Abstract
One of the fundamental biochemical defects underlying the complications of diabetic cardiovascular system is elevation of diacylglycerol (DAG) and its effects on protein kinase C (PKC) signaling. It has been noted that exercise training attenuates poor cardiac performance in Type 1 diabetes. However, the role of PKC signaling in exercise-induced alleviation of cardiac abnormalities in diabetes is not clear. We investigated the possibility that exercise training modulates PKC-βII signaling to elicit its beneficial effects on the diabetic heart. bio-breeding diabetic resistant rats, a model reminiscent of Type 1 diabetes in humans, were randomly assigned to four groups: 1) nonexercised nondiabetic (NN); 2) nonexercised diabetic (ND); 3) exercised nondiabetic; and 4) exercised diabetic. Treadmill training was initiated upon the onset of diabetes. At the end of 8 wk, left ventricular (LV) hemodynamic assessment revealed compromised function in ND compared with the NN group. LV myocardial histology revealed increased collagen deposition in ND compared with the NN group, while electron microscopy showed a reduction in the viable mitochondrial fraction. Although the PKC-βII levels and activity were unchanged in the diabetic heart, the DAG levels were increased. With exercise training, the deterioration of LV structure and function in diabetes was attenuated. Notably, improved cardiac performance in training was associated with a decrease in myocardial DAG levels in diabetes. Exercise-induced benefits on cardiac performance in diabetes may be mediated by prevention of an increase in myocardial DAG levels.
Keywords: diabetic cardiomyopathy, protein kinase C-βII, bio-breeding diabetes resistant rat
diabetes mellitus profoundly compromises the cardiovascular health of the individual (9). The major cause of mortality and morbidity in individuals with diabetes is cardiovascular disease (17). The presence of cardiomyopathy in individuals with longstanding diabetes was speculated in a 1972 report (45) and was later extended by the Framingham study, which described an excess in the number of insulin-treated diabetic individuals afflicted with cardiac failure, unaccounted by coronary heart disease (29). Later reports confirmed the presence of diabetic cardiomyopathy (DCM) in individuals with both Type 1 and Type 2 diabetes (4, 52). The cardiac effects of chronic hyperglycemia manifest as abnormalities of both active and passive cardiac functional apparatus (for e.g., myocardial mitochondria and interstitial collagen, respectively), ultimately resulting in cardiac failure (15, 57). The pathological condition of the myocardium preceding overt cardiac failure has been described as DCM (4).
Several studies have reported an elevation of tissue diacylglycerol (DAG) levels under hyperglycemic conditions (9, 27). A critical signaling step that links hyperglycemia to diabetic complications involves the DAG-induced activation of protein kinase C (PKC) (18, 35). In the diabetic heart, however, activation of PKC-βII isoform has been shown to underlie the pathogenesis of DCM (26). Cardiac PKC-βII activation resulted in cardiomyopathy with interstitial fibrosis and hypertrophy (7, 55). Consequently, increased DAG levels and PKC-βII activation in the diabetic heart have been considered to be the critical steps in the pathology of DCM.
Exercise training is of particular interest in both the prevention and treatment of DCM, as a number of studies clearly indicate a beneficial role for exercise in the amelioration of cardiac abnormalities in Type 1 diabetes (8, 13, 14, 46, 47). However, mechanism(s) of exercise-induced benefits in DCM remains elusive. In this study, we investigated, in a rat model of diabetes that is reminiscent of human autoimmune diabetes pathogenesis (41), the possibility of molecules involved in cardiac PKC-βII signaling, as mediators of exercise-induced effects in DCM. We hypothesized that exercise-induced cardiac benefits, if any, in the bio-breeding diabetes resistant rat (BBDR) model of autoimmune diabetes are mediated by components of PKC-βII signaling in the diabetic myocardium. We verified the effects of exercise training on the protein expression levels, activation, and activity of PKC-βII. We also verified the effects of exercise training on the major activator and inhibitor of PKC-βII, namely, DAG and sphingosine, respectively. Our investigation revealed that exercise training was associated with a remarkable decrease in the cardiac DAG level in diabetic rats, suggesting that the favorable changes in cardiac structure and function associated with exercise training in DCM could be mediated by the prevention of an otherwise significant increase in the DAG levels. Thus exercise may exert its beneficial effects by targeting a critical biochemical defect involved in the pathogenesis of DCM.
METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Animal maintenance and induction of Type 1 diabetes.
Thirty-two male BBDR rats (Biomedical Research Models), aged 23–25 days, were randomized to the following four groups (n = 8/group): 1) nonexercised nondiabetic (NN); 2) nonexercised diabetic (ND); 3) exercised nondiabetic (EN); and 4) exercised diabetic (ED). Animals were housed in standard rat cages (one rat per cage) in pathogen free environment maintained at 19°C. Standard rat chow and water were fed ad libitum. Autoimmune diabetes was induced in ND and ED groups by injection of the anti-RT6 monoclonal antibody DS4.23 hybridoma supernatant (kindly provided by Dr. Dale L. Greiner, University of Massachusetts Medical Center) 2 ml/day for 5 days/wk. The supernatant was combined with a nonspecific immune system activator polyinosinic-polycytidylic acid (Poly I:C, Sigma, St. Louis, MO; 5 μg/g body mass, 3 days/wk), as described previously (53) for injections. NN and EN animals were injected with vehicle. After confirmation of increased plasma glucose levels (≥200 mg/dl) for 3 consecutive days, the rats were considered diabetic. Plasma glucose levels were measured every day using a digital glucometer (Accucheck, Roche Diagnostics, Indianapolis, IN). Insulin was delivered to diabetic rats by a subcutaneous osmotic pump at a flow rate (0.25 μl/h) predetermined by the manufacturer(ALZET, Cupertino, CA) (34). Body mass was recorded twice per week in all groups. Glycated hemoglobin (HbA1c) levels were measured at the end of the experiment using antibody-based A1cNow meter (Metrika, Sunnyvale, CA), as described (37).
Exercise training protocol.
The rats from EN and ED group were trained using our moderate-endurance treadmill training protocol (51), comparable to other reports (13, 14). Training was initiated in the diabetic rats a day following insulin pump implantation. Rats in the exercise groups were trained on a treadmill with custom-built running tracks for 8 wk. The training intensity and duration began at 15 m/min for 5 min on day 1 and progressed to 20 m/min for 50 min by the end of week 2. The rats maintained the intensity and duration of 20 m/min for 60 min/day for the remaining 6 wk (5 days/wk). To rule out the confounding effects of nontraining factors in the training environment, all animals in the NN and ND groups were handled every day and subjected to the sound of the running treadmill by placing their cages next to the exercising animals.
Physical activity challenge.
After 8 wk of training, rats were evaluated for their ability to sustain a physical activity challenge, as our laboratory described in details previously (37). Briefly, on culmination of exercise training, the efficacy of metabolically active lean tissue (skeletal and cardiac muscles) to sustain a physical challenge was tested in all groups of animals. The rats were scored on a 30-min running challenge. The rats ran for 5-min intervals at the following speeds: 5, 10, 15, 20, 25, and 30 m/min. The following activity capacity scores were assigned: 0, no run; 0.5, complete run for 5 min; 1.0, complete run for 10 min; 1.5, complete run for 15 min; 2.0, complete run for 20 min; 2.5, complete run for 25 min; and 3.0, complete run for 30 min. In case of incomplete runs during a step, the full score corresponding to the lower step was assigned. An electric prodder was used during this procedure, and a run was scored incomplete when the animal spent 30 continuous seconds on the prodder.
Cardiac physiological evaluation.
At the end of 8 wk of diabetes, steady-state left ventricular (LV) function was evaluated via closed-chest carotid arterial catheterization in rats under pentobarbital sodium anesthesia (40 mg/kg ip). The rats were wrapped in warm towels to maintain their body temperature and were allowed to breathe spontaneously during the procedure. A 40-mm-long incision was made in the carotid triangle for right common carotid artery exposure. The Millar microtip conductance catheter was introduced via a carotid incision secured by proximal and distal sutures. The catheter was gently advanced while continuously monitoring the pressure changes to ascertain its entry in to the LV. Following the introduction of the catheter, we allowed time for stabilization of breathing before recording pressure and volume (PV) data. While the catheter was secure in the LV, the steady-state PV data were recorded with the Power Lab MPVS-400 system (ADInstruments, Colorado Springs, CO). The conductance catheter approach specified by the Millar Instruments small-animal cardiac catheterization and measurement manual was utilized. A multivolume cuvette standard with blood obtained from BBDR rats (from a prior pilot study) was used to obtain the conversion factor via linear correlation curve fit. Immediately after data collection, the rats were euthanized with an overdose of pentobarbital sodium.
