Abstract
The incidence of myocardial infarction rises sharply at menopause, implicating a potential role for estrogen (E2) loss in age-related increases in ischemic injury. We aimed to identify quantitative changes to the cardiac mitochondrial proteome of aging females, based on the hypothesis that E2 deficiency exacerbates age-dependent disruptions in mitochondrial proteins. Mitochondria isolated from left ventricles of adult (6 mo) and aged (24 mo) F344 ovary-intact or ovariectomized (OVX) rats were labeled with 8plex isobaric tags for relative and absolute quantification (iTRAQ; n = 5–6/group). Groups studied were adult, adult OVX, aged, and aged OVX. In vivo coronary artery ligation and in vitro mitochondrial respiration studies were also performed in a subset of rats. We identified 965 proteins across groups and significant directional changes in 67 proteins of aged and/or aged OVX; 32 proteins were unique to aged OVX. Notably, only six proteins were similarly altered in adult OVX (voltage-dependent ion channel 1, adenine nucleotide translocator 1, cytochrome c oxidase subunits VIIc and VIc, catalase, and myosin binding protein C). Proteins affected by aging were primarily related to cellular metabolism, oxidative stress, and cell death. The largest change occurred in monoamine oxidase-A (MAO-A), a source of oxidative stress. While acute MAO-A inhibition induced mild uncoupling in aged mitochondria, reductions in infarct size were not observed. Age-dependent alterations in mitochondrial signaling indicate a highly selective myocardial response to E2 deficiency. The combined proteomic and functional approaches described here offer possibility of new protein targets for experimentation and therapeutic intervention in the aged female population.
Keywords: monoamine oxidase-A, respiration, ischemia, iTRAQ, cardioprotection
aging and estrogen (E2) deficiency are each associated with reduced ischemic tolerance in the female heart (80). However, the failure of exogenous hormone replacement therapy to reduce cardiovascular risk in older postmenopausal women (27, 50, 54) has resulted in a renewed search for therapeutic targets and strategies for the treatment of ischemic heart disease in the aged female population. The specific mechanistic disruptions in cardiac mitochondrial signaling that produce enhanced susceptibility to ischemic injury in the aged female heart, however, remain poorly understood. While data from our laboratory and others (29, 30) have correlated age- and E2-dependent declines in ischemic tolerance with altered expression and localization of cardioprotective signaling proteins, such as extracellular signal-regulated kinase 1/2 (ERK1/2), Akt, protein kinase Cε, and glycogen synthase kinase-3β, the breadth and extent of protein changes involved in this process are not known. Due to the pivotal role of mitochondria in the maintenance of cell survival (35, 36, 53, 83), we have focused our study on this subproteome. Importantly, quantification of the mitochondrial subproteome as it adapts to advancing age and E2 deficiency will allow us to uncover proteins associated with disruptions in cardiac signaling contributing to age-associated declines in ischemic tolerance.
Traditional discovery-based proteomic approaches using two-dimensional gel electrophoresis and isotope coded affinity tags have identified mitochondrial proteins that contribute to physiological and pathological cardiac phenotypes in adult animals (3, 5, 17, 59, 74). Another emerging proteomics approach includes isobaric tags for relative and absolute quantification (iTRAQ), where peptide labeling is achieved via the formation of an amide bond between the peptide reactive group of the iTRAQ tag and amine groups of target peptides (namely the NH2 terminus and lysine side chains). The newest version of iTRAQ allows eight different samples to be analyzed simultaneously and provides quantitative ratios between individual samples of experimental groups so that proteins contributing to reductions in ischemic tolerance involved with aging and/or E2 deficiency can be readily identified.
It is important to note that previous application of discovery-based approaches have been limited to hearts isolated from aged males (15, 16, 23, 39) or adult E2-deficient females (12, 26, 64). Here, we describe for the first time, a targeted mitochondrial application of the iTRAQ 8plex proteomics approach to characterize alterations in mitochondrial signals occurring with age-associated E2 deficiency in the female rat myocardium. Possible targets for therapeutic intervention in the aged female population are described, and of particular interest were adaptive changes in proteins associated with metabolism, oxidative stress, and cell death. Subsequently, functional studies were performed on isolated mitochondria and hearts in vivo following pharmacological manipulation of the iTRAQ-identified protein, monoamine oxidase A (MAO-A).
METHODS
Animal care.
Adult (6 mo) and aged (23–24 mo) female Fisher 344 (F344) rats were supplied by Harlan Sprague-Dawley (Indianapolis, IN) and Taconic (Hudson, NY). Rats were housed under a 12:12 h light-dark cycle and received food and water ad libitum. All animal handling and utilization protocols were reviewed and approved by the Penn State University Institutional Animal Care and Use Committee and this investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). While circulating E2 is significantly reduced in the ovary-intact aged rat (28, 55), bilateral ovariectomy (OVX) surgeries were performed on a subset of aged (performed at 22 mo) rats according to standard procedures to more appropriately mimic the point at which cardiovascular morbidity and mortality is likely to become manifest in aged women (61). For proteomic and western blot studies only, an adult OVX group (performed at 5 mo) was utilized to garner important mechanistic insights regarding the effects of aging distinct from E2 deficiency on key signaling proteins. Animals were allowed to recover for 4–6 wk prior to the date of experimental study, and successful surgery was confirmed at the time of death by measuring uterine weight and circulating E2 levels by radioimmunoassay. Ovary-intact animals were used as age-matched controls.
Heart preparation.
Animals were anesthetized by injection of pentobarbital sodium (40 mg/kg body wt ip), and hearts were rapidly excised by midline thoracotomy and immediately rinsed in ice cold (4°C) saline. Within 60 s of excision, hearts were secured via aortic cannulation to a Langendorff apparatus and perfused at constant pressure (85 mmHg), temperature (37°C), and pH (7.4) with a modified Krebs-Henseleit bicarbonate buffer as previously described (30, 43, 55) for an equilibration period of 30 min to assure washout of blood proteins. Hearts were paced at 260 beats/min and a fluid-filled latex balloon inserted into the left ventricle (LV) was inflated to yield an end diastolic pressure of 5–6 mmHg (Gould Instrument Systems, Valley View, OH). The LV was isolated, weighed, halved, and snap-frozen in liquid N2. All isolated LV sections were stored at −80°C until tissue preparation.
Tissue sample preparation.
For proteomics studies, isolated mitochondrial protein homogenates were prepared exactly as described previously (29), except that Tris·HCl in the homogenization buffers were replaced with equivalent concentrations of HEPES (pH 7.4). This substitution was necessary to provide a primary amine-free buffer for subsequent iTRAQ labeling. All protein concentrations were determined by the method of Bradford (11).
iTRAQ sample preparation.
LV mitochondrial protein homogenates were prepared for proteomics analysis using the iTRAQ 8plex reagent kit (Applied Biosystems). Sample preparation protocols were optimized in a series of qualitative MudPIT (multidimensional protein identification technology) proteomics experiments. Specifically, comparisons were conducted in LV mitochondrial homogenates (n = 5 separate experiments on n = 5 different adult hearts) to optimize the iTRAQ sample preparation protocol. Results are summarized in Table 1. The T5 sample, in which ∼550 nonredundant proteins were identified at instantaneous false discovery rate (FDR) < 5.0%, represents the protocol that was decided upon for use in subsequent iTRAQ quantitative proteomics experiments for the following reasons: 1) the substitution of Tris·HCl with HEPES in the protein isolation buffer, which is required for compatibility with the sample buffer for iTRAQ labeling, did not affect the number of protein IDs obtained (T2 vs. T1 samples); 2) acetone precipitation dramatically reduced the number of protein IDs (T3 sample), thus sample clean-up by acetone precipitation was unnecessary; and 3) a protein-trypsin ratio (by mass) of 10:1, which is recommended in iTRAQ product literature, resulted in efficient sample digestion and protein IDs (T5 sample). Given the estimate that cardiac mitochondria contain ∼1,000–2,000 proteins (48, 68), this work covers 25–50% of the mitochondrial proteome with defensible confidence rates. Minimal cytosolic contamination of the isolated mitochondrial fraction has been previously demonstrated by us for the protein homogenization techniques employed here (6, 29).
Table 1.
Summary of qualitative MudPIT experiments conducted in LV mitochondrial protein homogenates for the optimization of iTRAQ sample preparation protocols
| Sample | Source | Protein Mass | Protein Isolation Protocol | Acetone Precipitation Performed | Trypsin Mass (protein ratio) | # Protein IDs |
|---|---|---|---|---|---|---|
| T1 | Adult | 600 μg | standard homog. buffer w/20 mM Tris-HCl | no | 10 μg (60:1) | 512 |
| T2 | Adult | 600 μg | modified homog. buffer subs/20 mM HEPES | no | 10 μg (60:1) | 555 |
| T3 | Adult | 600 μg | modified homog. buffer subs/20 mM HEPES | yes | 10 μg (60:1) | 296 |
| T4 | Adult | 600 μg | modified homog. buffer subs/20 mM HEPES | no | 60 μg (10:1) | 443 |
| T5 | Adult | 600 μg | modified homog. buffer subs/20 mM HEPES | no | 60 μg (10:1) | 541 |
MudPIT, multidimensional protein identification technology; LV, left ventricle; iTRAQ, isobaric tags for relative and absolute quantification. See text for additional details.
Individual iTRAQ 8plex analyses were conducted according to established procedures in our laboratory (45). Individual mitochondrial samples isolated from each experimental group was randomly assigned to each 8plex run [adult (n = 5), adult OVX (n = 5), aged ovary-intact (n = 6), and aged OVX (n = 5)]. Briefly, 100 μg of protein from each of eight mitochondrial samples (isolated from eight individual LV) was individually aliquoted, concentrated, and resuspended in 20 μl triethylammonium bicarbonate buffer, followed by parallel denaturing in 0.1% SDS, reduction in 5 mM Tris-(2-carboxyethyl) phosphine, and alkylation in 3.5 mM iodoacetamide. Trifluoroethanol was added to 5% concentration by volume, and samples were digested in 10 μg sequencing grade modified trypsin (Promega) overnight at 48°C. Each of the eight iTRAQ reagents (113–119 and 121 m/z) were reconstituted in 50 μl isopropanol, and each mitochondrial sample was labeled with a unique reagent by incubation for 2 h. at room temperature. The labeled samples were combined and washed 3× in Nano-Pure ddH2O and were resuspended in 500 μl strong cation exchange (SCX) loading buffer (12 mM ammonium formate in 25% acetonitrile, pH 2.5–3.0). Two-dimensional liquid chromatography (LC) separation and tandem mass spectrometry (MS/MS) analysis of the prepared samples were conducted at the Penn State Hershey Proteomics Core facility, specifically utilizing SCX LC separation, 15X nanoflow C18 reversed-phase LC separation, matrix-assisted laser desorption/ionization plate spotting, and tandem time-of-flight mass analysis.
iTRAQ data analysis.
