Abstract
The molecular mechanisms responsible for impaired insulin action have yet to be fully identified. Rodent models demonstrate a strong relationship between insulin resistance and an elevation in skeletal muscle inducible nitric oxide synthase (iNOS) expression; the purpose of this investigation was to explore this potential relationship in humans. Sedentary men and women were recruited to participate (means ± SE: nonobese, body mass index = 25.5 ± 0.3 kg/m2, n = 13; obese, body mass index = 36.6 ± 0.4 kg/m2, n = 14). Insulin sensitivity was measured using an intravenous glucose tolerance test with the subsequent modeling of an insulin sensitivity index (SI). Skeletal muscle was obtained from the vastus lateralis, and iNOS, endothelial nitric oxide synthase (eNOS), and neuronal nitric oxide synthase (nNOS) content were determined by Western blot. SI was significantly lower in the obese compared with the nonobese group (∼43%; P < 0.05), yet skeletal muscle iNOS protein expression was not different between nonobese and obese groups. Skeletal muscle eNOS protein was significantly higher in the nonobese than the obese group, and skeletal muscle nNOS protein tended to be higher (P = 0.054) in the obese compared with the nonobese group. Alternative analysis based on SI (high and low tertile) indicated that the most insulin-resistant group did not have significantly more skeletal muscle iNOS protein than the most insulin-sensitive group. In conclusion, human insulin resistance does not appear to be associated with an elevation in skeletal muscle iNOS protein in middle-aged individuals under fasting conditions.
Keywords: insulin resistance, nitric oxide, minimal model, obesity, iNOS, eNOS, nNOS
peripheral insulin resistance is central to the etiology of Type 2 diabetes and cardiovascular disease (10, 30, 36), and understanding insulin resistance is thus invaluable for prevention and treatment. Considerable evidence suggests that intact insulin signal transduction in skeletal muscle is required for optimal whole-body insulin-mediated glucose transport (15, 54, 59), and defects in insulin signaling have been observed in the skeletal muscle of insulin-resistant humans (4, 9, 25, 28, 32). Specifically, insulin-stimulated insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation, phosphatidylinositol-3 kinase (PI3K) activity, and Akt-2 activity have been reported to be impaired in the muscle of obese individuals or patients with Type 2 diabetes (9, 25). To date, the intracellular mechanisms responsible for these impairments remain unclear.
Inducible nitric oxide (NO) synthase (iNOS) recently has been linked to insulin resistance (27). Genetically obese diabetic (ob/ob) mice (21, 43, 52), Zucker diabetic fatty rats (43), high fat-fed mice (43), and high fat-fed rats (12) all have significantly greater skeletal muscle iNOS expression compared with their respective controls without an associated increase in endothelial nitric oxide (NO) synthase (eNOS) or neuronal nitric oxide synthase (nNOS). Perreault and Marette found that high fat-fed mice with a specific disruption in the iNOS gene (iNOS−/−), which nullified iNOS activity, were protected from the decrements in both skeletal muscle insulin signaling and insulin-mediated glucose transport observed in high fat-fed wild-type mice (43). Similarly, genetically obese diabetic (ob/ob) mice with an additional disruption of the iNOS gene (iNOS−/− ob/ob), which eliminated iNOS activity, had significantly greater insulin-stimulated skeletal muscle IRS-1 tyrosine phosphorylation, PI3K activity, and glucose transport than iNOS+/+ ob/ob mice (52). The authors proposed that the production of NO by iNOS was responsible for inducing insulin resistance in the wild-type animals (43, 52). A possible mechanism for this impairment in insulin signaling by iNOS was recently described as a cause-and-effect relationship between an elevation in S-nitrosation (the addition of NO to the thiol group of a cysteine residue) of Akt and a reduction in Akt activity (12, 58). This S-nitrosation-mediated reduction in Akt activity significantly reduced insulin-mediated glucose transport (12, 58). These data thus provide an explicit means by which excessive NO generated from iNOS could impair insulin signaling and insulin-mediated glucose transport in skeletal muscle (12, 58).
In humans, the relationship between iNOS and insulin action has yet to be sufficiently investigated. Relatively few publications report iNOS expression in human skeletal muscle (2, 38–40, 47, 55), and the only investigation to relate skeletal muscle iNOS expression to metabolism found that patients with uncontrolled Type 2 diabetes had approximately four times greater skeletal muscle iNOS protein than control subjects (55). The purpose of the present study was to determine whether skeletal muscle iNOS was higher in obese than in nonobese and higher in insulin-resistant compared with insulin-sensitive subjects. It was hypothesized that the insulin-resistant, obese subjects would have greater skeletal muscle iNOS protein content than nonobese individuals.
METHODS
Subjects.
Men and women between the ages of 40 and 65 yr were recruited as part of a larger investigation of cardiovascular disease. All subjects were sedentary (not currently engaged in any regular exercise program), and all women were postmenopausal. Exclusion criteria included the use of medications that could alter carbohydrate metabolism and evidence of diabetes, hypertension, heart disease, or orthopedic conditions prohibiting exercise. From this cohort of subjects, 27 subjects were selected a posteriori based on tissue sample availability and with the explicit objective to obtain two distinct groups based on body mass index (BMI). This selection process resulted in a nonobese group (n = 13; BMI of <27.2 kg/m2) and an obese group (n = 14; BMI of >33.5 kg/m2). Both the determination of insulin sensitivity and all skeletal muscle tissue analyses were performed after this selection process. These data were alternatively analyzed in an effort to describe the relationship between insulin sensitivity and skeletal muscle NOS protein content irrespective of obesity. For this, the population was arbitrarily divided into tertile groups based on insulin sensitivity index (SI), and variables were statistically compared between the high tertile group (n = 9) and the low tertile group (n = 9). The research protocol was approved by the institutional review boards at East Carolina University and Duke University, and all subjects provided written, informed consent.
Anthropometrics.
A digital electronic scale was utilized to measure body mass to the nearest 0.1 kg. Height was assessed with a stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Exercise testing.
All subjects performed a standardized maximal exercise test on a treadmill with 12-lead ECG monitoring and expired gas analysis to determine the level of aerobic fitness. Ventilation, oxygen consumption (V̇o2), and CO2 consumption (V̇co2) were continuously monitored throughout the test via open-circuit spirometry (model 2900 U, SensorMedics, Yorba Linda, CA; or TrueMax 2400, Parvomedics, Sandy, UT). The protocol utilized increased the workload by approximately one metabolic equivalent per each 2-min stage. The peak V̇o2 (V̇o2peak), an indicator of aerobic fitness, was determined from the values generated during the final 40 s of the exercise test. Both the nonobese and obese groups achieved a mean peak respiratory exchange ratio of >1.15.
Intravenous glucose tolerance test.
