Abstract
The passive pharyngeal critical closing pressure (Pcrit) is measured using a series of pressure drops. However, pressure drops also lower end-expiratory lung volume (EELV), which independently affects Pcrit. We describe a technique to measure Pcrit at a constant EELV. Continuous positive airway pressure (CPAP)-treated obstructive sleep apnea (OSA) patients and controls were instrumented with an epiglottic catheter, magnetometers (to measure change in EELV), and nasal mask/pneumotachograph and slept supine on nasal CPAP. Pcrit was measured in standard fashion and using our novel “biphasic technique” in which expiratory pressure only was lowered for 1 min before the inspiratory pressure was dropped; this allowed EELV to decrease to the drop level before performing the pressure drop. Seven OSA and three controls were studied. The biphasic technique successfully lowered EELV before the inspiratory pressure drop. Pcrit was similar between the standard and biphasic techniques (−0.4 ± 2.6 vs. −0.6 ± 2.3 cmH2O, respectively, P = 0.84). Interestingly, we noted three different patterns of flow limitation: 1) classic Starling resistor type: flow fixed and independent of downstream pressure; 2) negative effort dependence within breaths: substantial decrease in flow, sometimes with complete collapse, as downstream pressure decreased; and 3) and negative effort dependence across breaths: progressive reductions in peak flow as respiratory effort on successive breaths increased. Overall, EELV changes do not influence standard passive Pcrit measurements if breaths 3–5 of pressure drops are used. These results also highlight the importance of inspiratory collapse in OSA pathogenesis. The cause of negative effort dependence within and across breaths is not known and requires further study.
Keywords: obstructive sleep apnea, pharyngeal critical closing pressure, Starling resistor
obstructive sleep apnea (OSA) is a common disease characterized by repetitive collapse of the upper airway during sleep, leading to arousals, sleep fragmentation, and intermittent hypoxemia. Although OSA may have multiple contributing factors, poor upper airway anatomy is likely the dominant factor in most cases (13). Anatomy has been quantified in many ways during wakefulness and sleep, but one of the more useful methods has been the measurement of upper airway collapsibility (6). The pressure required to collapse the upper airway is the pharyngeal closing pressure, or Pcrit, and this can be thought of as the equivalent pressure surrounding the upper airway lumen (26). Higher pressures indicate greater collapsibility, and Pcrit has been shown to correlate with OSA and OSA severity (5).
Pcrit can be measured in a relatively passive airway (minimal upper airway muscle activity) by abruptly lowering the airway or mask pressure to subtherapeutic levels of continuous positive airway pressure (CPAP) for three to five breaths. Although relatively easy to perform, this method of determining upper airway collapsibility is imperfect. The reduction in mask pressure also causes a decrease in end-expiratory lung volume (EELV), which falls in relation to the pressure change (18). EELV is known to independently affect Pcrit (18, 27, 29). Increased lung volume stabilizes the upper airway, possibly through increased caudal traction, with a reduced (more negative, less collapsible) Pcrit. Conversely, decreased lung volume can potentiate collapse and an increased (less negative, more collapsible) Pcrit. Our group and others have attempted to measure Pcrit independently of changes in EELV. However, these methods are cumbersome (often using a head-out rigid shell or “iron lung”) and anticipate, but do not prevent, the changes in lung volume that occur during the pressure drop. Thus an easy methodology to change airway pressure while lung volume is constant could eliminate a confounding variable in the true measurement of Pcrit.
Such a technique would also answer a second related question. To account for changes in EELV, breaths 1 and 2 of a pressure drop are often discarded, and only peak flows during breaths three to five (if flow limited) are used in the determination of Pcrit. Although EELV may equilibrate rapidly (within 1–2 breaths), the effects of EELV on the airway might not be immediate, i.e., there may be lingering effects on breaths 3 and 4 through the temporal behavior of tissue stress adaptation (either relaxation or recovery). Comparison of Pcrit measurements made in a standard fashion and with EELV held constant could answer this question. Phrased another way, if EELV were held constant for all five breaths of the pressure drop, rather than decreasing over the first few breaths, would the measured Pcrit be the same?
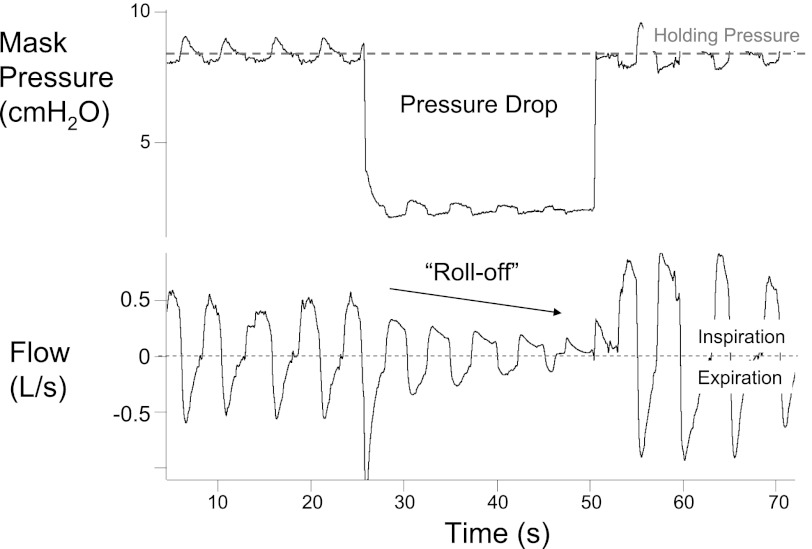
During a pressure drop, breath-by-breath peak inspiratory flows typically “roll-off,” that is, peak inspiratory flow decreases with each successive breath, until reaching an approximate steady state (see Fig. 1). It is assumed, but not known, that the initial roll-off in peak flow during breaths 1–3 is related to the change in lung volume that occurs with the pressure drop (24). However, we have observed similar roll-offs in peak flow during more prolonged pressure drops, well after any changes in EELV would be expected to have occurred (30). Moreover, these “late” roll-offs in flow occur while minute ventilation is below eupnea and ventilatory drive (Pco2) is increased, which should stimulate upper airway muscle activity to improve flow (11, 28). This observation suggests that the initial roll-off in flow might not be due solely to changes in EELV. Thus increasing respiratory effort or some other unidentified mechanism may be contributing to both this early and late decrement in peak flow.
Fig. 1.
Example of progressive decrease in peak flow during a pressure drop from our laboratory.