Protein identifications (IDs) and protein quantitation were determined from MS/MS spectra using the Paragon algorithm (66) of the ProteinPilot v3.0 software package (ABI-MDSSciex), searching the NCBInr (National Center for Biotechnology Information) protein database concatenated to a reversed version of itself (as a Decoy database). Only those proteins identified at an instantaneous (local) FDR < 5%, which was determined by analyzing the number of Decoy (reversed) database hits using the Proteomics System Performance Evaluation Pipeline beta software tool (ABI-MDSSciex) and had a ProteinPilot confidence score of 95% or better (unused score > 1.3, see below) were considered positive identifications (22, 82). Additionally, PANTHER (Protein Analysis Through Evolutionary Relationships) analysis (70) was conducted to classify identified proteins by linking their UniProt IDs to specific biological processes (using the Batch ID Search function of the PANTHER web application; http://www.pantherdb.org/batchIdSearch.jsp).
Western blotting.
Confirmatory Western blotting was performed according to well-established procedures in our laboratory (42). Briefly, equal amounts of mitochondrial protein sample were electrophoresed on SDS-polyacrylamide gels (Bio-Rad). Membranes were probed with primary antibody against MAO-A (1:900, Santa Cruz Biotechnology; overnight at 4°C). Immunoreactive bands were visualized by enhanced chemiluminescence (GE Amersham) and minor differences in protein loading were corrected for by SYPRO Ruby staining (Invitrogen) as described previously (58). Densitometry analysis was performed using Scion Image (NIH). All Western blot analyses were expressed relative to the adult control group.
Mitochondrial isolation and respiration.
Rats (n = 3–4/group) were anesthetized with 50 mg/kg pentobarbital, and hearts were excised by midline thoracotomy and secured to a Langendorff apparatus. Following a brief (3 min) perfusion with a modified Krebs-Henseleit buffer as described above, hearts were removed from the apparatus, and ventricles were isolated and homogenized in 3 ml of STE buffer [300 mM sucrose, 10 mM Tris (pH 7.4), 2 mM EGTA (pH 7.4)]. Homogenates were diluted in STE buffer supplemented with 0.5% BSA and centrifuged at 2,000 g. The resultant supernatant was centrifuged at 10,000 g, and the mitochondrial pellet was washed twice prior to resuspension in 300 μl STE buffer. Protein concentration was determined by the method of Bradford and did not differ between groups.
Respiration of isolated mitochondria was measured at 30°C using a Clarke-type electrode attached to a YSI oxygraph (Yellow Springs, OH) as described previously (31). Briefly, mitochondria were incubated in a buffer containing (in mM): 125 KCl, 20 MOPS, 10 Tris, 2 MgCl2, 2 KH2PO4, 0.5 EGTA, pH 7.2. Complex I respiration was measured in the presence of 2.5 mM α-ketoglutarate + 1 mM malate, while Complex II respiration was measured in the presence of 1 μM rotenone + 2.5 mM succinate. The MAO-A inhibitor clorgyline (5 μM; Sigma-Aldrich, St. Louis, MO) was added during state 2 respiration 2 min prior to initiating state 3 respiration with 1 mM ADP.
Coronary artery ligation and MAO-A inhibition.
Coronary artery ligation (CAL) was used to generate ischemia and reperfusion (I/R) injury (35 or 47 min I, 2 h R) as previously described (8), with and without the MAO-A inhibitor clorgyline (5 mg/kg in aged or aged OVX or 10 mg/kg in aged, ip) administered 60 min prior to CAL. Briefly, aged rats were anesthetized with pentobarbital [50 mg/kg body weight (BW), ip]. In the left lateral third intercostal space, a 1.5 cm incision was made into the chest cavity to expose the heart and the pericardium was opened. A snare was used to ligate the left coronary artery 3 mm from its origin. Both ends of a 6.0 prolene suture were fed through PE-90 tubing and secured with a hemostat. Following ligation, the hemostat was removed, releasing the ligature, and the chest cavity was sutured. Ventilation was maintained until the rats were able to breathe on their own and allowed to recover on a heating pad. Additional experiments were conducted in which 5 mg/kg clorgyline was administered (ip) 30 min prior to 35 min CAL, followed by 2 h reperfusion.
Triphenyltetrazolium chloride staining.
To assess infarct size following I/R, hearts were isolated and stained with triphenyltetrazolium chloride (TTC) as previously reported (56). Prior to euthanasia, rats were reanesthetized with pentobarbital, and hearts were removed by midline thoracotomy and perfused with a modified Krebs-Henseleit buffer for 60–90 s at 85 mmHg and 37°C. The coronary artery was religated and the not-at-risk myocardium was then perfused with 200–300 μl Evans blue (1% in saline). Hearts were removed, and LV isolated, weighed, and frozen at −20°C for 30 min. Heart slices were stained with 1% TTC in phosphate buffer (pH 7.4) for 10 min at 37°C and fixed in 10% formalin overnight at room temperature. Images of individual heart slices were obtained with an Olympus DP72 camera attached to an Olympus Stereomicroscope SZ61 (Olympus, Center Valley, PA). Infarct size was determined by ImageJ software (NIH; http://rsb.info.nih.gov/ij/index.html). Data are expressed as infarct relative to area at risk (AAR).
Statistical analyses.
Statistical analysis of iTRAQ quantitative protein data was automated by the Paragon algorithm of the ProteinPilot v3.0 software package and is based on the contributing peptide ratios, P values, and error factors. The error factor is used to provide an indication of the variance when presenting ratio data. The true ratio for each protein comparison is expected to be found between (reported ratio * error factor) and (reported ratio ÷ error factor). The unused and total protein scores reported for proteins of interest are reflective of a unique strength of ProteinPilot (Paragon algorithm), which is the handling of evidence for multiple protein identifications from a single identified peptide (redundant protein IDs or the “protein grouping problem” of protein identification). The Paragon algorithm applies a more stringent approach to protein identification from MS/MS spectra than most other software tools, in that a single identified peptide may not be used as evidence for more than one protein identification. The total protein score is thus a measurement of all the peptide evidence for a protein and is analogous to protein scores reported by other protein identification software. The unused protein score is unique to ProteinPilot analysis and is a measurement of all the peptide evidence for a protein that is not better explained by a higher ranking protein. The unused protein score is the true indicator of protein confidence (unused score = 1.3 is equivalent to 95% confidence).
Accordingly, quantitative protein ratios were expressed relative to a pooled adult control sample (made by pooling equal amounts of protein sample from three adult ovary-intact rat hearts also analyzed independently), which were included in each of the six iTRAQ 8plex analyses. The mean quantitation ratio across all identified proteins for each of these three individual control samples was not different from 1.0 (0.995, 0.984, and 0.984), indicating very low variability between samples that comprised the pooled control. Subsequent iTRAQ runs were comparative and consisted of the pooled adult control sample and samples from each experimental group.
For Western blotting, morphological, respiration, and infarct size results, all data are presented as means ± standard error and were analyzed using the Statistical Analysis System general linear models procedure. Group comparisons were analyzed by ANOVA. All post hoc comparisons were analyzed by the Tukey-Kramer or Duncan method. An α-level of P < 0.05 was defined as statistically significant for all comparisons.
RESULTS
Baseline morphology and function.
Baseline morphology for adult and aged, ovary-intact and OVX rats are presented in Table 2. Rat BW and LV weight were significantly increased by both age and OVX (P < 0.0001). Circulating E2 levels were significantly reduced by aging and further reduced by OVX (P < 0.01). No significant differences were observed in LV weight/BW ratio.
Table 2.
Morphology and characteristics of ovary-intact and OVX adult and aged rats
| Adult Intact | Adult OVX | Aged Intact | Aged OVX | |
|---|---|---|---|---|
| Rat wt, g | 183 ± 3 | 218 ± 4† | 260 ± 7* | 283 ± 10*† |
| LV wt, mg | 0.50 ± 0.02 | 0.61 ± 0.02† | 0.81 ± 0.02* | 0.81 ± 0.02*† |
| LV/Rat wt, mg/g | 2.73 ± 0.01 | 2.80 ± 0.01 | 3.12 ± 0.03 | 2.86 ± 0.01 |
| Estradiol, pg/ml | 21.37 ± 1.01 | 5.71 ± 0.38*† | 14.59 ± 1.44* | 7.64 ± 1.08*† |
| n | 5 | 5 | 6 | 5 |
Adult rats, 6 mo;aged rats, 23–24 mo. OVX, ovariectomized.
Different from adult intact,
different from aged intact (P < 0.05).
iTRAQ analysis of LV mitochondrial proteins.
