Abstract
Pregnancy is associated with ventricular hypertrophy and volume overload. Here we investigated whether late pregnancy is associated with cardiac structural and hemodynamic changes, and if these changes are reversed postpartum. Female mice (C57BL/6) were used in nonpregnant diestrus (NP), late-pregnant (LP), or 7-day postpartum (PP7) stages. Echocardiography and cardiac catheterization were performed to monitor cardiac hemodynamics. Transcript expression of proangiogenic vascular endothelial growth factor, cardiac fetal gene osteopontin, cardiac extracellular matrix-degrading enzymes matrix metalloproteinase-2, and a disintegrin and metalloproteinase-15 and -17 were assessed by RT-PCR. Masson trichrome staining for cardiac fibrosis and endothelial marker CD31 immunostaining for angiogenesis were performed. Heart hypertrophy in LP was fully reversed in PP7 (heart weight: NP = 114 ± 4 mg; LP = 147 ± 2 mg; PP7 = 117 ± 8 mg, P < 0.05 for LP vs. PP7). LP had elevated left ventricular (LV) pressure (119 ± 5 mmHg in LP vs. 92 ± 7 mmHg in NP, P < 0.05) that was restored at PP7 (95 ± 8 mmHg, P < 0.001 vs. LP). LP had increased LV contractility (maximal rate of increase of LV pressure = 6,664 ± 297 mmHg/s in LP vs. 4,294 ± 568 mmHg/s in NP, P < 0.01) that was restored at PP7 (5,313 ± 636 mmHg/s, P < 0.05 vs. LP). LV ejection fraction was reduced in LP (LP = 58 ± 1% vs. NP = 70 ± 4%, P < 0.001) and was already restored at PP1 (77 ± 2%, P < 0.001 vs. LP). Myocardial angiogenesis was significantly increased in LP (capillary density = 1.25 ± 0.02 vs. 0.95 ± 0.01 capillaries/myocyte in NP, P < 0.001) and was fully restored in PP7 (0.98 ± 0.01, P < 0.001 vs. LP). Vascular endothelial growth factor was upregulated in LP (LP = 1.4 ± 0.1 vs. NP = 1 ± 0.1, normalized to NP, P < 0.001) and was restored in PP7 (PP7 = 0.83 ± 0.1, P < 0.001 vs. LP). There was no increase in cardiac fibrosis in LP. Matrix metalloproteinase-2 transcript levels were downregulated in LP (LP = 0.47 ± 0.03 vs. NP = 1 ± 0.01, normalized to NP, P < 0.001) and was restored at PP7 (0.70 ± 0.1, P < 0.001 vs. LP). In conclusion, pregnancy-induced heart hypertrophy is associated with transient cardiac dysfunction, increased cardiac angiogenesis, lack of fibrosis, and decreased expression of remodeling enzymes that are reversed postpartum.
Keywords: pregnancy, hypertrophy, angiogenesis, fibrosis
in some physiological conditions, such as exercise and pregnancy, the heart undergoes hypertrophy as an adaptive response, which is potentially reversible and is without significant long-term detrimental effects on cardiac function. During pregnancy, healthy women develop ventricular hypertrophy and cardiac dysfunction as a result of transient volume overload, as well as increased stretch and force demand (8, 9).
It has already been shown that the molecular and functional signature of physiological heart hypertrophy during pregnancy differs from that of pathological hypertrophy (9). The precise underlying molecular mechanisms of pregnancy-induced hypertrophy are still not clearly understood. Systolic function is preserved throughout most of pregnancy through a fall in afterload, but decreases near term as a result of decreased contractility and diminished preload (7).
In general, physiological hypertrophic conditions in the heart are associated with either normal or increased myocardial angiogenesis (12). Physiological hypertrophy during pregnancy also requires the proportional growth of the capillary network (11). This increase in cardiac angiogenesis should help to meet the demands for increased blood volume and myocardial hypertrophy during pregnancy.
In pathological heart hypertrophy, the involvement of cardiac extracellular matrix (ECM) in the progression to decompensated heart failure is well established. Fibrosis, metalloproteinase upregulation, and reactivation of matricellular fetal genes are key steps in the progression of adverse cardiac ECM remodeling (2, 4). However, little is known about the involvement of the cardiac ECM in pregnancy-induced heart hypertrophy.