Here we propose a technique to measure Pcrit at a constant EELV, termed the “biphasic” technique: pressure is dropped first only in expiration to allow EELV to fall and equilibrate, and then inspiratory pressure is also dropped and inspiratory flow is assessed to determine Pcrit. By comparing the traditionally measured Pcrit with the Pcrit measured using the biphasic technique, we sought to determine whether changes in EELV that occur during the pressure drop affect the Pcrit and whether waiting until breaths 3–5 adequately accounts for EELV changes. In addition, this technique allowed us to test the hypothesis that a progressive fall in EELV is the cause of the roll-off in inspiratory flow after a pressure drop. Specifically, if the roll-off after a pressure drop is due to changes in EELV, we would expect no roll-off in flow while EELV is held constant. By contrast, persistence of the roll-off would suggest that factors other than changes in EELV are leading to decreases in flow.
METHODS
Ethical approval.
All subjects gave written, informed consent before participation in this study, which was approved by the Human Research Committee of the Brigham and Women's Hospital and conformed to the standards set by the Declaration of Helsinki.
Subjects.
A total of 13 subjects (10 OSA, 3 controls) were recruited for the study. Subjects included previously diagnosed OSA patients using CPAP therapy for >3 mo and healthy controls not known to have a sleep disorder. No study subject had ever smoked, had any other respiratory disorder, or took medications known to affect respiratory or airway/muscle function. Two patients were on medication for the treatment of hyperlipidemia, one was on medication for treatment of hypertension, one used a proton-pump inhibitor, and one was on a stable dose of thyroid hormone replacement. While some of the OSA subjects in this study participated in other studies conducted in our laboratory, none of the findings of the present study have been previously published.
Equipment.
The study consisted of a single overnight experiment. Patients arrived 2 h before their usual bedtime to be instrumented. Wakefulness and sleep stages were determined using standard electroencephalogram, chin electromyogram, and electro-oculogram. Airway pressure was measured at the level of the epiglottis using a pressure-tipped catheter (Millar MPC-550, Millar Instruments, Houston, TX) passed through the nose and advanced 1.5 to 2 cm below the base of the tongue under direct visualization. Before insertion, both nostrils were sprayed with 0.05% oxymetazoline hydrochloride, a decongestant, and the more patent nostril was then anesthetized with 4% lidocaine topical spray. A nasal mask (Profile Lite or GoldSeal, Respironics, Murraysville, PA) was placed over the nose and airflow was measured with a pneumotachograph (model 3700A; Hans Rudolph, Kansas City, MO) and a differential pressure transducer (model MP45; Validyne, Northridge, CA). Two pairs of magnetometers (EOL Eberhard, Oberwil, Switzerland) were placed on the front and back of the subject along the midline at the level of the sternum and just above the umbilicus. Calibration was performed during wakefulness by comparison with tidal volumes recorded by the pneumotachograph. Expired CO2 was continuously recorded from a catheter placed in the nostril with a capnograph (Vacumed, Ventura, CA). Arterial blood oxygen saturation via pulse oximetry (BCI, Waukesha, WI) and the electrocardiogram were monitored throughout the study for safety purposes. During the night, positive pressure was provided using either a modified CPAP machine (Respironics, Murraysville, PA) capable of providing positive or negative pressure, and able to switch rapidly between settings, or a commercially available BiPAP Synchrony device (see below).
Data were acquired on a 1401 plus interface and Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
Protocol.
When subjects were awake and lying comfortably in bed, magnet calibration was performed. CPAP was then applied, and subjects were allowed an opportunity to sleep. CPAP was initially set at the therapeutically prescribed level for OSA patients and at 4 cmH2O for controls, and it was increased if needed during sleep to eliminate flow limitation (flattened inspiratory flow, dissociation between mask and epiglottic pressure), snoring, or chest-abdomen paradox. This level of CPAP is referred to as the holding pressure. Once stable non-rapid-eye-movement sleep had been achieved, passive Pcrit measurements were made using either standard pressure drops or biphasic drops (described below) in random order. Repeated Pcrit measurements were made using both methodologies as many times as possible during stable non-rapid-eye-movement sleep. Patients were given time to reenter stable sleep after brief arousals, but if they awoke the measurement was aborted until stable sleep resumed. Patients slept supine.
Standard pressure drops.
With the use of the modified CPAP machine, standard Pcrit measurements were made by abruptly dropping the airway pressure for three to five breaths from the holding pressure to progressively lower CPAP levels, typically starting 1 or 2 cmH2O below the holding pressure and progressing in decrements of 0.5 or 1 cmH2O per drop. If necessary, negative airway pressure was applied. Pressure drops were separated by at least 1 min.
Biphasic pressure drops.
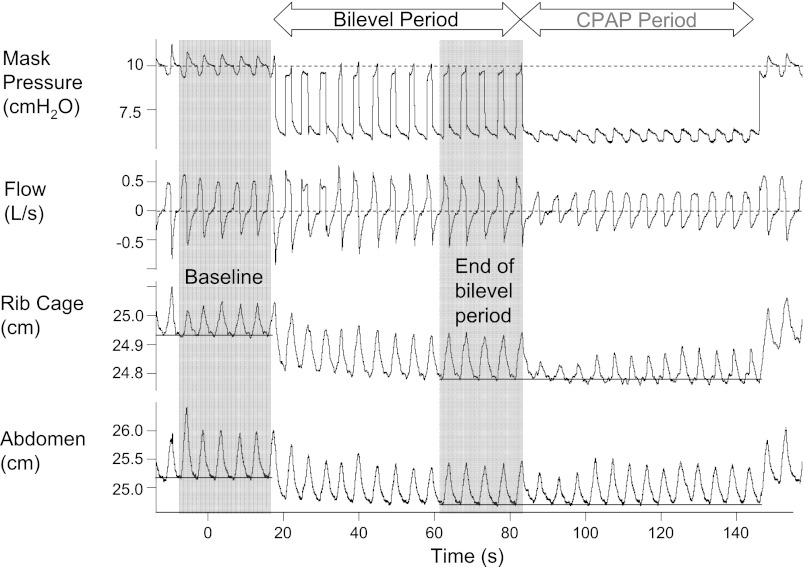
At the start of the biphasic pressure drop, the expiratory pressure only was reduced to the drop pressure of interest for 1 min. The inspiratory pressure remained at the holding pressure during this “bilevel period.” During the bilevel period, EELV should fall yet ventilation should be maintained. After 1 min, the inspiratory pressure was also decreased to the drop pressure, the “CPAP period” (see Fig. 2). The drop pressure was initially 1–2 cm H2O below the holding pressure and was decreased with each successive drop by 0.5 to 1 cmH2O. For pressure drops to 4 cmH2O or above, the BiPAP Synchrony was used in spontaneous (S) mode. During the bilevel period, inspiratory positive airway pressure was set at the holding pressure and expiratory positive airway pressure set to the drop pressure. At the end of the bilevel period, the ventilator mode was switched to CPAP and set at the drop level. As commercially available systems do not allow an expiratory positive airway pressure of <4 cmH2O, for pressure drops below this level, we used the modified CPAP machine to switch manually between holding and drop pressures during the bilevel period. Pressure was increased to the holding pressure just after the start of inspiration and decreased to the drop pressure as inspiratory flow approached zero. The CPAP period was continued until stable flow limitation developed for ∼1 min or apnea or arousal occurred, at which point pressure was returned to the holding pressure.