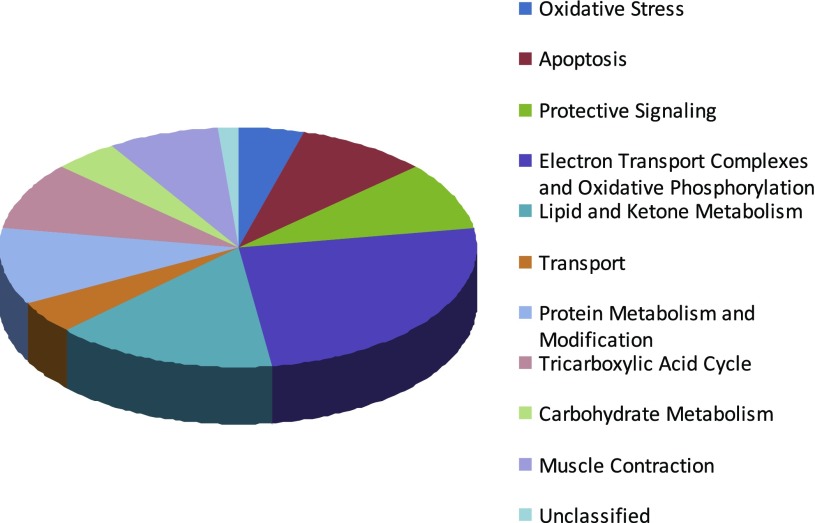
For the quantitative assessment of cardiac mitochondrial protein abundance in aging and E2 deficiency, six iTRAQ 8plex proteomics analyses were conducted (n = 24 samples plus biological replicates). A total of 965 proteins were identified in the iTRAQ analyses of mitochondrial homogenates (see Supplemental Table S1 for all protein IDs and summary of functional groupings).1 Further analysis revealed 425 proteins (44%) were identified in all six iTRAQ analyses and 656 (65%) were identified in at least four analyses, the minimum requirement to be included in the analysis to identify group changes. The associated biological processes determined by PANTHER analysis (70) for those proteins changed with age or aged OVX are shown in Fig. 1.
Fig. 1.
PANTHER (Protein Analysis Through Evolutionary Relationships) biological function representation of 67 nonredundant proteins that differed significantly with age-associated estrogen (E2) deficiency identified by isobaric tags for relative and absolute quantification (iTRAQ) 8plex analyses (instantaneous false discovery rate <5.0% and unused score >1.3). Data based on adult (n = 5), aged ovary-intact (n = 6), and aged ovariectomized (OVX, n = 5) female F344 rats.
Quantitative protein ratios were expressed relative to a pooled adult control sample and proteins of interest were those that differed significantly in the aged ovary-intact, aged OVX and/or adult OVX rat heart relative to the adult ovary-intact rat heart (Tables 3 and 4). A total of 67 proteins demonstrated significant directional changes in aged and aged OVX groups (P < 0.05), with 32 of these proteins being unique to the aged OVX group. Interestingly, only six of these proteins were significantly altered in the adult OVX group [VDAC1 (voltage-dependent ion channel 1), ANT1 (adenine nucleotide translocator, solute carrier family 25, member 4), Cox7c (cytochrome c oxidase, subunit VIIc), catalase, and Cox6c, and myosin binding protein C; Tables 3 and 4]. About 50% (36) of the identified proteins altered in aged OVX relative to adult hearts are involved in mitochondrial ATP production (20 of these being increased and 16 being decreased). Reductions were primarily observed in electron transport chain complexes (Tables 3 and 4). Examples of proteins that increased in abundance included heat shock family proteins (Hsp60, Hsp8, and mtHsp70) and the antioxidant enzyme superoxide dismutase 2 (SOD2, MnSOD), which tended to be involved in cardioprotection. However, the antioxidant enzyme catalase exhibited decreased levels in aged and adult OVX hearts.
Table 3.
Cardiac mitochondrial proteins with increased abundance in aging and E2 deficiency
| Unused Score | Total Score | % Coverage | Accession # | Protein Identified | Quantitation Ratio Aged/Adult | P Value | Error Factor | Quantitation Ratio Adult OVX/Adult | P Value | Error Factor | Quantiation Ratio Aged OVX/Adult | P Value | Error Factor | PANTHER Biological Process |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15.3 | 15.3 | 50.9 | gi 8394331 | superoxide dismutase 2, mitochondrial (SOD2) | 1.22 | 0.0038 | 1.13 | 1.02 | 0.43 | 1.21 | 1.38 | 0.0031 | 1.21 | antioxidation and free radical removal |
| 4.0 | 4.0 | 19.7 | gi 38511566 | 3-hydroxy-3-methylglutaryl-Coenzyme A lyase | 1.07 | 0.6542 | 4.07 | 1.31 | 0.47 | 18.95 | 1.47 | 0.0185 | 1.15 | metabolism: Acyl-CoA metabolism |
| 32.4 | 32.7 | 58.3 | gi 6978431 | acyl-Coenzyme A dehydrogenase, long-chain | 1.20 | 0.0142 | 1.12 | 1.05 | 0.29 | 1.09 | 1.25 | 0.0003 | 1.09 | metabolism: Acyl-CoA metabolism; electron transport |
| 23.7 | 23.7 | 51.3 | gi 8392833 | acyl-Coenzyme A dehydrogenase, medium chain | 1.19 | 0.0084 | 1.12 | 1.06 | 0.34 | 1.10 | 1.19 | 0.0066 | 1.09 | metabolism: Acyl-CoA metabolism; electron transport |
| 20.7 | 20.7 | 51.0 | gi 48734846 | acyl-Coenzyme A dehydrogenase, short chain | 0.83 | 0.0500 | 1.17 | 1.01 | 0.50 | 1.20 | 1.22 | 0.0149 | 1.13 | metabolism: Acyl-CoA metabolism; electron transport |
| 30.5 | 30.5 | 60.2 | gi 6980972 | glutamate oxaloacetate transaminase 2 | 1.10 | 0.0370 | 1.09 | 1.06 | 0.50 | 1.16 | 1.19 | 0.0287 | 1.09 | metabolism: amino acid metabolism |
| 28.3 | 28.3 | 51.0 | gi 81885266 | dihydrolipoyl dehydrogenase, mitochondrial precursor (dihydrolipoamide dehydrogenase) | 1.20 | 0.0146 | 1.12 | 1.07 | 0.31 | 1.13 | 1.30 | 0.0001 | 1.10 | metabolism: electron transport |
| 30.8 | 30.8 | 44.3 | gi 55605 | aldehyde dehydrogenase preprotein | 1.25 | 0.0127 | 1.12 | 1.05 | 0.35 | 1.11 | 1.26 | 0.0020 | 1.13 | metabolism: other metabolism |
| 14.5 | 14.5 | 95.7 | gi 6981260 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 5 | 1.08 | 0.4442 | 1.23 | 1.10 | 0.32 | 1.19 | 1.14 | 0.0094 | 1.10 | metabolism: oxidative phosphorylation |
| 13.3 | 13.3 | 36.5 | gi 157820787 | NADH dehydrogenase (ubiquinone) 1, alpha/beta subcomplex, 1 | 1.11 | 0.0916 | 1.13 | 1.32 | 0.42 | 1.26 | 1.25 | 0.0105 | 1.16 | metabolism: oxidative phosphorylation |
| 14.1 | 14.1 | 45.8 | gi 57164133 | NADH dehydrogenase (ubiquinone) 1, subcomplex unknown, 2 | 1.07 | 0.2319 | 1.12 | 1.13 | 0.24 | 1.16 | 1.15 | 0.0095 | 1.10 | metabolism: oxidative phosphorylation |
| 33.8 | 33.8 | 90.5 | gi 6978725 | cytochrome c, somatic | 1.20 | 0.0066 | 1.14 | 1.20 | 0.32 | 1.17 | 1.12 | 0.0617 | 1.13 | metabolism: oxidative phosphorylation; apoptotic processes |
| 58.2 | 58.2 | 43.5 | gi 81883712 | 2-oxoglutarate dehydrogenase E1 component, mitochondrial precursor (Alpha-ketoglutarate dehydrogenase) | 1.03 | 0.5217 | 1.09 | 1.08 | 0.16 | 1.09 | 1.22 | 0.0004 | 1.09 | metabolism: tricarboxylic acid pathway |
| 78.6 | 78.6 | 70.4 | gi 60391194 | aconitate hydratase, mitochondrial precursor (Citrate hydro-lyase) (Aconitase) | 1.09 | 0.1683 | 1.10 | 0.99 | 0.47 | 1.08 | 1.09 | 0.0146 | 1.07 | metabolism: tricarboxylic acid pathway |
| 31.4 | 31.4 | 48.3 | gi 81902084 | citrate synthase, mitochondrial precursor | 1.05 | 0.0722 | 1.11 | 1.05 | 0.30 | 1.10 | 1.18 | 0.0024 | 1.09 | metabolism: tricarboxylic acid pathway |