Our laboratory has previously characterized some of the molecular and functional signature of pregnancy-induced physiological heart hypertrophy (8). Here we investigated whether there were structural and hemodynamic changes associated with pregnancy-induced heart hypertrophy and if these changes were reversed postpartum. We also explored the involvement of the cardiac ECM in pregnancy-induced hypertrophy by studying myocardial fibrosis, expression of metalloproteinases, matrix metalloproteinase (MMP)-2, a disintegrin and metalloproteinase (ADAM)-15, and ADAM-17, and matricellular fetal gene osteopontin (OPN). These ECM enzymes are well established for increased activity during pathological heart failure (10, 18, 31). We found that heart hypertrophy in late pregnancy was associated with mild cardiac hemodynamic dysfunction without fibrosis. Pregnancy was also associated with increased angiogenesis. Interestingly, ECM protein OPN was unchanged and matrix-degrading enzymes MMP-2, ADAM-15, and ADAM-17 were downregulated in the hearts from the late pregnant mice. These structural and hemodynamic changes were reversed by day 7 postpartum.
METHODS
Animals.
Young adult female (3–4 mo) mice (C57BL/6) were used in nonpregnant diestrus stage (NP, n = 10), late pregnant (LP, day 19–20 of pregnancy, n = 10), or 7 days postpartum (PP7, n = 8). Protocols received institutional review and committee approval. The investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996).
Cardiac hemodynamics.
Echocardiography and cardiac catheterization were performed to monitor cardiac hemodynamics. Mice were anesthetized with inhaled isoflurane (2% for induction and 1.5% for maintenance) for echocardiography. B-mode and M-mode echocardiography were performed (VisualSonics Vevo 770, 30-MHz linear transducer). M-mode echocardiography was used to measure left ventricular (LV) ejection fraction (LVEF). The LV pressure was also measured directly by inserting a catheter (1.4F Millar SPR-671) connected to a pressure transducer (Power Laboratories, ADInstruments) into the LV right before death. For cardiac catheterization, the mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg) intraperitoneally. The animals were placed on a controlled warming pad to keep the body temperature constant at 37°C. After a tracheotomy was performed, a cannula was inserted, and the animals were mechanically ventilated. After a midsternal thoracotomy, mice were placed under a stereomicroscope (Zeiss, Hamburg, Germany), and a pressure-conductance catheter was introduced via the apex into the LV. The catheter was connected to a signal processor (ADInstruments) and LV pressures, maximal rate of increase of LV pressure (dP/dtmax), and decline of LV pressure (dP/dtmin) were recorded digitally. After the pressures were recorded, hearts were removed rapidly under deep anesthesia for preservation of protein integrity.
Gross histological evaluation and hypertrophy measurements.
The hearts were dissected and weighed to determine heart hypertrophy. The body weight (BW; g) of the mice was recorded. The weight ratio of the heart to BW was also calculated and compared between the groups.
Real-time PCR.
Total RNA from heart was isolated using Trizol (Invitrogen) and reverse transcribed with gene-specific primers using Omniscript RT kit (Qiagen). GAPDH was used as internal reference gene.
Primers.
The sequences of the primers are as follows: OPN forward primer 5′-CAACGGCCGAGGTGA-3′; OPN reverse primer 5′-ATGGCTGCCCTTTCCGTTGTT-3′; ADAM-15 forward primer 5′-CCCTATGTCCTCTTTGTGTGGA-3′; ADAM-15 reverse primer 5′-GCAGAACTCAGGCAGATCACAA-3′; ADAM-17 forward primer 5′-GCGTTGACACTGACAACTCGT-3′; ADAM-17 reverse primer 5′-CAGCTGGTCAATGAAATCCCAAA-3′; vascular endothelial growth factor (VEGF)a forward primer 5′-ACACGGTGGTGGAAGAAGAG-3′; VEGFa reverse primer 5′-CAAGTCTCCTGGGGACAGAA-3′; MMP-2 forward primer 5′-CCCCATGAAGCCTTGTTTACCA-3′; MMP-2 reverse primer 5′-TGGAAGCGGAACGGGAACTTG-3′; GAPDH forward primer 5′-CCTGCACCACCAACTGCTTAG-3′; GAPDH reverse primer 5′-ATGACCTTGCCCACAGCCTTG-3′.
