Abstract
Scaling relationships have been formulated to investigate the influence of collagen fibril diameter (D) on age-related variations in the strain energy density of tendon. Transmission electron microscopy was used to quantify D in tail tendon from 1.7- to 35.3-mo-old (C57BL/6) male mice. Frequency histograms of D for all age groups were modeled as two normally distributed subpopulations with smaller (DD1) and larger (DD2) mean Ds, respectively. Both DD1 and DD2 increase from 1.6 to 4.0 mo but decrease thereafter. From tensile tests to rupture, two strain energy densities were calculated: 1) uE [from initial loading until the yield stress (σY)], which contributes primarily to tendon resilience, and 2) uF [from σY through the maximum stress (σU) until rupture], which relates primarily to resistance of the tendons to rupture. As measured by the normalized strain energy densities uE/σY and uF/σU, both the resilience and resistance to rupture increase with increasing age and peak at 23.0 and 4.0 mo, respectively, before decreasing thereafter. Multiple regression analysis reveals that increases in uE/σY (resilience energy) are associated with decreases in DD1 and increases in DD2, whereas uF/σU (rupture energy) is associated with increases in DD1 alone. These findings support a model where age-related variations in tendon resilience and resistance to rupture can be directed by subtle changes in the bimodal distribution of Ds.
Keywords: stress transfer, strain energy density, work of fracture, finite mixture model
tendons may be regarded as biological fiber composites comprising highly parallel collagen fibrils, strong and stiff in tension, reinforcing a weak, hydrated extracellular matrix (ECM) rich in proteoglycans (PGs) (28). Increasingly, studies on tendon biological organizations from the molecular level (2, 7, 8, 29, 41) to the fibrillar level (2, 21b, 22, 25, 27) are revealing new insights for how fibrils respond to external loads. In principle, this is important because the insights could facilitate the development of a basic understanding of the structure-property relationship of tendon addressing the influence of fibrillar structure, e.g., size and morphology (22, 26), and alteration to the structure by specific genes (5, 11, 40, 55) on the age variation in the mechanical properties of tendon, e.g., strength and stiffness (9, 21a, 49, 52). In practice, the qualitative arguments (5, 12, 34, 37, 45, 54, 55) that have been developed for explaining the structure-property relationship of tendon are not validated adequately by quantitative models (11, 26, 40).
The influence of the spread of fibril lateral sizes on the mechanical property of tendon is one of the key aspects for understanding the structure-property relationship. Studies have revealed that the frequency histograms of the fibril diameter (D), a parameter for the fibril lateral size, of tendon from postnatal growth stages to old age feature non-Gaussian profiles that may be described as bimodal or even trimodal (11, 30, 37, 54, 55). Except in tissues from very young animals (namely mice at birth until, e.g., 2 wk old) that feature near-Gaussian profiles (37), the non-Gaussian profiles from growth to old age preclude any straight-forward analyses of D arising from such a population (30). Accordingly, studies using quantitative (regression) models (11, 40), which relate the mean D of the frequency histogram to the specimen mechanical property, have yielded conflicting findings. For instance, we note that the mean D correlates to the structural strength (maximum force) and structural modulus of the force-displacement curve but not to the material strength (maximum stress) and material modulus of the stress-strain curve (11, 40). Thus the justification for existing regression models is limited. Additionally, so far, only data from the postnatal growth phase of mice have been evaluated (11, 40), and the applicability of the structure-property relationship seems to be much less established for a wider age range, spanning from maturation to old age.
The intent of this paper is to present an investigation into the structure-property relationship of tendon to clarify the conflicting findings of D-mediated variations in mechanical property of tendons during aging. To this end, we provide an elegant demonstration of an energy approach for modeling the influence of collagen fibril lateral size to the age variation strain energy components for resilience and resistance to rupture of tail tendons from C57BL/6 mice. Unlike previous studies on the structure-property relationship, which focus on the age-related variations in the mechanical property at specific points of the loading process, the energy approach establishes a strategic framework for a comprehensive understanding of the various mechanical parameters throughout the entire loading process. Hypotheses are proposed to evaluate the significance of the structure-property relationships.
MATERIALS AND METHODS
An energy approach to modeling the structure-property relationship of tendon.
Consider tension to be applied to a tendon in the axial direction of an assembly of parallel collagen fibrils. Figure 1 shows a single fibril embedded in and reinforcing ECM of the tendon. Such regions are observed in many tissues, such as ligament and articular cartilage; application of tensile stress also tends to orient fibrils (28). Loading begins with the elastic stress-transfer stage (22). According to a shear-lag analysis (20, 22), as interfibrillar ECM deforms elastically, adhesion at the fibril/PG-matrix interface causes the fibrils to be recruited into tension. The fibrils stretch elastically, and the fibril-fibril lateral spacing decreases as the fibrils are increasingly drawn closer together. In the process of fibril deformation, intermolecular sliding of tropocollagen (18, 47) is resisted predominantly by the forces of interaction associated with covalent cross-links; a variety of other forces arising from hydrogen bonding, van der Waals, and electrostatics could also play an important part in the molecular mechanics of collagen, although the precise mechanism is not clear. Additionally, hydrogen bonding, van der Waals, and electrostatics forces are responsible for regulating the stress transfer to the fibrils (39); these forces account for the mechanics of interaction of decoron with collagen (36) as well as that of glycosaminoglycan (GAG) side chains associated with decorin PGs bound on adjacent fibrils (16, 20a, 39) and the other PGs in interfibrillar ECM (20a). As loading progresses from the elastic stress-transfer stage to mode β, a transitional stage (20), stress transfer occurs by sliding friction between the fibril and the PG-matrix; at a molecular level, this corresponds to a continuous disruption and formation of GAG interaction. The frictional force is parameterized by the corresponding shear stress (τβ), which is constant throughout the interface. Mode β marks the transition from the elastic to the plastic stress-transfer stage (20, 22), where the fibril continues to deform elastically as interfibrillar ECM deforms plastically and slides over the fibril/PG-matrix interface. After the plastic stress-transfer stage, the loading regime enters the failure stage, where macroscopic, permanent deformation of interfibrillar matrix corresponds at the molecular level to an extensive disruption of GAG interactions arising from appreciable displacement of the side chains. However, substantial interpenetration of the GAG side chains also occurs as the distance of adjacent fibrils decreases, and so, the frictional force increases correspondingly. Accordingly, the frictional shear stress, τRP (>τβ), is constant throughout the fibril/PG-matrix interface during fibril rupture and fibril pull-out from the ECM (Fig. 2B).
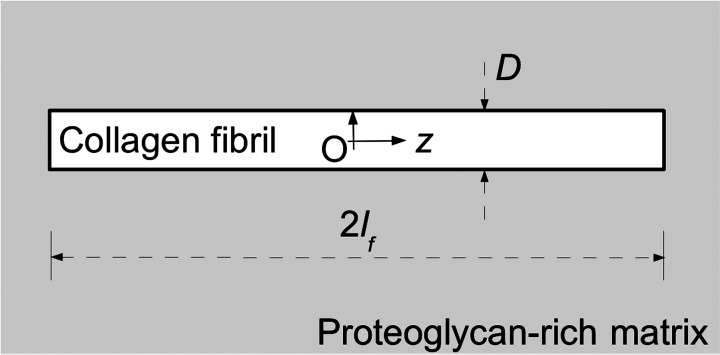
Fig. 1.
Model of a collagen fibril embedded in a coaxial cylinder of hydrated PG-rich matrix (shaded region). Here, 2lf denotes the length of the fibril and D its diameter. The fibril center (O) defines the origin of the cylindrical polar coordinate system; the fibril axis defines the z-axis.
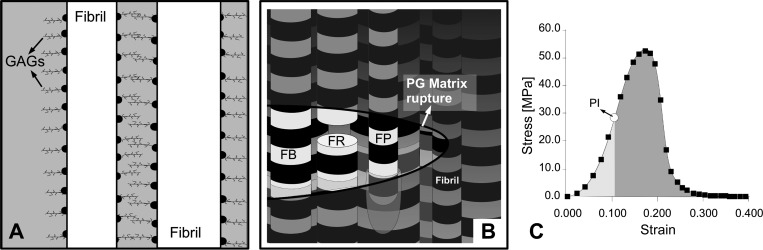
Fig. 2.