| 24.1 | 24.1 | 50.0 | gi 149025179 | dihydrolipoamide S-succinyltransferase (E2 component of 2-oxo-glutarate complex), isoform CRA_a | 1.30 | 0.0108 | 1.19 | 0.99 | 0.65 | 1.26 | 1.42 | 0.0167 | 1.29 | metabolism: tricarboxyli c acid pathway |
| 22.0 | 22.0 | 49.7 | gi 68565369 | isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial precursor (Isocitric dehydrogenase) | 1.06 | 0.17 | 1.16 | 1.00 | 0.57 | 1.14 | 1.15 | 0.0048 | 1.10 | metabolism: tricarboxylic acid pathway |
| 47.7 | 47.8 | 76.8 | gi 42476181 | malate dehydrogenase, mitochondrial | 1.18 | 0.0101 | 1.11 | 1.04 | 0.38 | 1.08 | 1.21 | 0.0025 | 1.08 | metabolism: tricarboxylic acid pathway |
| 27.4 | 27.4 | 85.34 | gi 205495 | cardiac myosin light chain 2 | 1.84 | 0.0300 | 1.24 | 1.05 | 0.20 | 1.09 | 1.40 | 0.0400 | 1.26 | muscle contraction |
| 50.2 | 50.2 | 65.9 | gi 8392836 | acetyl-coenzyme A acetyltransferase 1 | 1.19 | 0.0084 | 1.12 | 1.06 | 0.34 | 1.10 | 1.19 | 0.0066 | 1.09 | protein acetylation |
| 56.5 | 56.6 | 69.8 | gi 56383 | heat shock protein (Hsp60) precursor | 1.12 | 0.0042 | 1.08 | 1.03 | 0.23 | 1.10 | 1.16 | 0.0043 | 1.10 | protein folding; protein complex assembly |
| 3.9 | 4.0 | 20.6 | gi 71051777 | heat shock protein 8 | 1.07 | 0.3258 | 1.24 | 1.30 | 0.12 | 1.54 | 1.34 | 0.0479 | 1.41 | protein folding; protein complex assembly; stress response |
| 2.9 | 2.9 | 7.1 | gi 81884348 | cytosol aminopeptidase (Leucine aminopeptidase) | 1.35 | 0.4766 | 8.74 | 1.18 | 0.15 | 1.68 | 1.42 | 0.0348 | 1.28 | proteolysis |
| 12.5 | 12.5 | 26.3 | gi 50402096 | elongation factor 1-alpha 2 (Statin S1) | 1.36 | 0.0064 | 1.22 | 0.99 | 0.51 | 1.23 | 1.36 | 0.0212 | 1.29 | translational regulation |
| 31.9 | 31.9 | 79.5 | gi 8810245 | voltage-dependent anion channel 1 (VDAC 1) | 1.41 | <0.0001 | 1.13 | 1.21 | 0.02 | 1.16 | 1.29 | 0.0005 | 1.14 | transport: anion transport |
| 7.9 | 8.0 | 8.1 | gi 6981424 | prosaposin | 1.61 | 0.0891 | 1.80 | 0.94 | 0.63 | 1.39 | 1.55 | 0.0357 | 1.48 | transport: lipid and fatty acid transport |
| 54.7 | 54.7 | 74.2 | gi 38014819 | solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 (Slc25a4) | 1.08 | 0.0210 | 1.07 | 1.15 | 0.05 | 1.11 | 1.14 | 0.0024 | 1.09 | transport: nucleoside, nucleotide and nucleic acid transport |
| 18.7 | 18.7 | 39.0 | gi 9798638 | glyceraldehyde-3-phosphate dehydrogenase (Gapdh) | 1,33 | 0.0786 | 1.26 | 1.11 | 0.91 | 1.20 | 1.40 | 0.0179 | 1.30 | unclassified |
| 13.7 | 13.7 | 67.1 | gi 68341999 | low molecular mass ubiquinone-binding protein | 1.21 | 0.0514 | 1.16 | 1.08 | 0.26 | 1.12 | 1.12 | 0.0344 | 1.11 | unclassified |
| 24.7 | 24.7 | 65.0 | gi 83302472 | ES1 protein homolog, mitochondrial precursor | 1.28 | 0.0260 | 1.16 | 1.08 | 0.29 | 1.15 | 1.25 | 0.0127 | 1.18 | no PANTHER hit |
| 14.9 | 14.9 | 61.3 | gi 535069 | muscle LIM protein | 1.59 | 0.0500 | 1.95 | 1.06 | 0.64 | 1.38 | 1.60 | 0.0500 | 1.47 | muscle development |
| 38.6 | 38.6 | 31.6 | gi 57824043 | myosin binding protein C, cardiac | 1.46 | 0.0191 | 1.16 | 1.17 | 0.01 | 1.14 | 1.33 | 0.0368 | 1.12 | muscle development/contraction |
| 30.5 | 30.5 | 30.5 | gi 34933197 | PREDICTED: similar to Amine oxidase [flavin-containing] A (Monoamine oxidase type A) (MAO-A) | 1.91 | 0.0002 | 1.20 | 0.91 | 0.37 | 1.20 | 1.85 | 0.0079 | 1.21 | oxidant stress |
| 17.4 | 17.5 | 34.7 | gi 109484674 | PREDICTED: similar to dihydrolipoamide S-acetyltransferase (E2 component of pyruvate dehydrogenase complex) | 1.21 | 0.0266 | 1.12 | 0.99 | 0.76 | 1.14 | 1.29 | 0.0097 | 1.12 | metabolism: CHO metabolism |
| 18.9 | 18.9 | 46.5 | gi 56090293 | pyruvate dehydrogenase (lipoamide) beta | 0.97 | 0.7241 | 1.17 | 1.01 | 0.64 | 1.18 | 1.21 | 0.0096 | 1.15 | metabolism: CHO metabolism |
| 29.8 | 29.8 | 59.5 | gi 71051030 | pyruvate dehydrogenase E1 alpha 1 | 1.03 | 0.6054 | 1.11 | 0.99 | 0.69 | 1.09 | 1.18 | 0.0005 | 1.09 | metabolism: CHO metabolism |
| 2.9 | 2.9 | 25.4 | gi 149058974 | rCG44669 (cytochrome c oxidase, subunit VIIc; Cox7c) | 1.19 | 0.2121 | 1.35 | 1.42 | 0.05 | 1.30 | 1.26 | 0.0480 | 1.26 | unclassified |
| 29.6 | 29.7 | 56.0 | gi 149016520 | rCG50966 (3-oxoacid-CoA transferase 1 (OXCT1/SCOT) | 1.12 | 0.3615 | 1.27 | 1.08 | 0.46 | 1.23 | 1.33 | <0.0001 | 1.12 | metabolism: ketone metabolism |
| 60.9 | 60.9 | 67.6 | gi 116242506 | stress-70 protein, mitochondrial precursor (75 kDa glucose-regulated protein) (Heat shock 70 kDa protein 9) | 1.07 | 0.1432 | 1.12 | 1.02 | 0.39 | 1.10 | 1.13 | 0.0300 | 1.09 | protein folding; protein complex ass embly |
| 14.9 | 14.9 | 38.5 | gi 157820845 | Tu translation elongation factor, mitochondrial | 1.11 | 0.1522 | 1.16 | 0.96 | 0.40 | 1.20 | 1.29 | 0.0010 | 1.14 | no PANTHER hit |
| 32.4 | 32.4 | 55.1 | gi 55741544 | ubiquinol cytochrome c reductase core protein 2 | 1.07 | 0.2253 | 1.11 | 1.07 | 0.27 | 1.12 | 1.14 | 0.0108 | 1.10 | no PANTHER hit |
Proteins of interest were identified from 6 iTRAQ 8plex analyses and are grouped according to associated biological processes. Boldface indicates that the difference in expression was significant relative to adult ovary-intact control.
Table 4.
Cardiac mitochondrial proteins with decreased abundance in aging and E2 deficiency
| Unused Score | Total Score | % Coverage | Accession # | Protein Identified | Quantitation Ratio Aged/Adult | P Value | Error Factor | Quantitation Ratio Adult OVX/Adult | P Value | Error Factor | Quantitation Ratio Aged OVX/Adult | P Value | Error Factor | PANTHER Biological Process |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17.9 | 17.9 | 42.9 | gi 6978607 | catalase | 0.83 | 0.0458 | 1.20 | 0.82 | 0.02 | 1.11 | 0.84 | 0.0748 | 1.17 | antioxidation and free radical removal |
| 40.0 | 40.0 | 66.9 | gi 56541110 | acyl-Coenzyme A dehydrogenase, very long chain | 1.02 | 0.8014 | 1.17 | 1.03 | 0.64 | 1.14 | 0.89 | 0.0237 | 1.11 | metabolism: Acyl-CoA metabolism; electron transport |