Fig. 2.
Example of the biphasic drop in a single subject, illustrating the change in lung volumes. During the biphasic drop, only the expiratory pressure is dropped from the holding pressure for 1 min (the bilevel period). Later the inspiratory pressure is also lowered to the same drop pressure [the continuous positive airway pressure (CPAP) period]. At the start of the bilevel period, there is an immediate decrease in lung volume (as measured by rib cage and abdominal magnotometers). A new equilibrium is reached by the end of the bilevel period. Compared with the end of the bilevel period, there is no further change in lung volume during the CPAP period.
Analysis.
Data were analyzed on a breath-by-breath basis using custom-designed semiautomated software. Analyzed variables included the following: inspiratory time, expiratory time, tidal volume, peak flow, mask pressure, and rib cage and abdominal anterior-posterior distance (provided continuously by each respective magnetometer pair). For each breath, magnetometer distances were measured just before inspiratory flow. If flow was obstructed, then the first negative deflection in epiglottic pressure was used to define the end of expiration and the start of the breath.
To determine Pcrit, mask pressure and peak inspiratory flow from breaths 3–5 during each pressure drop series were plotted only if the breaths were flow limited. breaths 3–5 of the CPAP period, if flow limited, were used to determine the Pcrit using the biphasic technique. A linear regression model of peak inspiratory flow vs. mask pressure was used to extrapolate the pressure at zero flow, defined as Pcrit (20). The multiple Pcrit values in each condition were averaged to determine a Pcrit using standard pressure drops and a Pcrit using biphasic pressure drops.
Changes in EELV were determined using a previously validated relationship between magnetometer readings and lung volume (1). During each breath of a standard pressure drop, the magnetometer distances at the start of inspiration were compared with the average of these distances during the five breaths immediately preceding that drop. The differences in distance and the previously determined calibration factor were used to measure acute changes in lung volume during Pcrit measurement. For each pressure drop, the lung volume changes during breaths 3–5 were plotted against the change in mask pressure (measured at end expiration) from the holding pressure, and a linear regression model was fitted to the data.
For the biphasic pressure drops, the five breaths immediately preceding the drop were also used as baseline. The last five breaths during the bilevel period were used to assess changes in lung volume, tidal volume, and CO2 that occurred during the bilevel period. Similar to the standard pressure drops, the relationship between the change in lung volume for a given change in expiratory pressure was determined. Once the inspiratory pressure was also decreased to the drop pressure, EELV was assessed for further change during the breaths of the CPAP period.
Statistical analysis.
Paired Student's t-test was used to compare Pcrit values obtained using standard and biphasic pressure drops. Tidal volume and end-tidal CO2 at baseline and at the end of the biphasic period were similarly compared. A P value of <0.05 was considered statistically significant. Values are presented as means ± SD unless otherwise indicated.
RESULTS
Subject characteristics.
Complete data could not be obtained in three subjects due to poor sleep or major mask/mouth leaks. A single OSA subject's abdominal girth exceeded the upper limit of the magnetometer range, and changes in lung volume are not reported for this individual. The anthropometric and polysomnographic data for the 10 remaining subjects (7 OSA, 3 controls) in whom data could be obtained are shown in Table 1.
Table 1.
Subject characteristics
| Diagnosis | n (M/F) | Age, yr | BMI, kg/m2 | AHI, events/hour | CPAP Rx, cmH2O | Holding Pressure, cmH2O |
|---|---|---|---|---|---|---|
| OSA | 2/5 | 49.7 ± 8.0 | 39.2 ± 5.7 | 35.9 ± 13.6 | 9.1 ± 1.7 | 9.3 ± 2.2 |
| Control | 2/1 | 47.7 ± 10.6 | 26.5 ± 5.0 | 4.6 ± 2.7 | 6.0 ± 1.7 |
Values are means ± SD. M/F, male/female; BMI, body mass index; AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea.
Lung volumes.
As expected, during standard pressure drops, EELV fell in proportion to the change in mask pressure. EELV during breaths 3–5 fell on average 55 ± 12 ml for each 1 cmH2O drop from the holding pressure. Similar (P value nonsignificant) changes in EELV were seen when only the expiratory pressure was dropped during the bilevel period; an example from a single subject is shown in Fig. 2. By the end of the bilevel period, EELV fell by 59 ± 18 ml for each 1 cmH2O drop in the expiratory pressure below the holding pressure. Importantly, once inspiratory pressure was also reduced to the drop pressure at the start of the CPAP period, further changes in lung volume were negligible. EELV decreased only an additional 6.3 ± 2.8 ml for each cmH2O drop in inspiratory pressure.
Pcrit.
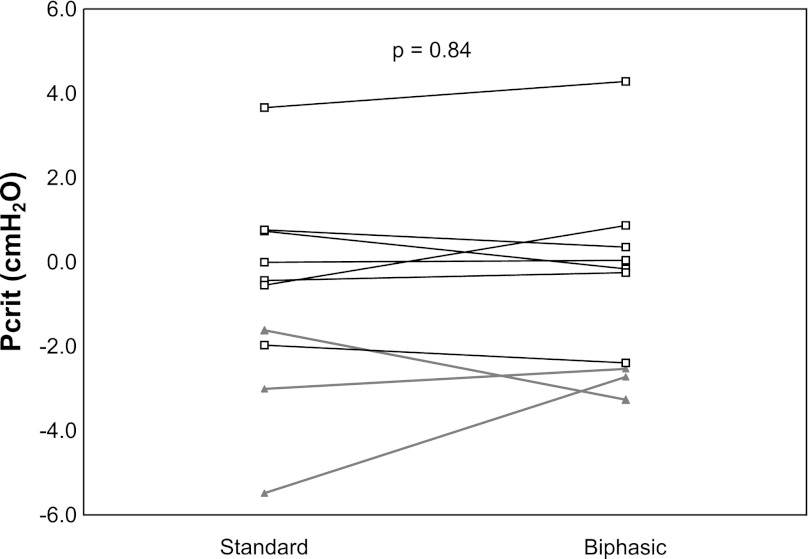
On average, standard Pcrit measurements were made 3.4 ± 1.3 times per subject, and biphasic measurements were made 2.0 ± 1.2 times per subject. Pcrit measured using the standard and biphasic technique are shown in Fig. 3. There was no difference in Pcrit between the two techniques (P = 0.84). During the biphasic measurements, at the end of the bilevel period, tidal volume was slightly greater than baseline (561 ± 13 vs. 537 ± 12 ml, nonsignificant), and end-tidal CO2 was slightly lower (39.6 ± 2.8 vs. 40.6 ± 2.8 mmHg, nonsignificant).