Collagen fibril reinforcement in ECM. A, top row: interaction of glycosaminoglycans (GAGs) between 2 collagen fibrils. B: schematic of fibrillar failures in ECM. Rupture of interfibrillar ECM is depicted to have occurred with the crack tip propagating from left to right. Fibrils are represented by long rods featuring bands (representing the 67-nm periodicity). FB, fibril bridging the ruptured site in interfibrillar ECM; FR, fibril rupture; and FP, fibril pull-out. C, bottom row: a typical stress-strain plot from a mouse tail tendon. On the stress-strain curve, the point of inflection (PI) is where yielding has started. The areas of the lighter and darker shaded regions under the stress-strain curve correspond to uE and uF, respectively; the strain energy density to fracture u0 = uE + uF.
Application of the principles of essential work of fracture to a fiber composite (53) yields the following partitioned components of the energy stored in the tendon: the nonessential energy (uE), which contributes primarily to tendon resilience (regulated by fibrils undergoing elastic deformation), and the essential energy (uF), which relates primarily to resistance of the tendon to rupture (regulated by fibril rupture, leading to defibrillation and rupture of the interfibrillar matrix). On the stress-strain curve (Fig. 2C), uE corresponds to the area under the curve from the origin to the point of inflection (PI; which marks the limit of the linear region), whereas uF corresponds to the area under the curve from the PI to the point of fracture. From the scaling relationships, we find that uE = μβ(D) (see Eq. A6), and uF = μR(D) + μP(D) (by combining Eqs. A8 and A10; see below). Here, μ represents the strain energy density (symbol in adjacent parentheses indicates the argument of μ), and subscripts β, R, and P denote mode β, fibril rupture, and fibril pull-out, respectively. In principle, the equations for uE and uF would be valid if all of the fibrils in the tendon featured the same D. In reality, we find that the population of fibrils possesses a spread of D with a non-Gaussian profile. According to the finite mixture law (35), this profile may be described by two or more normally distributed subpopulations. The argument that follows hereafter has been developed using the bimodal distribution. [This is not unrealistic, because it has been reported that the minimum number of subpopulations for the mouse tail tendon from growth to old age is two (37).] Let D1 and D2 represent the normal distributions of the respective subpopulations with the smaller (DD1) and larger (DD2) mean Ds. We find that
| (1) |
From Eq. 1, we arrive at
| (2) |
| (3) |
where cE1 and cE2 are constants of proportionality for Eq. A6 (see below), and cRP1 and cRP2 describe the summation of the constants of proportionality cR1 and cR2 (see Eq. A8) and cP1 and cP2 (see Eq. A10); i.e.
| (4) |
We note that cE1 and cE2 are expressed in terms of the fibril yield stress (σfy), fibril stiffness (Ef), τβ, and a scale factor for length (L; see Eq. A6); cR1 and cR2 are expressed in terms of the fibril fracture stress (σfu), Ef, τRP, and L (see Eq. A8); cP1 and cP2 are expressed in terms of only σfu, τRP, and L (see Eq. A10).
According to the postulates of Parry et al. (37), in the small-strain regime, where the elasticity of the tissue predominates, creep inhibition is accomplished by the presence of small D fibrils (creep could otherwise yield a nonrecoverable strain). In the high-stress regime, for the tissue to withstand high stress, the strength of the tissue is accomplished by the presence of large D fibrils. By adapting these postulates for our energy-based argument for investigating how the bimodal distribution of D directs tendon resilience and resistance to rupture in tendons from growth to old age, we hypothesize that when the tendon is acted on by an external load: 1) at small deformation, fibrils of all diameters are recruited into action, absorbing strain energy and contributing to elastic tensile deformation (the resilience hypothesis), and 2) at very large deformation up until tissue fracture, larger D fibrils are responsible for regulating the strain energy absorption to resist rupture (the resistance-to-rupture hypothesis). In this study, using in vitro data from a mouse model, we will test these hypotheses by evaluating the mathematical models of Eqs. 2 and 3 to find out how much of the age-related variations in the respective strain energy density components can be explained by a linear relationship with increasing DD1 and DD2.
Tissue preparation and mechanical testing.
The tissue preparations, load-deformation curves, and transmission electron microscopy (TEM) obtained by Goh et al. (21a) were the starting point for the re-analysis of data necessary for the modeling presented below. All procedures (21a) were approved by the UK Home Office and accorded with the UK Animals (Scientific Procedures) Act 1986. In summary, tail tendons were obtained from three to four male C57BL/6 mice at 1.7, 2.6, 4.0, 11.5, 23.0, 29.0, 31.5, and 35.3 mo of age, and 12 mm-long sections of the tendon were subjected to tensile tests in PBS (pH 7.2) at a displacement rate of 0.067 mm/s. Starting with the original records of the stress-strain data of each sample (21a), the areas under the stress-strain curve (Fig. 2C), corresponding to the parameters u0, uE, and uF, were determined. In addition, we have determined σY and σU from the original stress-strain data (21a). Five to 10 samples/tail were tested; averaging the values of the respective parameter for all samples/animal and for all animals within that age group yielded the representative value (within SE) for each age group.
The occurrence of yield in the tendon is associated with the point of inflexion lying between the toe region and the point of maximum stress on the stress-strain curve (Fig. 2C). Within this region, we find the gradient increases, peaks at the inflexion point, and decreases with increasing strain. To identify the inflexion point, we fitted an appropriate polynomial equation to the stress-strain data points from the origin to the maximum stress and evaluated the polynomial equation to determine the stress vs. strain corresponding to the peak gradient (21a).
TEM.
Images from TEM obtained by Goh et al. (21a) were re-analyzed. Briefly, the micrographs were taken of ultrathin sections of fixed and resin-embedded tendons on a Tecnai BioTWIN instrument (FEI, Eindhoven, The Netherlands) at an accelerating voltage of 80 kV. Digitized micrographs of near-transverse sections were used to measure D. The micrographs for measurements of D were at an instrumental magnification of ×15,000. The precise magnification was determined using a diffraction grating replica (2,160 lines/mm). Each sample area is an entire micrograph corresponding to a specimen rectangle of 4 μm × 5 μm. The area from each tendon sample was an average of 10 specimen rectangles with all fibrils in a near-transverse section; these rectangular areas were selected randomly through a survey over widely separate locations across the tendon sample. The cross-section of each fibril was manually traced and the area computed using the Semper5 image analysis package (Synoptics, Cambridge, UK). Following approaches reported elsewhere (11, 30, 37), D was derived from the fibril cross-sectional area by modeling the shape of the fibril as circular. Data from all animals were then combined and considered representative of the collagen fibril profile in fascicles from that age group.
Finite mixture modeling.
Consider the lateral size population of the fibrils to be a heterogeneous mixture of a few normally distributed subpopulations for the purpose of modeling the non-Gaussian profiles of the frequency histograms of D (35). By inspection, the smallest number of subpopulations in the mixture is two, and we shall refer to these subpopulations by D1 and D2 for simplicity. Finite mixture modeling was implemented using MatLab (version 7; MathWorks, Natick, MA). To determine the optimal solution to the mean and SD of D for the respective subpopulations, a simulated annealing (SA) optimization algorithm was developed to evaluate for possible profiles that best fit the primary distribution [for a similar approach using SA, see Goh et al. (21)]. To execute the SA algorithm, the “temperature” parameter was assigned a value of 0.5 with a reduction factor of 0.9. During each run, the maximum number of configurations that the SA algorithm could explore was fixed at 100; the number of temperature steps to be executed was fixed at 100; and the number of successes allowable before looping to the next temperature steps was fixed at 10. The configuration space addressed the fibril subpopulations D1 and D2. The Gaussian profile of these subpopulations was defined by the amplitude (which described the proportion of fibrils in the subpopulation), mean, and SD; the magnitudes of these parameters were assigned from a predefined range of values using an algorithm for randomizing the selection of values. An initial run was executed to obtain a preliminary range of values for the mean and SD, respectively, followed by a refinement run by narrowing the range of values. Linear regression was implemented as part of the the SA approach to fit the profiles of D1 and D2 to the primary distribution.
Statistical analysis.