Single peak was detected from the first derivative of fluorescence vs. temperature plots (melting curve), indicating amplification of a single product. Agarose gel electrophoresis at the end of the reaction also confirmed the amplification of a single product of the expected size. Controls were as follows: 1) reaction cocktail without reverse transcriptase tested in a regular 40 cycle PCR; and 2) H2O instead of cDNA tested in parallel to real-time PCR.
Histochemistry and imaging for fibrosis.
Whole hearts were fixed in 4% paraformaldehyde in 0.1 M Na2HPO4 and 23 mM NaHPO4 (pH 7.4) for 4 h on ice. The tissue was then immersed in ice-cold 20% sucrose in 0.1 M Na2HPO4 and 23 mM NaHPO4 (pH 7.4) overnight to cryoprotect the tissue and mounted using OCT, and transversal 6- to 7-μm sections were obtained with a cryostat. Tissue sections were stained with Masson trichrome staining. Briefly, the slides were deparaffinized and rinsed in deionized water and mordant in preheated Bouin's solution at 56°C for 15 min. Slides were allowed to cool in tap water and washed in running tap water to remove the yellow color from sections. Sections were stained in working Weigert's iron hematoxylin solution for 5 min and washed in running tap water for 5 min. After being rinsed in deionized water, sections were stained in Biebrich scarlet-acid fucshin for 5 min, followed by another rinse in deionized water. Slides were placed in working phosphotungstic/phosphomolybdic acid solution for 5 min and then placed in aniline blue solution for 5 min. Then slides were placed in 1% acetic acid for 2 min, rinsed, dehydrated through alcohol, cleared in xylene, and mounted. The images were acquired using light microscopes (Axiovert 135, Zeiss, and Nikon Eclipse E 400).
Immunofluorescence staining.
Heart cross sections (6–7 μm) were fixed in acetone for 15 min at −20°C. The sections were then washed with PBS + 0.1% Triton three times and incubated with 10% normal goat serum in PBS + 0.1% Triton for 30 min to block the background. The sections were then incubated with primary antibody in PBS + 0.1% Triton + 1% normal goat serum at 4°C overnight. The sections were then washed with PBS + 0.1% Triton three times and incubated with the appropriate secondary antibody in PBS + 0.1% Triton + 1% normal goat serum at room temperature for 1 h. After the secondary antibodies were washed with PBS + 0.1% Triton three times, the sections were incubated with wheat germ agglutinin (WGA, 1:200 dilution) in PBS + 0.1% Triton + 1% normal goat serum for 1 h at room temperature. The sections were then washed with PBS three times and mounted using Prolong gold (Molecular Probes) for imaging. The images were acquired using a high-resolution laser scanning confocal microscope (Olympus).
Heart capillary density quantification.
Capillary density in heart sections was quantified as the number of capillaries per cardiomyocytes using CD31 (endothelial cell marker) and WGA immunostaining. WGA binds to saccharides of cardiomyocyte membrane (29, 33). Capillary density was quantified from at least five high-power fields (×60) per slide, two slides per animal (3–4 animals per group). Sections were randomly selected, and the observers were blinded.
Reagents.
Primary antibody used was anti-PECAM (CD31, Millipore, 1:200). Secondary antibody used was goat anti-rabbit-IgG-Alexa Fluor 488 (1:1,000) for immunofluorescence. WGA Alexa Fluor 488 conjugate was purchased from Invitrogen. Masson trichrome staining was performed using trichrome stain (Masson) kit (Sigma-Aldrich).
Statistical analysis.
One-way ANOVA tests were used to compare between groups using SPSS13.0 for Windows (SPSS, Chicago, IL). When significant differences were detected, individual mean values were compared by post hoc tests (Bonferroni) that allowed for multiple comparisons. P values < 0.05 were considered statistically significant. Values are expressed as means ± SE.
RESULTS
Pregnancy-induced cardiac hypertrophy is reversed postpartum.