A 3-h intravenous glucose tolerance test (IVGTT) was performed in the morning hours after a 12-h fast to determine insulin sensitivity based on the Bergman minimal model calculations (6). A 50% dextrose bolus (0.3 g/kg body mass) was administered after fasting samples were collected, and insulin (0.025 U/kg body mass) was then injected at minute 20. Blood samples were collected at minutes 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180. Blood samples were collected in tubes with EDTA. Plasma samples were stored at −80°C until later analyses. Plasma glucose analyses were performed using the YSI 2300 Stat Plus System (Yellow Springs, OH), whereas a chemiluminescent enzyme immunoassay (ACCESS Immunoassay System, Beckman Coulter, Brea, CA) was utilized to measure plasma insulin. SI was calculated from plasma glucose and insulin values with the software program MINMOD millennium (version 5.10, 2002) (7).
Skeletal muscle biopsy.
Skeletal muscle was acquired from vastus lateralis of the quadriceps femoris muscle group by the percutaneous needle muscle biopsy technique (19). All biopsies were performed in the morning after a 12-h overnight fast and preceded the IVGTT. Muscle samples were frozen in liquid nitrogen and stored at −80°C until later analyses.
Tissue homogenization and evaluation of protein content.
Frozen muscle was homogenized on ice with a ground glass homogenizer in a buffer solution [1% Triton X-100, 50 mM HEPES (pH = 7.4), 4 mM EGTA, 10 mM EDTA, 15 mM tetrasodium pyrophosphate, 100 mM β-glycerolphosphate, 25 mM sodium fluoride, 5 mM Na3VO4, as well as a commercial protease inhibitor cocktail (P8340, Sigma, St. Louis, MO)]. Homogenized samples remained on ice for ∼1 h and were then placed on a rotating wheel at 4°C for an additional hour to enhance the solubilization of proteins. Samples were centrifuged (16,000 g) at 4°C for 10 min, after which the supernatant was collected. Homogenate samples were diluted 1:5 in homogenization buffer before total protein content was assessed in triplicate by bicinchoninic acid (BCA) assay kits (Pierce Biotechnology, Rockford, IL) with bovine serum albumin as the standard.
Western blot analyses.
Muscle homogenates were mixed (1:1) with ×2 lamelli buffer (4% SDS, 20% glycerol, 20% β-mercaptoethanol, 0.004% bromphenol blue, and 0.125 M Tris·HCl, pH of ∼6.8) and brought to a standard protein concentration with deionized, distilled water. Samples were then boiled for 5 min, and when eNOS, nNOS and iNOS were measured, proteins were separated by 5% SDS-PAGE at 175 V for ∼1 h. For 3-nitrotyrosine analysis, proteins were separated by 4–20% SDS-PAGE at 175 V for ∼75 min. Each gel was balanced with the same number of samples from nonobese and obese subjects, and each gel was also evenly balanced based on SI. For all analyses, every sample loaded onto polyacrylamide gels had a total protein content of 60 μg. Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA) at 100 V for ∼2 h at 4°C in transfer buffer (25 mM Tris base, 192 mM glycine, 20% methanol). Transfer was verified by Ponceau S staining. Membranes were blocked for 90 min at room temperature in either 5% nonfat dry milk (nNOS) or 5% BSA (iNOS, eNOS, 3-nitrotyrosine) mixed in a solution of Tris-buffered saline with Tween-20 (TBS-T); 20 mM Tris base, 150 mM NaCl, 0.1% Tween-20; pH 7.5. For immunodetection, membranes were incubated overnight at 4°C with primary antibody diluted in blocking buffer. Primary antibody dilutions were: 1:500 for eNOS (Upstate Cell Signaling Solutions, Lake Placid, NY); 1:750 for iNOS (BD Biosciences, San Jose, CA); 1:1,000 for nNOS (Santa Cruz Biotechnology), and a final antibody concentration of 0.5 μg/ml for 3-nitrotyrosine (Upstate Cell Signaling Solutions). Then, after successive washes in TBS-T, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Millipore, Billerica, MA) in blocking buffer for 1 h at room temperature followed by washes with TBS-T. Immunogenic detection was accomplished with enhanced chemiluminescent substrate (Pierce Biotechnology), and bands were visualized by autoradiography. The relative quantification was determined by the assessment of the integrated optical density of each band performed using LabWorks 4.6 software (UVP, LLC, Upland, CA). When possible, commercial Western blot stripper buffer was utilized so that PVDF membranes could be reprobed for different protein targets. Total protein was quantified with Ponceau S according to Aldridge and colleagues (3), and for all target blots (i.e., iNOS, nNOS, eNOS, and 3-nitrotyrosine) there were no significant differences in total protein between the obese and nonobese groups as well as the low tertile SI and high tertile SI groups. Optical density units for each band were normalized to the average signal of the bands on each respective membrane.
Western blot analyses yielded two distinct skeletal muscle iNOS bands (see Fig. 2A), and these bands were aligned to a similar doublet observed in the iNOS-positive control, mouse macrophages stimulated with lipopolysaccharide and interferon-γ. This doublet is consistent with previous reports that analyzed human skeletal muscle (38, 44), rat placenta (45), macrophages (56), primary bronchial epithelial cells (31), cultured neonatal rat cardiomyocytes (51), as well as HEK293 and RT4 cells (31). Vodovotz et al. reported two distinct iNOS variants (cytosolic ∼130 kDa and particulate ∼135 kDa) in stimulated macrophages with each variant markedly contributing to the total iNOS activity (31). Due to this existing evidence, we believe that it is appropriate and necessary to represent skeletal muscle iNOS protein content as the aggregate signal of the observed doublet. Therefore, all results are presented with total iNOS protein represented as the sum of the signal of both iNOS bands. Additionally, analysis of the data using either of the bands, instead of the sum of the signal from both, resulted in the same statistical outcomes.
Fig. 2.