The nature of the tensile test data was checked to ensure that it satisfied the normality and homogeneity of variance of residuals (6) with respect to age—DD1 and DD2. One-way ANOVA, complemented by the Fisher least square difference (LSD) test (6), was carried out to evaluate for significant differences in the age variations of u0, uE/σY, and uF/σU. The Fisher LSD test was used to compare the mean of one age group with the mean of another for statistical difference (at an individual error rate of 0.05) by generating (and comparing) seven sets of confidence intervals from the eight age groups used in this study. Multiple regression analysis (1, 6) was used to assess for significant correlation of DD1 and DD2 with uE/σY and uF/σU, respectively; the results were reported as means ± SE. Significance was defined as P < 0.05. Multicollinearity of DD1 vs. DD2 was assessed using the Pearson correlation coefficient test (1). All statistical analyses were performed using Minitab commercial software (version 14; Minitab, State College, PA).
RESULTS
Strain energy density.
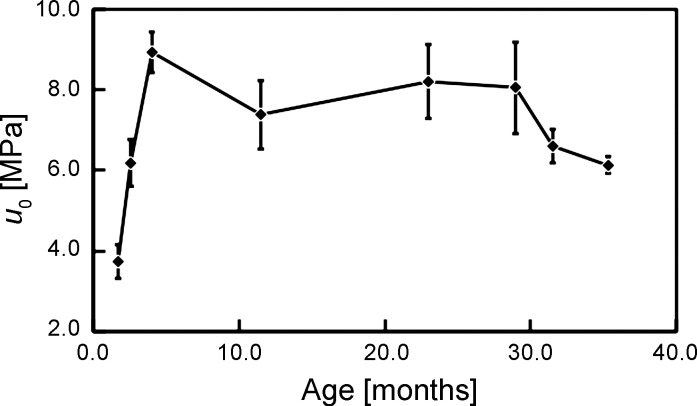
Figure 3 shows the plot of u0 vs. age. The ANOVA test reveals that not all of the means of u0 among the different age groups are equal (P = 0.001; F = 5.84). The Fisher LSD test shows that statistical differences occur between the means only for: 1) 1.7 mo vs. the respective age group from 2.6 to 35.3 mo, 2) 2.6 vs. 4.0 and 23.0 mo, 3) 4.0 vs. 31.5 and 35.3 mo, and 4) 23.0 vs. 35.3 mo. Correspondingly, the plot of u0 vs. age describes an increasing u0 from 1.7 to 4.0 mo, fluctuation of u0 with no appreciable trend from 4.0 to 29.0 mo, and a decreasing u0 from 29.0 to 35.3 mo.
Fig. 3.
Graph of strain energy density to fracture (u0) vs. age. The vertical bars represent SE.
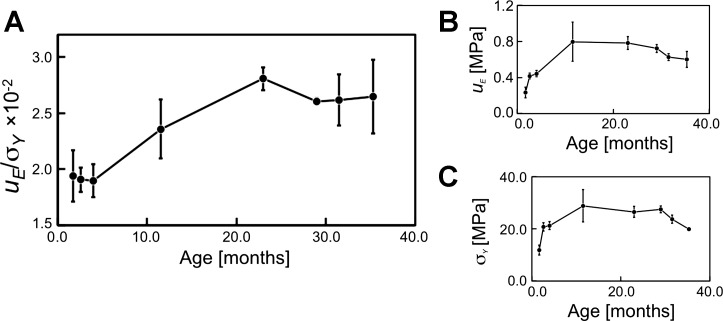
Figure 4A shows the normalized strain energy density (uE/σY) vs. age. Included in this figure are plots of the respective parameters—uE (Fig. 4B) and σY (Fig. 4C)—vs. age for informational purpose. The ANOVA test reveals that not all of the means of uE/σY are equal (P = 0.009; F = 3.90). The Fisher LSD test shows that statistical differences occur between the means for: 1) 1.7 mo, 2) 2.6 mo, and 3) 4.0 mo vs. the respective age group from 23.0 to 35.3 mo. Correspondingly, the plot of uE/σY vs. age describes fluctuations in uE/σY from 1.7 to 4.0 mo with no appreciable trend; thereafter, uE/σY increases from 4.0 to 23.0 mo and fluctuates from 23.0 to 35.3 mo.
Fig. 4.
Graphs of (A) normalized strain energy density (uE/σY), (B) strain energy density contributing to tendon resilience (uE), and (C) stress generated by the tendon at yield point (σY) vs. age. The vertical bars represent SE.
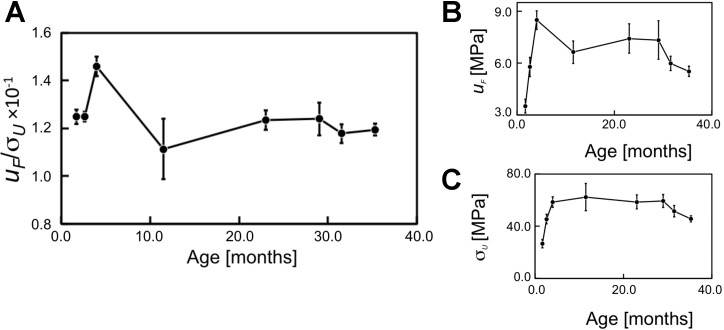
Figure 5A shows the normalized strain energy density (uF/σU) vs. age. Also included in this figure are plots of the respective parameters—uF (Fig. 5B) and σU (Fig. 5C)—vs. age for information. [Interestingly, it is observed that the plots of σY (Fig. 4C) and σU (Fig. 5C) vs. age reveal very similar profiles to u0 vs. age (Fig. 3).] The ANOVA test reveals that not all of the means of uF/σU are equal (P = 0.049; F = 2.59). The Fisher LSD test shows that statistical differences occur between the means for: 1) 1.7 vs. 4.0 mo, 2) 2.6 vs. 11.5 mo, and 3) 4.0 mo vs. the respective age groups from 11.5 to 35.3 mo. Correspondingly, the plot of uF/σU vs. age reveals no appreciable change in uF/σU from 1.7 to 2.6 mo, increase in uF/σU from 2.6 at 4.0 mo, decrease in uF/σU from 4.0 to 11.5 mo, and fluctuations with no appreciable change thereafter. Clearly, the trend exhibited by uF/σU is a contrast to that exhibited by the uE/σY. We note that whereas results from the Fisher test show that the variation in u0 with respect to age is accounted for by four out of the seven sets of statistical differences between the means, the variation in uE/σY (and also uF/σU) is accounted for by three out of the seven sets of statistical differences between the means.
Fig. 5.
Graphs of (A) normalized strain energy density (uF/σU), (B) strain energy density contributing to tendon resistance to rupture (uF), and (C) strength of the tendon (σU) vs. age. The vertical bars represent SE.
Fibril diameter distribution.
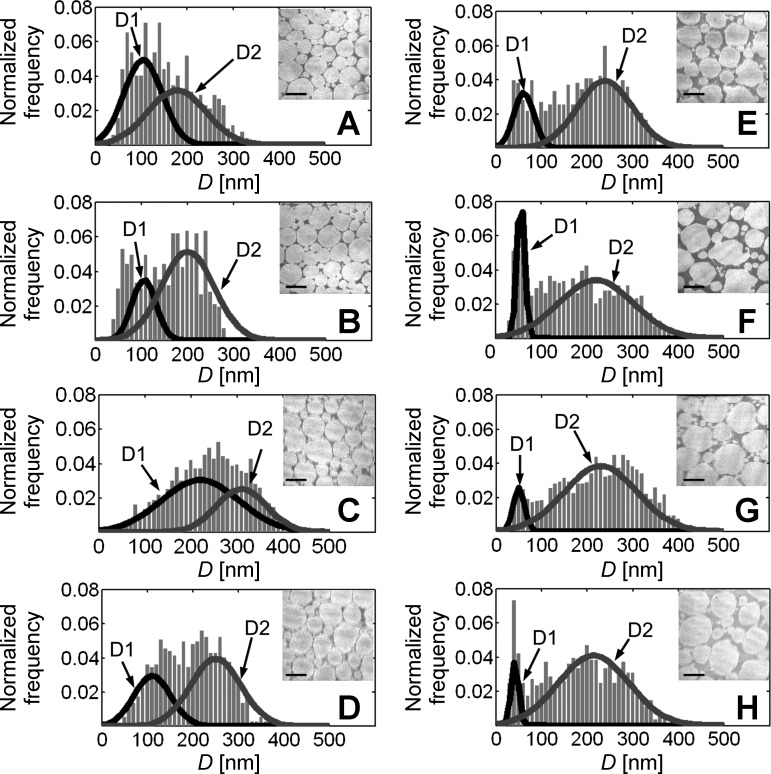
Figure 6 shows an array of histograms of normalized frequency vs. D and the corresponding (representative) TEMs of the cross-section of tendon for age group 1.7-35.3 mo. To the best of our knowledge, there has been no systematic study to evaluate similar histograms of frequency vs. D of the C57BL/6 tail tendon from growth to old age. However, we note that our results for the growth phase reveal similar profiles to those reported elsewhere for 3- and 8-wk-old mice (11). By inspection, it is observed that the maximum D in each histogram fluctuates with age. At 1.7 mo, the spread of D values has a maximum D of ∼300 nm. At 4.0 mo thereafter, the maximum D fluctuates at close to 400 nm. Qualitatively, these observations implicate that the tendon of young mice is dominated by fibrils with small D (with a fairly regular morphology), but as the mice grow older, larger D fibrils (with increased morphological irregularity) dominate.