The pressure and volume data were analyzed with Chart 5.1.1 software (ADInstruments, Colorado Springs, CO). The values for heart rate, stroke volume, ejection fraction, LV output, maximum change in pressure over time (dP/dtmax; +dP/dt) and minimum change in pressure over time (dP/dtmin; −dP/dt) were averaged from at least three consecutive cardiac cycles (60).
Citrate synthase activity assay.
An increase in the skeletal muscle citrate synthase (CS) activity was used as a marker of exercise training. The CS activity assay was performed as described (51). Briefly, left soleus muscle tissue (∼200 mg) was homogenized with 20 pestle strokes in glass-Teflon homogenizer on ice in the homogenization buffer [containing 0.1 M Tris, pH 8.1, 0.15 M NaCl, 0.1% Triton X-100, 1 mM ethylenediaminetetraacetate (EDTA) and 0.2 mM phenylmethylsulfonyl fluoride] using tissue mass (mg)-to-buffer volume (μl) ratio 1:20. The homogenate was centrifuged at +4°C at 16,000 g for 15 min. The supernatant was removed and stored at −80°C. Protein concentration in the extracts was measured using Protein Assay Reagent (Bio-Rad, Hercules, CA), and the CS activity in the extracts was assayed in the multiwell plate format as follows. Each well of the 96-well plate contained a final total volume of 200 μl of the reaction mix, and the reagents were added in the following order: 20 μl 5,5′-dithio-bis(2-nitrobenzoic acid) (Sigma), 5 mM in 1 M Tris·HCl, pH 8.1; 130 μL H2O; 30 μl acetyl coenzyme A (Sigma), 10 mM in H2O; 10 μl muscle extract; and 10 μl oxaloacetic acid (Sigma), 50 mM in 0.1 M Tris·HCl, pH 8.1. Final concentrations of the reagents were as follows: 0.5 mM 5,5′-dithio-bis(2-nitrobenzoic acid), 1.5 mM acetyl coenzyme A, and 2.5 mM oxaloacetic acid. The oxaloacetic acid was added last, to start the reaction immediately before absorbance recording. The reaction was monitored by measuring the absorbance in each well at 405-nm wavelength every 20 s for 3 min using a MRXII Microplate Reader and Kinetic software package (Dynex Technologies, Chantilly, VA). The linear portion of the reaction curve was used to calculate the CS activity, which was expressed in 405-nm absorbance per minute per milligram total protein. All assays were done in duplicates, and each extract was assayed for the CS activity at least three times.
Histology.
To determine the characteristics of the passive components of cardiac pump function, histological analyses for interstitial collagen were performed. LV tissue from ∼3 mm above the heart apex was transferred to 4% paraformaldehyde for fixation, followed by paraffin embedding, sectioning, and staining with picrosirius red to detect interstitial collagen deposits with light microscopy (36). Images were acquired with a Nikon eclipse TE300 microscope equipped with a spot RTKE camera (Diagnostic Instruments, Sterling Heights, MI). Three fields of view captured at random from every specimen obtained from the LV of each animal were analyzed for histological measurements. Every LV was included in the analysis, and more than five sections from each LV were obtained for analysis.
Transmission electron microscopy.
To determine the characteristics of the active components of the cardiac pump function, myocardial mitochondrial analyses by transmission electron microscopy was performed. To ensure mitochondrial integrity, the LV tissue was obtained from the beating heart. Sample preparation for ultrastructural analyses was performed as in our laboratory's previous study (46). Samples were rinsed in cold PBS and placed in 2% gluteraldehyde at +4°C. This process took <10 s from biopsy of the beating heart to fixation. The tissue was rinsed in buffer and postfixed with 1% osmium tetroxide. Tissue was rinsed with distilled water before undergoing a graded ethanol dehydration series and was infiltrated using a mixture of one-half propylene oxide and one-half resin overnight. Twenty-four hours later, the tissue was embedded in Epon 812 resin (Electron Microscopy Sciences, Ft. Washington, PA). Eighty-nanometer sections were cut on an LKB Nova Ultratome and were placed on acid treated grids, which were stained using a double lead stain technique with 0.5% lead citrate and 7% uranyl acetate. Images were captured using a JEM 100 CXII transmission electron microscope at 80 kV. Six fields of view, captured at random from each specimen, were included for image analyses.
Image analyses.
Histological and ultrastructural profiles were analyzed with Image J, a freely downloadable Java-based environment (http://rsb.info.nih.gov/ij). The myocardial interstitial collagen fractional area was determined as the ratio of interstitial collagen area to the area of the field of view, expressed as a percentage. Mitochondrial fractional area was determined in a similar manner. Only viable mitochondria were included, as defined by intact inner and outer membranes (46).
LV protein extraction.
For total protein extract, LV tissue (∼30 mg) was homogenized using a glass Teflon homogenizer in an ice-cold extraction buffer (1:20 mass/volume) containing 10 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 20 mM Na2MoO4, 50 mM NaF, 0.2 mM Na3VO4, and 1% Triton X-100. Extraction buffer was supplemented with complete, Mini EDTA-Free Protease Inhibitor Cocktail tablets (Roche Applied Science, Indianapolis, IN). After centrifugation at 16,000 g for 15 min at 4°C, the supernatant was collected. Cytosolic and membrane protein fractions were extracted from ∼150 mg of LV tissue as described elsewhere (55). Protein concentration was determined using Protein Assay Reagent (Bio-Rad, Hercules, CA).
PKC βII immunoblotting.
Equal amounts of myocardial samples per lane (50–100 mg) were electrophoresed on 8% SDS-polyacrilamide gels, transferred onto polyvinylidene fluoride membranes, and processed according to a standard immunoblotting procedure (50). For PKC-βII detection, primary rabbit polyclonal antibody (catalog no. sc-210, Santa Cruz Biotechnology, Santa Cruz, CA) was used. Antibody binding was assessed by Pierce Supersignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). Rat cerebrum lysate (BD Biosciences, San Jose, CA) was used as a positive control. The specificity of the PKC-βII bands was verified using the antibody specific blocking peptide (catalog no. sc-210 P: Santa Cruz Biotechnology). Equal loading was confirmed with Ponceau's solution (Sigma) staining of membranes. Densitometric analysis was carried out with Adobe Photoshop CS2 software.
PKC-βII activity assay.
PKC-βII was partially purified by passing the fractions through a column with TSK Toyopearl DEAE-650M (Toyo Soda, Tokyo, Japan) and was eluted with 200 mM NaCl. PKC-βII activity was measured using phosphocellulose P81 (Whatman) paper assay (43, 44), as described elsewhere (11, 30). Phosphate incorporation into a hexadecameric peptide ERMRPRKRQGSVRRRV (synthesized and purified by SynBioSci, Livermore, CA), a PKC ϵ-pseudosubstrate (11), was measured at 30°C for 10 min in the reaction mixture containing 10 mM MOPS, pH 7.2, 12.5 mM β-glycerophosphate, 0.5 mM sodium orthovanadate, 0.5 mM dithiothreitol, 0.5 mM CaCl2, 10 mM Mg(CH3CO2)2, 0.2 mM [γ-32P]ATP (50 Ci/mol), 80 μg/ml phosphatidylserine (Sigma), 8 μg/ml DAG (Upstate Biotechnology, Lake Placid, NY), 0.3 μM protein kinase A inhibitor peptide (PKI; Upstate Biotechnology), 3.3 μM Ca2+/calmodulin-dependent protein kinase inhibitor (compound R24571, Upstate Biotechnology), and 95 μM peptide. PKI and compound R24571 were used to ensure that measured activity was due to PKC-βII. Aliquots (30 μl) were spotted onto P81 filters, washed with 0.5% H3PO4, and counted in a scintillation counter. To account for basal activity, including autophosphorylation, the reaction was performed in the absence of the substrate, and these values were subtracted from corresponding experimental values.
LV lipid analyses.
Samples for lipid analysis were prepared essentially as described (5). Briefly, 50 mg of fresh frozen tissue were cut in small pieces and homogenized in 500 μl of ice-cold buffer containing 50 mM Tris, pH 7.4, 0.25 M sucrose, 25 mM KCl, and 0.5 mM EDTA, using glass Teflon homogenizer for 40 s. After cooling on ice for 15 min, the samples were homogenized again for 20 s, and the homogenates were filtered through the gauze pad, assayed for protein concentration, and stored at −80°C until the analysis. Analysis for the levels of DAG and sphingosine was performed according to the methods presented elsewhere (5).