| 69.1 | 69.1 | 63.3 | gi 60688124 | hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein), alpha subunit | 0.83 | 0.0003 | 1.10 | 1.06 | 0.24 | 1.10 | 0.91 | 0.0235 | 1.09 | metaboli sm: carbohydrate metabolism; beta-oxidation |
| 38.3 | 38.3 | 66.3 | gi 52782765 | succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial precursor (Fp) | 0.96 | 0.3486 | 1.10 | 1.02 | 0.67 | 1.10 | 0.91 | 0.0153 | 1.08 | metabolism: carbohydrate metabolism; oxidative phosphoryl ation |
| 10.9 | 10.9 | 44.6 | gi 457929 | ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit (Atp5d) | 0.85 | 0.0151 | 1.13 | 1.04 | 0.45 | 1.10 | 0.80 | 0.0067 | 1.16 | metabolism: electron transport; cation trans port |
| 52.2 | 52.2 | 54.6 | gi 25742739 | acyl-CoA synthetase long-chain family member 1 | 0.96 | 0.4715 | 1.13 | 1.00 | 0.89 | 1.11 | 0.89 | 0.0437 | 1.10 | metabolism: fatty acid metabolism |
| 6.0 | 6.0 | 20.4 | gi 157818027 | acyl-CoA synthetase short-chain family member 1 | 0.69 | 0.0002 | 1.15 | 0.82 | 0.51 | 1.76 | 0.81 | 0.0004 | 1.09 | metabolism: fatty acid metabolism |
| 17.5 | 17.5 | 25.2 | gi 77993368 | acyl-CoA synthetase family member 2 | 0.76 | 0.0200 | 1.23 | 0.94 | 0.60 | 1.31 | 1.01 | 0.8000 | 1.29 | metabolism: fatty acid metabolism |
| 50.7 | 50.7 | 77.8 | gi 149027156 | acetyl-CoA acyltransferase 2 | 1.05 | 0.3595 | 1.23 | 0.98 | 0.7540 | 1.15 | 1.25 | 0.02 | 1.15 | metabolism: protein metabolism |
| 20.1 | 20.1 | 44.3 | gi 68837285 | d-beta-hydroxybutyrate dehydrogenase, mitochondrial precursor (3-hydroxybutyrate dehydrogenase) | 0.80 | 0.0217 | 1.20 | 0.95 | 0.53 | 1.21 | 0.76 | 0.0402 | 1.30 | metabolism: other metaboli sm |
| 5.7 | 5.7 | 41.4 | gi 157824071 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex 3 | 1.03 | 0.5880 | 1.13 | 0.92 | 0.60 | 1.36 | 0.83 | 0.0308 | 1.18 | metabolism: oxidative phosphorylation |
| 44.0 | 44.0 | 73.7 | gi 55741424 | NADH dehydrogenase (ubiquinone) flavoprotein 1, 51 kDa | 0.97 | 0.3527 | 1.07 | 1.02 | 0.55 | 1.08 | 0.89 | 0.0095 | 1.09 | metabolism: oxidative phosphorylation |
| 5.4 | 5.4 | 35.8 | gi 54145376 | ATP synthase F0 complex subunit 8 | 0.83 | 0.0201 | 1.16 | 0.97 | 0.58 | 1.22 | 0.80 | 0.0426 | 1.24 | metabolism: oxidative phosphorylation; cation transport |
| 36.0 | 36.0 | 62.5 | gi 81884378 | cytochrome b-c1 complex subunit 1, mitochondrial precursor (Complex III subunit 1) | 0.93 | 0.3321 | 1.14 | 0.98 | 0.61 | 1.12 | 0.89 | 0.0356 | 1.11 | metabolism: proteolysis; electron transport |
| 75.7 | 75.7 | 85.3 | gi 71681130 | subunit | 0.94 | 0.0781 | 1.08 | 1.05 | 0.45 | 1.09 | 0.90 | 0.0258 | 1.09 | metabolism: purine metabolism; electron transport |
| 4.1 | 4.1 | 11.8 | gi 53850596 | succinate dehydrogenase complex, subunit C | 0.88 | 0.3565 | 2.88 | 1.02 | 0.67 | 1.10 | 0.75 | 0.0005 | 1.00 | metabolism: tricarboxylic acid pathway; electron transport |
| 24.1 | 24.4 | 30.7 | gi 6978543 | ATPase, Na+/K+ transporting, alpha 1 polypeptide | 0.84 | 0.0574 | 1.15 | 0.97 | 0.65 | 1.15 | 0.78 | 0.0038 | 1.16 | transport: cation transport |
| 10.6 | 10.6 | 38.8 | gi 50927657 | ATPase, Na+/K+ transporting, beta 1 polypeptide | 0.78 | 0.0441 | 1.24 | 0.96 | 0.35 | 1.27 | 0.72 | 0.0135 | 1.19 | transport: cation transport |
| 50.0 | 50.0 | 37.3 | gi 8392935 | ATPase, Ca2+ transporting, myocardial, slow twitch 2 isoform a | 0.81 | 0.0131 | 1.07 | 0.92 | 0.19 | 1.10 | 0.74 | <0.0001 | 1.08 | transport: cation transport; calcium ion homeostasis |
| 25.1 | 25.1 | 40.1 | gi 47718004 | solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 3 (Slc25a3) | 0.93 | 0.0730 | 1.08 | 0.94 | 0.27 | 1.18 | 0.89 | 0.0091 | 1.09 | metabolism: electron tranport |
| 32.2 | 32.2 | 89.6 | gi 78099013 | myoglobin | 0.95 | 0.2943 | 1.10 | 1.15 | 0.26 | 1.14 | 0.90 | 0.0267 | 1.10 | transport; blood circulation and gas exchange |
| 7.6 | 7.6 | 84.3 | gi 34786049 | subunit | 0.96 | 0.3948 | 1.11 | 1.07 | 0.45 | 1.10 | 0.81 | 0.0087 | 1.16 | metabolism: electron tranport |
| 16.5 | 16.5 | 71.1 | gi 34849861 | cytochrome c oxidase, subunit Vlc (Cox6c) | 0.98 | 0.4443 | 1.06 | 0.89 | 0.01 | 1.13 | 0.90 | 0.0090 | 1.08 | metabolism: electron tranport |
| 2.2 | 3.2 | 18.3 | gi 871525 | calcium binding protein | 0.77 | 0.4549 | 18.80 | 0.75 | 0.29 | 6.58 | 0.73 | 0.0354 | 1.25 | no PANTHER hit |
| 13.8 | 13.8 | 54.6 | gi 599963 | mitochondrial oxidative phosphorylation coupling factor 6 | 0.86 | 0.0088 | 1.12 | 1.02 | 0.63 | 1.11 | 0.81 | 0.0025 | 1.14 | no PANTHER hit |
| 16.3 | 16.3 | 34.6 | gi 149042663 | sarcalumenin (predicted), isoform CRA_a | 0.80 | 0.0277 | 1.22 | 0.97 | 0.68 | 1.25 | 0.81 | 0.0240 | 1.19 | no PANTHER hit |
Proteins of interest were identified from 6 iTRAQ 8plex analyses and are grouped according to associated biological processes. Boldface indicates that the difference in expression was significant relative to adult ovary-intact control.
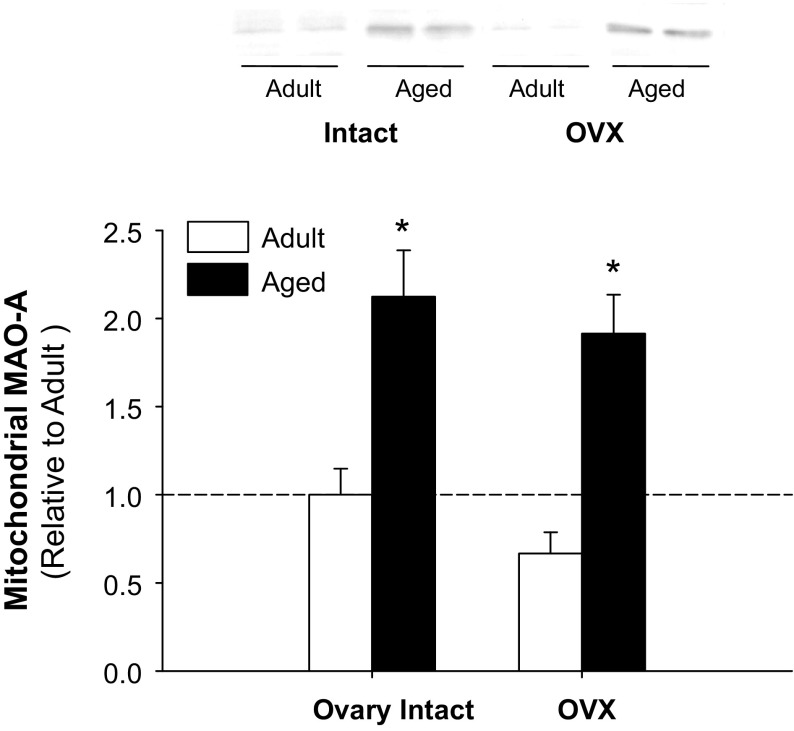
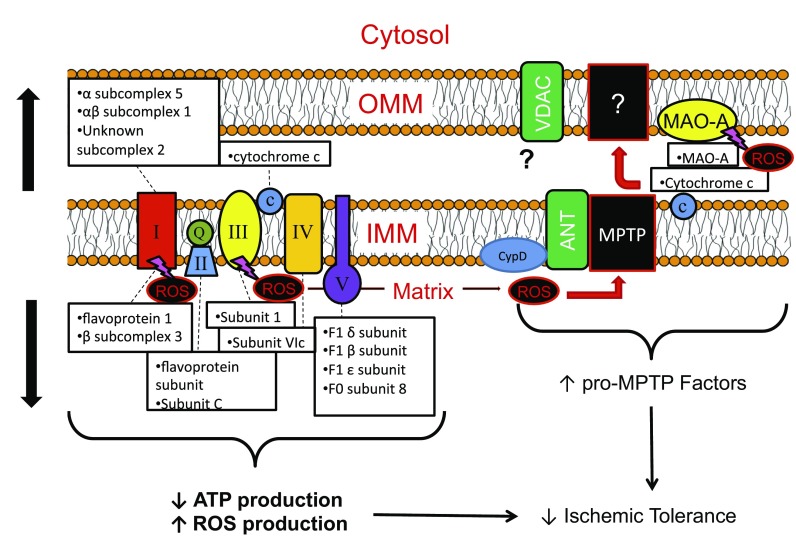
We also observed an increased quantity of mitochondrial proteins involved in the initiation of cell death in aged OVX and/or aged hearts, including cytochrome c and possible mitochondrial permeability transition pore (MPTP) regulatory proteins VDAC1 and ANT1 (Table 3). A >50% increase was observed in aged OVX for prosaposin (Table 3). The protein that demonstrated the greatest change in abundance in aged and aged OVX, however, was MAO-A (∼91% increase). The directional increase in aged and aged OVX was confirmed by Western blotting (Fig. 2); significant changes in MAO-A due to E2 deficiency in adult rats were not observed.
Fig. 2.
Mitochondrial monoamine oxidase-A (MAO-A) protein levels are increased with aging and age-associated E2 deficiency. Representative immunoblots are presented for mitochondrial MAO-A levels with E2 deficiency (OVX) in adult and aged hearts. *Age effect (P < 0.05; n = 4–5/group). Values are means ± SE; data are expressed relative to adult ovary-intact controls.
Mitochondrial respiration and infarct size following acute MAO-A inhibition.