Fig. 6.
Histograms of normalized frequency vs. D distribution and the corresponding (representative) transmission electron micrographs of the cross-sections of tendons from (A) 1.7-, (B) 2.6-, (C) 4.0-, (D) 11.5-, (E) 23.0-, (F) 29.0-, (G) 31.5-, and (H) 35.3-mo-old mice. The number of C57BL/6 mice tested for the respective age group was: (A) 3, (B) 3, (C) 3, (D) 4, (E) 3, (F) 4, (G) 4, and (H) 4 mice. The data were combined from all animals for each age group. Dark and light solid curves in each histogram represent the D1 and D2 fibril subpopulations, respectively. Original scale bars, 150 nm.
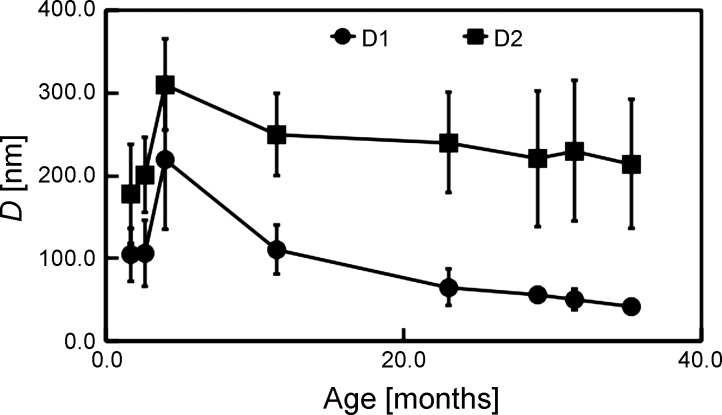
Plots of DD1 and DD2 vs. age (Fig. 7) reveal that the mean Ds are small at 1.7 mo but increase rapidly with age until 4.0 mo. Thereafter, DD1 decreases (more rapidly than DD2) with age. Interestingly, the SD of the DD2 is small at 1.7 mo, increases steadily with age until 29.0 mo, and shows no appreciable change from 29.0 to 35.3 mo. On the other hand, the SD of the DD1 is small at 1.7 mo, increases steadily with age until 4.0 mo, but decreases steadily with age thereafter. From the multicollinearity analysis of DD1 vs. DD2, we find that the Pearson correlation coefficient test = 0.599. We conclude that the DD1 and DD2 are not correlated with each other, with a cautionary note that the Pearson correlation coefficient test is marginally less than the tolerable threshold, i.e., 0.600 (1).
Fig. 7.
Graph of D vs. age. Here, circles and squares correspond to the D1 and D2 fibril subpopulations, respectively. Results are reported as means ± SD; vertical bars represent SD.
To the best of our knowledge, no quantitative models have been reported of fibril growth in vertebrate tissues that could account for all of the factors that control the fibril diameter distribution. Although this study was prompted by observations on the age-related variations in the mean D, the results on the age-related variations in the width of the bimodal distributions could be the focus for further investigation. Of considerable value are the following points relating to these results: 1) the spread of these sizes is complicated by the presence of both unipolar and bipolar fibrils as well as the contribution of interfibrillar fusion (27), and 2) the individual variations could play a part in contributing to the spread of D values. For instance, the smaller SD value of the DD1 during old age could implicate reduction in individual variability associated with the subpopulation.
Multiple regression analysis.
Regression analysis reveals strong evidence of a linear relationship between uE/σY and both DD1 and DD2. Here, it is observed that the two coefficients, cE1 and cE2, of Eq. 2 are different from zero (ANOVA, P = 0.000; F = 15.98). Table 1 summarizes the results of cE1 (P = 0.000) and cE2 (P = 0.000). The R2 value indicates that the predictors, i.e., the DD1 and DD2, can explain 54.5% of the variation in uE/σY; the remaining 45.5% of the variation in uE/σY arises from random arrangement of the points on the uE/σY vs. DD1 and DD2 space. The model's y intercept [=(4.4 ± 5.7) × 10−3] is not significantly different from zero (P = 0.452), suggesting that uE/σY will not be significantly different from zero when DD1 and DD2 are zero.
Table 1.
Multiple regression analysis
| Coefficient | Predicted Value × 10−4 (nm−1) |
|---|---|
| cE1 | −1.2 ± 0.2* |
| cE2 | 1.3 ± 0.3* |
| cRP1 | 1.8 ± 0.8* |
| cRP2 | −0.7 ± 1.2 |
Here, the predicted values of cE1 and cE2 refer to the coefficients of Eq. 2 [constants of proportionality (see Eq. A6)], and those of cRP1 and cRP2 refer to the coefficients of Eq. 3 [summation of the constants of proportionality cR (see Eq. A8) and cP (see Eq. A10)].Values are means ± SE.
Significantly different, P < 0.05.
Similarly, regression analysis reveals strong evidence of a linear relationship between uF/σU and DD1 and DD2. The analysis suggests that at least one coefficient, i.e., cRP1, cRP2, or both, is different from zero (ANOVA, P = 0.000; F = 4.74). However, further examination shows that only cRP1 is significantly different from zero (P = 0.040), indicating that only DD1 is significantly related to uF/σU. The R2 value indicates that the predictors can only explain 29.2% of the variation in uF/σU; in other words, the remaining 70.8% of the variation in uF/σU arises from random arrangement of the points on the uF/σU vs. DD1 and DD2 space. Interestingly, the model's y intercept [=(1.2 ± 0.2) × 10−1] is significantly different from zero (P = 0.000), suggesting that uF/σU will be significantly greater than zero when DD1 is zero. However, this must be meaningless, since we cannot have a nonzero value for uF/σU when DD1 is zero. It may well be that if our sample included smaller values of DD1, then we would find that the relationship was curved. Note also that the magnitude of cRP1 is one order of magnitude greater than that of cE1; this is consistent with the (order of magnitude) difference between uF/σU and uE/σY. The results of the derived coefficients cRP1 and cRP2 are summarized in Table 1.
DISCUSSION
Aging changes in the fracture toughness of tendon reflect phases of development and influence from different biological organizational levels.
The variations in u0 as a function of age may be divided into three phases corresponding to postnatal growth, maturation, and old age (32, 51). These phases of aging are not unique to u0 but have been established previously for σU and E (21a). For the model tendon used in this study, growth is characterized by a rapid increase in u0 until maturation; the transition from postnatal growth to maturation occurs at ∼4 mo. Throughout the maturation phase, u0 fluctuates with no appreciable trend. The transition from maturation to old age occurs for ∼23 mo; thereafter, u0 decreases with increasing age during old age. Overall, this suggests that tendon appears to be optimally toughened at maturation. Viidik (51) reported that the u0 of rat tail tendon (spanning 1.0–35.0 mo) features trends similar to those reported here, albeit that there is an absence of justifications for the underlying statistical significance of the data in Viidik's work. It is necessary, however, to consider the molecular, cellular-ECM, physiological, and population levels of biological organization to understand the effects of one level on the next-higher level in the presence of aging (17). This study argues that at the physiological level, age variation in u0 is the result of changes in uE and uF. In turn, uE and uF are influenced by the structural changes at the ECM level. The implications of this will be discussed further in the next paragraph, where we address the use of the scaling relationships for evaluating, at the ECM level, the influence of collagen fibril lateral size on the strain energy density absorbed by the tendon in the presence of aging.