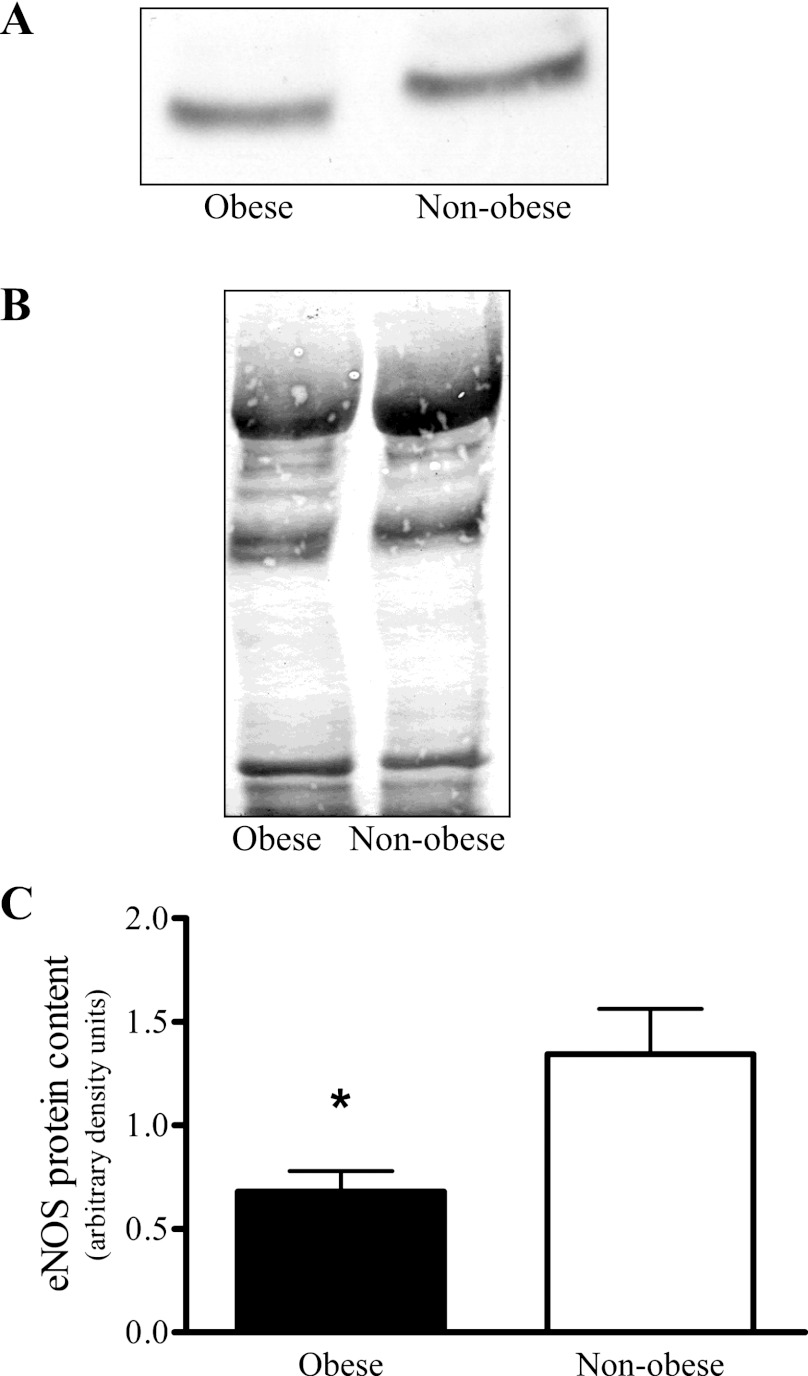
Total skeletal muscle inducible NO synthase (iNOS) protein content determined by Western blot analyses from the vastus lateralis muscle of nonobese (n = 13) and obese (n = 14) sedentary subjects. Representative iNOS Western blot including the iNOS-positive control [(+)iNOS], stimulated mouse macrophage (A), representative Ponceau S stained blot for total protein quantification (B), and comparison between nonobese and obese total skeletal muscle iNOS protein content (C). No statistically significant difference was observed.
Adipokine multiplexed analysis.
Circulating adipokine concentrations were analyzed to assess the inflammatory status of the subjects using two commercially available multiplexed bead-based assays (Millipore, Billerica, MA). These assays are antibody sandwich assays using capture and detector (secondary) antibodies and streptavidin-phycoerythrin (SA-PE) as the reporter signal. The median fluorescent intensity (MFI) of SA-PE from a minimum of 50 beads/events is reported and converted to a concentration using five-parameter logistics and modeling software. All samples were analyzed on a Luminex 200 System (Luminex, Austin, TX) using Masterplex QT 2010 software (MiraiBio, Hitachi Solutions America, South San Francisco, CA). The assays used were the human cytokine/chemokine (MPXHCYTO-60K) for IL-1β, IL-1ra, IL-6, MCP-1, and TNF-α, and human serum adipokine (Panel A; HADK1-61K-A) for adiponectin and resistin. Assays for IL-1 β, IL-1ra, IL-6, MCP-1, and TNF-α, had reported sensitivities of 0.4, 2.9, 0.3, 0.9, and 0.1 pg/ml, respectively, whereas adiponectin and resistin had sensitivities of 145.4 and 6.7 pg/ml, respectively. Overnight-fasted plasma samples drawn before the start of the IVGTT were run in duplicate and within one plate each. Basal plasma samples for IL-1ra and IL-6 did not meet the assay sensitivity and, therefore, were excluded from the analysis.
Statistical analysis.
The software package Statview 5.0.1 (SAS Institute, Cary, NC) was used for statistical analyses. Values are expressed as means ± SE. Subject characteristics and skeletal muscle variables were compared with unpaired Student's t-tests. Stepwise linear regression analysis was performed to predict SI from potential independent variables. Alpha level was set at 0.05 for all analyses.
RESULTS
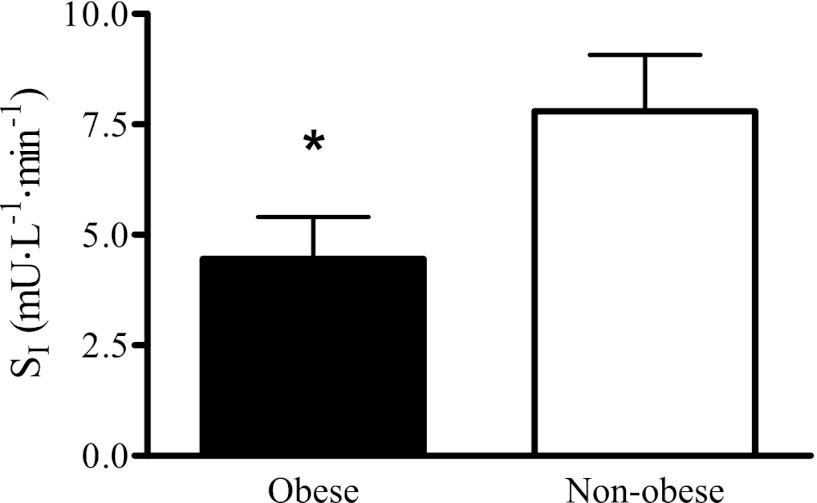
Subject characteristics are listed in Table 1. Body mass, time to exhaustion, and V̇o2 peak were significantly different between the nonobese and obese groups. There were trends for both low-density lipoprotein (P = 0.13) and fasting insulin (P = 0.10) to be higher in the obese compared with the nonobese group. The obese group had a significantly lower SI than the nonobese group, indicating that they were more insulin resistant (Fig. 1). No significant differences in blood plasma adipokines emerged between a subset of the high tertile SI and low tertile SI groups (Table 2), with the exception of adiponectin, which was higher in the high tertile SI group (P < 0.05).
Table 1.