Statistical analyses.
Statistical analysis was performed with SPSS (version 15.0). Group differences on parameters were tested with a factorial (two-way) ANOVA. Two factors with two levels were specified as follows: factor A, health status (level 1: nondiabetic; level 2: diabetic), and factor B: physical activity status (level 1: nonexercised; level 2: exercised). In the presence of insignificant interaction effects, further analysis on the main effects was performed using simple one-way ANOVA at each level of factors A and B. In the presence of group differences, post hoc pairwise multiple comparisons were performed using Tukey's honestly significant difference test with the level of significance held at P ≤ 0.05. When significant interaction effects were observed in the presence of significant main effects (ordinal), a simple one-way ANOVA was performed with Tukey's honestly significant difference post hoc tests. In the presence of significant, disordinal interactions, the interpretation of data from one-way ANOVA was preceded with caution that the main effects may not be necessarily significantly different on marginal means. Data were presented as means ± SE.
RESULTS
Gravimetry and glucometry.
Table 1 summarizes the results from gravimetry and glucometry of rats with 8-wk diabetes and exercise training. With insulin treatment, the ND group was able to maintain 80% of the NN group's mean body mass. Meanwhile, the heart-to-body mass ratio of ND, EN, and ED groups was increased (23, 18, and 24%, respectively) compared with the NN group, suggestive of cardiac hypertrophy in the former three groups. The results from heart-to-body mass ratio, however, were not an unbiased indicator of cardiac hypertrophy in the animals due to the catabolic flux-induced body mass loss in diabetes. Meanwhile, taken together with complementary data (e.g., activity capacity and microscopy), it provides information on the cardiac health of animals in this study.
Table 1.
Glucometry and gravimetry of rats at the end of 8 wk of diabetes and exercise training
| NN | ND | EN | ED | |
|---|---|---|---|---|
| Body mass, g | 379 ± 13b,d | 302 ± 32a | 341 ± 19 | 311 ± 39a |
| Heart mass, g | 1.05 ± 0.05 | 1.03 ± 0.09 | 1.12 ± 0.09 | 1.07 ± 0.11 |
| Heart/body mass, mg/g | 2.78 ± 0.09b,c,d | 3.43 ± 0.14a | 3.27 ± 0.15a | 3.44 ± 0.22a |
| Plasma glucose, mg/dl | 122 ± 12b,d | 546 ± 63a,c | 111 ± 21b,d | 482 ± 171a,c |
| HbA1c, % | 4.8 ± 0.4b,d | 10.8 ± 1.6a,c,d | 5.2 ± 0.2b,d | 7.8 ± 0.4a,b,c |
Values are means ± SE; n = 8 rats/group. NN, nonexercised nondiabetic; ND, nonexercised diabetic; EN, exercised nondiabetic; ED, exercised diabetic. Significant difference from
NN,
ND,
EN, and
ED: P ≤ 0.05. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity) was significant for body mass (P = 0.021), heart/body mass (P = 0.001), and HbA1c measurements (P = 0.001), while it was not significant for heart mass and plasma glucose measures.
Insulin therapy alone was unable to restore the glucose homeostasis in the ND group, as demonstrated by their HbA1c levels. However, with the combination of exercise training and insulin treatment, there was a 28% decrease in HbA1c levels in diabetic animals compared with the ND group, illustrating the beneficial role of exercise on plasma glucose regulation in BBDR rats.
Skeletal muscle CS activity.
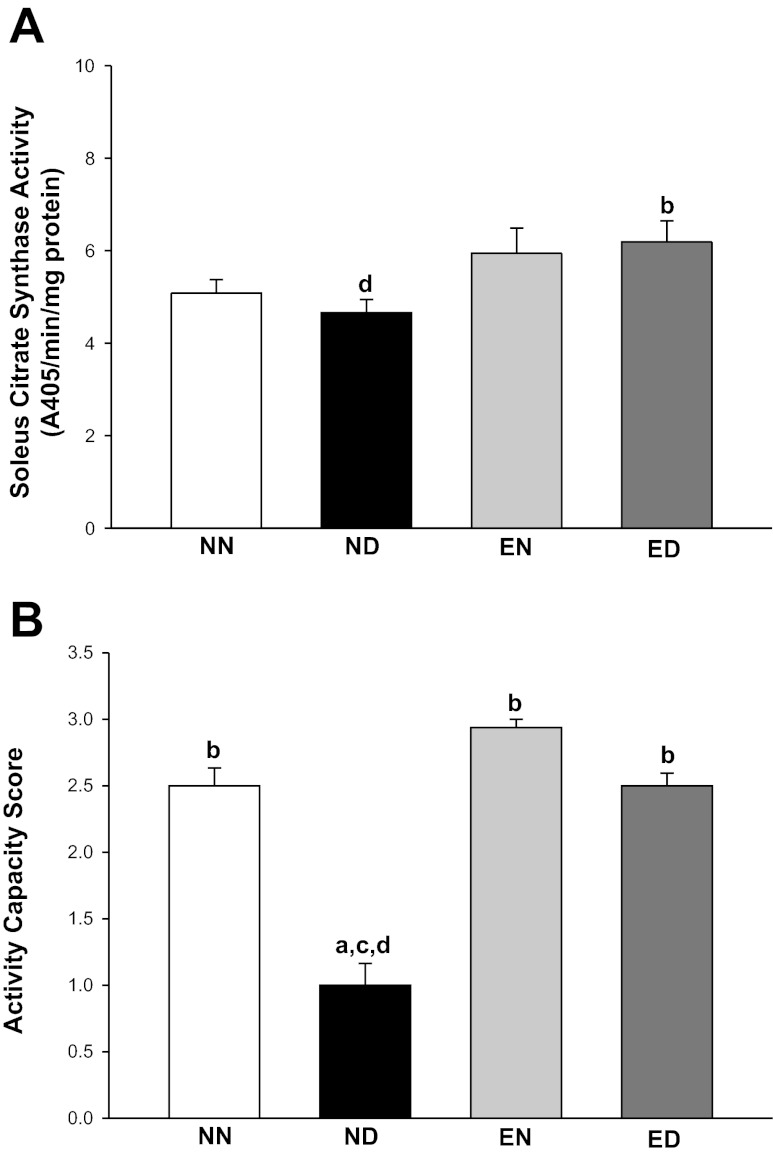
The soleus CS activity was increased 32% in the ED group compared with the ND group, thus marking the training effect in diabetes (Fig. 1A).
Fig. 1.
Exercise training increased skeletal muscle citrate synthase activity and activity capacity in diabetes. A: citrate synthase activity levels determined in the soleus muscles of rats from all groups. The activity of mitochondrial oxidative enzyme was measured to mark the exercise training effect. The enzyme activity was expressed as 405-nm absorbance (A405) per minute per milligram protein. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was not significant. B: activity capacity scores assigned to all four groups of rats after the cessation of exercise training. The scoring method was employed to complement the results from A and to partly determine the ability of metabolically active lean tissue to sustain an increased physical challenge in the presence of cardiac hypertrophy. Higher scores indicate better performance. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was significant (P = 0.001). NN, nonexercised nondiabetic; ND, nonexercised diabetic; EN, exercised nondiabetic; ED, exercised diabetic. Values are means ± SE. Significant difference from a NN, b ND, c EN, and d ED: P ≤ 0.05.
Physical activity challenge.
The mean activity capacity score (Fig. 1B) was 2.5 in the NN and ED groups and 3.0 in the EN group, whereas it was 1.0 in the ND group, the latter indicating compromised ability to sustain physical activity in the presence of diabetes and the absence of exercise training.
LV hemodynamics.
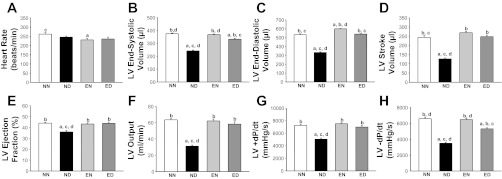
The LV hemodynamic parameters are summarized in Fig. 2. There was no significant difference in the heart rate between the NN, ND, and ED groups. However, we observed a 12% decrease in the heart rate of the EN group compared with the NN group.
Fig. 2.
Exercise training improved left ventricular (LV) hemodynamics in diabetes. Steady-state LV hemodynamic data from all groups of rats are shown. A: heart rate. B: end-systolic volume. C: end-diastolic volume. D: stroke volume. E: ejection fraction. F: output. G: maximum change in pressure over time (+dP/dt). H: minimum change in pressure over time (−dP/dt). Values are means ± SE. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was significant (P = 0.001) for all hemodynamic measures with the exception of A. Significant difference from a NN, b ND, c EN, and d ED: P ≤ 0.05.
