Abstract
We previously reported the unexpected finding that 4 wk of exposure to intermittent hypoxia (IH), which simulates the hypoxic stress of obstructive sleep apnea, improved LV cardiac function in healthy, lean C57BL/6J mice. The purpose of the present study was to assess the impact of 4 wk of IH on cardiac function in a transgenic murine model that exhibits a natural history of heart failure. We hypothesized that IH exposure would exacerbate cardiac decompensation in heart failure. Adult male FVB (wild type) and transgenic mice with cardiac overexpression of tumor necrosis factor α (TNF-αTG) at 10–12 wk of age were exposed to 4 wk of IH (nadir inspired oxygen 5–6% at 60 cycles/h for 12 h during light period) or intermittent air (IA) as control. Cardiac function was assessed by echocardiography and pressure-volume loop analyses, and mRNA and protein expression were performed on ventricular homogenates. TNF-αTG mice exposed to IA exhibited impaired LV contractility and increased LV dilation associated with markedly elevated cardiac expression of atrial natriuretic peptide and brain natriuretic peptide compared with wild-type mice. When wild-type FVB mice were exposed to IH, they exhibited increases in arterial pressure and dP/dtmax, consistent with our previous report in C57BL/6J mice. Surprisingly, we found that TNF-αTG mice exposed to IH showed a reduction in end-diastolic volume (38.7 ± 3.8 to 22.2 ± 2.1 ul; P < 0.01) and an increase in ejection fraction (29.4 ± 2.5 to 41.9 ± 3.1%; P < 0.05). In contrast to our previous study in C56Bl/6J mice, neither FVB nor TNF-αTG mice exhibited an upregulation in β-adrenergic expression or cAMP in response to IH exposure. We conclude that 4 wk of exposure to IH in mice induces adaptive responses that improve cardiac function in not only healthy animals but also in animals with underlying heart failure.
Keywords: blood pressure, echocardiography, ejection fraction, heart failure, sympathetic activation, tumor necrosis factor
exposure to hypoxia can lead to adaptive or pathological effects, depending on the severity, pattern, and duration of exposure. Two extremes serve to highlight the dichotomy of responses that hypoxia can evoke. Sustained periods of continuous 10% hypoxia simulating altitude (∼5,000 m) for ≥3 wk is a classic model for induction of pulmonary hypertension and right heart hypertrophy in rodents (26). In contrast, a small number of brief periods (minutes) of exposure to nonlethal hypoxia or cardiac ischemia, separated by periods of normoxia or repurfusion, protect the heart from subsequent ischemic insult (27). More recently, a third form of hypoxic paradigm has emerged in the literature: modeling the acute intermittent hypoxic episodes associated with airway obstruction that characterize sleep apnea (29).
The patterns and duration of exposure to intermittent hypoxia (IH) have varied considerably between laboratories for a variety of technical and biological reasons. The periods of IH have ranged from 15 s to 2 min and occur at rates from 20 to 120 IH events/h throughout the light or sleeping period of rodents (17, 31). When the IH stimulus is applied for weeks to months in rodents, a range of pathologies emerge that reproduce several features of the metabolic syndrome, including hypertension (1), insulin resistance (16, 28), and hyperlipidemia (22). Some studies have also shown that IH can impair cardiac contractility and induce left ventricular (LV) dilation (5, 6). However, we recently showed, using a highly sensitive pressure-volume (P-V) loop approach, that 4 wk of exposure to IH in lean, healthy C57BL/6J mice surprisingly resulted in increased LV contractility, which was at least in part mediated by activation of cardiac β-adrenergic pathways (25). A potential corollary of our findings is that IH may induce adaptive or compensatory physiological changes, whereas any pathological effects of IH may require the presence of underlying co-morbidity.
We, therefore, examined the effect of IH in a transgenic tumor necrosis factor α (TNF-α) mouse model that exhibits a natural history of heart failure. Failing human myocardium expresses abundant quantities of TNF-α (13, 34), and a mouse model with cardiac overexpression of TNF-α on an FVB background developed at the University of Pittsburgh exhibits a phenotype of congestive heart failure that occurs as early as 6 wk of age (18, 19). The purpose of our present study was threefold: 1) to determine whether 4 wk of IH exposure in wild-type FVB mice replicated our previous observation of increased LV contractility in the C57BL/6J strain; 2) to determine whether the development of heart failure in TNF-α transgenic (TNF-αTG) mice was exacerbated by 4 wk of IH exposure; 3) to determine whether any changes in cardiac function in FVB or TNF-αTG mice exposed to IH were associated with altered β-adrenergic signaling. Our overall hypothesis was that IH accelerates the progression of LV dysfunction in mice genetically predisposed to heart failure.
METHODS
Animals.
Two groups of mice were used for this study: 1) male TNF-αTG mice with cardiac-specific over expression of tumor necrosis factor alpha and 2) FVB wild-type littermates for use as controls (18, 19). All mice were male, were 10–12 wk old, and were obtained from Dr. Charlie McTiernan (Cardiovascular Institute, University of Pittsburgh, Pittsburgh, PA) for use in the study. All studies were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh Medical Center and complied with the American Physiological Society Guidelines for Animal Studies.
Protocol.
Mice were housed in regular cages that were customized to deliver either an IH stimulus or an intermittent room air (IA) stimulus as a control, using previously described techniques (29). Our approach in customizing the cages was to allow the animals to live in their normal environment continuously throughout the protocol. In brief, a gas control delivery system regulated the flow of room air, nitrogen, and oxygen into the customized cages housing the mice. A series of programmable solenoids and flow regulators enabled the manipulation of inspired oxygen from 20.9 to 5.0–6.0% over a 30-s period with a rapid reoxygenation to room air levels using a burst of 100% O2 in the succeeding 30-s period. Hypoxic events occurred at a rate of one event per minute throughout the 12-h light period from 8 AM to 8 PM. During the 12-h dark period from 8 PM to 8 AM, the animals were maintained in a constant undisturbed room-air environment. Control animals (sham exposure) received the same gas-flow exposure as the IH animals but using only room air. Animals were exposed to 28 consecutive days of either IH or IA before the terminal experiment assessing P-V loop function.