Subject characteristics
| Nonobese | Obese | |
|---|---|---|
| n (men/women) | 7/6 | 8/6 |
| Age, yr | 51.2 ± 1.7 | 50.9 ± 1.5 |
| Height, cm | 171.5 ± 2.0 | 163.9 ± 5.1 |
| Mass, kg | 75.1 ± 2.2 | 101.5 ± 3.2* |
| Body mass index, kg/m2 | 25.5 ± 0.3 | 35.6 ± 0.4* |
| Cholesterol, mg/dl | 178.5 ± 11.2 | 190.0 ± 7.4 |
| HDL-C, mg/dl | 50.8 ± 4.6 | 46.4 ± 3.8 |
| LDL-C, mg/dl | 110.9 ± 6.9 | 125.1 ± 6.0 |
| Triglyceride, mg/dl | 99.9 ± 8.7 | 119.4 ± 12.7 |
| Insulin, μU/ml | 7.3 ± 1.1 | 11.9 ± 2.4 |
| Glucose, mg/dl | 86.3 ± 2.0 | 93.3 ± 4.0 |
| Time to exhaustion, s | 862.2 ± 61.7 | 618.7 ± 45.2* |
| V̇o2 peak, liter/min | 2.4 ± 0.2 | 2.5 ± 0.2 |
| V̇o2 peak, ml · kg−1 · min−1 | 30.9 ± 1.7 | 24.6 ± 1.3* |
Data are means ± SE.
Significant difference between nonobese and obese groups (P < 0.05).
Fig. 1.
Insulin sensitivity index (SI) of nonobese (n = 13) and obese (n = 14) sedentary men and women (40–65 yr) determined from intravenous glucose tolerance tests with subsequent analyses by the Bergman minimal model. *Significant difference between nonobese and obese groups (P < 0.05).
Table 2.
Subject characteristics for groups generated according to each subject's SI, high vs. low tertile
| Low Tertile SI | High Tertile SI | |
|---|---|---|
| n (obese/nonobese) | 7/2 | 4/5 |
| n (men/women) | 7/2 | 3/6 |
| Age, yr | 48.6 ± 2.1 | 52.9 ± 1.6 |
| Height, cm | 166.7 ± 7.8 | 166.4 ± 2.2 |
| Mass, kg | 101.6 ± 5.3 | 82.2 ± 4.1* |
| Body mass index, kg/m2 | 33.5 ± 1.5 | 29.9 ± 2.0 |
| V̇o2 peak, liter/min | 2.7 ± 0.2 | 2.2 ± 0.1 |
| V̇o2 peak, ml · kg−1 · min−1 | 26.6 ± 2.1 | 26.9 ± 2.3 |
| Insulin, μU/ml) | 14.0 ± 3.4 | 6.3 ± 1.3 |
| Glucose, mg/dl | 93.0 ± 6.1 | 87.4 ± 2.6 |
| SI, mU · liter−1 · min−1 | 2.0 ± 0.4 | 11.2 ± 1.1* |
| IL-1β, pg/ml | 9.6 ± 3.8 | 5.0 ± 2.9 |
| MCP-1, pg/ml | 232.6 ± 17.1 | 314.3 ± 58.0 |
| TNF-α, pg/ml | 4.8 ± 0.9 | 5.2 ± 0.7 |
| Resistin, ng/ml | 49.0 ± 3.6 | 58.6 ± 0.8 |
| Adiponectin, μg/ml | 15.9 ± 1.9 | 30.4 ± 3.9* |
Subject characteristics for groups generated according to each subject's insulin sensitivity index (SI), high vs. low tertile (n = 9/group). For the multiplexed analytes analyzed from blood plasma, sample sizes were n = 8/group, except for adiponectin analysis (high tertile SI, n = 7; low tertile SI, n = 8). Data are means ± SE.
Significant difference between high and low tertile SI groups (P < 0.05).
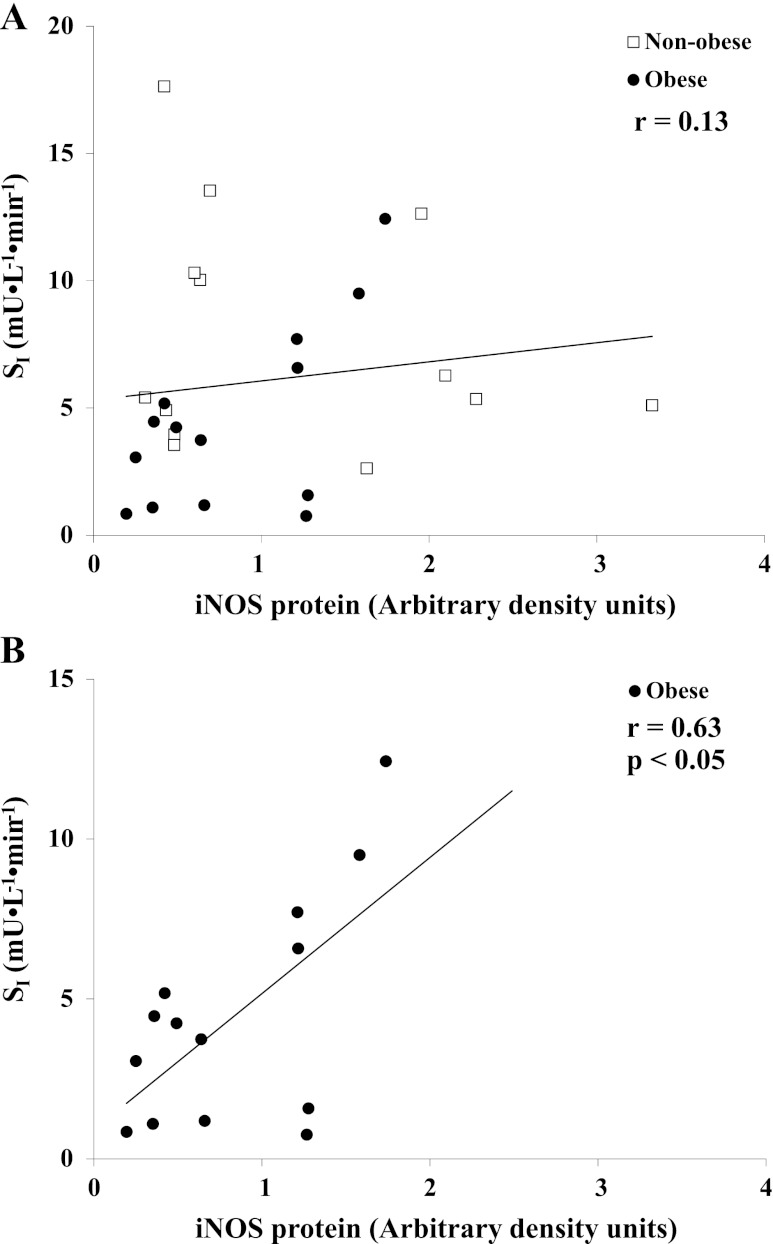
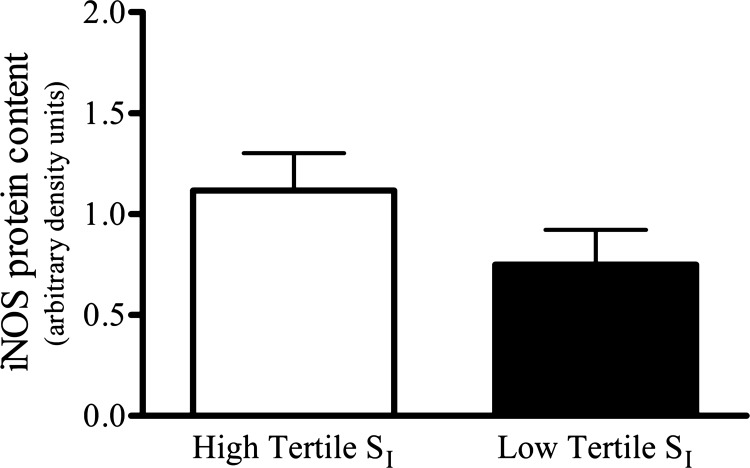
No significant difference was found when the skeletal muscle iNOS protein of nonobese and obese groups were compared (P = 0.25; Fig. 2C). Linear regression analyses revealed no significant relationship (r = 0.13; P = 0.51) between SI and skeletal muscle iNOS protein content (Fig. 3A); however, upon closer inspection and analysis of the data, a significant positive correlation between skeletal muscle iNOS protein content and SI existed among only the obese subjects (n = 14; r = 0.63; P < 0.05; Fig. 3B). Comparisons of skeletal muscle iNOS protein content were also made between reconfigured groups generated according to each subject's SI. The high and low tertiles according to SI (n = 9/group) had SI means ± SE of 11.2 ± 1.1 and 2.0 ± 0.4 mU·liter−1·min−1, respectively, and skeletal muscle iNOS protein was not statistically different in the high compared with the low tertile SI group (P = 0.17; Fig. 4).