While the stroke volume decreased by 48% in the ND group compared with the NN group, exercise training prevented this deficit in the diabetic rats. Accordingly, the ejection fraction showed an 18% decrease in the ND group compared with the NN group and improved in diabetes, with training. The basal decrease in ejection fraction across all four groups (relative to the 55–70% standard in humans) could be attributed to the use of pentobarbital sodium anesthesia, which has the effect of attenuating steady-state LV ejection fraction in rodents (25, 59).
The LV output in the ND group decreased by 51% compared with that in the NN group. Both the +dP/dt and −dP/dt showed a decrease (30 and 46%, respectively) in the ND group compared with the NN group. This indicated that both the active (contractile) and passive (relaxation) components of cardiac pump function were compromised in the diabetic heart. With training, however, diabetic rats were able to overcome these deficits in +dP/dt and −dP/dt.
Significant interaction effect between the health status (factor A) and physical activity status (factor B) was observed on all hemodynamic measures, except heart rate, suggesting the differential effects of exercise training on nondiabetic and diabetic rats.
LV myocardial histology.
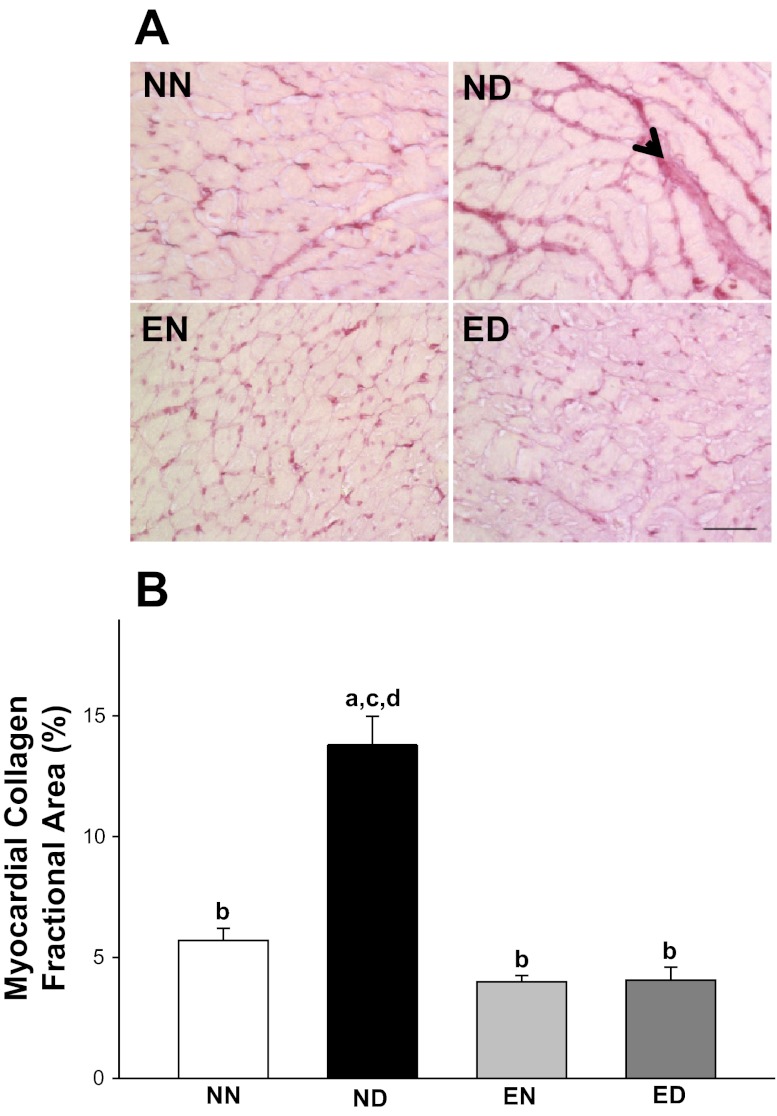
Upon screening of collagen, a structural component of passive cardiac pump function, nonuniformly distributed dense picrosirius red staining indicative of thick collagen weaves dominated the histological profiles of ND myocardium (Fig. 3). The collagen distribution was uniformly sparse and pericellular in the NN, EN, and ED groups. The collagen fractional area increased 2.4-fold in the ND group compared with the NN group. With training, the collagen fractional area in the diabetic myocardium returned to levels (4.1 ± 2.8%) comparable to control (5.7 ± 2.7%). Significant interaction effects between the health status and physical activity status of the animals was observed with respect to collagen deposition in the myocardium.
Fig. 3.
Exercise improved passive cardiac function in diabetes by preventing the accumulation of myocardial collagen. A: collagen-specific picrosirius red stained histological profiles of LV myocardium from all rat groups. The collagen distribution is highlighted by an arrowhead. Scale bar: 50 μm. B: LV myocardial collagen fractional area expressed as percent collagen fractional area in all four experimental groups. Values are means ± SE. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was significant (P = 0.001). Significant difference from a NN, b ND, c EN, and d ED: P ≤ 0.05.
LV myocardial ultrastructure.
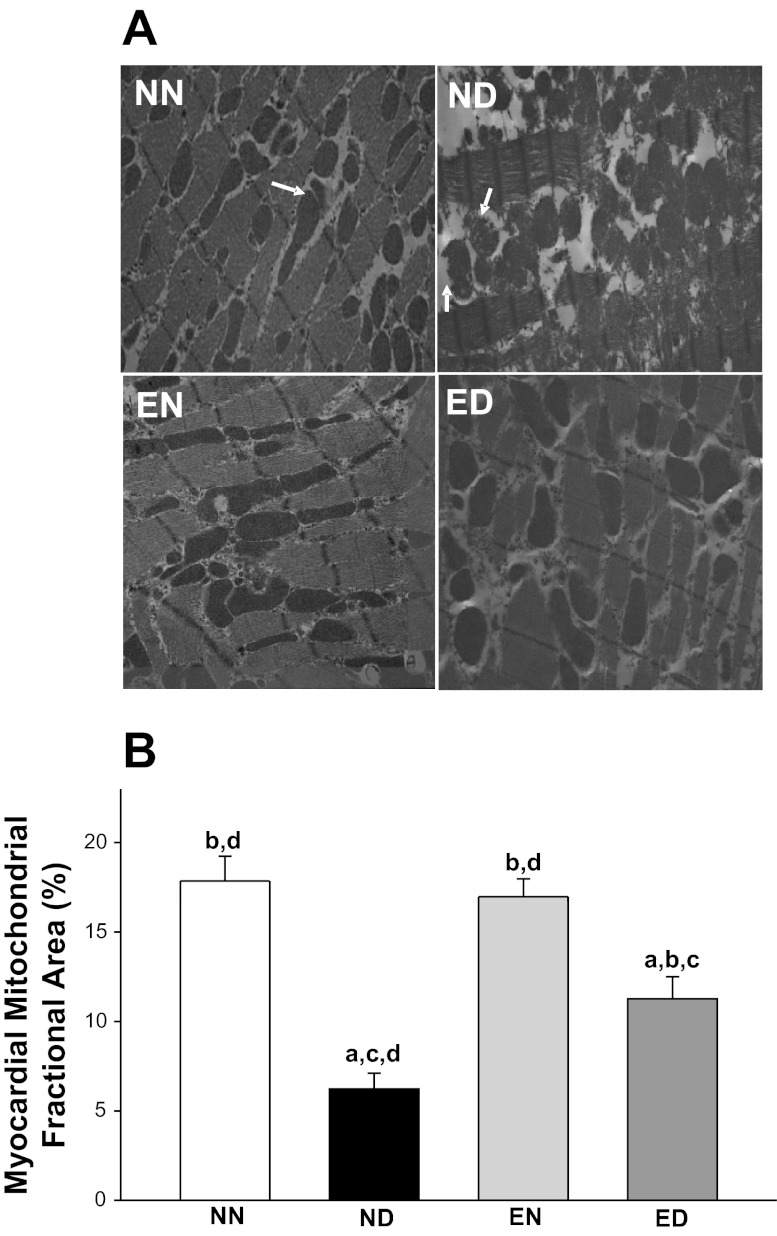
To quantify the structural components fundamental to active cardiac pump function, we analyzed the distribution and characteristics of mitochondria. The viable mitochondrial fractional area in the ND group decreased 65% compared with that in the NN group (Fig. 4). With training, the reduction in viable mitochondrial fractional area in the diabetic myocardium was attenuated by an 81% increase compared with the ND rats. A significant interaction effect between the health status and physical activity status of animals was observed with respect to LV myocardial mitochondrial fractional area.