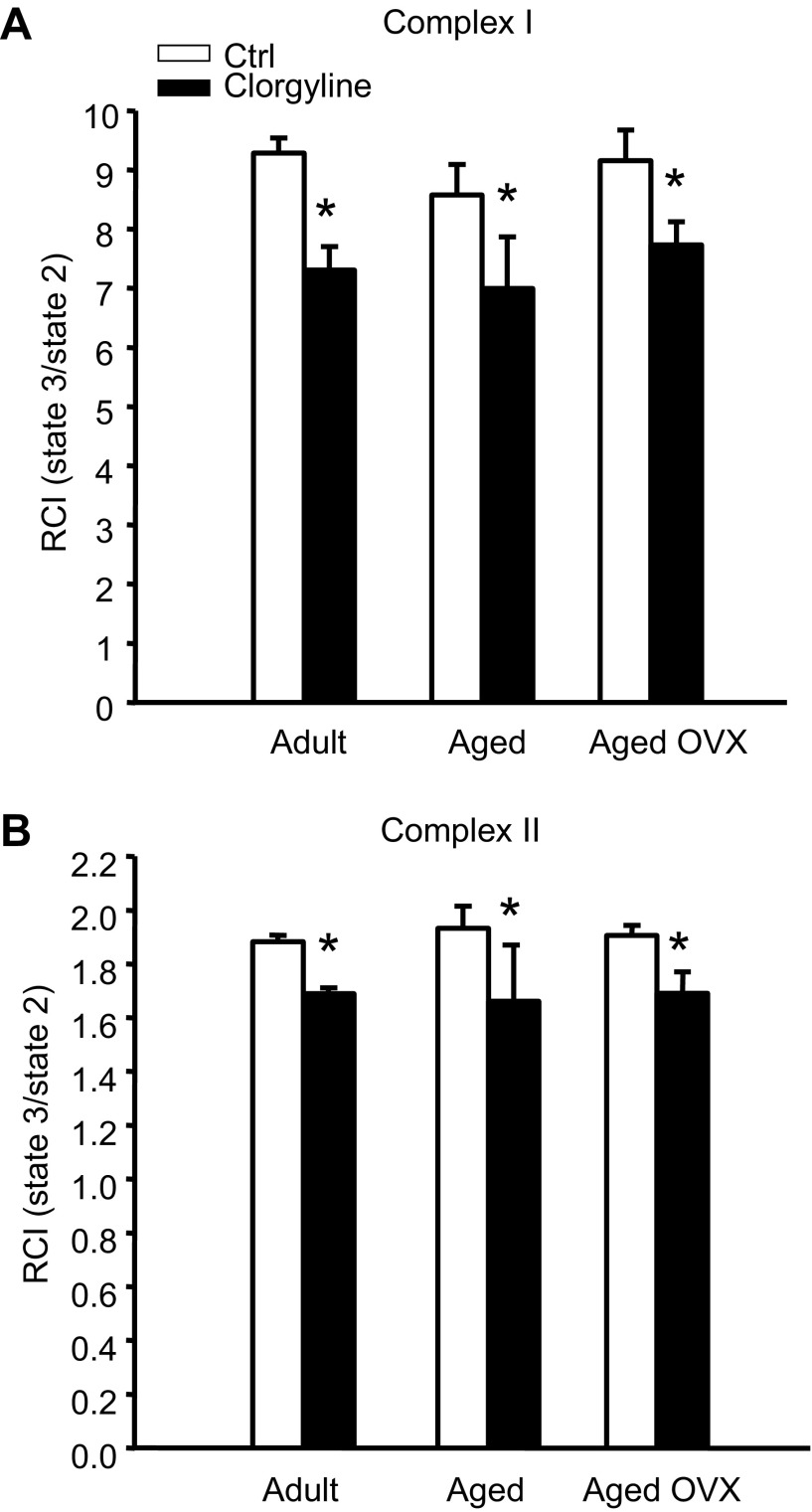
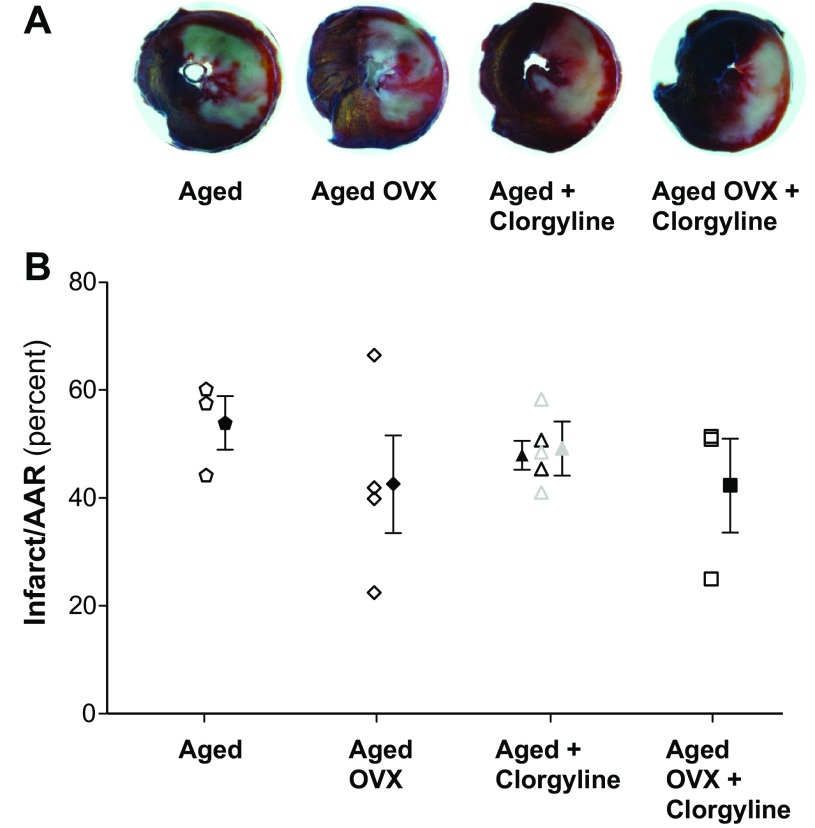
Since iTRAQ screening revealed the greatest increase in MAO-A in aged female rat mitochondria, we sought to determine whether the MAO-A specific inhibitor clorgyline had differential effects on mitochondrial respiration. Accordingly, succinate/rotenone (complex II) and oxoglutarate/malate (complex I)-stimulated respiration were assessed in ventricular mitochondria isolated from adult (6 mo), aged (24 mo), or aged OVX female rats. Figure 3 demonstrates that clorgyline decreased the respiratory control index (RCI) by 10–20% (P < 0.01) in all groups (primarily due to an increased state 2 respiration with no significant clorgyline effect on state 3 respiration), indicative of mild mitochondrial uncoupling and cardioprotection prior to ischemia. Accordingly, CAL studies were undertaken to determine the effects of acute MAO-A inhibition on infarct size reduction in aged rats. Mean AAR was ∼36% and not significantly different across experimental groups or treatments. When matched for AAR, infarct size following 47 min CAL was not different between vehicle and either 5 or 10 mg/kg clorgyline, 60 min prior to CAL in aged or aged OVX (Fig. 4). Additional studies were conducted in which 5 mg/kg clorgyline was administered (ip) 30 min prior to 35 min CAL, followed by 2 h R or in isolated perfused hearts, and no improvement in infarct size or functional recovery was observed (data not shown).
Fig. 3.
MAO-A inhibition reduces mitochondrial respiratory control index (RCI) in hearts from adult, aged, and aged OVX female F344 rats. RCI was determined in the presence of 2.5 mM α-ketoglutarate + 1 mM malate (Complex I; A), or 1 μM rotenone + 2.5 mM succinate (Complex II; B) following treatment with 5 μM clorgyline during state 2 respiration. All values are displayed as mean RCI (state 3/state 2) ± SE from n = 2–4 independent experiments. *Main effect of clorgyline P < 0.005; n = 3–4/group.
Fig. 4.
Infarct size is not affected by acute MAO inhibition in aged or aged OVX rats. Coronary artery ligation (CAL) was used to generate ischemia and reperfusion (I/R) injury (47 min I: 2 h R), with and without the MAO-A inhibitor clorgyline [5 mg/kg (black symbols) in aged (n = 5) or aged OVX (n = 3)] or 10 mg/kg clorgyline in aged (gray symbols; n = 3) administered 60 min (ip) prior to CAL. CAL studies were also performed using 35 min I: 2 h R in aged (n = 4) following 5 mg/kg clorgyline and no infarct sparing effect was observed (data not shown). Infarct size is expressed as a percentage of area at risk (AAR).
DISCUSSION
The aim of the current study was to identify, using a novel high-throughput quantitative iTRAQ 8plex proteomics approach, alterations in mitochondrial proteins that may contribute to increased I/R injury and disease risk with E2 deficiency in aged female rats. Here, we identified 965 proteins across groups and significant directional changes in 67 proteins with aged and/or aged OVX, and 32 unique to aged OVX. Notably only six proteins were similarly altered in adult OVX. Proteins affected by aging were primarily related to cellular metabolism, oxidative stress, and cell death, with the largest change seen in MAO-A, a source of oxidative stress. Translational studies revealed that while acute MAO-A inhibition induced mild uncoupling in isolated aged mitochondria, reductions in infarct size were not observed. To the best of our knowledge, this is the first application of an iTRAQ proteomics approach to study mitochondrial mechanisms of I/R injury and disease in the aged female heart.
About 50% of the identified proteins altered in aged OVX relative to adult hearts are involved in mitochondrial ATP production (Tables 3 and 4). Age-associated reductions in cardiac mitochondrial ATP production have been previously reported in male rodents, including declines in the rate of oxidative phosphorylation and the activity of electron transport chain (ETC) complexes III and IV (47). A recent report on age-associated alterations in male rat cardiac mitochondrial gene transcripts noted widespread downregulation of ETC complex RNA as well as decreased complex I and IV activity (60), while proteomic profiling in aged male mouse hearts demonstrated reduced expression of several mitochondrial ETC complex subunits (14). Here, we demonstrate primarily reduced quantity of protein subunits of ETC complex I (NADH dehydrogenase), II (succinate dehydrogenase), III (cytochrome bc1 complex), IV (cytochrome c oxidase), and V (F0F1 ATPase) in aged, E2-deficient hearts, and bidirectional changes in proteins involved in fatty acid substrate metabolism (acyl Co-A synthetase subunits). Increases were primarily observed, in contrast, for proteins involved in carbohydrate and amino acid metabolism (pyruvate dehydrogenase subunits) and enzymes of the tricarboxylic acid cycle. An explanation for the differential regulation of upstream and downstream metabolic processes is not immediately evident, although one hypothesis may be that chronic reductions in ATP production due to reduced ETC and oxidative phosphorylation (62) capacity lead to compensatory upregulation of substrate-level and tricarboxylic acid cycle enzymes. Moreover, observed increases in Hsp60 and mtHsp70 in aged OVX are consistent with previous studies in aged male hearts (18) and may be related to alterations in mitochondrial matrix protein import of nuclear-encoded enzymes, which may or may not be balanced by changes in proteolysis. Measurement of the activity and/or phosphorylation status (19) of these enzymes is indicated for a more comprehensive characterization of metabolic alterations and substrate utilization in the aged female heart and represents the focus of ongoing studies in our laboratory.
Dysregulated mitochondrial metabolism has been suggested as an explanation for impaired ischemic tolerance in the aged heart (34, 44, 60) and may contribute to I/R injury through multiple mechanisms (see Fig. 5 for model summary). First, the reduced capacity for ATP production upon reperfusion leads to impaired cellular and mitochondrial volume regulation (due to insufficient ATP for active ion transport pumps), swelling, lysis, and initiation of necrotic and apoptotic cell death (21). We observed ∼20% downregulation of the Na+/K+ ATPase and Ca2+ ATPase pumps in aged OVX hearts, which may further contribute to these detrimental events. Additionally, metabolic dysregulation is thought to contribute to cellular injury through increased mitochondrial reactive oxygen species (ROS) production in the aged heart (47). Complex III, for example, has been identified as a major source of age-associated increases in mitochondrial superoxide radical (O2.) production both at baseline and in response to I/R (47). High levels of ROS are generated during I/R from additional sources both within and outside the mitochondria, including ETC complex I (7, 72), the xanthine oxidase system (79), and vascular NADPH oxidase (2), and contribute to cellular injury through lipid peroxidation, protein oxidation, enzyme inactivation, and DNA damage (10). Furthermore, ROS can induce opening of the MPTP and therefore initiation of cell death by the facilitation of mitochondrial Ca2+ overload and/or the oxidation of thiol groups of ANT, a possible MPTP regulatory protein (38, 41, 83).