Predictions from the scaling relationships (Eqs. 2 and 3) suggest a new meaning for the influence of collagen fibril lateral size on resilience and resistance to rupture in connective tissue from growth to old age. Applications of the energy argument, in contrast to the previous stress argument (9, 10, 26, 37, 54), have allowed a deeper understanding of the influence of the absorbed energy on the depth of disruption of the fibrillar fine structure as quantified by the scaling relationships. Multiple regression analysis of uE/σY vs. DD1 and DD2 (Eq. 2) indicates support for the resilience hypothesis, which holds that fibrils of all sizes are responsible for directing the absorption of the strain energy imparted to the deforming tissue at initial loading. From Table 1, the results suggest that an increasing DD2 contributes to increasing the tissue resilience, but an increasing DD1 contributes to decreasing the tissue resilience. On the other hand, the resistance-to-rupture hypothesis is rejected based on evidence from multiple regression analysis of the uF/σU vs. DD1 and DD2 (Eq. 3). Alternatively, we speculate that the smaller D fibrils direct further absorption of strain energy by the deforming tissue until fracture. Compared with fibrils with small D, the fine structure of fibrils with large D will have to be disrupted to a greater depth for fracture to occur. Thus the failure of ECM favors fibrils with smaller Ds; these fibrils fracture easily because of the lower strain energy absorbed. Similarly, fibrils with small D will also debond easily, owing to the smaller area available between the fibril-PG interface for molecular interactions.
Although these findings lend to possible broader applicability of the scaling relationships to the other connective tissues of similar concern, e.g., aorta (3) and cornea (13), we highlight the following cautionary notes. First, considering that tail tendons are useful for aging studies (11, 21a, 40, 49, 52)—they are not weight bearing and may be influenced by systemic effects of aging (21a, 51)—we acknowledge that the findings may not apply to all tendons (or to connective tissues of all species). For instance, the different limb tendons that are directly involved in locomotion would experience a different loading environment and could respond differently to aging (9, 10, 26). Second, although the findings suggest that fibril lateral size influences the age variation in the strain energy densities, we do not rule out the possibility of the fibril lateral size and strain energy densities responding independently to collagen cross-linking (4, 5) or an unknown third factor. Further discussion is out of the scope of this paper, but this issue has been targeted for investigation in future studies.
Fibril/PG-matrix interactions.
The transfer of stress by τβ and τRP from the interfibrillar matrix to the fibril is central to the theoretical framework of the scaling relationships. This section presents simple order-of-magnitude estimates of the ratio of τRP to τβ, which is a parameterization of the relative stress transfer. Let Em and σm be the stiffness and average stress in interfibrillar ECM, respectively. Applying the rule of mixtures to modeling the influence of the fibril volume fraction (Vf) on E and σU leads to E = [Ef − Em]Vf + Em, and σU = [σfu − σm]Vf + σm (21a); linear regression analysis reveals that the y intercepts from the respective relationships are both negative and not significant (P > 0.05). Thus the predominance of the role of collagen for regulating Ef and σU can be satisfied to order of magnitude (see appendix) by setting these y intercepts to zero. One then finds that E = EfVf, and σU = σfuVf; additionally, σY = σfyVf. Linear regression analysis of E = EfVf (P < 0.001; F = 768.9), σY = σfyVf (P < 0.001; F = 440.6), and σU = σfuVf (P < 0.001; F = 571.8) yields the gradients (i.e., Ef, σfy, and σfu) of the respective equations (Table 2). We note that the order of magnitude estimates for Ef falls with the range of 0.3–1.6 GPa derived from experiments (14, 44); the order of magnitude estimate for σfy is smaller than the lower limit (70 MPa) obtained by experiment (44); and the order of magnitude estimate for σfu is smaller than the cyclic loading fracture stress (200 MPa) reported by Shen et al. (44). To develop our analysis further, we note that cR1, cP1, and cE1 are related to σfy, σfu, τβ, τRP, and Ef, according to Eqs. A6, A8, and A10 (see below). From Eqs. 2 to 4, we find that cRP1/cE1 = [cR1 + cP1]/cE1 leads to a mathematical expression for the ratio of τRP to τβ; i.e., τRP/τβ = Kσfu{σfu + [3/4]Ef}/[σfy]2, where K = cE1/cRP1. By considering the upper (mean + SE) and lower (mean − SE) limits of cE1, cRP1, Ef, σfy, and σfu (Tables 1 and 2), we obtain an estimate of τRP/τβ in the range of 15–87. This estimate is important in terms of the mechanism of stress transfer by frictional sliding (as quantified by τβ and τRP, respectively) for small and large deformation effects; the argument demonstrates that τRP is 10–102 times (to order of magnitude) higher than τβ.
Table 2.
List of derived parameters for the order of magnitude analysis of τRP/τβ
| Parameter | Predicted Value (MPa) |
|---|---|
| Ef | 728.8 ± 29.7 |
| σfy | 29.0 ± 1.5 |
| σfu | 65.8 ± 3.0 |
Values are means ± SE.
The τRP/τβ analysis lends to further predictions of τβ and τRP. These parameters can be estimated to order of magnitude according to two contentions as follow. First, from fibril pull-out tests carried out using an atomic force microscope (25), the force to pull out a fibril is of order of magnitude σfu[πD2/4] = 10−7 N for typical fibrils having D = 10−7 m and length not exceeding the critical value of 2lc = 10−6 m (34). Second, from shear-sliding analysis (see appendix), we have 2lc/D = σfu/2τRP; the shear action τRP is of order of magnitude σfu[πD2/4]/{πlcD} = 105 Pa. Given that τRP/τβ ranges from 10 to 102 (from the argument established in the previous paragraph), it follows that τβ ranges from 103 to 104 Pa. Of note, this range of estimates for τβ is important since it encompasses estimates derived from alternative arguments. For instance, the force of interaction between GAG side chains from decorin PGs bound on adjacent fibrils is of order of magnitude 10−11 N (33). It can be assumed that the fibrils are packed tetragonally so that the distribution of PGs over the fibril surface is such that there are four PGs for every 68 nm along the fibril (39). By this argument, it follows that the shear stress is 4(4 × 10−11 N)/[π(10−7 m)(68 × 10−9 m)] ≈ 7,489 Pa, which lies within the estimated limits of τβ. Both decorin and biglycan PGs compete for collagen binding and the possibility of targeting identical or adjacent binding sites on the fibril (42, 43). If the population of decorin PGs were to decrease, this could lead to an increased binding of biglycan to fibrils to compensate for the lack of decorin (55) and to regulate the stress transfer (maintaining the values of τβ and τRP) throughout the different phases of aging. To summarize, the argument presented in this paragraph reveals that in tendon from postnatal growth to old age, the fibril/PG-matrix interface experiences weaker GAG interactions during initial loading than at subsequent stages of loading following yielding.
Possible model limitations.
Our energy argument has enabled the formulation of the scaling relationships for studying the age variation in structure-property relationship in tendon. As we weigh the implications of our approach, it is important to acknowledge the following possible model limitations.
To begin, we note that tendon is predominantly composed of type I collagen (4). This is a trimeric biomacromolecule featuring a Gly-X1-X2 amino acid sequence pattern and a standard triple-helix throughout their ∼1,000 residue triple-helix domain (Gly = glycyl residue; X1 = proline residue; X2 = 4-hydroxyproline residue). So far, it is not known if type I collagen triple-helix features crystal imperfections. Tendon also contains small amounts of one or more of the other minor collagens, such as type XXII fibril-associated collagen, which is located on the surface of fibrils at the myotendinous junction (31); it features interruptions (i.e., crystal imperfections) in the amino acid sequence pattern. An interrupted domain may involve one (or more) missing residue, in which case, the Gly residues in the interruptions would be separated by one (or more) non-Gly residue (48). Furthermore, the residues within the interruptions are predominantly hydrophobic; some of these residues also possess a net charge (48). We speculate that these interruptions may contribute to the microinhomogeneity of strains within the fibrils. Consequently, plastic microstrains could develop within these domains even when the tendon is nominally within the elastic limit (15). At a molecular level, we note that σfy is the measure of the force of attraction arising from covalent bonds within (and between) collagen molecules/unit volume; Ef is the measure of the rate of change of the force of attraction/unit volume with respect to strain; and σfu is the measure of the force of attraction/unit volume for resisting bond disruption within (and between) collagen molecules. In the energy argument leading to the scaling (structure-property) relationships, we have assumed that these fibrillar mechanical parameters, i.e., σfy, σfu, and Ef, are constants of age and that fibril rupture occurs at the fibril center, where the stress is highest (see appendix). However, variation in the proportions of type I and type XXII collagens has been difficult to assess with age. Indeed, if the proportion of type XXII collagen increases with age relative to type I collagen, these fibrillar mechanical properties would be affected by changes to the density and orientation of the crystal imperfections (44, 46). These imperfections could influence the mechanical stability of the fibril by regulating the proportion of the energy absorbed by the deforming fibril; the strain energy increases as the tendon deforms with increasing strain. From the theory of local average bonding (46), it is predicted that eventually, a crack will be initiated when a bond within the domain of the imperfection is disrupted, and this will propagate within the fibril as the nearby bonds become energetically unstable. We do not rule out the possibility of the influence of collagen imperfections on σfy, σfu, and Ef with increasing age. These discussions are important because they implicate the effects of structure on the mechanical properties of fibrils as reported by Shen et al. (44) and provide the basis for directing future studies on the structural effects on the mechanics of fibrils in the presence of aging.