Fig. 4.
Exercise improved active cardiac function in diabetes by preventing myocardial mitochondrial damage. A: the presentation of left ventricular myocardial mitochondria (arrows) and their distribution pattern in all rat groups. The arrows in the ND profile mark a few of the many nonviable mitochondria with damaged membranes. Transmission electron microscopy images magnification: ×7,200. B: quantification of LV myocardial mitochondrial fractional area expressed as percent viable mitochondrial fractional area in all four experimental rat groups. Only viable mitochondria, defined as electron-dense mitochondria with intact outer and inner membranes, were considered for analysis. Values are means ± SE. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was significant (P = 0.022). Significant difference from a NN, b ND, c EN, and d ED: P ≤ 0.05.
LV PKC-βII protein levels and activity.
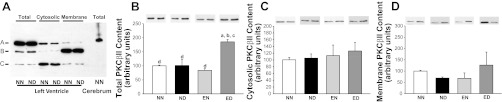
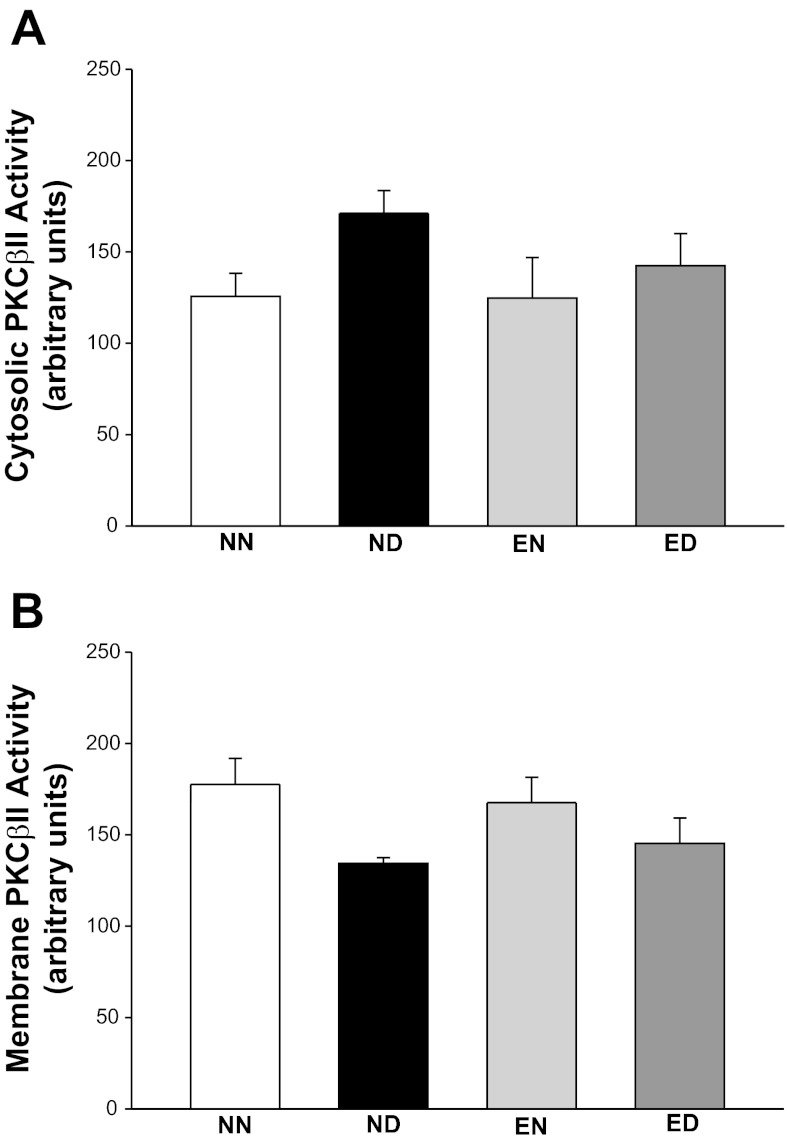
Immunoblotting analysis revealed that the PKC-βII antibody detected protein bands with different apparent molecular masses in rat LV extracts (total, cytosolic, and membrane protein fractions) (Fig. 5A). We observed three bands in the total protein extracts with apparent molecular masses of 80, 78, and 76 kDa, similar to findings from human ventricular tissue (48). The 80-kDa band comigrated with the single band of 80 kDa from the rat cerebrum total protein lysate (positive control) (19, 23). The three forms of PKC-βII observed in the rat LV extracts differ by their phosphorylation state that affects their electrophoretic mobility (31, 42). The 76-kDa form represents a newly synthesized protein that is not phosphorylated or phosphorylated at threonine 500. This form is not active toward PKC substrates (31). Its autophosphorylation at threonine 641 results in the 78-kDa form that is capable of further autophosphorylation at serine 660, yielding the 80-kDa form. The 80-kDa form represents a “mature” protein that is fully phosphorylated and capable of translocating to membrane (42) marking PKC-βII activation. Noteworthy, all three bands were specific PKC-βII bands, as confirmed using the antibody-specific blocking peptide (not shown). Of the three forms, the mature 80-kDa form is active both in vivo and in vitro; thus we included only this form in our further analyses, similar to other reports (26). The total PKC-βII protein level was indistinguishable between the NN, ND, and EN groups (Fig. 5B). Training mediated an 82% increase in the protein levels of ED compared with the ND group. Since PKC-βII activation is indirectly evaluated by its distribution between cytosol and membrane (26), we analyzed the PKC-βII levels in cytosolic and membrane fractions. We noticed no difference in the cytosolic and membrane levels of PKC-βII between groups (Fig. 5, C and D). Moreover, the PKC-βII activity in cytosolic and membrane LV fractions was also not different between groups (Fig. 6, A and B). No interaction effect was observed between the main factors with respect to PKC-βII activity, as well as cytosolic/membrane protein expression levels.
Fig. 5.
Fractional LV PKC-βII content was unaffected in the presence of diabetes and exercise. Immunoblotting analysis of PKC-βII in total protein extracts and cytosolic and membrane fractions obtained from the NN BBDR rat LV tissue is shown. A: presentation of PKC-βII immunoreactive bands in the LV tissue from NN and ND rats is shown. A, B, and C correspond to PKC-βII isoforms migrating at 80, 78, and 76 kDa, respectively. Rat cerebrum lysate was used as a positive control. B–D: representative immunoblots of the “mature” (80-kDa) PKC-βII band in LV total protein extracts (B) and cytosolic (C) and membrane (D) fractions from all four rat groups (top) and its quantitation by densitometry (bottom) are presented. Values are means ± SE. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was significant (P = 0.002) for the total and was not significant for the cytosolic and membrane fractions. Significant difference from a NN, b ND, c EN, and d ED: P ≤ 0.05.
Fig. 6.
Fractional LV PKC-βII activity was unaffected in the presence of diabetes and exercise. LV PKC-βII activity in cytosolic (A) and membrane (B) fractions of all rat groups measured on activity of the protein with a synthetic pseudosubstrate is shown. Values are means ± SE. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was not significant.
LV PKC-βII activator and inhibitor levels.
