mapping studies is somewhat controversial because wild-derived strains are so genetically dissimilar to classical inbred strains (44). In the present study, performing association mapping with and without inclusion of wild-derived strains markedly changed the number of significant associations (Tables 2 and 3). Lightfoot et al. (20) also observed an effect of the wild-derived strains on association mapping for wheel running traits. Twelve QTL were identified for wheel running phenotypes in male and female mice from 38 inbred and wild-derived strains. This number was notably reduced after removing the wild-derived strains. Although the use of a large number of inbred strains and a high-density SNP map is recommended for genome-wide association mapping (41), the effect of wild-derived strains on association mapping observed by Lightfoot and by us suggests that it would be prudent to conduct analyses with and without these strains to identify significant associations and the effect of population structure on the genetic architecture of complex traits.
QTL were identified for exercise capacity by using EMMA to perform association mapping. One criticism of genome-wide association mapping approaches including single SNP marker and haplotype mapping, which relies on a three-SNP window to define haplotype blocks, is that it can be affected by population structure, sparse SNP data, regions of low polymorphisms, or high haplotype diversity, potentially resulting in a large number of false positives (41). Although EMMA also has limitations (45), it was selected for association mapping because it incorporates a kinship matrix that corrects for relatedness among inbred strains (16). This should correct for differences in population structure due to the interrelatedness of inbred strains, which has been shown to reduce the number of false positive associations compared with other mapping approaches (16). Although EMMA has been used successfully to identify significant associations for a variety of physiological and disease-related traits (3, 9, 16), traditional linkage analysis needs to be performed to confirm results from mouse genome-wide association studies (23, 37).
In summary, we performed a large strain survey for exercise capacity using a graded treadmill test. This study demonstrated that a large strain survey and association mapping could be used to identify new QTL for exercise capacity. As with most genome-wide association studies, replication studies are needed to confirm our findings. One option is to use the strain survey data presented here, coupled with large SNP databases to identify the most appropriate strains for traditional linkage analysis to confirm these QTL. In addition, the narrow QTL regions identified here contain a small number of potential candidate genes that can be systematically studied to confirm their role in modulating exercise capacity. Future studies confirming these QTL and identifying the physiological relationship between candidate gene and exercise capacity are required to establish this gene or genetic variant as being the underlying cause of differences in exercise capacity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-085918 (M. P. Massett).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M.C. and M.P.M. conception and design of research; S.M.C. performed experiments; S.M.C. and M.P.M. analyzed data; S.M.C. and M.P.M. interpreted results of experiments; S.M.C. and M.P.M. prepared figures; S.M.C. and M.P.M. drafted manuscript; S.M.C. and M.P.M. edited and revised manuscript; S.M.C. and M.P.M. approved final version of manuscript.
REFERENCES
- 1.American Physiological Society Resource Book for the Design of Animal Exercise Protocols. Bethesda, MD: American Physiological Society, 2006 [Google Scholar]
- 2.Barbato JC, Koch LG, Darvish A, Cicila GT, Metting PJ, Britton SL. Spectrum of aerobic endurance running performance in eleven inbred strains of rats. J Appl Physiol 85: 530–536, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Berndt A, Cario CL, Silva KA, Kennedy VE, Harrison DE, Paigen B, Sundberg JP. Identification of fat4 and tsc22d1 as novel candidate genes for spontaneous pulmonary adenomas. Cancer Res 71: 5779–5791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard C, Rankinen T, Chagnon YC, Rice T, Perusse L, Gagnon J, Borecki I, An P, Leon AS, Skinner JS, Wilmore JH, Province M, Rao DC. Genomic scan for maximal oxygen uptake and its response to training in the HERITAGE Family Study. J Appl Physiol 88: 551–559, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, Wehrens XH, De Windt LJ. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem 283: 22295–22303, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bray MS, Hagberg JM, Perusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C. The human gene map for performance and health-related fitness phenotypes: the 2006–2007 update. Med Sci Sports Exerc 41: 35–73, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cheverud JM, Vaughn TT, Pletscher LS, Peripato AC, Adams ES, Erikson CF, King-Ellison KJ. Genetic architecture of adiposity in the cross of LG/J and SM/J inbred mice. Mamm Genome 12: 3–12, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Comi GP, Fortunato F, Lucchiari S, Bordoni A, Prelle A, Jann S, Keller A, Ciscato P, Galbiati S, Chiveri L, Torrente Y, Scarlato G, Bresolin N. Beta-enolase deficiency, a new metabolic myopathy of distal glycolysis. Ann Neurol 50: 202–207, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Farber CR, Bennett BJ, Orozco L, Zou W, Lira A, Kostem E, Kang HM, Furlotte N, Berberyan A, Ghazalpour A, Suwanwela J, Drake TA, Eskin E, Wang QT, Teitelbaum SL, Lusis AJ. Mouse genome-wide association and systems genetics identify asxl2 as a regulator of bone mineral density and osteoclastogenesis. PLoS Genet 7: e1002038, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448: 1050–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Garland T, Jr, Gleeson TT, Aronovitz BA, Richardson CS, Dohm MR. Maximal sprint speeds and muscle fiber composition of wild and laboratory house mice. Physiol Behav 58: 869–876, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, Ahluwalia MK, Higuchi R, Peltz G. In silico mapping of