Fig. 3.
Passive Pcrit measured in standard fashion and using the biphasic technique in OSA (□) and controls (▲).
Early roll off in flow goes away when EELV remains essentially constant.
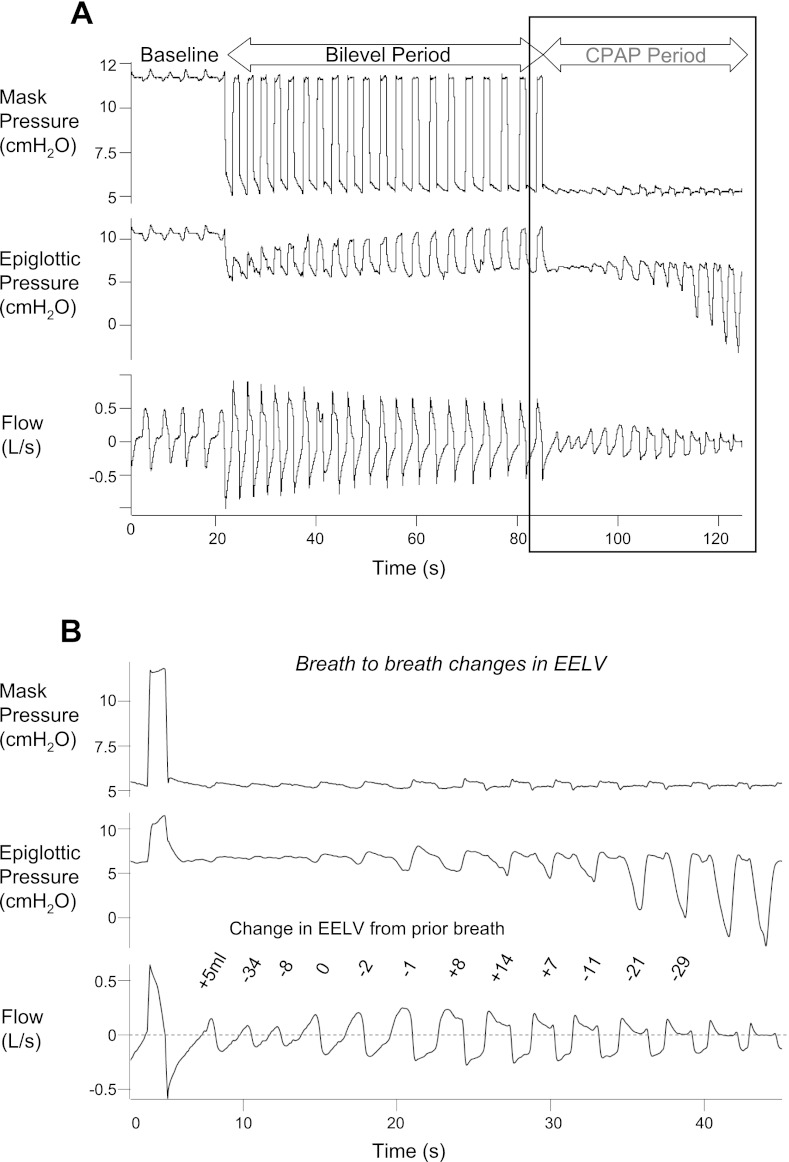
As expected, most standard pressure drops showed an initial roll-off in peak flow during breaths 1–3. With biphasic drops, with little change in EELV during the CPAP period, there was no initial roll-off. Rather, flow initially increased during the first few breaths after the transition from the biphasic drop to the CPAP period. However, after these first few (>3) breaths, there was often a late roll-off or decrease in peak or mean flow and the development of flow limitation, despite little or no changes in EELV (Fig. 4).
Fig. 4.
Changes in end-expiratory lung volume (EELV) during the bilevel period and CPAP periods. A: by the end of the bilevel period, EELV has fallen ∼410 ml from baseline. B: during the CPAP period, after a 1–2 breath further decline of ∼30 ml, EELV changes very little on a breath by breath basis, even as flow initially increases then decreases.
Patterns of late flow limitation.
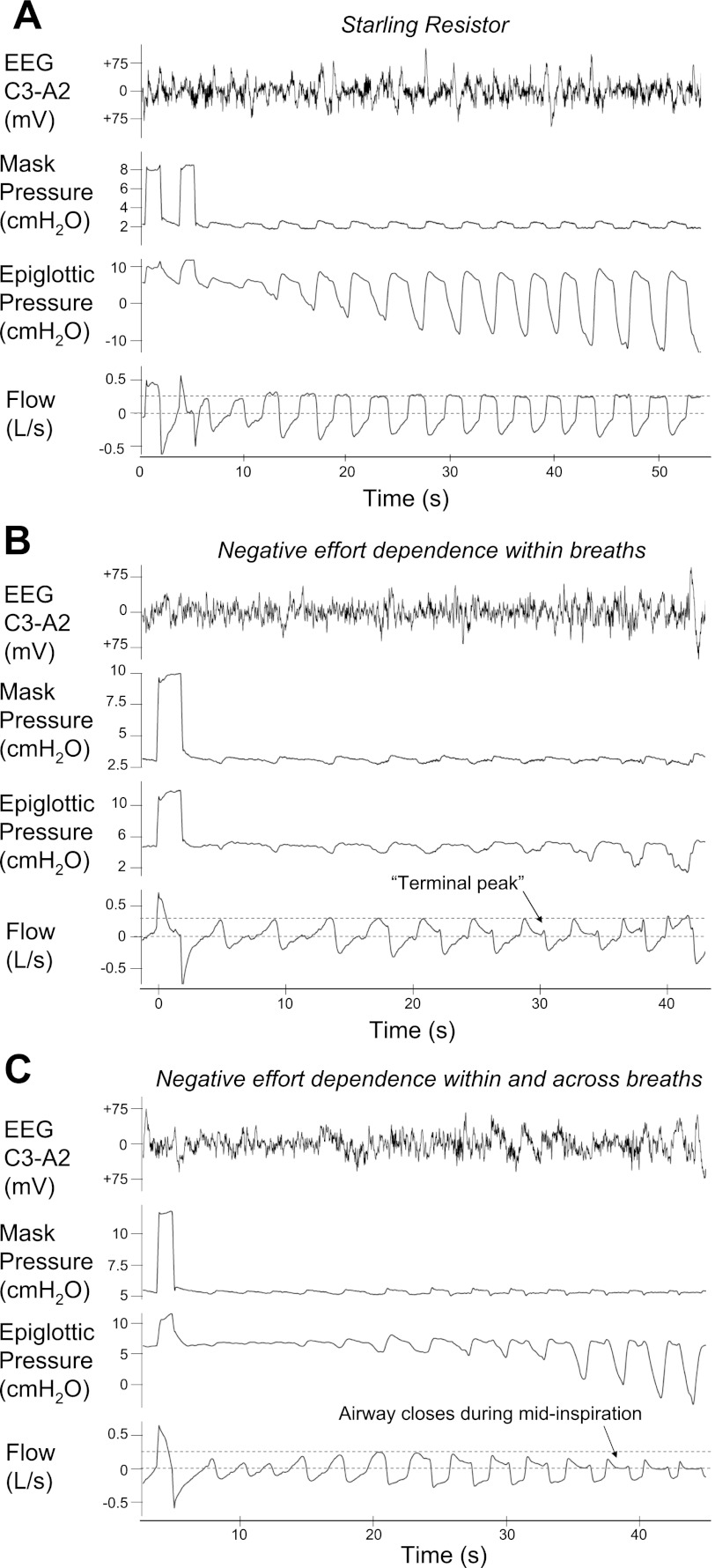
An interesting and unexpected finding was that when flow limitation developed, it did so in one of three different patterns (Fig. 5). In three subjects (2 OSA, 1 control), flow within a breath initially increased then became constant and independent of changes in downstream pressure (Fig. 3A), as predicted by the Starling resistor model. Successive breaths were identical (inspiratory time, peak flow, and mean flow) and min ventilation was stable (Table 2). In four subjects (2 OSA, 2 control), flow within a breath increased but subsequently decreased as the downstream pressure continued to decrease. The peak flows of successive breaths were nearly identical (Fig. 3B); however, mean flow was progressively lower with each breath and ventilation was only preserved through an increase in the respiratory duty cycle. We have called this pattern “negative effort dependence within breaths only.” The remaining three subjects (all OSA) exhibited what we have termed “negative effort dependence both within breaths and across breaths.” Within breaths, flow initially rose with increasing effort but later decreased. With each subsequent breath, the peak flow fell further (Fig. 3C). Mean flow and minute ventilation also decreased, despite an increase in the duty cycle. Importantly, for the range of drop pressures tested in each subject the pattern of flow limitation was consistent across the night. Again, regardless of flow patterns, EELV remained fairly constant during the prolonged subtherapeutic CPAP periods.
Fig. 5.