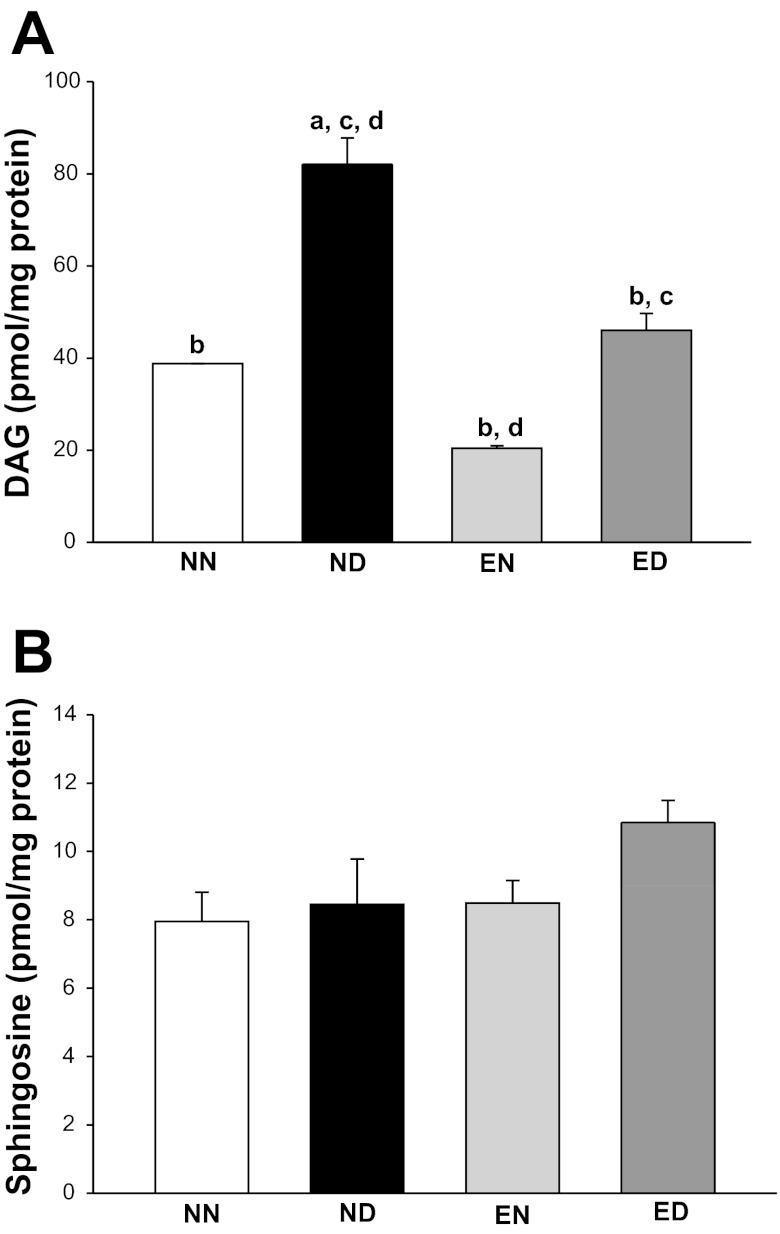
The level of PKC-βII activator, DAG, increased 115% in the diabetic LV compared with NN group, and this increase was prevented with exercise training (Fig. 7A). Moreover, we noticed that the level of sphingosine, a potent inhibitor of PKC-βII, was unchanged in the LV of diabetic animals and demonstrated no difference with exercise training (Fig. 7B).
Fig. 7.
Exercise prevented the increase in cardiac diacylglycerol (DAG) in diabetes, but did not change sphingosine levels. High-performance liquid chromatography-tandem mass spectrometry analysis of LV DAG (A) and sphingosine (B) in all rat groups is shown. Values are means ± SE. Factorial (2 × 2) ANOVA interaction effect (health status × physical activity status) was not significant for both myocardial DAG and sphingosine levels. Significant difference from a NN, b ND, c EN, and d ED: P ≤ 0.05.
DISCUSSION
The main findings of present study on the BBDR rat model of Type 1 diabetes are as follows: 1) manifestation of abnormal cardiac structure and function; 2) prevention of cardiac abnormalities with endurance exercise training; and 3) decrease in myocardial DAG levels as a possible mechanism mediating the training-induced cardiac benefits in Type 1 diabetes.
We chose the rat model to study DCM pathogenesis and intervention, since rats are inherently resistant to the development of atherosclerosis in the absence of high-fat diet. As a result, rats have become the model of choice for studying DCM pathology, without the confounding effects of cardiac macrovascular disease (24). BBDR rats circulate normal numbers of CD4+, CD8+, and ART2+ T cells and develop diabetes only under conditions that mimic an interaction of a viral perturbant with their genetic loci susceptible for diabetes through autoimmune pathogenesis, hence modeling human Type 1 diabetes effectively (39, 41). Although invaluable in our understanding of diabetic complications, the chemotoxin-induced models (e.g., the streptozotocin- or alloxan-induced diabetes) suffer from a disadvantage in modeling humans with Type 1 diabetes due to the absence of autoimmune pathogenesis and compensatory mechanisms that allow for survival without insulin dependence. The aforementioned features preclude the results obtained from chemotoxin-induced models of Type 1 diabetes for efficient translational and therapeutic utilization. With the utility of BBDR rats, however, these limitations were overcome. The manifestation of structural features like myocardial interstitial fibrosis and decrease in viable myocardial mitochondrial density, along with compromised cardiac pump function, showed that the BBDR rat model could be successfully utilized to investigate the pathophysiology of diabetic heart.
With training, rats in the ED group demonstrated an increased activity of soleus CS compared with their nonexercised counterparts. It was shown in our laboratory's earlier studies that the training protocol used in this study was sufficient to improve cardiac structure and function in diabetes compared with nonexercised animals (46, 51). The activity capacity scores, complemented by the histological results for collagen accumulation in the myocardium of the ED group, suggested a cardiac training effect, although peripheral adaptation effects could not be ruled out.
The ND group showed a significant compromise in LV pump function, as demonstrated by the decrease in stroke volume and ejection fraction compared with the NN group. These results in BBDR rats were reminiscent of the cardiac performance data, obtained noninvasively, from Wistar rats at similar duration of diabetes (2). In addition to cardiac cycle volumetry, the catheterization procedure allowed us to quantify the instantaneous rates of change of both maximum and minimum LV pressure (+dP/dt and −dP/dt, respectively) in the ND rats, which were significantly diminished compared with NN rats. The latter result suggests diminution of both systolic and diastolic LV function in BBDR diabetic rats and, consequently, the possibility of failing active and passive components of the cardiac pump. With exercise, however, the diabetic rats were able to retain most of their cardiac pump function comparable to the nondiabetic rats.
The abnormal LV physiological parameters due to the sedentary mode of life in diabetes were improved following exercise training. It is possible that some degree of cardiac dysfunction in BBDR rats was perhaps a result of the catabolic flux, unlike in individuals with Type 1 diabetes (10), thus limiting direct translation of our present study. However, despite the basal catabolic milieu, exercise training improved cardiac function. Stroke volume, ejection fraction, LV output, and +dP/dt benefited the most from endurance training. These results clearly underscore the role of endurance training in benefiting the diabetic heart beyond that attainable by insulin therapy alone. Moreover, the results also corroborate the findings of previous reports on the beneficial role of exercise training on the diabetic heart in other animal models of Type 1 diabetes (8, 13, 14, 32, 33). We noted a decrease in the steady-state ejection fraction in both diabetic and nondiabetic groups. Our results were consistent with previous reports of decreased ejection fraction in rats anesthetized with pentobarbital sodium under both diabetic (38) and nondiabetic conditions (1, 40). Meanwhile the interaction effects of main factors in our study also pointed out to the differential effects of training on cardiac hemodynamics in the diabetic vs. nondiabetic animals. Interestingly, exercise-mediated improvement of LV end-systolic volume, end-diastolic volume, stroke volume, ejection fraction, output, and pressure differential was dependent on the diabetic status. The improvement in cardiac physiology conferred by exercise was accompanied by improvement in myocardial histology and ultrastructure as well.
Interstitial collagen accumulation in the myocardial tissue is one of the cardinal features of DCM (15) and serves as the marker for detrimental passive performance of the cardiac pump (57). The passive components (interstitial fibrous proteins) of the cardiac pump confer the myocardium with resilient properties and are the major determinants of −dP/dt during the cardiac cycle. Our results indicated an increase in the distribution of interstitial myocardial collagen in the ND group. The −dP/dt values of the ND group, along with the abundance of collagen distribution, were suggestive of a nonresilient and stiff LV myocardium, reminiscent of our laboratory's earlier structural MRI report (36). With endurance training, the structural defect in collagen accumulation in diabetes was attenuated, along with the −dP/dt value.
The mitochondria have been implicated as the ultimate target of diabetic cardiovascular complications (9). Specifically, the diabetic heart manifests both structural and functional abnormalities of mitochondria (16, 46). By quantifying the viable fraction of myocardial mitochondria, we were able to verify one of the major organellar structural correlates that may underlie the LV functional deficits exhibited by the ND group. The ejection fraction and +dP/dt, both parameters representative of the functional efficacy of active cardiac pump, were decreased in the ND group compared with the NN group. It is possible that the significant decrease in viable mitochondrial fractional area in the ND group underlies the deficits of active cardiac pump function manifested by the diabetic LV under a sedentary condition. Endurance training rescued the diabetic LV from the abovementioned functional deficits. The functional restoration occurred in the presence of an increase in the viable mitochondrial fractional area in trained diabetic rats. Taken together, these results verify the role of exercise-mediated benefits on both functional and structural correlates of cardiac pump. Hence, we proceeded to investigate, at the molecular level, the correlate(s) of exercise-induced benefits on cardiac function in Type 1 diabetes.