Fig. 5.
Summary of proposed changes in mitochondrial electron transport complex and proapoptotic proteins that may contribute to reductions in ischemic tolerance with age-associated E2 deficiency. Abbreviations: ANT, adenine nucleotide translocator 1; ATP, adenosine triphosphate; CypD, cyclophilin D; IMM, inner mitochondrial membrane; MAO-A, monoamine oxidase-A; MPTP, mitochondrial permeability transition pore; OMM, outer mitochondrial membrane; ROS, reactive oxygen species; VDAC, voltage-dependent anion channel.
Indicative of possible increased ROS production in the aged, E2-deficient heart, we observed altered expression of several mitochondrial proteins involved in the oxidative stress response. A large increase (>90%) MAO-A, which is found in the outer mitochondrial membrane and represents a potent source of hydrogen peroxide (H2O2) during I/R (9, 71), was noted in both aged and aged OVX hearts but not adult OVX. Given recent evidence that MAO-A inhibition can reduce I/R injury in adult hearts (for review see Refs. 20, 37), we sought to determine effects of acute MAO-A inhibition on mitochondrial respiration and subsequent I/R injury in the aged, E2-deficient heart. While we observed reduced RCIs in isolated mitochondria following MAO-A inhibition with clorgyline (which would be predictive of mild mitochondrial uncoupling and cardioprotection), acute MAO-A inhibition at varying doses and durations of exposure prior to I/R injury in vivo was unable to produce an infarct sparing effect in the aged female rat heart. We observed a similar lack of efficacy in isolated perfused hearts when clorgyline was delivered 15 min prior to I/R (data not shown), suggesting that the aged female heart is refractory to protection by MAO-A inhibition. The mechanism of reduced cardioprotective efficacy of MAO-A inhibition in aged animals previously demonstrated in adult animals (9) is not immediately evident but, combined with the well-characterized refractoriness of the aged heart to ischemic intervention (34), likely includes an inability of age-associated changes in antioxidant machinery to combat overproduction of ROS associated with senescence.
In this regard, SOD2 (MnSOD), the mitochondrial SOD isoform that catalyzes the conversion of the strongly reactive O2. radical to less reactive H2O2 and molecular O2 (32), was increased by nearly 40% in aged OVX. It is likely that these increases represent compensatory adaptations to chronically increased ROS production in the aged female heart (47), and interestingly, our observation of increased SOD2 expression is in contrast to studies in male F344 rats demonstrating age-related increases in cardiac SOD2 activity (33, 57) but unaltered SOD2 expression (2, 73). In contrast to our observations of increased heat shock protein and SOD2 levels, the antioxidant enzyme catalase, which neutralizes H2O2 to H2O and O2, exhibited decreased abundance in aged hearts. It has been reported, however, that catalase does not significantly contribute to the removal of H2O2 in cardiac mitochondria, even under strong oxidative conditions such as those achieved in I/R (4). This function has instead been attributed to glutathione peroxidase (4), which demonstrated an insignificant trend for increased levels in aged and aged OVX hearts (data not shown).
Despite these apparently compensatory increases in mitochondrial antioxidant enzyme abundance in the aged female heart, it is likely that the large bursts of ROS produced during I/R (34, 44, 75) overwhelm these defenses and contribute to the increased infarct size observed in aging and E2 deficiency (30, 55) and decreased efficacy of MAO-inhibition on infarct sparing. In this regard, we observed increased levels in aged and aged OVX hearts of mitochondrial proteins involved in the initiation of cell death, including cytochrome c and possible MPTP regulatory proteins VDAC1 and ANT1 (Fig. 5). The increased quantity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme that has been reported to play a proapoptotic role in the mitochondria through induction of the MPTP (69), was also noted. In contrast, the significant increase in prosaposin, which is involved in fatty acid transport but has also been demonstrated to play an antiapoptotic role in a variety of cell types (46), may represent an additional compensatory mechanism to combat increased oxidative stress in the aged heart (65).
Limitations.
A potential limitation of the current study is the purity of the isolated mitochondrial sample and use of frozen tissue for proteomic analyses. We have previously demonstrated that cytosolic contamination of the mitochondrial fraction is <2% (29), and no direct indications of plasma membrane contamination (i.e., proteins such as ecto-5′-nucleotidase) were observed herein (Supplemental Table S1). However, use of frozen tissue may have spuriously introduced damage to mitochondrial structure and or proteins. Another potential limitation of the current studies, and all rodent studies that utilize an OVX model, includes the complicated nature of the menopausal transition in rodents (49, 51) and questionable relevance of this model to mimic human reproductive senescence. Clearly, the study of nonhuman primates has been purported as the model perhaps most applicable to the study of human menopause (40, 77, 78). The onset of senile anestrous is variable in rats (1, 13, 52, 63, 67), resulting in a state of persistent estrous followed by persistent diestrous, whereby sustained E2 levels are similar in magnitude to diestrous in adult animals. However, notable similarities between menopause and “estropause” (for recent review see Refs. 13, 52, 81) include cessation of estrous cyclicity (∼16 mo in F344 rats) and a progressive deterioration in hypothalamic-pituitary-gonadal axis function thereafter (67) until senile anestrous. Interestingly, the menopausal transition in humans is also characterized by elevated E2 levels (24, 25, 76). Nevertheless, use of age-appropriate rats in conjunction with OVX, as we use here, represents an often overlooked but critical design consideration of rodent studies to recapitulate postmenopausal E2 deficiency in a meaningful way (i.e., differential responses of OVX in adult vs. aged rats that do not recapitulate reproductive senescence). Finally, the effects of estrogen replacement on cardiovascular health in older women, particularly at the time of the menopausal transition, remains incompletely characterized and awaits additional clinical and experimental data (54). In this regard, effects of E2 replacement on the mitochondrial proteome of adult and aged rats, as well as following I/R injury is worthy of future study.
In summary, a targeted mitochondrial approach incorporating a discovery-based, state-of-the-art iTRAQ proteomic analysis was used to characterize alterations in protective signaling due to age-associated E2 deficiency. We provide novel evidence for an environment of increased mitochondrial oxidative stress in the aged, E2-deficient rat heart, which 1) is associated with altered mitochondrial metabolism, 2) is associated with possible compensatory adaptations in antioxidant enzymes (SOD2 and Hsp), and 3) is likely to contribute to greater cell death in I/R injury through upregulated MPTP regulatory proteins (VDAC1 and ANT1) and enzymes (MAO-A) that is not observed in hearts isolated from adult OVX. Functional studies also highlight the therapeutic challenges of developing efficacious strategies to protect the aged female myocardium from I/R injury. Importantly, ischemic heart disease remains the leading cause of morbidity and mortality in U.S. women, and the data described here provide valuable insight into possible experimental targets for intervention utilizing a model of female aging.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-091097, HL-091097-01A2S1, and AA-019403 (to D. H. Korzick) and the Intramural Research Program of the NIH, National Institute on Aging (E. G. Lakatta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.S.L., S.J.J., J.C.H., V.L., and D.H.K. performed experiments; T.S.L., S.J.J., J.C.H., V.L., and D.H.K. analyzed data; T.S.L., J.C.H., J.E.V.E., E.G.L., and D.H.K. interpreted results of experiments; T.S.L., S.J.J., J.C.H., V.L., and D.H.K. prepared figures; T.S.L. drafted manuscript; T.S.L., S.J.J., J.C.H., V.L., J.E.V.E., E.G.L., and D.H.K. approved final version of manuscript; J.C.H., J.E.V.E., E.G.L., and D.H.K. edited and revised manuscript; D.H.K. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
Gratitude is expressed to Rick Ball for surgical expertise and completion of CAL surgeries.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Aasum E, Cooper M, Severson DL, Larsen TS. Effect of BM 17.0744, a PPARα ligand, on the metabolism of perfused hearts from control and diabetic mice. Can J Physiol Pharmacol 83: 183–190, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol 285: H1015–H1022, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Agnetti G, Kane LA, Guarnieri C, Caldarera CM, Van Eyk JE. Proteomic technologies in the study of kinases: novel tools for the investigation of PKC in the heart. Pharmacol Res 55: 511–522, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antunes F, Han D, Cadenas E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H2O2 detoxification in in vivo conditions. Free Radic Biol Med 33: 1260–1267, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE. Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res 99: 706–714, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res 90: 390–397, 2002. [DOI] [PubMed] [Google Scholar]
- 7. Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem 279: 17197–17204, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking-based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 108: 908–916, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112: 3297–3305, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Bognar Z, Kalai T, Palfi A, Hanto K, Bognar B, Mark L, Szabo Z, Tapodi A, Radnai B, Sarszegi Z, Szanto A, Gallyas F, Jr, Hideg K, Sumegi B, Varbiro G. A novel SOD-mimetic permeability transition inhibitor agent protects ischemic heart by inhibiting both apoptotic and necrotic cell death. Free Radic Biol Med 41: 835–848, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 12. Budas GR, Churchill EN, Disatnik M, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res 88: 83–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med (Maywood) 229: 977–987, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Chakravarti B, Oseguera M, Dalal N, Fathy P, Mallik B, Raval A, Chakravarti DN. Proteomic profiling of aging in the mouse heart: altered expression of mitochondrial proteins. Arch Biochem Biophys 474: 22–31, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Chakravarti B, Oseguera M, Dalal N, Fathy P, Mallik B, Raval A, Chakravarti DN. Proteomic profiling of aging in the mouse heart: Altered expression of mitochondrial proteins. Arch Biochem Biophys 474: 22–31, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Chang J, Van Remmen H, Cornell J, Richardson A, Ward WF. Comparative proteomics: characterization of a two-dimensional gel electrophoresis system to study the effect of aging on mitochondrial proteins. Mech Ageing Dev 124: 33–41, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Budas GR, Churchill EN, Disatnik M, Hurley TD, Mochly-Rosen D. Activation of aldehyde ischemic damage to the heart. Science 321: 1493–1495, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craig EE, Hood DA. Influence of aging on protein import into cardiac mitochondria. Am J Physiol Heart Circ Physiol 272: H2983–H2988, 1997. [DOI] [PubMed] [Google Scholar]