Another concern addresses the changes in the intermolecular cross-links of collagen with age. According to Bailey (4), there are two major cross-link processes: 1) the initial stabilization of the fibrils through lysyl oxidase during growth and maturation and 2) the subsequent process arising from the reaction with glucose or its metabolites that occurs with age (during old age), as the turnover of the collagen is reduced to a minimum. Bailey (4) argued that the divalent dehydro-hydroxylysinonorleucine (deH-HLNL) cross-link, which binds two collagen molecules end to end along the length of the fibril, predominates in young tendon (i.e., the first cross-link process). At maturation, deH-HLNL could react with histidine to form the trivalent (mature) cross-link, which is known as histidino-hydroxylysinonorleucine (HHL) (4). In addition, deH-HLNL may react with another cross-link or lysyl aldehyde from an adjacent fibril to form interfibrillar cross-links. The presence of these interfibrillar cross-links may contribute to increasing uF as more energy would be required for fibril pull-out. During maturation, as the rate of turnover of collagen decreases, the proportion of new collagen stabilized by the deH-HLNL decreases, thus leading to a build-up of the HHL (4). An appreciable increase in HHL density with age may lead to a corresponding increase in the magnitudes of σfy, σfu, and Ef; this could challenge our underlying assumption that these parameters are age invariant (21a). Following maturation, whereas there is little change in the concentration of HHL and deH-HLNL, new intermolecular cross-links could result from the production of advanced glycation end-products (AGEs) by the glycation process [i.e., the second cross-link process (4, 10)]. The higher concentration of cross-links in the tendon at old age (vs. maturation) suggests a possible mechanism for maintaining—by counteracting the decrease in collagen concentration—the mechanical properties of tendon (10). Nevertheless, as acknowledged in an earlier study (21a), how the cross-links, i.e., HHL, deH-HLNL, and AGEs, cooperate to influence σfy, σfu, and Ef during old age is not well understood. The scaling relationships of Eqs. 2 and 3 do not take into account any possible influence of changing cross-link profiles or densities with age.
We highlight three recent findings that support the arguments addressing the influence of covalent cross-link on the molecular mechanics of collagen: 1) the presence of the cross-link (C-terminal) between two collagen molecules serves to resist intermolecular slippage during deformation (50); 2) the overlap regions act as a buffer by preventing the stress from concentrating along the portion of the strands containing the cross-link (50); and 3) rupture and sliding of tropocollagen molecules in deforming fibrils are strongly influenced by fibrillar length, width, and cross-linking density (18, 47). Thus increasing fibrillar width leads to increase in the stiffness, maximum stress generated, and strain energy absorbed within the fibril (47); accordingly, the scaling relationships have predicted how these parameters interplay to regulate uE (Eq. 2) and uF (Eq. 3).
Conclusion.
There have been several studies to establish the structure-property relationship of connective tissues in the presence of aging since the work of Parry et al. (37). These studies have included qualitative and quantitative models. The different approaches are inevitable because of the continuing improvement in the techniques used in these studies, such as the finite mixture modeling (35) of the fibril size distribution (30) and decorin-deficient mouse model for studying the altered fibril structure (11, 40, 55), as a result of experience in the application of these techniques to an increasingly wide range of problems, such as the regulation of fibrillogenesis and tendon development (55). To this end, our study has made a number of contributions to understanding the structure-property relationship of tendon in the presence of aging. It has centrally addressed an energy-based argument to formulate the scaling relationships for modeling the influence of D on the strain energy absorbed to fracture by the tendon. These findings indicate the important contributions of the D1 and D2 fibril subpopulations to age variations in tendon resilience. An increase in the DD2 and a decrease in the DD1 contribute to an increase in uE/σY and vice versa. On the other hand, only D1 contributes to age varations in tendon resistance to rupture. Accordingly, the larger the DD1, the higher is the uF/σU and vice versa. This study provides important first evidence of a possible role for the bimodal D distribution in directing age-related variations in tendon resilience and resistance to rupture.
GRANTS
Support for this work was provided by grants from the European Union Framework Programme QLK6-CT-2001-00175 and Merlion-France Programme 5.03.07 under the French Ministere des Affaires Etrangeres et Europeennes and Collier Charitable Trust COLCHAGA09. D. F. Holmes and Y. Lu were funded by the Wellcome Trust 091840/Z/10/Z (to K. E. Kadler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.L.G., P.P.P., D.F.H., K.E.K., T.J.W., and D.B. conception and design of research; K.L.G., P.P.P., D.F.H., and Y.L. performed experiments; K.L.G. and D.F.H. analyzed data; K.L.G., P.P.P., D.F.H., D.B., and K.E.K. interpreted results of experiments; K.L.G. and D.F.H. prepared figures; K.L.G. and D.F.H. drafted manuscript; K.L.G., P.P.P., D.F.H., K.E.K., T.J.W., and D.B. edited and revised manuscript; K.L.G., P.P.P., D.F.H., K.E.K., T.J.W., D.B., and Y.L. approved final version of manuscript.
Glossary
- D
Collagen fibril diameter
- DD1, DD2
Mean Ds of collagen fibril subpopulations, D1 and D2, respectively
- E
Elastic modulus (stiffness) of tendon
- ECM
Extracellular matrix
- Ef, Em
Elastic moduli of collagen fibril and interfibrillar matrix, respectively
- Gβ
Work of elastic deformation of a fibril during transitional stage (mode β)
- GP
Work of fibril pull-out from ECM
- GR
Work of fibril rupture
- L
Scale factor for length
- lz
Embedded length of a fibril prior to pull-out
- N
Number of fibrils/unit cross-sectional area of tendon
- PG
Proteoglycan
- uE, uF
Strain energy densities contributing to tendon resilience and resistance to rupture, respectively
- Uf, Um
Strain energies for elastic deformation of a fibril and intefibrillar matrix, respectively
- u0
Strain energy density to fracture of tendon
- Vf
Volume fraction of collagen fibrils in ECM
- z
Axial ordinate of the cylindrical polar coordinate system
- δ
Axial deformation in fibril
- εf
Axial strain in fibril
- μβ
Strain energy density for elastic deformation of fibrils
- μP
Strain energy density for fibril pull-out from ECM
- μR
Strain energy density for rupture of fibrils
- σf
Axial stress in the fibril as a function of distance, z
- σm
Average stress in interfibrillar ECM
- σU
Maximum stress (strength) of tendon
- σY
Stress in the tendon at the point of yielding
- σfu
Breaking stress of collagen fibril
- σfy
Yield stress at the fibril center when the tendon yields at σY
- τβ
Shear stress generated at the fibril/PG-matrix interface during the transitional stage (mode β)
- τRP
Shear stress generated at the fibril/PG-matrix interface during fibril rupture or pull-out
- 2lc
Critical length of collagen fibril
- 2lf
Collagen fibril length
APPENDIX
Modeling the fibril elastic deformation scaling relationship.