Activation of PKC-βII has been shown as an underlying factor, resulting in cardiac hypertrophy and fibrosis under hyperglycemic conditions (20, 26, 35, 49, 55, 56). Hence, we hypothesized that the exercise-induced benefits on diabetic myocardium are mediated by the modulation of molecules involved in PKC-βII signaling. PKC-βII, one of the conventional isoforms of PKC, is membrane bound for its activation (31). As a result, previous studies have used the fractional distribution of the protein in myocardial tissue as an indicator of its activation in vivo in the diabetic heart (26). The isoform-specific activity has been measured in vitro by the capacity of PKC-βII to phosphorylate its substrates (26).
We looked at the levels, fractional distribution, and activity of PKC-βII as possible mediators of exercise-induced benefits in the diabetic myocardium. Diabetes did not change the total PKC-βII protein levels, while exercise training produced an increase in its levels. Fractionation of myocardial PKC-βII into cytosolic and membrane components did not reveal any difference in protein content and activity between groups. Studies by others have shown that the PKC-βII protein levels either increased (26, 58) or did not change (28) in the myocardial membrane fraction in diabetes. In addition, the cytosolic PKC-βII protein levels were found unchanged (19, 26, 28, 58) or even increased (21) in diabetes. Moreover, PKC-βII activity was increased in membrane fraction only (26), or in both cytosolic and membrane fractions (19) of the diabetic myocardium. This apparent inconsistency may be due to differences in models used and duration of diabetes. Furthermore, the PKC-βII activity assay performed in vitro could not necessarily be an accurate reflection of the situation in vivo, when the amount of PKC-βII protein (cytosolic or membrane) present in control and diabetic cardiac tissue may be the same, but its activation may depend on the amount of its physiological activator (DAG). The in vitro activity assay of semipurified PKC-βII reflects the total activatable PKC-βII when an exogenous activator is added in excess. Thus it parallels the amount of protein evaluated by immunoblotting and, as such, confirms the results of the immunoblotting in this study. Meanwhile, as indicated by the results in our study, diabetes- or exercise-induced regulation of PKC-βII protein expression, membrane translocation, and in vitro measured activity did not occur in BBDR rat myocardium.
PKC activation is dependent on the availability of DAG (and hence its levels); meanwhile, PKC activity is not solely dependent on the availability of its activator (DAG), but on the availability of PKC-βII substrates as well. Catalytically incompetent PKC is cytosolic and is bound to a pseudosubstrate in vivo. DAG is required for the binding of PKC to membrane and detachment of the pseudosubstrate from the catalytic site (activation of PKC) to make the kinase competent and accessible to substrates. We considered the possibility that DAG, the key activator of PKC-βII in diabetes, was modulated by exercise training. Widespread increase in DAG synthesis occurs as a result of hyperglycemia in vascular tissues (27), including the heart (26). Chronic hyperglycemia leads to increased DAG accumulation, mainly through de novo synthesis (56). DAG-induced activation of PKC-βII has been attributed to the vascular complications of diabetes (9, 35, 55, 56). Previous studies suggested the possibility of modulation of skeletal muscle DAG content in the context of exercise-mediated insulin signaling benefits on skeletal muscle (12, 54). However, the aforementioned studies involved electrical stimulation of normal skeletal muscles that may not be applicable to diabetic cardiac muscle with exercise training. Our results indicated that the myocardial DAG content was increased in diabetic rats compared with the nondiabetic group. Importantly, this increase was prevented by exercise training. Interestingly, exercise also resulted in an associative decrease in the myocardial DAG of nondiabetic rats, thus demonstrating no interaction effect between the main factors.
Sphingosine, one of the breakdown products of sphingolipids, is a potent inhibitor of PKC-βII (22). Our results indicated no changes in the myocardial sphingosine content in diabetic rats. Similarly, studies on obese individuals with Type 2 diabetes showed no changes in cardiac sphingosine levels (3). Exercise has been shown to mediate an increase in skeletal muscle sphingosine in healthy rats (6). However, the role of exercise in modulating cardiac sphingosine levels has not been studied. Our results showed no change in myocardial sphingosine content with diabetes and exercise training, suggesting that sphingosine was not a likely mediator of exercise-induced benefits in diabetes.
Our study revealed that neither diabetes nor exercise significantly affected the fractional LV PKC-βII content and activity in BBDR rats. Although our biochemical results were in contrast to some other reports studying various other animal models of Type 1 diabetes (26, 58), they were, on the other hand, not unique to our study (19, 28), as mentioned earlier. The hemodynamic, histological, and ultrastructural data obtained in our study, meanwhile, indicated the deterioration of cardiac structure and function with diabetes in the absence of exercise. Collectively, these results were suggestive of compromised homeostasis at the molecular level that perhaps is not the result of a change in PKC-βII content in BBDR rats. Meanwhile, on investigation for possible biochemical changes upstream (activation) and downstream (inhibition) of PKC-βII, which may underlie the pathological changes of the diabetic heart and their alleviation by exercise training, DAG (the activator of PKC-βII) emerged as a likely molecular candidate and a possible mediator of exercise-induced benefits in the diabetic heart. Exercise decreased DAG and fibrosis without impacting PKC-βII levels and activity.
In summary, our results indicated that it was the differential availability of the PKC-βII activator (DAG) and not the PKC-βII protein content/activity that was implicated in the cardiac abnormalities of BBDR rats with autoimmune diabetes. It is possible that the functional benefits conferred by prevention of myocardial mitochondrial damage in diabetes with exercise complement the benefits of decrease in myocardial DAG. These results are suggestive of an in vivo scenario in which the increased myocardial DAG content in diabetes leads to the complications (9) and its decrease, by association, with exercise training ameliorates cardiac dysfunction. The causal role of exercise-induced decrease in DAG content in ameliorating diabetic cardiac dysfunction awaits further investigation.
GRANTS
This work was funded by American Heart Association (AHA) Scientist Development Award to I. V. Smirnova, and AHA Predoctoral Fellowship Award to R. Loganathan. Partial support was provided by the National Institutes of Health Grant P20 RR016475 from the Idea Networks for Biomedical Excellence Program of the National Center for Research Resources. Core facility equipment and services were supported by National Institute of Child Health and Human Development Grant HD02528.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L. and I.V.S. conception and design of research; R.L., L.N., I.G.B., and I.V.S. performed experiments; R.L., L.N., I.G.B., and I.V.S. analyzed data; R.L., L.N., I.G.B., and I.V.S. interpreted results of experiments; R.L., L.N., and I.V.S. prepared figures; R.L. drafted manuscript; R.L. and I.V.S. edited and revised manuscript; R.L., L.N., I.G.B., and I.V.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Anton Fedosyuk for exercising rats and assistance with diabetes induction, Rosetta Barkley for histological slide preparations, Barabara Fegley for assistance with transmission electron microscopy, and Eileen Roach (deceased) for assistance with light microscopy. We are grateful to Dr. Lisa Stehno-Bittel and the anonymous reviewers for providing critical comments on the manuscript. We thank Dr. Dale L. Greiner (University of Massachusetts Medical Center, Worcester, MA) for kindly donating the anti-RT6 monoclonal antibody DS4.23 hybridoma supernatant, and Dr. Jacek Bielawski (Medical University of South Carolina, Charleston, SC) for assistance with lipid analysis.
Present addresses: R. Loganathan, Department of Cell Biology, Johns Hopkins University School of Medicine, Baltimore, MD; I. G. Boulatnikov, University of Kansas Medical Center Kidney Institute, Kansas City, KS.