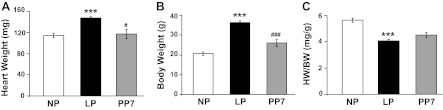
We assessed myocardial hypertrophy in the LP and postpartum stages. As expected, late pregnancy was associated with significant heart hypertrophy [heart weight (HW) in LP = 147 ± 2 mg vs. NP = 114 ± 4, P < 0.001, Fig. 1A] that was reversed at PP7 (HW = 117 ± 8 mg, P < 0.05 vs. LP). Conversely, HW-to-BW ratio (HW/BW) was decreased in LP, despite the cardiac hypertrophy (HW/BW in LP = 4.08 ± 0.12 mg vs. 5.64 ± 0.15 mg/g in NP, P < 0.001, Fig. 1C). This decrease was due to the significantly elevated BW during late pregnancy (BW = 36.3 ± 0.89 g in LP vs. 20.61 ± 0.83 g in NP, P < 0.001). The increase in BW was reversed at PP7 (26.00 ± 1.77 g, P < 0.001 vs. LP, Fig. 1B). The HW/BW was not completely reversed by PP7 [HW/BW = 4.52 ± 0.21 in PP7, P = nonsignificant (NS) vs. LP, and P = NS vs. NP, Fig. 1C].
Fig. 1.
Pregnancy is associated with cardiac hypertrophy. Bar graphs show heart weight (HW) (mg; A), body weight (BW) (g; B), and HW/BW (mg/g; C) in nonpregnant (NP; open bars), late pregnant (LP; solid bars), and 7-day postpartum (PP7; shaded bars) mice. Values are means ± SE (n = 4–10 animals/group). ***P < 0.001 vs. NP. #P < 0.05 vs. LP. ###P < 0.001 vs. LP.
Pregnancy results in mild cardiac hemodynamic dysfunction that is restored postpartum.
We monitored the cardiac hemodynamics in LP, as well as postpartum stages. LP was associated with modestly elevated LV pressure (119 ± 5 mmHg in LP vs. 92 ± 7 mmHg in NP, P < 0.05) that was restored at PP7 (95 ± 8 mmHg, P < 0.001 vs. LP, Fig. 2A). LP was also associated with increased LV contractility (dP/dtmax = 6,664 ± 297 mmHg/s in LP vs. 4,294 ± 568 mmHg/s in NP, P < 0.01) that was restored at PP7 (5,313 ± 636 mmHg/s, P < 0.05 vs. LP, Fig. 2B). Late pregnancy resulted in a mildly depressed LVEF (LP = 58 ± 1% vs. NP = 70 ± 4%, P < 0.001) that was restored as early as PP1 (77 ± 2%, P < 0.001 vs. LP).
Fig. 2.
Late pregnancy results in transient mild cardiac hemodynamic dysfunction. Bar graphs show left ventricular developed pressure (LVP; A), maximal rate of increase of LV pressure (dP/dtmax; B), and decline in LV pressure (dP/dtmin; C) in NP (open bars), LP (solid bars), and PP7 (shaded bars) mice. Values are means ± SE (n = 4–10 animals/group). *P < 0.05 vs. NP. **P < 0.01 vs. NP. #P < 0.05 vs. LP.
Pregnancy-induced cardiac hypertrophy is associated with increased angiogenesis that is reversed postpartum.
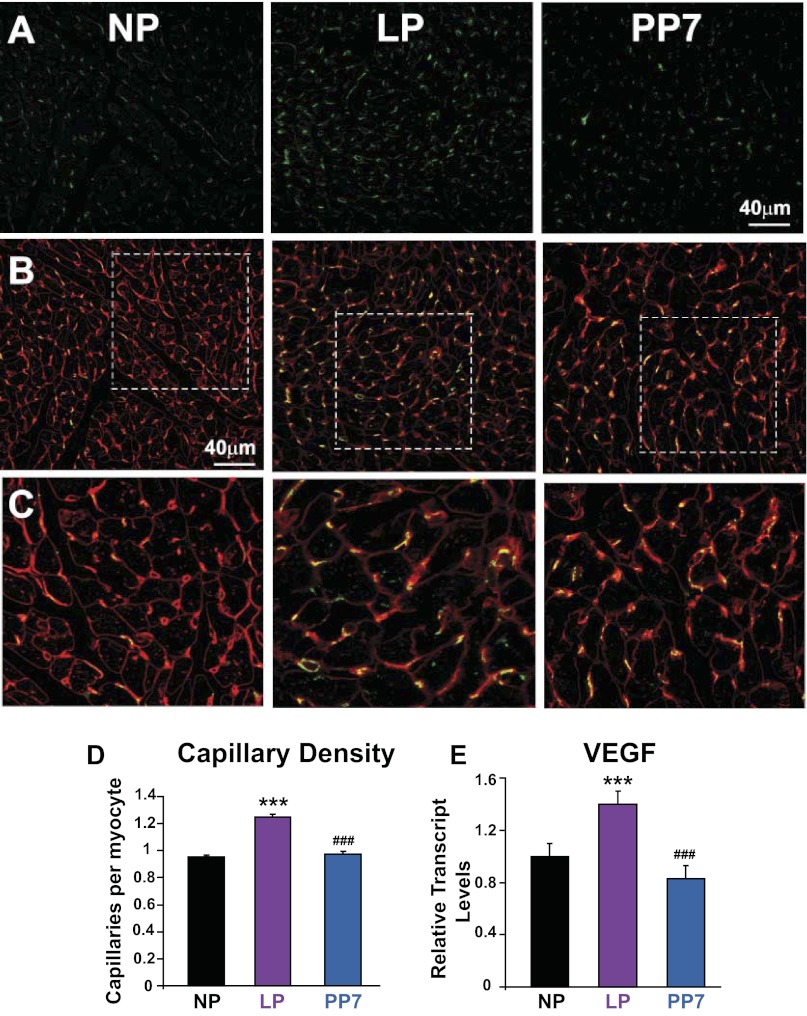