Echocardiography.
Transthoracic echocardiography was performed under light (∼1%) isoflurane inhalation anesthesia with the animals breathing spontaneously. Measurements were performed after 27 days of exposure to IH or IA, 1 day before P-V loop analysis and subsequent death. Short axis M- and B-mode images of the left ventricle (LV) were obtained using a VisualSonics 770 machine with a 25-MHz linear transducer to determine heart rate (HR), end-diastolic dimension (EDD), end-systolic dimension (ESD), diastolic anterior wall thickness, diastolic posterior wall thickness, % fractional shortening (%FS), which was calculated as %FS = 100[(EDD −ESD)/EDD]. Using Teichholz's formula (32), end-diastolic volume [7.0/(2.4 + LVEDD) × LVEDD3], end-systolic volume [7.0/(2.4 + LVESD) × LVESD3], and ejection fraction (%) {100 × [(LVEDV− LVESV)/LVEDV]} were calculated. Parameters were averaged over 10–20 cardiac cycles.
Left ventricular P-V loop analyses.
Mice were removed from the IH or IA exposure chambers and anesthetized with 1–2% isoflurane in room air via facemask and placed on a heating pad with the temperature set to 37.5°C. The anesthetized animal was placed in a supine position, and a 10- to 15-mm incision was made in the anterior midline of the neck to expose the trachea. The left external jugular vein was dissected free and catheterized with PE-10 tubing. The trachea was cannulated, and the animal was attached to a positive-pressure volume-controlled rodent ventilator (MiniVent, Harvard Apparatus, Holliston, MA) that incorporated isoflurane inhalation anesthesia. Tidal volume was set at 200–250 μl and ventilator rate at 110–130 breaths/min. The ventilator setting and level of anesthesia were adjusted to maintain the animal in an anesthetized state without any spontaneous breathing efforts.
The right carotid artery was dissected and exposed, and a Millar Mikro-Tip conductance catheter (model PVR-1045, tip size of 1F, Millar Instruments, Houston, US) was introduced into the artery and advanced into the LV via the aortic valve (2). Once steady-state hemodynamics were achieved, P-V loops were recorded and processed using an MVPS-400 system (Millar Instruments, Houston, TX). For all animals, parallel conductance (VP) was determined individually using a 10- to 12-μl bolus of 15% saline given through the venous catheter (12). The cuvette calibration method (Millar Instruments) was used to calculate the absolute volume data. For the cuvette calibration, four separate groups of male transgenic mice with cardiac overexpression of TNF or their control littermates (3 mice per group, total n = 12) were exposed to IH or IA as detailed above, and the calibration data were subsequently applied to the corresponding groups. With the use of PVAN 3.6 software (Millar Instruments), the P-V loop data were processed to compute cardiac parameters as described previously (2, 12). At the end of the experiment, the animals were killed under anesthesia and mechanical ventilation to obtain a terminal blood draw, and the heart was excised and snap frozen with liquid nitrogen.
Identification of transgenic mice using Southern blot analysis.
After offspring were born, the tails were biopsied at the age of 10 days. Genomic DNA was isolated, and transgenic mice were identified by PCR with a sense primer (5′-CCA CAT TCT TCA GGA TTC TCT-3′) specific to the α-MHC promoter exon 2 and an antisense primer (5′-CAG CCT TGT CCC TTG AAG AGA-3′) specific to the TNF-α cDNA nucleotides 579 to 599, as described previously (19).
Quantitative real-time RT-PCR analysis.
Total RNA was isolated from ventricular tissue homogenates of heart from both control and transgenic mice exposed to IH or IA using the RNeasy mini-kit (Quiagen) per manufacturer's instructions. DNA was removed from the samples via a DNAse step during RNA purification in the RNeasy kit. Reverse transcription was performed on 1 μg of total RNA using the Reverse IT First Stand Synthesis Kit (Abgene). Real-time RT-PCR was subsequently performed using the DNA binding dye SYBR Green3 and previously validated primers and conditions as described for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β1, β2, and β3-adrenergic receptors, brain natriuretic peptide (BNP), and atrial natriuretic peptide (ANP) (11, 15, 24, 25).
cAMP measurements.
Frozen ventricular tissue was cut, weighed, and homogenized on ice (1:10 vol) in 0.1 M HCl. Samples were then spun at 10,000 rpm for 5 min, and the supernatant was collected for soluble protein and cAMP. Total protein was measured in each sample using the Bio-Rad protein assay kit. Equal amounts of protein were then assayed for cAMP using a cAMP Direct Immunoassay Kit (BioVision) per the manufacturer's instructions.
Statistical analyses.
Statistical differences between strain (FVB vs. TNF-αTG) and hypoxia (IA vs. IH) were determined by two-way ANOVA, and differences between each of the four group means was determined by one-way ANOVA, with statistical significance between individual means determined by Newman-Keuls post hoc analyses. For RT-PCR measuring the mRNA, the comparative 2[−ΔΔC(T)] method was used (23). Differences were considered significant at P < 0.05. Data are expressed as means ± SE.
RESULTS
Body and organ weights.