Fig. 3.
The relationship between SI and total skeletal muscle iNOS protein content in nonobese (n = 13) and obese (n = 14) sedentary subjects (A) and the relationship between SI and total skeletal muscle iNOS protein content in exclusively the obese subjects (B). Although no statistically significant relationship was observed for the entire cohort, isolated analysis of the obese subjects found a significant correlation (P < 0.05).
Fig. 4.
Comparison of total skeletal muscle iNOS protein content between groups generated according to each subject's SI, high vs. low tertile (n = 9/group). No statistically significant difference was observed.
Individual 3-nitrotyrosine bands were quantified with no difference in total 3-nitrotyrosine protein found between the nonobese and obese groups (1.02 ± 0.05 vs. 0.98 ± 0.05; P = 0.60), nor were there differences in 3-nitrotyrosine protein content between the groups generated based on SI (see Fig. 10). No significant association (r = 0.08; P = 0.68) was found between total skeletal muscle iNOS protein and total 3-nitrotyrosine protein.
Fig. 10.
Skeletal muscle 3-nitrotyrosine protein content determined by Western blot analyses from the vastus lateralis muscle of nonobese (n = 13) and obese (n = 14) sedentary subjects. Representative 3-nitrotyrosine Western blot. No statistically significant difference was observed.
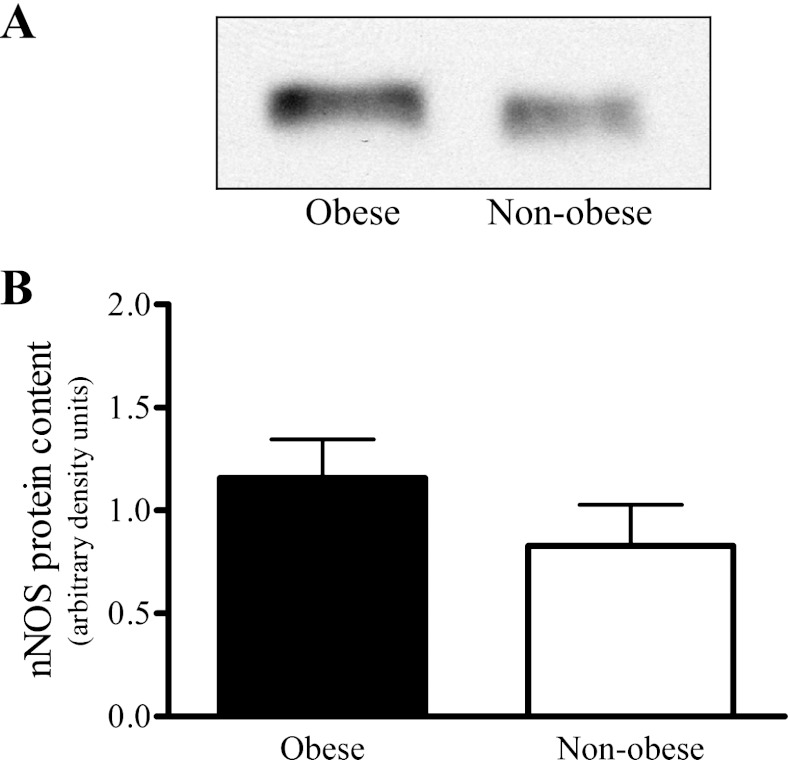
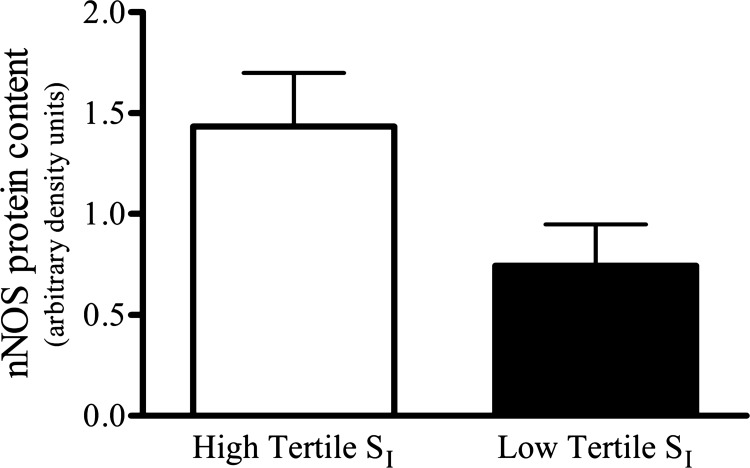
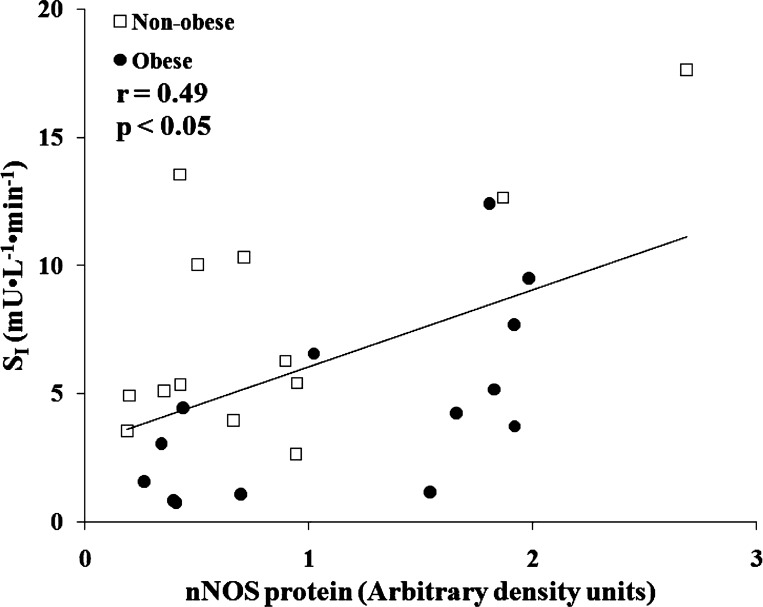
Skeletal muscle eNOS protein was significantly greater in the nonobese than in the obese group (Fig. 5C), but no differences were observed between the groups established based on SI (Fig. 6). The nonobese and obese groups did not vary in skeletal muscle nNOS protein content (Fig. 7B); however, the skeletal muscle nNOS protein tended (P = 0.054) to be less in the low compared with high tertile SI group (Fig. 8). Correlation analysis (Pearson product) revealed a significant relationship (r = 0.49; P < 0.05) between SI and nNOS protein (Fig. 9), but not between SI and eNOS protein (r = 0.04; P = 0.83).