Consider a long fibril (of length 2lf and diameter D) embedded within the ECM (Fig. 1) in the direction of an applied load acting on the tendon. As loading progresses from the elastic stress-transfer stage to a transitional stage, mode β (20), the frictional sliding action at the fibril/PG-matrix interface generates τβ, which is constant of distance (z) along the fibril axis (20). Here z = 0 corresponds to the fibril center, and z = lf corresponds to the fibril end. In response to the shear action, the fibril deforms, and an axial tensile stress (σf), a function of z, is generated within the fibril (20)
| (A1) |
Thus σf is maximum at z = 0. Assuming symmetric loading, the elastic energy (Uf) stored in an infinitesimal length (Δz) on one-half of the fibril is
| (A2) |
Substituting the expression of σf from Eq. A1 into Eq. A2 gives ΔUf = 2πlf2{τβ2/Ef}[1 − z/lf]2Δz. On the other hand, work done by the fibril element against interfibrillar ECM is
| (A3) |
where δ, the axial deformation in the fibril, is a function of z given by
| (A4) |
and εf is the corresponding elastic strain in the fibril (also a function of z), which relates linearly to σf such that σf = Efεf. Substituting the expression for σf (Eq. A1) into σf = Efεf, we find that εf = 4[lf/D]{τβ/Ef}[1 − z/lf]. As the fibril end-face (z = lf) is free of stress (20), thus εf and δ are reduced to zero at this point. Evaluating the integral in Eq. A4 leads to δ = ΔUf = 2[lf2/D]{τβ/Ef}[1 − z/lf]2; upon substituting the expression of δ into Eq. A3, we find ΔUm = 2πlf2{τβ2/Ef}[1 − z/lf]2Δz = ΔUf. It follows that ΔUf + ΔUm = 2ΔUf. Alternatively, summing the integrals of the respective infinitesimal elements, ΔUf and ΔUm, from z = lf to 0 leads to the total work done, i.e., Uf + Um = [4/3]πlf3τβ2/Ef. We assume that the distribution of the fibrils is a truly random one. This implies that all cross-sections of the tissue normal to the tensile axis are identical, i.e., that in any small length of the tissue, the number of fibril centers/unit area of cross-section must be constant. Let N be the number of fibrils/unit cross-sectional area of the composite; the volume fraction of the fibrils is approximated by Vf ≈ NπD2/4. The work of elastic deformation up to the point of yielding of the tendon, i.e., Gβ = N[Uf + Um] = {4/3}Nπlf3[τβ2/Ef]. At z = 0, assuming the fibrils have yielded, we have σf = σfy, and lf = D[σfy/τβ]/4 (Eq. A1). Thus we find
| (A5) |
Comparing the dimensions of Gβ (Eq. A5) and the associated strain energy density (μβ), we find that Gβ scales to μβ if we arbitrarily divide Eq. A5 throughout by a scale factor (L), which to order of magnitude, may be identified with the “grip-to-grip” length of the test sample (which can be fixed for all samples). By applying the rule of mixtures (21a) to modeling σY, assuming that to order of magnitude, σfyVf >> σm[1 − Vf] at PI (Fig. 2C), it follows that σY ≈ σfyVf. Replacing σfyVf by σY in Eq. A5 and recalling from previous studies that τβ (3), σfy, and Ef (21a) are constants and independent of age, we find
| (A6) |
where cE = [σfy]2/{6Efτβ}.
We highlight two important findings concerning fibrillar yielding in light of recent reports. First, the occurrence of yield has been explained by observing the point of deviation from linearity of the stress-strain curve in a single fibril test using a microelectromechanical system (44). Second, at the fibrillar level, yielding addresses a change in the mechanism of stress transfer. We attribute the change to two possible processes: 1) disruption of the bonds between adjacent fibrils and between the fibrils and PG matrix, where a sufficiently large, relative displacement of the fibrils occurs (36, 39), and 2) yielding in fibrils as slippage between tropocollagen molecules occurs (18, 47).
Modeling the fibril rupture scaling relationship.
As the applied load increases, eventually, microcracks initiate in interfibrillar ECM (Fig. 2B). Consider fibrils bridging a crack region of interfibrillar ECM (Fig. 2B). Around the crack site, ECM can no longer take up load effectively, and the bulk of the load is transferred to the fibrils. As the crack opens, τRP is generated as ECM shear slides over the fibrils; strain energy accumulates in each fibril as it deforms in response to τRP. For fibrils whose lengths are ≥2lc, eventually the stored energy reaches a level that is sufficient to fracture the fibril at the point, i.e., z = 0 of maximum σf = σfu (=4τRPlc/D; from Eq. A1). Suppose we apply a conservative assumption that one out of every two fibrils randomly selected from the ECM has a length ≥2lc; thus 50% of the population of fibrils across the crack could rupture, whereas the rest would be pulled out. Consider the elastic energy stored within a region from the crack region of interfibrillar ECM of the yielded fibril. On each side of the crack, the plane retracts and slips relative to the fibril (Fig. 2B). For an element (of infinitesimal length Δz) along this region, the energy takes the same form as Eq. A2. Recalling an argument from the elastic deformation analysis, we can determine the corresponding εf from σf = Efεf when σf = σfu. The elastic energy ΔUf absorbed by the small element is obtained by substituting the expression of σf into Eq. A2 to give ΔUf = 2πlf2{τRP2/Ef}[1 − z/lf]2Δz (38). Following from Eq. A3, the work done by the element in sliding against ECM is given by ΔUm = πDτRPδΔz2 (38). Following from this expression of ΔUm and Eq. A4, we proceed to evaluate δ. Noting that the values of εf and δ arising from the cracked-induced stress are reduced to zero at z = lc, on evaluating the integral of Eq. A4, we find that δ = 2{τRP/Ef}[lc − z]2/D (38). The arguments leading up to here also implicate that ΔUm = ΔUf when fibrils (bridging interfibrillar ECM crack) rupture (38). The sum of ΔUm and ΔUf, by integrating from z = lc to 0, gives the total work, i.e., Uf + Um = {8πτRP2/Ef}∫lc0lc − z]2dz = [4/3]πlc3{τRP2/Ef} (38). Suppose the matrix crack extends across the cross-section of the tendon. We note that the number of ruptured fibril/unit cross-sectional area = N/2; the volume fraction of these fibrils is given by NπD2/8 (38). With the use of the mathematical model described by Eq. A1 but replacing lf by lc [=D(σfu/τRP)/4] and τβ by τRP, noting that σf = σfu at z = 0, the corresponding work of rupture of the fibril (38) GR [=2(N/2)(Uf + Um) = N(8/3)πlc3τRP2/Ef] becomes
| (A7) |
Similarly, GR (Eq. A7) scales to the corresponding strain energy density (μR) by arbitrarily dividing Eq. A7 throughout by L. Applying the rule of mixtures to model σU, in the limit σfuVf >> σm[1 − Vf] (21a), we find that σU ≈ σfuVf. Here, we have modeled σfu as an invariant of age (21a). Let cR = [σfu]2/{6Ef τRP}. Replacing σfuVf by σU in Eq. A7 leads to
| (A8) |
Modeling the fibril pull-out scaling relationship.