Epiglottic pressure vs. inspiratory flow in 3 subjects, exhibiting the different patterns of negative effort dependence. In all cases, flow initially increases with increasing effort. However, above a certain inspiratory effort, three patterns are seen. A: no negative effort dependence. Subject behaves as a perfect Starling resistor. B: negative effort dependence within a single breath. After reaching a maximum, flow decreases within a breath as epiglottic pressure becomes more negative. Note that each successive breath reaches the same peak inspiratory flow. C: negative effort within and across breaths. As in B, flow decreases within each separate breath, but the peak flow is also reduced with each successive breath.
Table 2.
Quantification of respiratory variables during the three patterns of flow limitation
| Breath 1 | Breath 2 | Breath 3 | Breath 4 | Breath 5 | Breath 6 | |
|---|---|---|---|---|---|---|
| Starling resistor | ||||||
| Ti, s | 1.83 | 1.78 | 1.97 | 1.79 | 1.86 | 1.79 |
| Ti/Ttot | 0.52 | 0.49 | 0.50 | 0.49 | 0.52 | 0.52 |
| Peak flow, l/s | 0.34 | 0.31 | 0.29 | 0.32 | 0.31 | 0.31 |
| Mean flow, l/s | 0.25 | 0.23 | 0.22 | 0.24 | 0.23 | 0.22 |
| Tidal volume, liter | 0.45 | 0.41 | 0.43 | 0.42 | 0.42 | 0.40 |
| Respiratory rate, beat/min | 17.05 | 16.48 | 15.28 | 16.23 | 16.82 | 17.36 |
| Instantaneous ventilation, l/min | 7.70 | 6.79 | 6.57 | 6.87 | 7.06 | 6.96 |
| Negative effort dependence within a breath only | ||||||
| Ti, s | 1.80 | 1.84 | 1.94 | 1.95 | 2.10 | 2.24 |
| Ti/Ttot | 0.38 | 0.40 | 0.40 | 0.47 | 0.48 | 0.49 |
| Peak flow, l/s | 0.27 | 0.26 | 0.25 | 0.25 | 0.23 | 0.24 |
| Mean flow, l/s | 0.21 | 0.18 | 0.18 | 0.17 | 0.15 | 0.14 |
| Tidal volume, liter | 0.38 | 0.34 | 0.35 | 0.34 | 0.32 | 0.32 |
| Respiratory rate, beat/min | 12.38 | 13.62 | 13.26 | 13.14 | 13.75 | 12.91 |
| Instantaneous ventilation, l/min | 4.69 | 4.56 | 4.68 | 4.42 | 4.35 | 4.07 |
| Negative effort dependence within and across breaths | ||||||
| Ti, s | 1.27 | 1.52 | 1.46 | 1.76 | 2.01 | 1.96 |
| Ti/Ttot | 0.41 | 0.55 | 0.55 | 0.59 | 0.61 | 0.67 |
| Peak flow, l/s | 0.20 | 0.10 | 0.06 | 0.06 | 0.01 | 0.01 |
| Mean flow, l/s | 0.10 | 0.09 | 0.08 | 0.06 | 0.03 | 0.02 |
| Tidal volume, liter | 0.16 | 0.17 | 0.15 | 0.15 | 0.09 | 0.03 |
| Respiratory rate, beat/min | 19.49 | 21.75 | 22.40 | 20.09 | 18.28 | 20.40 |
| Instantaneous ventilation, l/min | 3.08 | 3.74 | 3.34 | 2.93 | 1.55 | 0.55 |
Breaths 1–6 are highlighted breaths from Fig. 6, A–C. Flow-limited Starling resistor breaths have similar peak flow, similar mean flow, and preserved tidal volume and minute ventilation. Negative effort dependence within a breath only has similar peak flow, but decreasing mean flow; ventilation is relatively preserved through increase in the duty ratio (Ti/Ttot). In contrast, in negative effort dependence across breaths, the increase in the duty ratio does not compensate for decreasing peak and mean flows.
DISCUSSION
Our biphasic technique was designed to allow measurement of passive upper airway collapsibility after changes in EELV had already occurred. As expected, lung volume changes occurred mostly during the early bilevel period of the biphasic drops, and there was little additional change in EELV during the subsequent CPAP period. We have used this technique to make several novel physiological observations.