Body weight and organ weight data at the end of 4 wk of exposure to IH or IA is shown in Table 1. As expected, mice exposed to IH did not gain as much weight over the 4-wk exposure period compared with mice exposed to IA. Both absolute and body weight-adjusted heart weights were significantly higher (P < 0.001) in the TNF-αTG mice compared with control wild-type mice, consistent with the presence of heart failure. However, the degree of heart failure was not sufficient to induce an increase in absolute lung weight, although weight-adjusted lung weight was higher (P < 0.01) in TNF-αTG mice compared with wild-type mice. There was no evidence of IH causing an increase in heart weight or lung weight, although the lower body weight after IH resulted in an increased (P < 0.01) lung weight-to-body weight ratio relative to IA mice.
Table 1.
Body weight and organ weight in wild-type and TNF-αTG mice after either 4 wk of IH or IA exposure
| Wild-type IA | Wild-type IH | TNF-αTG IA | TNF-αTG IH | Strain Effect | IH Effect | Interaction | |
|---|---|---|---|---|---|---|---|
| Body weight day 0, g | 27.5 ± 0.7 | 26.7 ± 0.7 | 26.4 ± 0.3 | 26.1 ± 0.3 | |||
| Body weight day 28, g | 30.5 ± 0.8 | 26.4 ± 0.9† | 28.6 ± 0.7 | 24.5 ± 0.5† | P < 0.05 | P < 0.001 | |
| Heart weight, g | 0.121 ± 0.003 | 0.108 ± 0.003† | 0.177 ± 0.007 | 0.140 ± 0.004† | P < 0.001 | P < 0.001 | P < 0.025 |
| Lung weight, g | 0.155 ± 0.006 | 0.154 ± 0.005 | 0.159 ± 0.009 | 0.141 ± 0.004 | |||
| Liver weight, g | 1.371 ± 0.035 | 1.184 ± 0.052† | 1.398 ± 0.041 | 1.157 ± 0.04† | P < 0.001 | ||
| Heart weight/body wt, g/100 g | 0.40 ± 0.01 | 0.41 ± 0.01 | 0.62 ± 0.03 | 0.57 ± 0.01 | P < 0.001 | P < 0.05 | |