complex disease-related traits in mice. Science 292: 1915–1918, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Hagberg JM, Rankinen T, Loos RJ, Perusse L, Roth SM, Wolfarth B, Bouchard C. Advances in exercise, fitness, and performance genomics in 2010. Med Sci Sports Exerc 43: 743–752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med 193: 607–620, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol 153: 329–338, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics 178: 1709–1723, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby A, Kang HM, Wade CM, Cotsapas C, Kostem E, Han B, Furlotte N, Kang EY, Rivas M, Bogue MA, Frazer KA, Johnson FM, Beilharz EJ, Cox DR, Eskin E, Daly MJ. Fine mapping in 94 inbred mouse strains using a high-density haplotype resource. Genetics 185: 1081–1095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamande N, Mazo AM, Lucas M, Montarras D, Pinset C, Gros F, Legault-Demare L, Lazar M. Murine muscle-specific enolase: cDNA cloning, sequence, and developmental expression. Proc Natl Acad Sci USA 86: 4445–4449, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol 92: 2245–2255, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lightfoot JT, Leamy L, Pomp D, Turner MJ, Fodor AA, Knab A, Bowen RS, Ferguson D, Moore-Harrison T, Hamilton A. Strain screen and haplotype association mapping of wheel running in inbred mouse strains. J Appl Physiol 109: 623–634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lightfoot JT, Turner MJ, Debate KA, Kleeberger SR. Interstrain variation in murine aerobic capacity. Med Sci Sports Exerc 33: 2053–2057, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Lightfoot JT, Turner MJ, Knab AK, Jedlicka AE, Oshimura T, Marzec J, Gladwell W, Leamy LJ, Kleeberger SR. Quantitative trait loci associated with maximal exercise endurance in mice. J Appl Physiol 103: 105–110, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Manenti G, Galvan A, Pettinicchio A, Trincucci G, Spada E, Zolin A, Milani S, Gonzalez-Neira A, Dragani TA. Mouse genome-wide association mapping needs linkage analysis to avoid false-positive Loci. PLoS Genet 5: e1000331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol 288: R1006–R1013, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Massett MP, Fan R, Berk BC. Quantitative trait loci for exercise training responses in FVB/NJ and C57BL/6J mice. Physiol Genomics 40: 15–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res 14: 1806–1811, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pletcher MT, McClurg P, Batalov S, Su AI, Barnes SW, Lagler E, Korstanje R, Wang X, Nusskern D, Bogue MA, Mural RJ, Paigen B, Wiltshire T. Use of a dense single nucleotide polymorphism map for in silico mapping in the mouse. PLoS Biol 2: e393, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15: 707–747, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Reed DR, Li X, McDaniel AH, Lu K, Li S, Tordoff MG, Price RA, Bachmanov AA. Loci on chromosomes 2, 4, 9, and 16 for body weight, body length, and adiposity identified in a genome scan of an F2 intercross between the 129P3/J and C57BL/6ByJ mouse strains. Mamm Genome 14: 302–313, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rico-Sanz J, Rankinen T, Rice T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Quantitative trait loci for maximal exercise capacity phenotypes and their responses to training in the HERITAGE Family Study. Physiol Genomics 16: 256–260, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Rikke BA, Battaglia ME, Allison DB, Johnson TE. Murine weight loss exhibits significant genetic variation during dietary restriction. Physiol Genomics 27: 122–130, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Rocha JL, Eisen EJ, Van Vleck LD, Pomp D. A large-sample QTL study in mice: I. Growth. Mamm Genome 15: 83–99, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–9445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development 131: 2205–2218, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stylianou IM, Korstanje R, Li R, Sheehan S, Paigen B, Churchill GA. Quantitative trait locus analysis for obesity reveals multiple networks of interacting loci. Mamm Genome 17: 22–36, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Su WL, Sieberts SK, Kleinhanz RR, Lux K, Millstein J, Molony C, Schadt EE. Assessing the prospects of genome-wide association studies performed in inbred mice. Mamm Genome 21: 143–152, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Su Z, Li Y, James JC, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Genetic linkage of hyperglycemia, body weight and serum amyloid-P in an intercross between C57BL/6 and C3H apolipoprotein E-deficient mice. Hum Mol Genet 15: 1650–1658, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Suto J. Quantitative trait locus analysis of plasma cholesterol levels and body weight by controlling the effects of the Apoa2 allele in mice. J Vet Med Sci 69: 385–392, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Totsuka Y, Nagao Y, Horii T, Yonekawa H, Imai H, Hatta H, Izaike Y, Tokunaga T, Atomi Y. Physical performance and soleus muscle fiber composition in wild-derived and laboratory inbred mouse strains. J Appl Physiol 95: 720–727, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Tsaih SW, Korstanje R. Haplotype association mapping in mice. Methods Mol Biol 573: 213–222, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Ways JA, Cicila GT, Garrett MR, Koch LG. A genome scan for Loci associated with aerobic running capacity in rats. Genomics 80: 13–20, 2002 [DOI] [PubMed] [Google Scholar]
- 43.West DB, Goudey-Lefevre J, York B, Truett GE. Dietary obesity linked to genetic loci on chromosomes 9 and 15 in a polygenic mouse model. J Clin Invest 94: 1410–1416, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H, Wang JR, Didion JP, Buus RJ, Bell TA, Welsh CE, Bonhomme F, Yu AH, Nachman MW, Pialek J, Tucker P, Boursot P, McMillan L, Churchill GA, de Villena FP. Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet 43: 648–655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng M, Dill D, Peltz G. A better prognosis for genetic association studies in mice. Trends Genet 28: 62–69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]