Pcrit measured at constant EELV.
There was no difference between the Pcrit measured in the standard fashion and the Pcrit measured with the biphasic technique, using breaths 3–5 of each pressure drop (during the standard drop or during the CPAP period). The individual differences in Pcrit are within the generally accepted repeatability of the measurement (10, 17). This confirms prior work that suggests that most of the lung volume change occurs within the first two breaths (18). It also suggests that the effects of EELV on Pcrit are immediate. These data are consistent with the hypothesis that tracheal traction is the mechanism by which increased EELV directly exerts an increased mechanical caudal traction that stabilizes the upper airway.
Of note, we used the biphasic technique to shift the timing of the lung volume change associated with pressure drops. However, subjects were still maintained at a different EELV than their supine, sleeping functional residual capacity (FRC). Determining upper airway collapsibility at supine FRC during sleep is of interest but is difficult since patients with OSA are typically studied on positive pressure and are thus above FRC, while control subjects are studied using negative pressure and are below FRC. Because of the effect of EELV on Pcrit, these deviations from FRC cause the airway to appear artifactually more stable in OSA patients and more collapsible in controls. In future experiments the biphasic technique could be modified to assess Pcrit at or near FRC (provided there is not substantial expiratory obstruction): the expiratory pressure would be lowered to 0 cmH2O, and peak flow could be assessed at a variety of inspiratory pressures.
Early roll-off disappears when EELV remains essentially constant.
A second motivation for the current work was to study the early roll-off in peak flow that occurs during a standard pressure drop. Earlier work by Schwartz et al. (24) had implicated changes in lung volume as the cause of this roll-off. In their study, they compared the peak flow from breath 1 vs. breath 2 vs. breath 3 during a pressure drop to determine Pcrit, with simultaneous measurement of genioglossus EMG (24). They hypothesized that peak flow would improve from breaths 1–3 as muscle recruitment occurred. Surprisingly, they found that peak flow actually decreased. They concluded that lung volume changes, which were inferred from changes in esophageal pressure, were the most likely factor mediating this change. In our experiment, with EELV held constant, the early roll-off in peak flow disappeared. This finding seems to support the lung volume hypothesis proposed by Schwartz et al. However, it should be noted that during the bilevel period respiratory drive and effort were slightly reduced. As a result, the first few breaths of the subsequent CPAP period were often small and nonflow limited. Thus the disappearance of the early roll-off in peak flow could have been because of less effort producing less flow, as opposed to EELV-related changes in the mechanical properties of the airway limiting flow. Further studies are needed to confirm if the early roll-off is indeed due to changes in lung volume.
Late roll-off in flow even when EELV is constant.
During the initial breaths of the CPAP period, as respiratory effort increased, so too did peak inspiratory flow (see Fig. 5). However, when flow limitation developed in subsequent breaths three patterns were observed: 1) that predicted by the Starling resistor model, 2) negative effort dependence within breaths only, and 3) negative effort dependence both within breaths and across breaths. With the last two patterns of flow limitation, there was a late roll-off in mean inspiratory flow, despite constant EELV and increasing respiratory effort. This observation was unexpected since a prolonged drop to sub-therapeutic CPAP will cause hypercapnia and/or hypoxemia that should stimulate upper airway dilator muscles to stiffen or open the airway and thereby maintain or potentially increase (rather than decrease) inspiratory flows. Increasingly negative intrathoracic or intraluminal pressures are also expected to help maintain or increase flow, not decrease it, via reflex stimulation of the upper airway muscles (12, 21). Unlike a Starling resistor, in which flow becomes independent of downstream pressure (once more negative than some threshold), we saw that flow decreased with increasingly negative downstream pressure in most of our subjects. That is, more effort produced less flow (“negative effort dependence”), not only within a breath but also progressively over a series of breaths.
Negative effort dependence: patterns and possible causes.
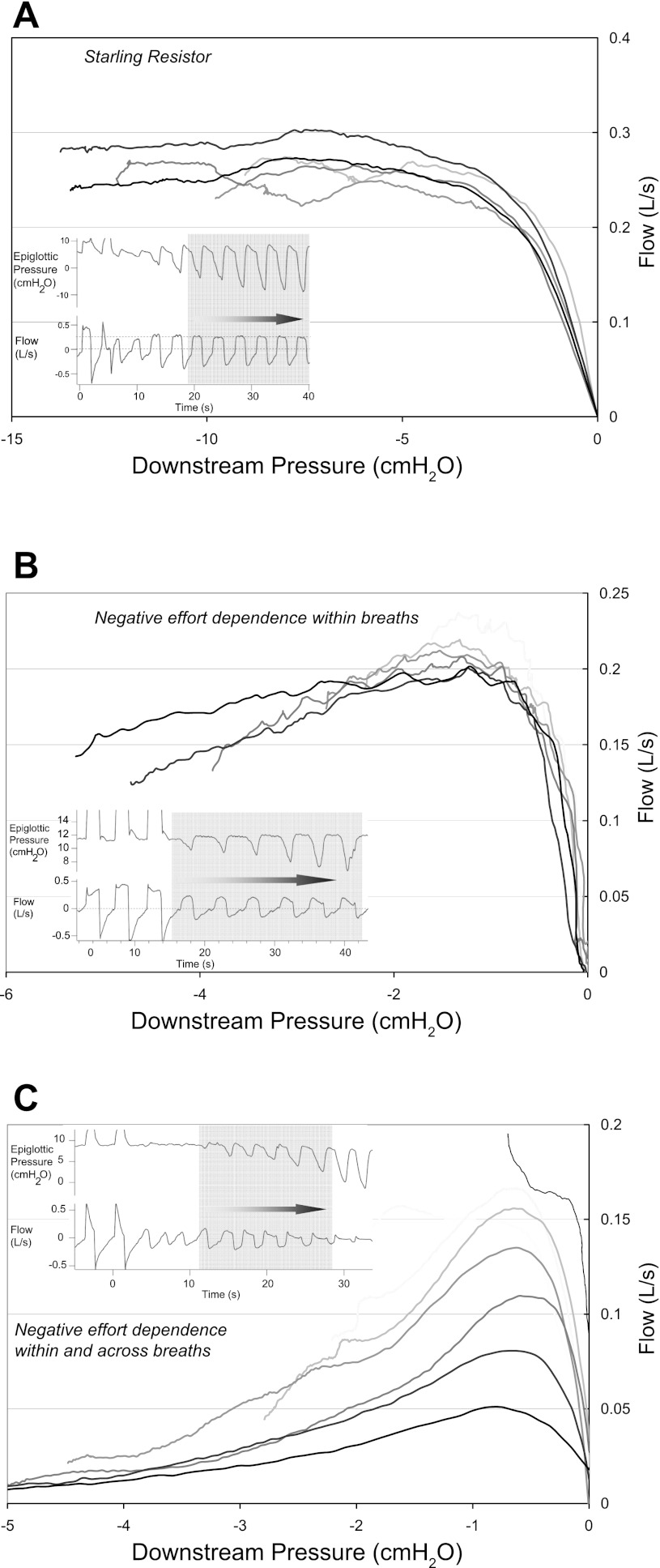
Two patterns of negative effort dependence were seen. In the first pattern, subjects exhibited negative effort dependence “within breaths only.” In these individuals, flow initially increased with increasing respiratory effort to a point. Instead of plateauing, flow then decreased as downstream pressure became more negative (see Fig. 5B). In some cases, flow even increased again during late inspiration as downstream pressure returned to baseline (“terminal peak”). Importantly, with each successive breath in these individuals, peak flow was the same, and the relationship between downstream pressure and peak flow was maintained across many breaths (Fig. 6B). These individuals were “sweeping out” the same pressure-flow curve on each breath. Because there is negative effort dependence in the pressure-flow curve, the mean flow rate with each successive breath decreases but the peak flow does not (see Table 2). Up to a point, these subjects were able to maintain a stable minute ventilation by prolonging the inspiratory time. These data suggest that the upper airway is narrowing during inspiration but that it returns to the same baseline level by the start of the next breath. In fact, the “terminal peak” suggests that some improvement takes place even during late inspiration, as downstream pressure subsides. Importantly, these results differ from imaging studies performed in OSA patients during wakefulness, which showed airway dilation during inspiration and the greatest narrowing at end expiration (23). Finally, as shown in Figs. 5B and 6B, the degree of negative effort dependence, as assessed by the difference between the peak flow and the flow during mid-inspiration, can be substantial. Although small amounts of negative effort dependence, with slight down-sloping of the pressure-flow curve of the upper airway have been previously been recognized, the individual variability and the potential magnitude of this effect has, we believe, been underappreciated (25). In some subjects, the magnitude is large enough to constitute a deviation from the Starling resistor as a model of the dynamics of the upper airway.