| Lung weight/body wt, g/100 g | 0.50 ± 0.01 | 0.58 ± 0.02† | 0.56 ± 0.03 | 0.61 ± 0.03 | P < 0.01 | ||
| Liver weight/body wt, g/100 g | 4.53 ± 0.14 | 4.49 ± 0.16 | 4.90 ± 0.12 | 4.71 ± 0.13 | P < 0.05 | ||
| Sample size, n | 17 | 15 | 13 | 13 |
Data are shown as mean ± SE. TNF-αTG, TNF-α transgenic mice; IH, intermittent hypoxia: IA, intermittent air. Strain effect, IH effect, and interaction were determined by ANOVA.
Significant difference between IA and IH groups by one-way ANOVA (P < 0.05).
Impaired baseline cardiac function in TNF-αTG.
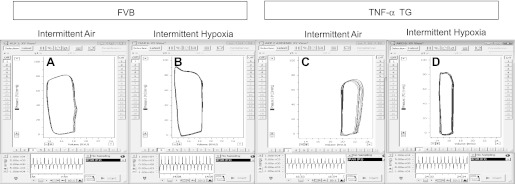
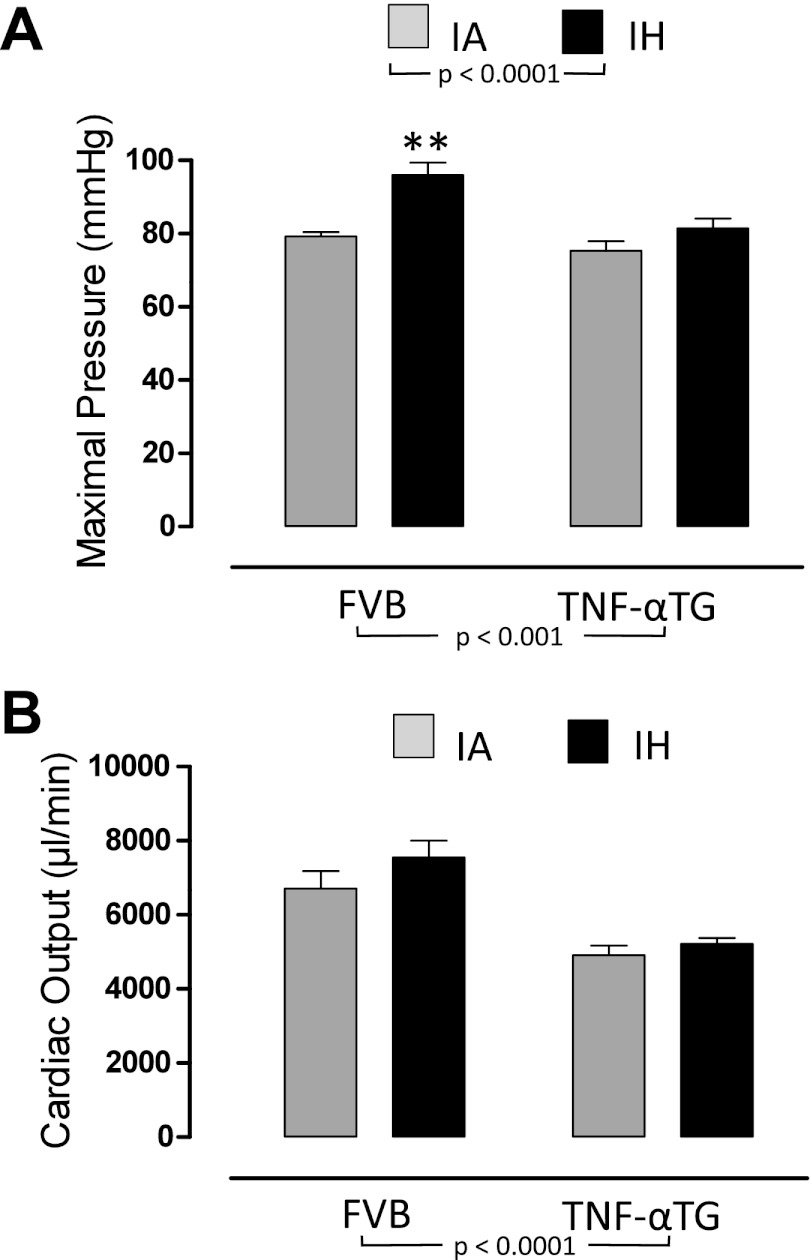
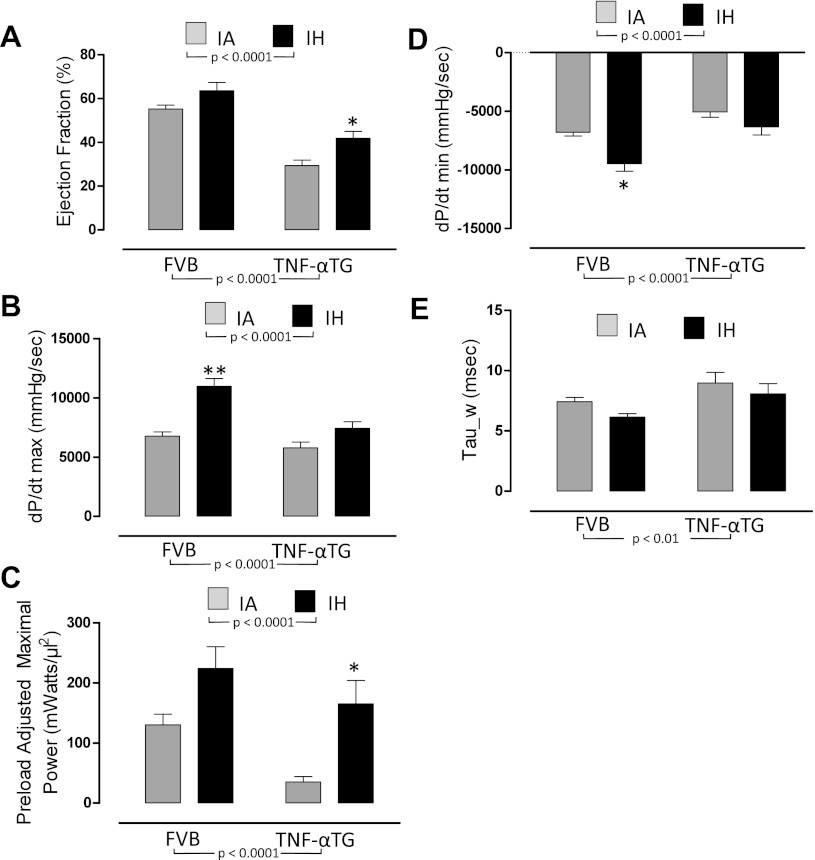
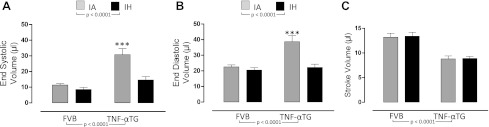
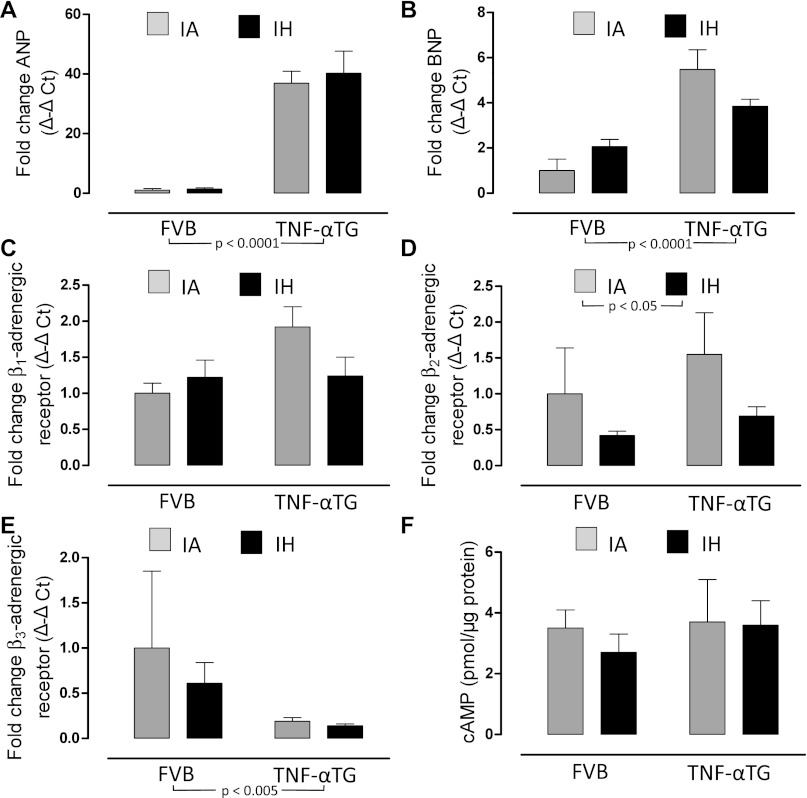
We determined, using P-V loop analyses (Fig. 1) and echocardiography, that TNF-αTG mice exhibit a reduced cardiac output (Fig. 2B), accompanied by impaired LV systolic (Fig. 3, A–C) and diastolic (Fig. 3, D and E) function and LV dilation (Fig. 4, A and B; Table 2), but no evidence of hypertrophy (Table 2). Biomarkers of heart failure (ANP and BNP) were elevated markedly in TNF-αTG mice compared with wild-type mice (Fig. 5, A and B). There were no differences in expression levels of β-adrenergic receptors or in cAMP levels between wild-type and TNF-αTG mice, although two-way ANOVA did show a significant independent effect of strain on β3-adrenergic receptor expression, with lower levels in TNF-αTG mice compared with wild-type mice (Fig. 5E). Overall, TNF-αTG mice under baseline conditions exhibit a phenotype of impaired LV contractility and relaxation associated with significant LV dilation.