We next examined the vascular status of the myocardium during pregnancy. Capillary density (capillaries/myocyte) was significantly increased in late pregnancy (density = 0.95 ± 0.01 in NP, 1.25 ± 0.02 in LP, P < 0.001, Fig. 3) and was fully restored in PP7 (density = 0.98 ± 0.01 capillaries/myocyte, P < 0.001 vs. LP, Fig. 3). Furthermore, VEGF, a well-established marker of angiogenesis, also showed elevated transcript levels in late pregnancy (1 ± 0.1 in NP, 1.4 ± 0.1 in LP, normalized to NP, P < 0.001, Fig. 3E). VEGF transcripts were restored by PP7 (0.83 ± 0.1 in PP7, P < 0.001 vs. NP, Fig. 3E).
Fig. 3.
Pregnancy leads to increased cardiac angiogenesis. A: immunofluorescence images of the heart showing CD31-immunolabeled capillaries (green) in NP, LP, and PP7. B: immunofluorescence images of the heart showing double labeling of CD31 (green) and wheat germ agglutinin (red) in NP, LP, and PP7. C: magnified views from B showing cardiomyocytes and capillaries. D: capillary density quantification based on the immunofluorescence images shown as capillaries per cardiomyocyte in NP (black bar), LP (purple bar), and PP7 (blue bar). E: relative transcript expression of proangiogenic vascular endothelial growth factor (VEGF) in NP (black bar), LP (purple bar), and PP7 (blue bar). Values are means ± SE (n = 4 animals/group). ***P < 0.001 vs. NP. ###P < 0.001 vs. LP.
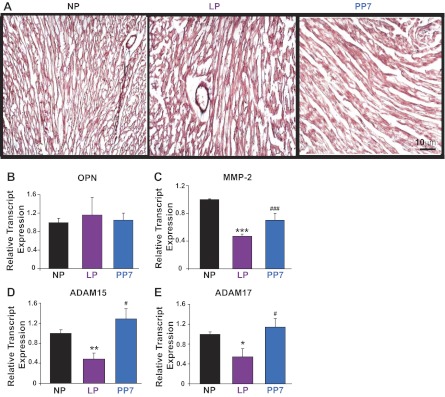
The pregnant heart does not undergo ECM remodeling.
Fibrosis, a well-documented change that normally accompanies cardiac stress, was completely absent in the pregnant heart (Fig. 4A). In addition, transcript levels of OPN, an ECM protein, were not significantly changed in late pregnancy compared with NP controls (1 ± 0.10 in NP, 1.16 ± 0.38 in LP, 1.05 ± 0.15 in PP7, normalized to NP, all P = NS, Fig. 4B). Transcript levels of ECM-degrading gelatinase enzyme MMP-2 were downregulated in late pregnancy and were restored at PP7 (1 ± 0.01 in NP, 0.47 ± 0.03 in LP, 0.70 ± 0.1 in PP7, normalized to NP, all P < 0.001, Fig. 4C). Similarly, transcript levels of disintegrin metalloproteinase ADAM-15 were significantly downregulated in LP hearts (1.00 ± 0.07 in NP, 0.48 ± 0.13 in LP, normalized to NP, P < 0.01, Fig. 4C), as were transcripts of ADAM-17 (1.00 ± 0.05 in NP, 0.54 ± 0.2 in LP, normalized to NP, P < 0.05, Fig. 4D). Their levels were restored 1 wk after delivery (1.45 ± 0.13 for ADAM-15, 1.15 ± 0.03 for ADAM-17, normalized to corresponding NP, all P < 0.05 vs. corresponding LP).