Across the crack within interfibrillar ECM, fibrils shorter than 2lc are not expected to rupture (38). Instead, as the applied load increases, eventually, these fibrils will be pulled out from the ECM when the surfaces of the crack are completely displaced apart (Fig. 2B). To calculate the work of pull-out, we assume on order of magnitude that the interfacial shear stress generated is τRP and is constant during pull-out. Let lz be the embedded length of a fibril. The force needed to pull the fibril out is equal to the force resisted by the fibril, i.e., πD2σf/4 = πDlz τRP, where σf is a function of fibril axial distance z whose origin has now been designated to begin at the point adjacent to the crack plane. At any instance during the pull-out process, the profile of σf along the embedded length is such that it decreases linearly with distance; consequently, consistent with the earlier assumption of a stress-free end-face, we find σf = 0 at the fibril end. Consider an infinitesimally small element along the embedded fibril at distance z from the cracked plane. The work of pull-out is Uf = ∫lz0 πD τRP zdz = {1/2}πDlz2 τRP (38). We note that the number of fibrils pulled out across the unit area of crack is N/2 (38). Let the number of fibrils, with embedded lengths ranging from lz and lz + Δlz on one side of the crack, which are involved in the pull-out process, be {N/2}Δlz/2lz (38). Since we are considering one side of the crack, the work of fibril pull-out becomes GP/2. The work can be determined by summing the values of Uf for the fibrils being pulled out; i.e., GP/2 = ∫0lz[N/2]Ufdlz/2lz = VfDlf2 τRP2/24 (38). Thus GP attains a maximum value when lf = lc [=D(σfu/τRP)/4] (38), which is
| (A9) |
GP scales to the corresponding strain energy density (μP) by arbitrarily dividing Eq. A9 throughout by L. Let cP = σfu/{8τRP}. Recalling an earlier argument, which invokes the rule of mixtures to establish the lower limit of σU = σfuVf, it follows that replacing σfuVf by σU in Eq. A9 leads to
| (A10) |
REFERENCES
- 1. Allison PD. Multiple Regression: A Primer. London: Pine Forge, 1999 [Google Scholar]
- 2. Annovazzi L, Genna F. An engineering, multiscale constitutive model for fiber-forming collagen in tension. J Biomed Mater Res A 92: 254–266, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Astrand H, Stalhand J, Karlsson J, Karlsson M, Sonesson B, Lanne T. In vivo estimation of the contribution of elastin and collagen to the mechanical properties in the human abdominal aorta: effect of age and sex. J Appl Physiol 110: 176–187, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122: 735–755, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bensamoun SF, Tsubone T, Subramaniam M, Hawse JR, Boumediene E, Spelsberg TC, An KN, Amadio PC. Age-dependent changes in the mechanical properties of tail tendons in TGF-β inducible early gene-1 knockout mice. J Appl Physiol 101: 1419–1424, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bland M. An Introduction to Medical Statistics (2nd ed.). Oxford, UK: Oxford University Press, 1995 [Google Scholar]
- 7. Buehler MJ. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci USA 103: 12285–12290, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cameron GJ, Cairns DE, Wess TJ. The variability in type I collagen helical pitch is reflected in the D periodic fibrillar structure. J Mol Biol 372: 1097–1107, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Carrol CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105: 1907–1915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjær M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107: 880–886, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Derwin KA, Soslowsky LJ. A quantitative investigation of structure function relationships in a tendon fascicle model. J Biomech Eng 121: 598–604, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res 20: 1315–1322, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Elsheikh A, Geraghty B, Rama P, Campanelli M, Meek KM. Characterization of age-related variation in corneal biomechanical properties. J R Soc Interface 7: 1475–1485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eppell SJ, Smith BN, Kahn H, Ballarini R. Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. J R Soc Interface 3: 117–121, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esin A, Jones WJD. A statistical approach to micro-plastic strain in metals. J Strain Anal Eng Design 1: 415–421, 1966 [Google Scholar]
- 16. Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol 28: 503–510, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. In: The Mouse in Biomedical Research (2nd ed.), edited by Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A. Waltham, MA: Elsevier, 2007 [Google Scholar]
- 18. Gautieri A, Vesentini S, Redaelli A, Buehler MJ. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett 11: 757–766, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Goh KL, Aspden RM, Hukins DWL. Review: finite element analysis of stress transfer in short-fibre composite materials. Compos Sci Technol 64: 1091–1100, 2004 [Google Scholar]
- 20a. Goh KL, Chen Y, Chou SM, Listrat A, Bechet D, Wess TJ. Effects of frozen storage temperature on the elasticity of tendons from a small murine model. Animal 4: 1613–1617, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Goh KL, Hiller J, Haston JL, Holmes DF, Kadler KE, Murdoch A, Meakin JR, Wess TJ. Analysis of collagen fibril diameter distribution in connective tissues using small-angle X-ray scattering. Biochim Biophys Acta 1722: 183–188, 2005 [DOI] [PubMed] [Google Scholar]
- 21a. Goh KL, Holmes DF, Lu HY, Richardson S, Kadler KE, Purslow PP, Wess TJ. Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J Biomech Eng 13: 021011, 2008 [DOI] [PubMed] [Google Scholar]
- 21b. Goh KL, Hukins DWL, Aspden RM. Critical length of collagen fibrils in extracellular matrix. J Theor Biol 223: 259–261, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Goh KL, Meakin JR, Aspden RM, Hukins DWL. Stress transfer in collagen fibrils reinforcing connective tissues: effects of collagen fibril slenderness and relative stiffness. J Theor Biol 245: 305–311, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Gutsmann T, Fantner GE, Kindt JH, Venturoni M, Danielsen S, Hansma PK. Force spectroscopy of collagen fibers to investigate their mechanical properties and structural organisation. Biophys J 86: 3186–3193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen P, Haraldsson BT, Aagaard P, Kovanen V, Avery NC, Qvortrup K, Larsen JO, Krogsgaard M, Kjaer M, Magnusson SP. Lower strength of the human posterior patellar tendon seems unrelated to mature collagen cross-linking and fibril morphology. J Appl Physiol 108: 47–52, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Holmes DF, Tait A, Hodson NW, Sherratt MJ, Kadler KE. Growth of collagen fibril seeds from embryonic tendon: fractured fibril ends nucleate new tip growth. J Mol Biol 399: 9–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hukins DWL, Aspden RM. Composition and properties of connective tissues. Trends Biochem Sci 10: 280–284, 1985 [Google Scholar]
- 29. Hulmes DJS, Wess TJ, Prockop DJ, Fratzl P. Radial packing, order, and disorder in collagen fibrils. Biophys J 68: 1661–1670, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones PN. On collagen fibril diameter distributions. Connect Tissue Res 26: 11–21, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci 120: 1955–1958, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kohn RR. Principles of Mammalian Aging. Upper Saddle River, NJ: Prentice-Hall, 1971 [Google Scholar]
- 33. Liu XH, Yeh ML, Lewis JL, Luo ZP. Direct measurement of the rupture force of single pair of decorin interactions. Biochem Biophys Res Commun 338: 1342–1345, 2005 [DOI] [PubMed] [Google Scholar]
- 34. McBride DJ, Trelstad RL, Silver FH. Structural and mechanical assessment of developing chick tendon. Int J Biol Macromol 10: 194–200, 1988 [Google Scholar]
- 35. McLachlan GJ, Peel D. Finite Mixture Models. New York: Wiley, 2000 [Google Scholar]
- 36. Orgel JPRO, Eid A, Antipova O, Bella J, Scott JE. Decorin core protein (Decoron) shape complements collagen fibril surface structure and mediates its binding. PloS ONE 4: e7028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parry DAD, Barnes GRG, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci 203: 305–321, 1978 [DOI] [PubMed] [Google Scholar]
- 38. Piggott MR. Load Bearing Fibre Composites (1st ed.). Oxford, UK: Pergamon, 1980 [Google Scholar]
- 39. Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons—a computational study from molecular to microstructural level. J Biomech 36: 1555–1569, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ. Investigating tendon fascicle structure-function relationships in a transgenic-age mouse model using multiple regression models. Ann Biomed Eng 32: 924–931, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Sasaki N, Odama S. Stress-strain curve and Young's modulus of a collagen molecule as determined by the X-ray diffraction technique. J Biomech 29: 655–658, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Schonherr E, Hausser H, Beavan L, Kresse H. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J Biol Chem 270: 8877–8883, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem 270: 2776–2783, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Shen ZL, Dodge MR, Kahn H, Ballarini R, Eppell SJ. Stress-strain experiments on individual collagen fibrils. Biophys J 95: 3956–3963, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silver FH, Freeman JW, Horvath I, Landis WJ. Molecular basis for elastic energy storage in mineralized tendon. Biomacromolecules 2: 750–756, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Sun CQ. Thermo-mechanical behavior of low-dimensional systems: the local bond average approach. Prog Mater Sci 54: 179–307, 2009 [Google Scholar]
- 47. Tang Y, Ballarini R, Buehler MJ, Eppell SJ. Deformation micromechanisms of collagen fibrils under uniaxial tension. J R Soc Interface 7: 839–850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thiagarajan G, Li Y, Mohs A, Strafaci C, Popiel M, Baum J, Brodsky B. Common interruptions in the repeating tripeptide sequence of non-fibrillar collagens: sequence analysis and structural studies on triple-helix peptide models. J Mol Biol 376: 736–748, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Torp S, Arridge RGC, Armeniades CD, Baer E. Structure-property relationships in tendon as a function of age. In: Structure of Fibrous Biopolymers, edited by Atkins EDT, Keller A. London: Butterworths, 1975 [Google Scholar]
- 50. Uzel SGM, Buehler MJ. Molecular structure, mechanical behavior and failure mechanism of the C-terminal cross-link domain in type I collagen. J Mech Behav Biomed Mater 4: 153–161, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Viidik A. Age-related changes in connective tissues. In: Lectures on Gerontology: On Biology of Ageing, Part A, edited by Viidik A. London: Academic, 1982, vol. 1 [Google Scholar]
- 52. Vogel HG. Species differences of elastic and collagenous tissue—influence of maturation and age. Mech Ageing Dev 57: 15–24, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Wong SC, Mai YW. Essential fracture work of short fiber reinforced polymer blends. Polym Eng Sci 39: 356–364, 1999 [Google Scholar]
- 54. Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol 111: 999–1006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98: 1436–1449, 2006 [DOI] [PubMed] [Google Scholar]