Fig. 6.
Pressure-flow curves of successive flow-limited breaths. Each insert highlights the breaths that are plotted, with darker curves representing later breaths. A: each separate breath shows a similar curve, with flow reaching a maximum and then becoming independent of further decreases in downstream pressure. B: each separate breath shows a similar curve, with decreases in downstream pressure associated with decreased flow. C: here each separate breath sweeps out a different curve.
Historically, negative effort dependence has most often been described in the context of isovolumetric expiratory flow limitation (specifically in isovolume pressure flow relationships) in which flow, which usually plateaus, sometimes exhibits a slight decrease with increasing effort. This is found to hold even after accounting for artifacts due to gas compression. Mead et al. (14) used the “equal pressure point (EPP)” concept to explain expiratory flow limitation. They proposed that negative effort dependence could be produced by movement of the EPP back toward the thoracic outlet, i.e., downstream relative to expiratory flow. In particular, since flow limited expiratory flow is the ratio of driving pressure to resistance upstream of the EPP, and since driving pressure remains the same at a given lung volume, decreased flow can only be the result of increased upstream resistance due to lengthening of the upstream segment. However, in the upper airway, it seems unlikely that the EPP could move enough to increase upstream resistance sufficiently to decrease flow to the extent seen here. Indeed, it is not clear whether an EPP even exists in the upper airway, since upstream driving pressure (ambient pressure at the nares) and extraluminal tissue pressure are not linked as in the lower airways. Alternatively, Dawson and Elliott (3) proposed wave-speed limitation to account for expiratory flow limitation. To explain negative effort dependence, which again was quite small in their experiments, they suggested that airway narrowing downstream of the choke point could reduce wall curvature at the choke point. This would reduce stiffness and cross-sectional area at the choke point, thereby reducing maximum flow. Again, we believe that this mechanism would produce small amounts of negative effort dependence in contrast to the substantial amounts we find. Compared with the mechanism that produces mild reductions in flow during forced expiration, the mechanism underlying these profound levels of negative effort dependence seen in the upper airway must therefore involve other factors, and currently remains an open question.
In contrast to negative effort dependence within a breath, negative effort dependence “within and across breaths” has not previously been described. Here, peak flow decreases with each successive breath, i.e., each breath sweeps out a different, progressively deleterious, pressure-flow curve (Fig. 6C). Morrell et al. (15, 16) speculated that progressive narrowing of the upper airway was due to a progressive reduction in EELV that negatively affected upper airway mechanics. Our study, however, shows that the worsening of the upper airway anatomy does not require changes in EELV. Additionally, in their experiment, respiratory drive fluctuated, and thus the observed hypopneas and apneas may have partially resulted from reductions in drive and upper airway muscle activity following arousal and transient hyperventilation (7, 32). Here we have shown that progressive narrowing across breaths can occur without arousals and in the setting of an increasing respiratory drive, i.e., the progressive narrowing is not simply due to passive collapse of the airway. We even saw progressive collapse when nasal pressure was greater than the Pcrit (for example, the mask or upstream pressure in Fig. 6C is 5.5 cmH2O, with measured Pcrit lower at 3.7 cmH2O). Thus, we speculate that similar to within breath negative effort dependence, the airway collapses during inspiration but does not fully recover during expiration: with each successive breath there is a new, worse pressure-flow relationship of the upper airway. The cause of this incomplete recovery is unknown, but could be due one or more of the following. First, airway elastic recoil may be slow, or not quick enough to reexpand the airway before the next inspiration. This effect may be exacerbated when inspiratory time lengthens relative to expiratory time during flow limitation (8, 22). Second, the tongue or other structure may be pulled progressively further into the airway and fail to return to its original position during expiration. Third, repetitive collapse during each inspiration may induce changes in surfactant composition and surface tension that promote further collapse (9). Whatever the mechanism, minute ventilation is not maintained despite changes in the duty cycle (Table 2). In these subjects, flow limitation will eventually lead to an arousal since increasing respiratory effort only worsens ventilation.
We and others (30) have focused on understanding OSA as a multifactorial disorder, with different traits having variable importance in individual patients. The presence of one of the described patterns of negative effort dependence (or lack thereof) may be a separate factor, or may reflect some of these traits. Better characterization of such sleep apnea “phenotypes” and timing of collapse could help personalize OSA treatment. Although speculative, we hypothesize that those patients with negative effort dependence within breaths might do best with bilevel positive airway pressure and fail with nasal expiratory resistance valves, which are thought to prevent collapse primarily during expiration (2, 19). Alternatively, such patients might be minimally affected or even improved by sedative medications (such as benzodiazepines and narcotics) that reduce respiratory drive, compared with those with extremely poor airway anatomy whose OSA severity might worsen substantially (4). Further research is needed to determine the clinical relevance of our findings.