Fig. 4.
The pregnant heart is protected from extracellular matrix remodeling. A: Masson trichrome staining for cardiac fibrosis in NP, LP, and PP7 hearts. B–E: relative transcript levels of cardiac extracellular matrix components osteopontin (OPN), matrix metalloproteinase-2 (MMP-2), a disintegrin and metalloproteinase-15 and -17 (ADAM-15 and ADAM-17) in NP (black bars), LP (purple bars), and PP7 (blue bars) hearts. Values are means ± SE (n = 4 animals/group). *P < 0.05 vs. NP. **P < 0.01 vs. NP. ***P < 0.001 vs. NP. #P < 0.05 vs. LP. ###P < 0.001 vs. LP.
DISCUSSION
As the main findings of this study, we report that late pregnancy-induced physiological heart hypertrophy is associated with 1) transient increase in LV pressure, LV contractility, and decrease in LVEF; 2) increased cardiac angiogenesis; 3) absence of myocardial fibrosis and OPN accumulation in the ECM; and 4) decreased ECM-degrading enzymes such as MMP-2, ADAM-15, and ADAM-17. We observed that these changes were reversed postpartum. Based on these results, we speculate that physiological heart hypertrophy of pregnancy leaves minimal detrimental effects on the heart postpartum and is without cardiac ECM remodeling.
Cardiac hemodynamic changes in the LP heart.
We observed a transient increase in LV pressure and contractility and a decrease in LVEF in the LP hearts. The mild increases in LV pressure and contractility were restored PP7, coinciding with the reversal of cardiac hypertrophy, suggesting that the transient increase in LV pressure and contractility may have resulted as an adaptive response for cardiac hypertrophy. The decrease in LVEF at very late stage of pregnancy is in accordance with our laboratory's previous study (8) and another recently published experimental study (6). Pregnancy is known to be associated with increased volume overload and cardiac hypertrophy that may have led to the transient cardiac dysfunction late in pregnancy. Diastolic dysfunction of the heart generally results from increased myocardial fibrosis (5). We investigated the ECM of the hearts in this study, but did not find any increase in myocardial fibrosis in the LP heart. In addition, we did not find a reexpression of cardiac fetal gene OPN in the myocardium. The fact that the ejection fraction was restored as early as day 1 postpartum suggests that the transient mild decrease in ejection fraction in LP was not due to any major structural changes in the myocardium, as proven by the lack of ECM remodeling in LP hearts.
Increased angiogenesis in the LP heart.
Hypertrophic conditions in the heart lead to increased myocardial angiogenesis (11). A hallmark of the progression from compensated hypertrophy to decompensated heart failure is a decrease in angiogenesis (15). We report significantly increased capillary density and upregulation of VEGF in the LP heart, indicative of increased neoangiogenesis. Physiologically, cardiac angiogenesis is stimulated to meet the demands for increased cardiac output and blood volume during pregnancy. The increase in myocardial blood vessels could be a result of the increased demand for oxygen and nutrients of cardiomyocytes, which follows from the physiological hypertrophic state of the heart. Furthermore, pregnancy is associated with elevated estrogen levels, which could also have a proangiogenic effect (14). Ultimately, we see the capillary density and VEGF transcript levels return to NP levels, but this is coupled with a reversal of cardiac hypertrophy. By contrast, the progression to decompensated heart failure shows decreased angiogenesis, but maintenance of pathological hypertrophy (15). The coordination of these two events is a possible reason the pregnant heart regresses to a healthy state rather than progressing to failure.
Absence of myocardial fibrosis and OPN expression in the ECM in the LP hearts.