- 19. Deng N, Zhang J, Zong C, Wang Y, Lu H, Yang P, Wang W, Young GW, Wang Y, Korge P, Lotz C, Doran P, Liem DA, Apweiler R, Weiss JN, Duan H, Ping P. Phosphoproteome analysis reveals regulatory sites in major pathways of cardiac mitochondria. Mol Cell Proteom 10: M110.–000117., 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail Rev 12: 249–260, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Meth 4: 207–214, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Grant J, Bradshaw A, Schwacke J, Baicu C, Zile M, Schey K. Quantification of protein expression changes in the aging left ventricle of Rattus norvegicus. J Proteome Res 8: 4252–4263, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) Staging System. J Clin Endocrinol Metab 92: 3060–3067, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin N Am 33: 637–659, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Hamilton KL, Lin L, Wang Y, Knowlton AA. Effect of ovariectomy on cardiac gene expression: inflammation and changes in SOCS gene expression. Physiol Genomics 32: 254–263, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 280: 605–613, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Hunter J, Korzick D. Age- and sex-dependent alterations in PKC-ERK1/2 signaling in rat myocardium. Mech Ageing Dev 126: 535–550, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Hunter JC, Korzick DH. Age- and sex-dependent alterations in protein kinase C (PKC) and extracellular regulated kinase 1/2 (ERK1/2) in rat myocardium. Mech Ageing Dev 126: 535–550, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in aged rat myocardium: roles of PKCδ, PKCε, Akt, and GSK3β. Am J Physiol Regul Integr Comp Physiol 292: R800–R809, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, Karmazyn M. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res 77: 416–424, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Jin ZQ, Zhou HZ, Cecchini G, Gray MO, Karliner JS. MnSOD in mouse heart: acute responses to ischemic preconditioning and ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 288: H2986–H2994, 2005. [DOI] [PubMed] [Google Scholar]
- 33. Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 19: 419–421, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res 66: 233–244, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3 in cardioprotection. Circ Res 104: 1240–1252, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaludercic N, Carpi A, Menabò R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta 1813: 1323–1332, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanno T, Sato EE, Muranaka S, Fujita H, Fujiwara T, Utsumi T, Inoue M, Utsumi K. Oxidative stress underlies the mechanism for Ca2+-induced permeability transition of mitochondria. Free Radic Res 38: 27–35, 2004. [DOI] [PubMed] [Google Scholar]
- 39. Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol 288: H371–H381, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Keller ET, Zhang J, Yao Z, Qi Y. The impact of chronic estrogen deprivation on immunologic parameters in the ovariectomized rhesus monkey (Macaca mulatta) model of menopause. J Repr Immunol 50: 41, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta 1813: 616–622, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Korzick DH, Hunter JC, McDowell MK, Delp MD, Tickerhoof MM, Carson LD. Chronic exercise improves myocardial inotropic reserve capacity through alpha1-adrenergic and protein kinase C-dependent effects in Senescent rats. J Gerontol A Biol Sci Med Sci 59: 1089–1098, 2004. [DOI] [PubMed] [Google Scholar]
- 43. Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCε-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3β but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol 293: H2056–H2063, 2007. [DOI] [PubMed] [Google Scholar]
- 44. Lakatta EG, Sollott SJ. The “heartbreak” of older age. Mol Interv 2: 431–446, 2002. [DOI] [PubMed] [Google Scholar]
- 45. Lancaster TS, Jefferson SJ, Korzick DH. Local delivery of a PKCε-activating peptide limits ischemia reperfusion injury in the aged female rat heart. Am J Physiol Regul Integr Comp Physiol 301: R1242–R1249, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee TJ, Sartor O, Luftig RB, Koochekpour S. Saposin C promotes survival and prevents apoptosis via PI3K/Akt-dependent pathway in prostate cancer cells. Mol Cancer 3: 31, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys 420: 287–297, 2003. [DOI] [PubMed] [Google Scholar]
- 48. Lopes R, Solter PF, Sisson DD, Oyama MA, Prosek R. Characterization of canine mitochondrial protein expression in natural and induced forms of idiopathic dilated cardiomyopathy. Am J Vet Res 67: 963–970, 2006. [DOI] [PubMed] [Google Scholar]
- 49. Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropic and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod 21: 193–203, 1979. [DOI] [PubMed] [Google Scholar]
- 50. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349: 523–534, 2003. [DOI] [PubMed] [Google Scholar]
- 51. Moorthy K, Sharma D, Basir SF, Baquer NZ. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp Gerontol 40: 295–302, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci 26: 10332–10348, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol 69: 51–67, 2007. [DOI] [PubMed] [Google Scholar]
- 54. Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive Services Task Force recommendations. Ann Intern Med 157: 104–113, 2012. [DOI] [PubMed] [Google Scholar]
- 55. Novotny JL, Simpson AM, Tomicek NA, Lancaster TL, Korzick DH. Rapid estrogen receptor-α activation improves ischemic tolerance in aged female rats through a novel PKCε-dependent mechanism. Endocrinology 150: 889–896, 2009. [DOI] [PubMed] [Google Scholar]
- 56. Novotny JL, Simpson AM, Tomicek NJ, Lancaster TS, Korzick DH. Rapid estrogen receptor-alpha activation improves ischemic tolerance in aged female rats through a novel protein kinase C epsilon-dependent mechanism. Endocrinology 150: 889–896, 2009. [DOI] [PubMed] [Google Scholar]
- 57. Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282: R423–R430, 2002. [DOI] [PubMed] [Google Scholar]
- 58. Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res 81: 404–414, 1997. [DOI] [PubMed] [Google Scholar]
- 59. Ping PP. Getting to the heart of proteomics. N Engl J Med 360: 532–534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, Syed RH, Siddiqui SA, Raghavakaimal S, Terzic A, Jahangir A. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev 129: 304–312, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y; American Heart Association Statistics Committee Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115: e69–e171, 2007. [DOI] [PubMed] [Google Scholar]
- 62. Savitha S, Sivarajan K, Haripriya D, Kokilavani V, Panneerselvam C. Efficacy of levo carnitine and alpha lipoic acid in ameliorating the decline in mitochondrial enzymes during aging. Clin Nutr 24: 794–800, 2005. [DOI] [PubMed] [Google Scholar]
- 63. Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience 119: 821–830, 2003. [DOI] [PubMed] [Google Scholar]
- 64. Schnoes KK, Jaffe IZ, Lyer L, Dabreo A, Aronovitz M, Newfell B, Hansen U, Rosano G, Mendelsohn ME. Rapid recruitment of temporally distinct vascular gene sets by estrogen. Mol Endocrinol 22: 2544–2566, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J 17: 1355–1357, 2003. [DOI] [PubMed] [Google Scholar]
- 66. Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics 6: 1638–1655, 2007. [DOI] [PubMed] [Google Scholar]
- 67. Sone K, Yamamoto-Sawamura T, Kuwahara S, Nishijima K, Ohno T, Aoyama H, Tanaka S. Changes of estrous cycles with aging in female F344/n rats. Exp Anim 56: 139–148, 2007. [DOI] [PubMed] [Google Scholar]
- 68. Stojanovski D, Johnston AJ, Streimann I, Hoogenraad NJ, Ryan MT. Import of nuclear-encoded proteins into mitochondria. Exp Physiol 88: 57–64, 2003. [DOI] [PubMed] [Google Scholar]
- 69. Tarze A, Deniaud A, Le Bras M, Maillier E, Molle D, Larochette N, Zamzami N, Jan G, Kroemer G, Brenner C. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene 26: 2606–2620, 2007. [DOI] [PubMed] [Google Scholar]
- 70. Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13: 2129–2141, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Toninello A, Salvi M, Pietrangeli P, Mondovi B. Biogenic amines and apoptosis: minireview article. Amino Acids 26: 339–343, 2004. [DOI] [PubMed] [Google Scholar]
- 72. Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J 191: 421–427, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van der Loo B, Bachschmid M, Labugger R, Schildknecht S, Kilo J, Hahn R, Palacios-Callender M, Luscher TF. Expression and activity patterns of nitric oxide synthases and antioxidant enzymes reveal a substantial heterogeneity between cardiac and vascular aging in the rat. Biogerontology 6: 325–334, 2005. [DOI] [PubMed] [Google Scholar]
- 74. Van Eyk JE. Lessons from old and new kinases. Circ Res 94: 135–137, 2004. [DOI] [PubMed] [Google Scholar]
- 75. Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem 271: 29223–29230, 1996. [DOI] [PubMed] [Google Scholar]
- 76. Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 292: 2991–2996, 2004. [DOI] [PubMed] [Google Scholar]
- 77. Wood CE, Sitruk-Ware RL, Tsong YY, Register TC, Lees CJ, Cline JM. Effects of estradiol with oral or intravaginal progesterone on risk markers for breast cancer in a postmenopausal monkey model. Menopause 14: 639–647, 2007. [DOI] [PubMed] [Google Scholar]
- 78. Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med (Maywood) 230: 818–828, 2005. [DOI] [PubMed] [Google Scholar]
- 79. Xia Y, Khatchikian G, Zweier JL. Adenosine deaminase inhibition prevents free radical-mediated injury in the postischemic heart. J Biol Chem 271: 10096–10102, 1996. [DOI] [PubMed] [Google Scholar]
- 80. Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol 287: H165–H171, 2004. [DOI] [PubMed] [Google Scholar]
- 81. Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction 131: 403–414, 2006. [DOI] [PubMed] [Google Scholar]
- 82. Zhao Z, Stanley BA, Zhang W, Assmann SM. ABA-regulated G protein signaling in arabidopsis guard cells: a proteomic perspective. J Proteome Res 9: 1637–1647, 2010. [DOI] [PubMed] [Google Scholar]
- 83. Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res 83: 213–225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.