Fig. 1.
Left ventricular pressure-volume loops in individual mice. Representative tracings of left-ventricular steady-state pressure-volume loops (top) and maximum first derivative of ventricular pressure (dP/dtmax; bottom) in wild-type (FVB) mice exposed to intermittent air (IA; A) or intermittent hypoxia (IH; B) and TNF-α transgenic (TNF-αTG) mice exposed to intermittent air (C) or intermittent hypoxia (D).
Fig. 2.
The effects of 4 wk of exposure to IA or IH on global cardiovascular function in wild-type and TNF-αTG mice. Values are means ± SE for maximal pressure (A) and cardiac output (B) determined by steady-state pressure-volume loops in either wild-type (FVB) or TNF-α transgenic (TNF-αTG) mice after 4 wk of intermittent hypoxia (IH) or intermittent air (IA) exposure. Significant strain (FVB vs. TNF-αTG) effects and hypoxia (IA vs. IH) effects were determined by two-way ANOVA and reported on the figure; there were no significant interactions. Differences between IA and IH means within each strain were determined by one-way ANOVA. **Significant difference (P < 0.01).
Fig. 3.
The effects of 4 wk of exposure to IA or IH on left ventricular systolic function (A–C) and diastolic function (D and E) in wild-type and TNF-αTG mice. Values are means ± SE for variables defining left ventricular systolic function [ejection fraction (A); maximum first derivative of ventricular pressure (dP/dtmax; B); preload adjusted maximal power (C)] and left ventricular diastolic function [minimum first derivative of ventricular pressure (dP/dtmin; D); relaxation constant (Tau_w; E)]. Variables were determined by steady-state pressure-volume loops in either wild-type (FVB) or TNF-α transgenic (TNF-αTG) mice after 4 wk of IH or IA exposure. Significant strain (FVB vs. TNF-αTG) effects and hypoxia (IA vs. IH) effects were determined by two-way ANOVA and are reported on the figure; there was a significant interaction for dP/dtmax only (P < 0.05). Differences between IA and IH means within each strain were determined by one-way ANOVA. **Significant difference (P < 0.01).
Fig. 4.
The effects of 4 wk of exposure to IA or IH on left ventricular volumes. Values are means ± SE for end-systolic volume (A), end-diastolic volume (B), and stroke volume (C) determined by steady-state pressure-volume loops in either wild-type (FVB) or TNF-αTG mice after 4 wk of IH or IA exposure. Significant strain (FVB vs. TNF-αTG) effects and hypoxia (IA vs. IH) effects were determined by two-way ANOVA and reported on the figure; there was a significant interaction for dP/dtmax only (P < 0.05). Differences between IA and IH means within each strain were determined by one-way ANOVA. **Significant difference (P < 0.01).
Table 2.
Echocardiographic assessment of left ventricular dimensions in wild-type and TNF-αTG mice before 4 wk of IH or IA exposure
| Wild-type IA | Wild-type IH | TNF-αTG IA | TNF-αTG IH | Strain Effect | IH Effect | Interaction | |
|---|---|---|---|---|---|---|---|
| Heart rate, beats/min | 487 ± 13 | 486 ± 16 | 475 ± 12 | 455 ± 20 | |||
| End diastolic dimension, mm | 0.354 ± 0.01 | 0.348 ± 0.011 | 0.454 ± 0.016 | 0.448 ± 0.015 | P < 0.001 | ||
| End systolic dimension, mm | 0.189 ± 0.01 | 0.189 ± 0.010 | 0.336 ± 0.017 | 0.326 ± 0.019 | P < 0.001 | ||
| Fractional shortening, % | 46.7 ± 2.0 | 45.5 ± 2.4 | 26.5 ± 1.4 | 28.0 ± 2.2 | P < 0.001 | ||
| Posterior wall thickness, mm | 0.097 ± 0.006 | 0.091 ± 0.010 | 0.083 ± 0.004 | 0.097 ± 0.012 | |||
| Ejection fraction, % | 83.1 ± 1.8 | 81.9 ± 2.2 | 58.0 ± 2.2 | 59.8 ± 3.4 | P < 0.001 | ||
| Sample size, n | 14 | 11 | 12 | 13 |
Values are means ± SE. Strain effect, IH effect, and interaction were determined by ANOVA. As expected, for data collected at this preexposure time point, there was no independent effect of IH or any interaction.
Fig. 5.
Biochemical and molecular responses to 4 wk of exposure to IA or IH in the ventricle of wild-type and TNF-αTG mice. Values are means ± SE for fold change [Δ-Δ cycle threshold (CT)] in expression of atrial natriuretic peptide (ANP; A), brain natriuretic peptide (BNP; B), β1-adrenergic receptor (C), β2-adrenergic receptor (D), β3-adrenergic receptor (E), and protein levels of cAMP (F) from ventricular tissue homogenates of heart in either wild-type (FVB) or TNF-αTG mice after 4 wk of IH or IA exposure. Significant strain (FVB vs. TNF-αTG) effects and hypoxia (IA vs. IH) effects were determined by two-way ANOVA and are reported on the figure; there was a significant interaction for BNP only (P < 0.05). Differences between IA and IH means within each strain were determined by one-way ANOVA. **Significant difference (P < 0.01).