Fibrosis normally accompanies the inflammatory response during cardiac stress and inhibits contractile function and electrical signal conduction in the heart (3). However, recent reports indicate that the pregnant heart is protected from cardiac fibrosis (1, 16). We also found no fibrosis in the LP heart. Pedram et al. (20) recently showed that estrogen protects against and reverses cardiac fibrosis through estrogen receptor-β. Again, the high levels of estrogen during pregnancy shed some insight onto one possible mechanism for the protection against fibrosis afforded to the pregnant heart.
Generally cardiac hypertrophy is also associated with a reexpression and accumulation of the fetal gene program, consisting of numerous ECM proteins, such as OPN (13). OPN is expressed in the developing heart, but is mostly absent in a healthy adult heart (27). OPN is known to reactivate in the heart in response to cardiac stress (32, 34). OPN also plays a role in the inflammatory response in the heart, although its exact role in inflammation is still not fully understood (22). We did not find a significant change in the expression of OPN in the LP hearts, indicating a lack of matricellular fetal gene reactivation, a tenet of ECM remodeling.
Decreased expression of ECM-degrading enzymes in the LP hearts.
Among the ECM-associated proteins, MMPs and ADAMs have been well documented for their direct role in causing adverse remodeling associated with heart failure (2–4). Degradation of the cardiac ECM leads to a loss in contractility, a phenomenon known as myocyte slippage (21). Gelatinase MMP-2, in particular, is one of the best characterized metalloproteinases responsible for this progression to heart failure (23). Although there was significant myocardial hypertrophy in the LP heart, surprisingly there was a decrease in the expression of ECM-degrading enzyme MMP-2.
We also explored two members of the ADAM family, ADAM-15 and ADAM-17. The ADAM family, like the related MMP family, is responsible for breaking down ECM-anchoring proteins, but, in addition to the metalloproteinase function, ADAMs also have a disintegrin domain (3). Integrins play a role in ECM interactions between cardiac myocytes and fibroblasts and also provide overall structural integrity and mediate cell-cell communication in the heart (3). Among their many functions, integrins mediate cardio-protective signaling in the failing heart (24–26). ADAM-15 and ADAM-17 are emerging as two of the most important ADAMs present in the heart during LV dysfunction, as not all ADAMs show altered regulation during cardiac stress (10, 18, 30). Again, we observe a decrease in expression of these ADAMs during late pregnancy, which is restored postpartum.
The downregulation of these three matrix-degrading enzymes, which are critical to the progression of heart failure, seems to indicate that the pregnant heart is partially protected from adverse cardiac ECM remodeling. Since estrogen is known to suppress the activity of ECM-degrading metalloproteinases (19), the decreased expression of these enzymes could well be due to the increased levels of estrogen at the LP stage. The hormonal regulation of metalloproteinases sheds some insight onto the possible mechanisms of this protection from adverse remodeling. Experimental volume-overloaded hearts result in elevated metalloproteinase activity, further indicating a fundamental difference between pathological volume overload and pregnancy (17, 28). This decrease in these ECM-degrading enzymes, coupled with an absence of myocardial fibrosis and a lack of OPN reexpression, could play a role in the lack of transition from compensated hypertrophy to heart failure.
Conclusions.
The LP heart shows signs of significant hypertrophy, along with mild hemodynamic dysfunction. During late pregnancy, the heart also shows increased angiogenesis and seems to be protected from adverse cardiac ECM remodeling, including absence of cardiac fibrosis and downregulation of ECM-degrading enzymes. The high levels of estrogen during late pregnancy could play a role in creating this unique molecular signature. The hypertrophy and dysfunction of the pregnant heart is rapidly reversed postpartum, and matrix-degrading enzymes are restored to NP levels.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL089876 and HL089876S1 (M. Eghbali).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.U. and M.E. conception and design of research; S.U., R.D.N., A.I., M.A., and H.M. performed experiments; S.U., R.D.N., A.I., M.A., and H.M. analyzed data; S.U., R.D.N., A.I., M.A., H.M., and M.E. interpreted results of experiments; S.U., R.D.N., and M.E. prepared figures; S.U. and R.D.N. drafted manuscript; S.U., R.D.N., and M.E. edited and revised manuscript; S.U., R.D.N., A.I., M.A., H.M., and M.E. approved final version of manuscript.