Limitations.
Limitations of our study include first the small number of subjects studied. We also did not study subjects with untreated OSA: the relationship between EELV and Pcrit might be different in untreated OSA (perhaps with upper airway edema) compared with treated OSA. Second, we do not have a measure of upper airway muscle activity or direct visualization of the motion of the tongue or other pharyngeal structures. Third, this technique requires the use of bilevel PAP/pressure support, which will increase minute ventilation and thus reduce respiratory drive. However, most of the increase is transient (31), and after 1 min of pressure support ventilation the changes in tidal volume and end-tidal CO2 were minimal compared with baseline. Furthermore, the slight hyperventilation that occurs would tend to make the upper airway muscles more “passive” and thus is less likely to change the passive Pcrit substantially. Finally, the biphasic technique was slightly less well tolerated than the standard passive Pcrit pressure drops; however, it is likely better tolerated than the use of an iron lung to control EELV using extrathoracic pressure.
Conclusion.
EELV changes do not influence standard passive Pcrit measurements if breaths 3–5 of a pressure drop are used. Progressive reductions in mean and peak flow can develop in the absence of EELV changes, and more negative downstream pressures during inspiration are associated, in some patients, with substantial decreases in flow. Although the cause remains unknown, the within breath negative effort dependence is associated with airway collapse during inspiration. However, negative effort dependence across breaths likely results from decreased airway size breath to breath due to incomplete recovery of airway dimension during expiration.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 5R01-HL-048531-16, R01 HL085188-02, R01-HL-090897-01A2, K24-HL-093218-01A1, K23-HL-105542, and P01-HL-095491 and American Heart Association Grants 0840159N and 0575028N. B. A. Edwards supported by the Thoracic Society of Australia and New Zealand/Allen and Hanbury's Respiratory Research Fellowship. S. A. Sands is supported by an American Heart Association fellowship (11POST7360012).
DISCLOSURES
D. J. Eckert is a consultant for Apnex Medical. A. Malhotra is a consultant for Philips Respironics, SHC, SGS, Apnex, Apnicure, and Pfizer. D. P. White is the chief medical officer for Philips Respironics. A. Wellman is a consultant for Philips Respironics, SOVA Pharmaceuticals and Apnex Medical. R. L. Owens is a consultant for Apnex Medical. All other authors do not have a financial relationship with a commercial entity that has an interest in the subject of this study.
AUTHOR CONTRIBUTIONS
Author contributions: R.L.O., B.A.E., D.J.E., A.M., and A.W. conception and design of research; R.L.O., B.A.E., D.J.E., and A.W. performed experiments; R.L.O., D.J.E., and A.W. analyzed data; R.L.O., B.A.E., J.P.B., D.J.E., D.P.W., A.M., and A.W. interpreted results of experiments; R.L.O., B.A.E., and A.W. prepared figures; R.L.O., S.A.S., and A.W. drafted manuscript; R.L.O., B.A.E., S.A.S., J.P.B., D.J.E., D.P.W., A.M., and A.W. edited and revised manuscript; R.L.O., B.A.E., S.A.S., J.P.B., D.J.E., D.P.W., A.M., and A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank sleep technicians Lauren Hess, Pamela DeYoung, and Erik Smales for laboratory assistance.
REFERENCES
- 1. Banzett RB, Mahan ST, Garner DM, Brughera A, Loring SH. A simple and reliable method to calibrate respiratory magnetometers and Respitrace. J Appl Physiol 79: 2169–2176, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Braga CW, Chen Q, Burschtin O, Rapoport DM, Ayappa I. Changes in lung volume and upper airway using MRI during application of nasal expiratory positive airway pressure in patients with sleep disordered breathing. J Appl Physiol 111: 1400–1409, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Dawson SV, Elliott EA. Wave-speed limitation on expiratory flow-a unifying concept. J Appl Physiol 43: 498–515, 1977 [DOI] [PubMed] [Google Scholar]
- 4. Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP, Malhotra A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 120: 505–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 110: 1077–1088, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol 57: 520–527, 1984 [DOI] [PubMed] [Google Scholar]
- 7. Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med 184: 1183–1191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 62: 861–867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkness JP, Christenson HK, Garlick SR, Parikh R, Kairaitis K, Wheatley JR, Amis TC. Decreased surface tension of upper airway mucosal lining liquid increases upper airway patency in anaesthetised rabbits. J Physiol 547: 603–611, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirkness JP, Peterson LA, Squier SB, McGinley BM, Schneider H, Meyer A, Schwartz AR, Smith PL, Patil SP. Performance characteristics of upper airway critical collapsing pressure measurements during sleep. Sleep 34: 459–467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RC, Schory K, Dover L, Fogel RB, White DP. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep 29: 470–477, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med 161: 1746–1749, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Malhotra A, White DP. Obstructive sleep apnoea. Lancet 360: 237–245, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol 22: 95–108, 1967 [DOI] [PubMed] [Google Scholar]
- 15. Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med 158: 1974–1981, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Morrell MJ, Badr MS. Effects of NREM sleep on dynamic within-breath changes in upper airway patency in humans. J Appl Physiol 84: 190–199, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Ong JS, Touyz G, Tanner S, Hillman DR, Eastwood PR, Walsh JH. Variability of human upper airway collapsibility during sleep and the influence of body posture and sleep stage. J Sleep Res 20: 533–537, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol 108: 445–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel AV, Hwang D, Masdeu MJ, Chen GM, Rapoport DM, Ayappa I. Predictors of response to a nasal expiratory resistor device and its potential mechanisms of action for treatment of obstructive sleep apnea. J Clin Sleep Med 7: 13–22, 2011 [PMC free article] [PubMed] [Google Scholar]
- 20. Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 170: 86–93, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Pierce R, White D, Malhotra A, Edwards JK, Kleverlaan D, Palmer L, Trinder J. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J 30: 345–353, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schneider H, Krishnan V, Pichard LE, Patil SP, Smith PL, Schwartz AR. Inspiratory duty cycle responses to flow limitation predict nocturnal hypoventilation. Eur Respir J 33: 1068–1076, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis 148: 1385–1400, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J Appl Physiol 66: 1626–1634, 1989 [DOI] [PubMed] [Google Scholar]
- 26. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol 64: 789–795, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Squier SB, Patil SP, Schneider H, Kirkness JP, Smith PL, Schwartz AR. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J Appl Physiol 109: 977–985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med 165: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 65: 2124–2131, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol 110: 1627–1637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 145: 114–120, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 169: 623–633, 2004 [DOI] [PubMed] [Google Scholar]