Fig. 5.
Skeletal muscle endothelial NO synthase (eNOS) protein content determined by Western blot analyses from the vastus lateralis muscle of nonobese (n = 13) and obese (n = 14) sedentary subjects. Representative eNOS Western blot (A), representative Ponceau S stained blot for total protein quantification (B), and comparison between nonobese and obese skeletal muscle eNOS protein content (C). *Significant difference between nonobese and obese (P < 0.05).
Fig. 6.
Comparison of skeletal muscle eNOS protein content between groups generated according to each subject's SI, high vs. low tertile (n = 9/group). No statistically significant difference was observed.
Fig. 7.
Skeletal muscle neuronal NO synthase (nNOS) protein content determined by Western blot analyses from the vastus lateralis muscle of nonobese (n = 13) and obese (n = 14) sedentary subjects. Representative nNOS Western blot (A), and comparison between nonobese and obese skeletal muscle nNOS protein content (B). The representative nNOS Western blot has an accompanying representative Ponceau S stained blot, which is shown in Fig. 5B. The representative eNOS and nNOS blots share the same Ponceau S blot because commercial stripper was utilized so that membranes could be reprobed. No statistically significant difference was observed.
Fig. 8.
Comparison of skeletal muscle nNOS protein content between groups generated according to each subject's SI, high vs. low tertile (n = 9/group). No statistically significant difference was observed.
Fig. 9.
Linear regression between SI and skeletal muscle nNOS protein content among nonobese (n = 13) and obese (n = 14) sedentary subjects. There was a significant positive association between SI and skeletal muscle nNOS protein content.
Stepwise linear regression analysis among numerous variables (age, body mass, BMI, time to exhaustion, V̇o2 peak, total cholesterol, HDL-C, LDL-C, triglycerides, fasting glucose, fasting insulin, and skeletal muscle nNOS, eNOS, and iNOS protein content) revealed that only body mass followed by skeletal muscle nNOS protein were independent predictors of SI, accounting for 29% and 25% of the variability in SI, respectively.
DISCUSSION
The most prominent finding in this investigation was that there was not significant elevation in skeletal muscle iNOS protein content despite evidence for insulin resistance in obese individuals. Additionally, there was no evidence for an association between iNOS content and insulin action in human skeletal muscle when we partitioned our population into tertiles based on relative insulin sensitivity. Together, these data show that human insulin resistance is not associated with an elevation in skeletal muscle iNOS protein in middle-aged individuals under fasting conditions. Skeletal muscle iNOS is therefore not necessarily increased in all states of insulin resistance.
The present iNOS and insulin sensitivity results contradict previous reports that showed that insulin resistance was related to an elevation in skeletal muscle iNOS expression (11, 12, 21, 43, 53, 55, 58). It is possible that methodological differences might explain this discrepancy since our study design contrasts sharply with that of these previous reports (12, 43, 52, 55, 58). The present study utilized obese, yet otherwise healthy, sedentary men and women, whereas the previous studies employed the use of rodents with a genetic dominance for Type 2 diabetes, 4- and 8-wk high-fat diet interventions on rodents, and humans with Type 2 diabetes (12, 43, 52, 55, 58). Torres et al. conducted the most relevant research for comparison to the present investigation but utilized a population of subjects with uncontrolled Type 2 diabetes, as indicated by a mean glycosylated hemoglobin level (HbA1c) of 11.2% (55). The elevated skeletal muscle iNOS protein content of these Type 2 diabetic patients might be explained by chronically elevated circulating glucose levels. In critically ill patients, Langouche et al. found that prevention of hyperglycemia with intensive insulin therapy resulted in significantly less skeletal muscle iNOS gene expression compared with patients who received typical insulin therapy (33). Although such a result fails to support a direct cause-and-effect relationship between hyperglycemia and the upregulation of iNOS expression, a follow-up study in a rabbit model of critical illness conclusively demonstrated that hyperglycemia was responsible for the elevation in iNOS gene expression in skeletal muscle (18). Thus the experimental design of these past investigations more likely depicts uncontrolled Type 2 diabetes rather than insulin resistance, and possibly any elevation in skeletal muscle iNOS protein could therefore be explained as a consequence rather than an etiological factor of Type 2 diabetes.
An alternative analysis was performed to describe the relationship between whole-body insulin action and skeletal muscle iNOS protein content irrespective of obesity. For this, the population was arbitrarily divided into tertile groups based on SI. The difference in SI between the high and the low tertile SI groups was considerable, with group means ± SE of 11.2 ± 1.1 and 2.0 ± 0.4 mU·liter−1·min−1, respectively, and a 3.03 mU·liter−1·min−1 magnitude of SI separation between the two closest points of the high and low tertile SI groups. This magnitude difference in SI is at least as great as previous studies that utilized an intravenous glucose tolerance test with subsequent minimal model analysis, which also reported a statistically significant difference in SI between cross-sectional groups (16, 20, 37). For example, Fonseca et al. reported an SI of 2.6 ± 1.6 and 4.3 ± 2.0 mU·liter−1·min−1 (means ± SE) for obese and normal weight subjects, respectively (20). These comparisons with the present study give us confidence to assert that the subjects within the high and low tertile SI groups represent two unequivocally different populations based on insulin sensitivity. The result of the analysis from these strictly defined SI groups did not support that iNOS protein is elevated in the skeletal muscle of insulin-resistant subjects.