REFERENCES
- 1. Aljabri MB, Songstad NT, Lund T, Serrano MC, Andreasen TV, Al-Saad S, Lindal S, Sitras V, Acharya G, Ytrehus K. Pregnancy protects against antiangiogenic and fibrogenic effects of angiotensin II in rat hearts. Acta Physiol (Oxf) 201: 445–456, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest 117: 568–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol 48: 474–482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown L. Cardiac extracellular matrix: a dynamic entity. Am J Physiol Heart Circ Physiol 289: H973–H974, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz 27: 92–98, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol 112: 1564–1575, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clapp JF, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol 80: 1469–1473, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96: 1208–1216, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Eghbali M, Wang Y, Toro L, Stefani E. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med 16: 285–291, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fedak PW, Moravec CS, McCarthy PM, Altamentova SM, Wong AP, Skrtic M, Verma S, Weisel RD, Li RK. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 113: 238–245, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQN, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128: 589–600, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev 72: 369–417, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Hwang DM, Dempsey AA, Lee CY, Liew CC. Identification of differentially expressed genes in cardiac hypertrophy by analysis of expressed sequence tags. Genomics 66: 1–14, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Iyer V, Klebba I, McCready J, Arendt LM, Betancur-Boissel M, Wu MF, Zhang X, Lewis MT, Kuperwasser C. Estrogen promotes ER-negative tumor growth and angiogenesis through mobilization of bone marrow-derived monocytes. Cancer Res 72: 2705–2713, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Katz MG, Fargnoli AS, Pritchette LA, Bridges CR. Gene delivery technologies for cardiac applications. Gene Ther 19: 659–669, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol 300: H931–H942, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res 89: 12–19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li JK, Du WJ, Jiang SL, Tian H. Expression of ADAM-15 in rat myocardial infarction. Int J Exp Pathol 90: 347–354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res 85: 719–728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol 24: 2152–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy HK, Tjahja IE, Campbell SE, Janicki JS, Hayden MR, Tyagi SC. Expression of matrix metalloproteinase activity in idiopathic dilated cardiomyopathy: a marker of cardiac dilatation. Mol Cell Biochem 264: 183–191, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 283: H1802–H1810, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol 47: 211–242, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res 90: 458–464, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Shai SY, Harpf AE, Ross RS. Integrins and the myocardium. Genet Eng (N Y) 24: 87–105, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Suryakumar G, Kasiganesan H, Balasubramanian S, Kuppuswamy D. Lack of beta3 integrin signaling contributes to calpain-mediated myocardial cell loss in pressure-overloaded myocardium. J Cardiovasc Pharmacol 55: 567–573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thayer JM, Giachelli CM, Mirkes PE, Schwartz SM. Expression of osteopontin in the head process late in gastrulation in the rat. J Exp Zool 272: 240–244, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Ulasova E, Gladden JD, Chen Y, Zheng J, Pat B, Bradley W, Powell P, Zmijewski JW, Zelickson BR, Ballinger SW, Darley-Usmar V, Dell'italia LJ. Loss of interstitial collagen causes structural and functional alterations of cardiomyocyte subsarcolemmal mitochondria in acute volume overload. J Mol Cell Cardiol 50: 147–156, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umar S, Lee JH, de Lange E, Iorga A, Partow-Navid R, Bapat A, van der Laarse A, Saggar R, Saggar R, Ypey DL, Karagueuzian HS, Eghbali M. Spontaneous ventricular fibrillation in right ventricular failure secondary to chronic pulmonary hypertension. Circ Arrhythm Electrophysiol 5: 181–190, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, Kassiri Z, Fernandez-Patron C. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension 54: 575–582, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Han DD, Wang HM, Liu M, Zhang XH, Wang HL. Downregulation of osteopontin is associated with fluoxetine amelioration of monocrotaline-induced pulmonary inflammation and vascular remodelling. Clin Exp Pharmacol Physiol 38: 365–372, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Williams EB, Halpert I, Wickline S, Davison G, Parks WC, Rottman JN. Osteopontin expression is increased in the heritable cardiomyopathy of Syrian hamsters. Circulation 92: 705–709, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Wright CS. Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J Mol Biol 178: 91–104, 1984 [DOI] [PubMed] [Google Scholar]
- 34. Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 44: 826–831, 2004 [DOI] [PubMed] [Google Scholar]