REFERENCES
- 1. Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan MI. Chronic alternate-day fasting results in reduced diastolic compliance and diminished systolic reserve in rats. J Card Fail 16: 843–853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Shafei AI, Wise RG, Gresham GA, Carpenter TA, Hall LD, Huang CL. Magnetic resonance imaging analysis of cardiac cycle events in diabetic rats: the effect of angiotensin-converting enzyme inhibition. J Physiol 538: 555–572, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baranowski M, Blachnio-Zabielska A, Hirnle T, Harasiuk D, Matlak K, Knapp M, Zabielski P, Gorski J. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. J Lipid Res 51: 74–80, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell DS. Diabetic cardiomyopathy. Diabetes Care 26: 2949–2951, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39: 82–91, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Blachnio-Zabielska A, Baranowski M, Zabielski P, Gorski J. Effect of exercise duration on the key pathways of ceramide metabolism in rat skeletal muscles. J Cell Biochem 105: 776–784, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation 99: 384–391, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Broderick TL, Poirier P, Gillis M. Exercise training restores abnormal myocardial glucose utilization and cardiac function in diabetes. Diabetes Metab Res Rev 21: 44–50, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Chaturvedi N, Stevens LK, Fuller JH. Mortality and morbidity associated with body weight in people with IDDM. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetes Care 18: 761–765, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Chiarini A, Whitfield JF, Armato U, Dal Pra I. Protein kinase C-beta II is an apoptotic lamin kinase in polyomavirus-transformed, etoposide-treated pyF111 rat fibroblasts. J Biol Chem 277: 18827–18839, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Cleland PJ, Appleby GJ, Rattigan S, Clark MG. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J Biol Chem 264: 17704–17711, 1989 [PubMed] [Google Scholar]
- 13. De Angelis KL, Oliveira AR, Dall'Ago P, Peixoto LR, Gadonski G, Lacchini S, Fernandes TG, Irigoyen MC. Effects of exercise training on autonomic and myocardial dysfunction in streptozotocin-diabetic rats. Braz J Med Biol Res 33: 635–641, 2000 [DOI] [PubMed] [Google Scholar]
- 14. DeBlieux PM, Barbee RW, McDonough KH, Shepherd RE. Exercise training improves cardiac performance in diabetic rats. Proc Soc Exp Biol Med 203: 209–213, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Dhalla NS, Pierce GN, Innes IR, Beamish RE. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1: 263–281, 1985 [PubMed] [Google Scholar]
- 16. Ferko M, Gvozdjakova A, Kucharska J, Mujkosova J, Waczulikova I, Styk J, Ravingerova T, Ziegelhoffer-Mihalovicova B, Ziegelhoffer A. Functional remodeling of heart mitochondria in acute diabetes: interrelationships between damage, endogenous protection and adaptation. Gen Physiol Biophys 25: 397–413, 2006 [PubMed] [Google Scholar]
- 17. Geiss LS, Herman WH, Goldschmid MG, DeStefano F, Eberhardt MS, Ford ES, German RR, Newman JM, Olson DR, Sepe SJ, et al. Surveillance for diabetes mellitus–United States, 1980–1989. MMWR CDC Surveill Summ 42: 1–20, 1993 [PubMed] [Google Scholar]
- 18. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319–1331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giles TD, Ouyang J, Kerut EK, Given MB, Allen GE, McIlwain EF, Greenberg SS. Changes in protein kinase C in early cardiomyopathy and in gracilis muscle in the BB/Wor diabetic rat. Am J Physiol Heart Circ Physiol 274: H295–H307, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Guo M, Wu MH, Korompai F, Yuan SY. Upregulation of PKC genes and isozymes in cardiovascular tissues during early stages of experimental diabetes. Physiol Genomics 12: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Gurusamy N, Watanabe K, Ma M, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Inactivation of 14–3-3 protein exacerbates cardiac hypertrophy and fibrosis through enhanced expression of protein kinase C beta 2 in experimental diabetes. Biol Pharm Bull 28: 957–962, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Hannun YA, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 243: 500–507, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Huang KP, Huang FL. Immunochemical characterization of rat brain protein kinase C. J Biol Chem 261: 14781–14787, 1986 [PubMed] [Google Scholar]
- 24. Iltis I, Kober F, Dalmasso C, Cozzone PJ, Bernard M. Noninvasive characterization of myocardial blood flow in diabetic, hypertensive, and diabetic-hypertensive rats using spin-labeling. MRI Microcirculation 12: 607–614, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Iltis I, Kober F, Dalmasso C, Lan C, Cozzone PJ, Bernard M. In vivo assessment of myocardial blood flow in rat heart using magnetic resonance imaging: effect of anesthesia. J Magn Reson Imaging 22: 242–247, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A 89: 11059–11063, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science 272: 728–731, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Jiang J, Yuen V, Xiang H, McNeill JH. Improvement in cardiac function of diabetic rats by bosentan is not associated with changes in the activation of PKC isoforms. Mol Cell Biochem 282: 177–185, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34: 29–34, 1974 [DOI] [PubMed] [Google Scholar]
- 30. Kazanietz MG, Areces LB, Bahador A, Mischak H, Goodnight J, Mushinski JF, Blumberg PM. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol 44: 298–307, 1993 [PubMed] [Google Scholar]
- 31. Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol 5: 1394–1403, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol 98: 461–467, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol 96: 1425–1432, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Kover KL, Geng Z, Hess D, Benjamin C, Moore WV. CD40/154 blockade and rejection of islet allografts in the streptozotocin and autoimmune diabetic rat. Pediatr Diabetes 2: 178–183, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 47: 859–866, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Loganathan R, Bilgen M, Al-Hafez B, Smirnova IV. Characterization of alterations in diabetic myocardial tissue using high resolution MRI. Int J Cardiovasc Imaging 22: 81–90, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Loganathan R, Bilgen M, Al-Hafez B, Zhero SV, Alenezy MD, Smirnova IV. Exercise training improves cardiac performance in diabetes: in vivo demonstration with quantitative cine-MRI analyses. J Appl Physiol 102: 665–672, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Malone JI, Schocken DD, Morrison AD, Gilbert-Barness E. Diabetic cardiomyopathy and carnitine deficiency. J Diabetes Complications 13: 86–90, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Mathews CE. Utility of murine models for the study of spontaneous autoimmune type 1 diabetes. Pediatr Diabetes 6: 165–177, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Ming Z, Legare DJ, Lautt WW. Absence of meal-induced insulin sensitization (AMIS) in aging rats is associated with cardiac dysfunction that is protected by antioxidants. J Appl Physiol 111: 704–714, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J 45: 278–291, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J 370: 361–371, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paudel HK, Xu YH, Jarrett HW, Carlson GM. The model calmodulin-binding peptide melittin inhibits phosphorylase kinase by interacting with its catalytic center. Biochemistry 32: 11865–11872, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Roskoski R., Jr Assays of protein kinase. Methods Enzymol 99: 3–6, 1983 [DOI] [PubMed] [Google Scholar]
- 45. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602, 1972 [DOI] [PubMed] [Google Scholar]
- 46. Searls YM, Smirnova IV, Fegley BR, Stehno-Bittel L. Exercise attenuates diabetes-induced ultrastructural changes in rat cardiac tissue. Med Sci Sports Exerc 36: 1863–1870, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Shao CH, Wehrens XH, Wyatt TA, Parbhu S, Rozanski GJ, Patel KP, Bidasee KR. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol 106: 1280–1292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shin HG, Barnett JV, Chang P, Reddy S, Drinkwater DC, Pierson RN, Wiley RG, Murray KT. Molecular heterogeneity of protein kinase C expression in human ventricle. Cardiovasc Res 48: 285–299, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Shizukuda Y, Reyland ME, Buttrick PM. Protein kinase C-delta modulates apoptosis induced by hyperglycemia in adult ventricular myocytes. Am J Physiol Heart Circ Physiol 282: H1625–H1634, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem 275: 9377–9384, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Smirnova IV, Kibiryeva N, Vidoni E, Bunag R, Stehno-Bittel L. Abnormal EKG stress test in rats with type 1 diabetes is deterred with low-intensity exercise programme. Acta Diabetol 43: 66–74, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Spector KS. Diabetic cardiomyopathy. Clin Cardiol 21: 885–887, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas VA, Woda BA, Handler ES, Greiner DL, Mordes JP, Rossini AA. Altered expression of diabetes in BB/Wor rats by exposure to viral pathogens. Diabetes 40: 255–258, 1991 [DOI] [PubMed] [Google Scholar]
- 54. Turinsky J, Bayly BP, O'Sullivan DM. 1,2-Diacylglycerol and ceramide levels in rat skeletal muscle and liver in vivo. Studies with insulin, exercise, muscle denervation, and vasopressin. J Biol Chem 265: 7933–7938, 1990 [PubMed] [Google Scholar]
- 55. Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, Walsh RA, King GL. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A 94: 9320–9325, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications. Diabet Med 18: 945–959, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Woodiwiss AJ, Kalk WJ, Norton GR. Habitual exercise attenuates myocardial stiffness in diabetes mellitus in rats. Am J Physiol Heart Circ Physiol 271: H2126–H2133, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Xia Z, Kuo KH, Nagareddy PR, Wang F, Guo Z, Guo T, Jiang J, McNeill JH. N-acetylcysteine attenuates PKCbeta2 overexpression and myocardial hypertrophy in streptozotocin-induced diabetic rats. Cardiovasc Res 73: 770–782, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol 277: H1967–H1974, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111: 2073–2085, 2005 [DOI] [PubMed] [Google Scholar]