FVB wild-type mice exhibit increased maximal pressure and dP/dtmax in response to IH.
FVB mice exposed to 4 wk of IH developed a >15 mmHg increase in maximal pressure, consistent with the development of sustained arterial hypertension (Fig. 2A). IH also induced a significant increase in dP/dtmax (Fig. 3B, left), but other indexes of contractility did not reach statistical significance. There was a more negative dP/dtmin after IH exposure (Fig. 3D), suggesting improved diastolic function, although an accompanying decrease in Tau_w did not reach statistical significance (Fig. 3E). Echocardiography did not show evidence of changes in cardiac function other than a decrease in end-distolic dimensions, and there was no evidence of LV hypertrophy in response to IH (Tables 2 and 3, posterior wall thickness). There were no changes in ANP or BNP expression in FVB mice exposed to 4 wk of IH (Fig. 5, A and B). Similarly, we saw no changes in β1, β2, β3-adrenergic expression or cAMP levels of the LV with IH exposure (Fig. 5, C–F).
Table 3.
Echocardiographic assessment of left ventricular dimensions in wild-type and TNF-αTG mice after 4 wk of IH or IA exposure
| Wild-type IA | Wild-type IH | TNF-αTG IA | TNF-αTG IH | Strain Effect | IH Effect | Interaction | |
|---|---|---|---|---|---|---|---|
| Heart rate, beats/min | 502 ± 12 | 511 ± 10 | 472 ± 8 | 487 ± 11 | P < 0.025 | ||
| End diastolic dimension, mm | 0.359 ± 0.01 | 0.314 ± 0.0188† | 0.468 ± 0.013 | 0.423 ± 0.010* | P < 0.001 | P < 0.001 | |
| End systolic dimension, mm | 0.208 ± 0.008 | 0.177 ± 0.01* | 0.343 ± 0.011 | 0.314 ± 0.010 | P < 0.001 | P < 0.005 | |
| Fractional shortening, % | 42.2 ± 1.7 | 43.9 ± 1.5 | 26.7 ± 0.9 | 25.8 ± 0.9 | P < 0.001 | ||
| Posterior wall thickness, mm | 0.092 ± 0.006 | 0.105 ± 0.005 | 0.093 ± 0.006 | 0.082 ± 0.005 | |||
| Ejection fraction, % | 78.9 ± 1.6 | 80.8 ± 2.0 | 58.7 ± 1.5 | 57.3 ± 1.6 | P < 0.001 | ||
| Sample size, n | 14 | 11 | 12 | 13 |
Values are means ± SE. Strain effect, IH effect, and interaction were determined by ANOVA. Significant difference between IA and IH groups by one-way ANOVA:
P < 0.05;
P < 0.01.
The impaired LV function in TNF-αTG mice is improved by IH exposure.
In TNF-αTG mice exposed to IH, there were no significant changes in maximal pressure or cardiac output (Fig. 2, A and B). However, there was evidence of improved systolic function, with IH inducing significant increases in ejection fraction and preload-adjusted maximal power (Fig. 3, A and C). There were no statistically significant effects of IH on diastolic function, but assessment of all four groups by two-way ANOVA showed a significant independent effect of IH for variables used to assess LV diastolic as well as systolic function (Fig. 3, A–E, IA vs. IH comparison; strong trend for Tau_w; P = 0.064). In TNF-αTG mice, IH exposure produced comparable and marked reductions in end-systolic and end-diastolic volume by P-V loop analyses (Fig. 4, A and B), with stroke volume unchanged compared with IA-exposed mice (Fig. 4C). Echocardiography also showed a decrease in the end-diastolic dimension in TNF-αTG mice exposed to IH (Table 3). Similar to wild-type mice, there were no changes in ANP, BNP, β1, β2, β3-adrenergic expression, or cAMP levels in TNF-αTG mice exposed to IH. However, by two-way ANOVA, there was a significant independent effect for IH to reduce β2-adrenergic expression compared with IA (Fig. 5D). Thus there was no evidence for an upregulation of β-adrenergic signaling contributing to the increases in LV cardiac contractility that occurred in both wild-type and TNF-αTG mice with 4 wk of exposure to IH.
DISCUSSION
We observed several new findings in the present study. First, we replicated in FVB mice the phenotype of increased dP/dtmax, without LV hypertrophy, and associated development of systemic arterial hypertension that we previously reported in C57BL/6J mice exposed to 4 wk of IH (25). However, in contrast to C57BL/6J mice, the increase in dP/dtmax in FVB mice was not associated with activation of β-adrenergic pathways. Second, we demonstrated for the first time using sensitive P-V loop analyses that TNF-αTG mice exhibited impaired LV systolic and diastolic function compared with wild-type mice under nonhypoxic conditions, accompanied by large increases in ANP and BNP expression. In contrast to our a priori hypothesis, 4 wk of exposure to IH did not cause any decrement in LV function in TNF-αTG mice. Surprisingly, 4 wk of exposure to IH increased several parameters of LV systolic function and markedly attenuated the magnitude of LV dilation. As in wild-type FVB mice, this unexpected improvement in LV function in the TNF-αTG mice was not associated with β-adrenergic activation.
FVB and C57BL/6J mice have similar adaptive cardiac responses to IH.