Recent investigations in animal models have also demonstrated a similar disconnect between skeletal muscle iNOS and insulin resistance (13, 14, 35). In mice, a 6-h intralipid infusion induced significant impairments in peripheral glucose disposal but failed to significantly increase skeletal muscle iNOS protein content (14). Lu and colleagues found that, compared with chow-fed mice, mice consuming a 60% high-fat diet for 18 wk had a significantly reduced glucose infusion rate during euglycemic hyperinsulinemic clamps; however, no difference in skeletal muscle iNOS gene expression was found between the chow-fed and high-fat-fed mice (35). In both of these examples, hepatic iNOS expression was significantly elevated compared with the control condition, and it was this hepatic iNOS expression that was purported to be responsible for the insulin-resistant state. It is possible that this mechanism might explain the results from the present study, but this would be purely speculative since no hepatic tissue was collected for analysis. The present investigation in humans extends the previous recent findings in animal models that have demonstrated that skeletal muscle iNOS is not associated with insulin resistance.
Total 3-nitrotyrosine protein content is an index of NO exposure (5) and has been shown to mirror iNOS expression in skeletal muscle (5, 24, 55). When iNOS protein is present within skeletal muscle, it is believed to be responsible for a large volume of NO production, both because it is predominately a transcriptionally regulated enzyme and because the specific activity of iNOS (∼800 nmol·min−1·mg−1) is significantly greater than eNOS (∼130 nmol·min−1·mg−1) or nNOS (∼400 nmol·min−1·mg−1) (22, 23, 41, 48). In the present study, we did not find total skeletal muscle 3-nitrotyrosine to be different between any of the groups. It is possible that no difference in 3-nitrotyrosine was found because the iNOS protein content difference between any two groups was insufficient to result in an appreciable difference in total 3-nitrotyrosine content. By comparison, Torres et al. reported a significant difference in total skeletal muscle 3-nitrotyrosine protein between Type 2 diabetics and control subjects, but the magnitude elevation in skeletal muscle iNOS protein (∼400%) suggested to be responsible for the elevation in 3-nitrotyrosine was far greater than in the present investigation (55).
Circulating plasma levels of various adipokines were measured to further describe the subjects. The adiponectin results are compatible with existing literature, which has found a positive relationship between insulin sensitivity and circulating adiponectin (46). Both IL-1β and TNF-α were chosen as targets because the expression of iNOS has been shown to increase in a variety of cultured skeletal muscle cells when exposed to a mixture of cytokines that included IL-1β and TNF-α (1, 42, 57). Similar to the skeletal muscle iNOS results, no difference in plasma IL-1β or TNF-α was found between the subset of the low tertile and high tertile SI groups. This parallel relationship of both TNF-α and IL-1β with iNOS is consistent with previous investigations, which found that elevated skeletal muscle IL-1β in chronic heart failure patients (1) and TNF-α in Type 2 diabetics (55) were associated with elevated skeletal muscle iNOS protein expression. The present data indicate that insulin resistance can occur in the absence of classic markers of inflammation (i.e., iNOS, TNF-α, IL-1β).
The present skeletal muscle eNOS data (Fig. 5C) confirm past findings from our laboratory observing that obese subjects have significantly less skeletal muscle eNOS protein than nonobese subjects (26). The endothelial cells of capillaries are the predominate cellular location for eNOS protein expression within human skeletal muscle (49), and since obese humans have a significantly lower capillary density (29), it would be logical to suggest this as an explanation as to why the skeletal muscle homogenates of obese subjects possessed significantly less eNOS protein. Given that skeletal muscle capillarization, a primary source of eNOS, and insulin sensitivity have a strong relationship (34), it was somewhat of a surprise that skeletal muscle eNOS protein and SI were not related and that the high insulin-sensitive tertile did not have significantly more eNOS protein than the low insulin-sensitive tertile (Fig. 6). This finding, however, is in agreement with a past report where skeletal muscle eNOS protein was not different between patients with Type 2 diabetes and control subjects (55). Although eNOS is necessary for normal systemic glucose homeostasis and insulin action in rodents (17), the available data in humans indicate that skeletal muscle eNOS protein may not play a meaningful role in peripheral insulin sensitivity.
Another novel finding from the present investigation was the positive linear relationship (r = 0.49; P < 0.05) between skeletal muscle nNOS protein and SI (Fig. 9). Stepwise linear regression analysis indicated that skeletal muscle nNOS was a significant independent predictor accounting for 25% of the variability in SI. Furthermore, nNOS protein tended (P = 0.054) to be less in the low compared with the high tertile SI group (Fig. 8). These results complement those reported by Bradley et al., where skeletal muscle nNOS expression in humans with impaired glucose homeostasis was found to be significantly lower than that of healthy subjects (8). However, a clear role for skeletal muscle nNOS in insulin action remains in question because Bradley et al. also found that the subjects with impaired glucose homeostasis significantly improved homeostasis model assessment (HOMA) scores after 4 wk of exercise training without a simultaneous increase in skeletal muscle nNOS (8). In mice, the importance of nNOS for proper insulin action was illustrated by significantly lower (∼16%) steady-state glucose infusion rates during hyperinsulinemic-euglycemic clamps in nNOS knockout mice compared with wild-type mice (50). Although skeletal muscle nNOS is certainly associated with and required for optimal insulin action, its exact role in this process is unclear.
In conclusion, we found that human insulin resistance was not associated with an elevation in skeletal muscle iNOS protein in a population of healthy, middle-aged men and women under the fasted condition. As expected, skeletal muscle eNOS protein was significantly higher in the nonobese compared with the obese subjects, although eNOS protein content was not correlated with insulin sensitivity. An additional novel finding was the positive relationship between skeletal muscle nNOS protein and whole-body insulin action; the explanation for this relationship is not yet evident and requires further research.
GRANTS
This study was supported in part by National Institutes of Health Grants R01 HL-57354 and R21 AG-19209-02.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.M.K., J.A.H., W.E.K., and R.C.H. conception and design of research; R.M.K., C.J.T., J.R.P., and M.D.C. performed experiments; R.M.K., J.A.H., C.J.T., J.R.P., M.D.C., and R.C.H. analyzed data; R.M.K., J.A.H., W.E.K., C.J.T., J.R.P., and R.C.H. interpreted results of experiments; R.M.K. prepared figures; R.M.K. drafted manuscript; R.M.K., J.A.H., W.E.K., C.J.T., J.R.P., M.D.C., and R.C.H. edited and revised manuscript; R.M.K., J.A.H., W.E.K., J.R.P., and R.C.H. approved final version of manuscript.