We previously reported that C57BL/6J mice exposed to IH for 4 wk exhibit an increase in cardiac contractility using the P-V loop analyses (25). We now show in another common inbred mouse strain, the FVB, that dP/dtmax is also increased by 4 wk of exposure to IH. In fact, the magnitude of the pressure increases would appear, if anything, to be greater in the FVB compared with the C57BL/6J strain. For example, in C57BL/6J mice, there was an 8% increase in maximal pressure and a 25% increase in dP/dtmax; comparable changes in FVB mice were 21% for maximal pressure and 62% for dP/dtmax. Thus the increase in dP/dtmax with 4 wk of IH exposure appears strain independent, but the magnitude of the response may be potentially genotype specific.
The mechanism for the improvement in dP/dtmax in FVB mice with exposure to IH does not appear related to activation of β-adrenergic pathways. In contrast, in our previous study in C57BL/6J mice, we showed that improved contractility after IH exposure was associated with an increase in cAMP levels and a trend for increased β1-adrenergic mRNA expression in the LV. Interestingly, although there were no changes in cAMP with IH in FVB mice, the level of cAMP under control IA conditions was almost double in FVB mice compared with our previous report in C57BL/6J mice (25), suggesting the FVB strain potentially exhibits a basal “hyperadrenergic” phenotype. Our own work in the FVB strain provides a physiological basis for an adrenergically hyperactive state.
In two separate studies, we assessed the blood pressure and heart rate variability responses to acute (minutes) hypoxic and hypercapnic exposure in six inbred mouse strains that included the FVB strain (3, 4). During exposure to hypoxia, the blood pressure fell in all strains of mice (e.g., 23.0 ± 3.8 mmHg in C57BL/6J mice) but was maintained at control levels in the FVB mice (−1.8 ± 2.1 mmHg). Furthermore, FVB mice significantly increased their blood pressure during combined hypoxic/hypercapnic exposure by 4.6 ± 1.2 mmHg, whereas in all other strains the blood pressure was at best maintained at control levels. Assessment of heart rate variability also demonstrated a unique pattern of autonomic response in the FVB mice. In response to hypoxic/hypercapnic exposure, FVB mice increased markers of sympathetic activation (low-frequency power increased from 0.9 ± 0.3 to 2.5 ± 0.3 ms2 and low-frequency/high-frequency power increased from 0.5 ± 0.3 to 2.0 ± 1.0 ms2). Although these blood pressure and heart rate variability changes represent acute responses to hypoxia and hypercapnia, they nevertheless suggest that the FVB strain is particularly sensitive to activation of its adrenergic systems controlling vascular and cardiac function. Consequently, the chronic increases in dP/dtmax that we report with 4 wk of IH exposure in FVB mice may represent an upregulation of pathways downstream from the β-adrenergic-G protein-cAMP signaling complex.
Improved cardiac function in TNF-αTG mice exposed to IH.
We confirmed the impaired cardiac contractility and LV dilation in TNF-αTG mice previously reported with MRI (19) and go on to show decreases in both systolic and diastolic function by P-V loop analyses. Our a priori hypothesis was that the vulnerable phenotype of the TNF-αTG mouse would make them susceptible to any pathological effects of IH. Unexpectedly, we found the opposite, with 4 wk of IH exposure producing an increase in systolic function and marked reductions in end-systolic and end-diastolic volume. Moreover, despite the progressive nature of heart failure in the TNF-αTG mouse, there were no deaths during the 4-wk period of hypoxia. Similar to the wild-type FVB mice, there was no evidence of LV hypertrophy or β-adrenergic activation in the TNF-αTG mice exposed to IH. However, the absence of changes in cardiac cAMP levels and β-adrenergic expression in response to IH does not necessarily exclude sympatho-activation, which is commonly reported with heart failure (10), potentially contributing to the contractility improvements we show in the TNF-αTG mice. For example, efferent sympathetic nerve activity or circulating catecholamines are also elevated by IH in healthy animals (1, 8, 9) and may similarly play a role in TNF-αTG mice. Alterations in ion channel transport, adaptive mitochondrial function, or reduced oxidant stress may also play a role in increasing cardiac contractility.
Overexpression of TNF-α in the heart is the genetic approach used to induce heart failure in our study. Clinical studies also show that worsening heart failure is associated with an increase in circulating TNF-α (21, 33). The role of TNF-α, however, may not necessarily be pathological, since recent studies have shown cardioprotective effects in ischemia-reperfusion through downstream mechanisms that include activation of the JAK/STAT pathway acting via TNF receptor type II (TNFR2) (14, 20). It is possible that IH, which is known to activate TNF-α (7, 30), may contribute to some of the beneficial cardiac outcomes we report in the present study through upregulation of TNFR2. Consequently, despite the genesis of our model of heart failure being dependent on overexpression of TNF-α in the heart, we cannot exclude the possibility that alterations in the TNF-α signaling axis in response to IH could play a protective cardiac role.
Finally, it is possible that the IH exposure may have improved vascularization in the compromised heart of the TNF-αTG mice in a comparable manner to the recent study of Xu et al. (35). In a study using rats, they showed that cardiac function was improved after myocardial infarction (ligation of the left anterior descending coronary artery) with exposure to daily 6-h periods of continuous 10% inspired oxygen for 4 wk. Taken together with our present study, these data suggest that 4 wk of hypoxia, whether it be intermittent (simulating sleep apnea) or continuous (simulating altitude), may induce adaptive changes.
What dose of hypoxia is adaptive to the heart?