REFERENCES
- 1. Adams V, Nehrhoff B, Spate U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res 54: 95–104, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Agusti A, Morla M, Sauleda J, Saus C, Busquets X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax 59: 483–487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172: 250–254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 54: 2351–2359, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Barreiro E, Comtois AS, Gea J, Laubach VE, Hussain SN. Protein tyrosine nitration in the ventilatory muscles: role of nitric oxide synthases. Am J Respir Cell Mol Biol 26: 438–446, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 6: 45–86, 1985 [DOI] [PubMed] [Google Scholar]
- 7. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5: 1003–1015, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bradley SJ, Kingwell BA, Canny BJ, McConell GK. Skeletal muscle neuronal nitric oxide synthase micro protein is reduced in people with impaired glucose homeostasis and is not normalized by exercise training. Metabolism 56: 1405–1411, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Brozinick JT, Jr, Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes 52: 935–941, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 114: 195–210, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Carvalho-Filho MA, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. Targeted disruption of iNOS prevents LPS-induced S-nitrosation of IRbeta/IRS-1 and Akt and insulin resistance in muscle of mice. Am J Physiol Endocrinol Metab 291: E476–E482, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 54: 959–967, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cha HN, Kim YW, Kim JY, Kim YD, Song IH, Min KN, Park SY. Lack of inducible nitric oxide synthase does not prevent aging-associated insulin resistance. Exp Gerontol 45: 711–718, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes 59: 861–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Clevenger CM, Parker Jones P, Tanaka H, Seals DR, DeSouza CA. Decline in insulin action with age in endurance-trained humans. J Appl Physiol 93: 2105–2111, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Cook S, Hugli O, Egli M, Vollenweider P, Burcelin R, Nicod P, Thorens B, Scherrer U. Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly 133: 360–363, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Ellger B, Langouche L, Richir M, Debaveye Y, Vanhorebeek I, Teerlink T, Van Leeuwen PA, Van den Berghe G. Modulation of regional nitric oxide metabolism: blood glucose control or insulin? Intensive Care Med 34: 1525–1533, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1982 [PubMed] [Google Scholar]
- 20. Fonseca VA, Fink LM, Kern PA. Insulin sensitivity and plasma homocysteine concentrations in non-diabetic obese and normal weight subjects. Atherosclerosis 167: 105–109, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes 54: 1340–1348, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Gerber NC, Nishida CR, Ortiz de Montellano PR. Characterization of human liver inducible nitric oxide synthase expressed in Escherichia coli. Arch Biochem Biophys 343: 249–253, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Gerber NC, Ortiz de Montellano PR. Neuronal nitric oxide synthase. Expression in Escherichia coli, irreversible inhibition by phenyldiazene, and active site topology. J Biol Chem 270: 17791–17796, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 42: 861–868, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95: 2195–2204, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hickner RC, Kemeny G, Stallings HW, Manning SM, McIver KL. Relationship between body composition and skeletal muscle eNOS. Int J Obes (Lond) 30: 308–312, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal 9: 319–329, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54: 1692–1697, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kern PA, Simsolo RB, Fournier M. Effect of weight loss on muscle fiber type, fiber size, capillarity, and succinate dehydrogenase activity in humans. J Clin Endocrinol Metab 84: 4185–4190, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res 102: 401–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kolodziejski PJ, Rashid MB, Eissa NT. Intracellular formation of “undisruptable” dimers of inducible nitric oxide synthase. Proc Natl Acad Sci USA 100: 14263–14268, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krook A, Bjornholm M, Galuska D, Jiang XJ, Fahlman R, Myers MG, Jr, Wallberg-Henriksson H, Zierath JR. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49: 284–292, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest 115: 2277–2286, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 415–424, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu M, Li P, Pferdekamper J, Fan W, Saberi M, Schenk S, Olefsky JM. Inducible nitric oxide synthase deficiency in myeloid cells does not prevent diet-induced insulin resistance. Mol Endocrinol 24: 1413–1422, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissen M, Isomaa B, Forsen B, Homstrom N, Saloranta C, Taskinen MR, Groop L, Tuomi T. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 54: 166–174, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Manetta J, Brun JF, Callis A, Mercier J, Prefaut C. Insulin and non-insulin-dependent glucose disposal in middle-aged and young athletes versus sedentary men. Metabolism 50: 349–354, 2001 [DOI] [PubMed] [Google Scholar]
- 38. McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA, Lee-Young RS. Skeletal muscle nNOS mu protein content is increased by exercise training in humans. Am J Physiol Regul Integr Comp Physiol 293: R821–R828, 2007 [DOI] [PubMed] [Google Scholar]
- 39. McIver KL, Evans C, Kraus RM, Ispas L, Sciotti VM, Hickner RC. NO-mediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain 120: 161–169, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Montes de Oca M, Torres SH, De Sanctis J, Mata A, Hernandez N, Talamo C. Skeletal muscle inflammation and nitric oxide in patients with COPD. Eur Respir J 26: 390–397, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Nishida CR, Ortiz de Montellano PR. Electron transfer and catalytic activity of nitric oxide synthases. Chimeric constructs of the neuronal, inducible, and endothelial isoforms. J Biol Chem 273: 5566–5571, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Okuda S, Kanda F, Kawahara Y, Chihara K. Regulation of inducible nitric oxide synthase expression in L6 rat skeletal muscle cells. Am J Physiol Cell Physiol 272: C35–C40, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med 7: 1138–1143, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Punkt K, Fritzsche M, Stockmar C, Hepp P, Josten C, Wellner M, Schering S, Buchwalow IB. Nitric oxide synthase in human skeletal muscles related to defined fibre types. Histochem Cell Biol 125: 567–573, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Purcell TL, Buhimschi IA, Given R, Chwalisz K, Garfield RE. Inducible nitric oxide synthase is present in the rat placenta at the fetal-maternal interface and decreases prior to labour. Mol Hum Reprod 3: 485–491, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med 14: 741–751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riede UN, Forstermann U, Drexler H. Inducible nitric oxide synthase in skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 32: 964–969, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Rodriguez-Crespo I, Ortiz de Montellano PR. Human endothelial nitric oxide synthase: expression in Escherichia coli, coexpression with calmodulin, and characterization. Arch Biochem Biophys 336: 151–156, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Rudnick J, Puttmann B, Tesch PA, Alkner B, Schoser BG, Salanova M, Kirsch K, Gunga HC, Schiffl G, Luck G, Blottner D. Differential expression of nitric oxide synthases (NOS 1–3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. FASEB J 18: 1228–1230, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 49: 684–687, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Slezak J, Buchwalow IB, Schulze W, Karczewski P, Wallukat G, Samoilova VE, Krause EG, Neumann J, Haller H. Cellular control of nitric oxide synthase expression and activity in rat cardiomyocytes. Antioxid Redox Signal 6: 345–352, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, Martyn JA, Kaneki M. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem 280: 14203–14211, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Sugita M, Kaneki M, Sugita H, Tokunaga E, Martyn Jeevendra AJ. Involvement of inducible nitric oxide synthase in stress-induced insulin resistance. Am Diabetes Assoc 66th Scientific Sessions A-411, 2006 [Google Scholar]
- 54. Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372: 182–186, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Torres SH, De Sanctis JB, de LBM, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol 181: 419–427, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Vodovotz Y, Russell D, Xie QW, Bogdan C, Nathan C. Vesicle membrane association of nitric oxide synthase in primary mouse macrophages. J Immunol 154: 2914–2925, 1995 [PubMed] [Google Scholar]
- 57. Williams G, Brown T, Becker L, Prager M, Giroir BP. Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol Regul Integr Comp Physiol 267: R1020–R1025, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem 280: 7511–7518, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem 270: 2107–2111, 1995 [DOI] [PubMed] [Google Scholar]