Several lines of evidence suggest that 4 wk of exposure to hypoxia may optimize physiological or adaptive responses. First, our own data in inbred C57BL/6J and FVB mice, as well as cardiac-compromised TNF-αTG mice, show significant improvements in LV function with 4 wk of exposure to IH. A different hypoxic pattern of daily 6-h exposure to continuous 10% inspired oxygen for 4 wk in rats with experimentally induced coronary artery ligation resulted in improvements in cardiac performance (35), as mentioned above. Similarly, our own unpublished observations in mice with experimental coronary artery ligation showed that 4 wk of IH exposure could also improve cardiac function. Finally, Park and Suzuki (27) demonstrated that the myocardial injury that occurs with experimentally induced cardiac ischemia is less in mice exposed to 4 wk of IH than in mice exposed to 1 or 2 wk of IH. Therefore, in murine models, 4 wk of exposure to IH may represent the “tipping point” between beneficial adaptive responses to hypoxia and detrimental pathological responses to hypoxia.
Published data suggest that longer periods of hypoxic exposure may not be beneficial. For example, Chen et al. (6) showed that there was a decline in cardiac function in mice exposed to IH for 8 wk and that in rats the ejection fraction declined from week 3 to week 5 of IH exposure (5). Thus there may be a very narrow window around 4 wk of hypoxic exposure in mice during which physiological adaptations occur, but longer periods of exposure may result in pathological cardiovascular outcomes.
In summary, FVB mice exhibit improvements in LV dP/dtmax in response to 4 wk of IH exposure, consistent with our previous report in C57BL/6J mice. Surprisingly, TNF-αTG mice, which exhibited impaired LV contractility and increased LV dilation, also demonstrated significant improvements in LV function after 4 wk of IH. Unlike C57BL/6J mice, neither the FVB nor the TNF-αTG mice showed an increase in β-adrenergic activation in response to IH, and we speculate that pathways downstream from the G protein-cAMP signaling complex may be upregulated or that alternative mechanisms, such as increased vascularization, are activated by IH. Whatever the mechanism, it is clear that a 4-wk exposure period to the paradigm of IH employed in our study can induce adaptive responses in both normal and compromised hearts.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grant HL-077785 and T32-HL-076124-07.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.N. and C.P.O. conception and design of research; J.N., L.C.R., and K.R.M. performed experiments; J.N., R.H.R., K.R.M., and C.P.O. analyzed data; J.N., R.H.R., E.M.D., L.C.R., K.R.M., and C.P.O. interpreted results of experiments; J.N. drafted manuscript; J.N., R.H.R., E.M.D., L.C.R., K.R.M., and C.P.O. approved final version of manuscript; R.H.R. and C.P.O. prepared figures; R.H.R., E.M.D., K.R.M., and C.P.O. edited and revised manuscript.
REFERENCES
- 1. Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83: 95–101, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Campen MJ, Tagaito Y, Jenkins TP, Balbir A, O'Donnell CP. Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. J Appl Physiol 99: 807–813, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Campen MJ, Tagaito Y, Li J, Balbir A, Tankersley CG, Smith P, Schwartz A, O'Donnell CP. Phenotypic variation in cardiovascular responses to acute hypoxic and hypercapnic exposure in mice. Physiol Genomics 20: 15–20, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chen L, Zhang J, Hu XJ, Philipson KD, Scharf SM. The Na+/Ca2+ exchanger-1 mediates left ventricular dysfunction in mice with chronic intermittent hypoxia. J Appl Physiol 109: 1675–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen LM, Kuo WW, Yang JJ, Wang SG, Yeh YL, Tsai FJ, Ho YJ, Chang MH, Huang CY, Lee SD. Eccentric cardiac hypertrophy was induced by long-term intermittent hypoxia in rats. Exp Physiol 92: 409–416, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol 119: 189–197, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20: 612–619, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Gaussin V, Tomlinson JE, Depre C, Engelhardt S, Antos CL, Takagi G, Hein L, Topper JN, Liggett SB, Olson EN, Lohse MJ, Vatner SF, Vatner DE. Common genomic response in different mouse models of beta-adrenergic-induced cardiomyopathy. Circulation 108: 2926–2933, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol Heart Circ Physiol 274: H1416–H1422, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH, Polak JM. Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet 347: 1151–1155, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation 109: 1892–1897, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia 48: 2386–2395, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension 37: 511–515, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Kubota T, McTiernan CF, Frye CS, Demetris AJ, Feldman AM. Cardiac-specific overexpression of tumor necrosis factor-alpha causes lethal myocarditis in transgenic mice. J Am Coll Cardiol 29: 29165, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81: 627–635, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 84: 201–208, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323: 236–241, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Li JG, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 24. McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, O'Doherty RM, McTiernan CF, O'Donnell CP. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res 77: 54–63, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Naghshin J, McGaffin KR, Witham WG, Mathier MA, Romano LC, Smith SH, Janczewski AM, Kirk JA, Shroff SG, O'Donnell CP. Chronic intermittent hypoxia increases left ventricular contractility in C57BL/6J mice. J Appl Physiol 107: 787–793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nattie EE, Bartlett D, Johnson K. Pulmonary-hypertension and right ventricular hypertrophy caused by intermittent hypoxia and hypercapnia in rat. Am Rev Respir Dis 118: 653–658, 1978 [DOI] [PubMed] [Google Scholar]
- 27. Park AM, Suzuki YJ. Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in mice. J Appl Physiol 102: 1806–1814, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 7: 7–16, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112: 2660–2667, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol 90: 2007–2013, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37: 7–11, 1976 [DOI] [PubMed] [Google Scholar]
- 33. Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 27: 1201–1206, 1996 [DOI] [PubMed] [Google Scholar]
- 34. TorreAmione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93: 704–711, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Xu WQ, Yu Z, Xie Y, Huang GQ, Shu XH, Zhu Y, Zhou ZN, Yang HT. Therapeutic effect of intermittent hypobaric hypoxia on myocardial infarction in rats. Basic Res Cardiol 106: 329–342, 2011 [DOI] [PubMed] [Google Scholar]