Abstract
Recurrent apnea with chronic intermittent hypoxia (CIH) is a major clinical problem in adult humans and infants born preterm. Patients with recurrent apnea exhibit heightened sympathetic activity as well as elevated plasma catecholamine levels, and these phenotypes are effectively recapitulated in rodent models of CIH. This article summarizes findings from studies addressing sympathetic activation in recurrent apnea patients and rodent models of CIH and the underlying cellular and molecular mechanisms. Available evidence suggests that augmented chemoreflex and attenuated baroreflex contribute to sympathetic activation by CIH. Studies on rodents showed that CIH augments the carotid body response to hypoxia and attenuates the carotid baroreceptor response to increased sinus pressures. Processing of afferent information from chemoreceptors at the central nervous system is also facilitated by CIH. Adult and neonatal rats exposed to CIH exhibit augmented catecholamine secretion from the adrenal medulla. Adrenal demedullation prevents the elevation of circulating catecholamines in CIH-exposed rodents. Reactive oxygen species (ROS)-mediated signaling is emerging as the major cellular mechanism triggering sympatho-adrenal activation by CIH. Molecular mechanisms underlying increased ROS generation by CIH seem to involve transcriptional dysregulation of genes encoding pro-and antioxidant enzymes by hypoxia-inducible factor-1 and -2, respectively.
Keywords: carotid chemoreceptor, carotid baroreceptor, sleep apneas, catecholamine secretion, reactive oxygen species, hypoxia-inducible factors
recurrent apnea is one of the most commonly occurring breathing disorders in adult humans and infants born preterm (45, 55). Apnea, i.e., cessation of breathing, can occur because of obstruction of the upper airway [obstructive sleep apnea (OSA)] or as a result of defective respiratory rhythm generation by the central nervous system (CNS; central sleep apnea). Human subjects with recurrent apnea exhibit elevated sympathetic nerve activity and increased circulating catecholamine levels (4, 16, 17, 22, 36, 38, 67). Apneas are associated with periodic decreases in arterial blood oxygen (O2) levels, resulting in chronic intermittent hypoxia (CIH). Studies on rodents demonstrated that exposure to CIH increases sympathetic activity similar to that seen in recurrent apnea patients [see Prabhakar et al. (56)], and they further provided important insights into the underlying mechanisms. In this article, we summarize findings from studies addressing the effects of CIH on sympathetic activity and the underlying cellular and molecular mechanisms.
SYMPATHETIC ACTIVITY IN HUMAN SUBJECTS WITH RECURRENT APNEA
Normal human subjects, during sleep, have low levels of sympathetic activity and reduced heart rate and blood pressures (26, 46, 68), and these sleep-state-dependent phenotypes are absent in OSA patients (43). Furthermore, adult patients with OSA exhibit elevated sympathetic activity during daytime, wherein apneas are absent (4, 22, 36, 67), and treatment with continuous positive airway pressure (CPAP) lowers sympathetic activity in these subjects (4, 28, 67). Circulating and urinary catecholamines (both norepinephrine and epinephrine) are elevated in OSA subjects (4, 16, 17, 38, 67), and CPAP treatment restores them to normal levels (71). However, whether the elevated plasma catecholamine levels arise from their release, either from the vasculature or from the adrenal medulla in response to increased sympathetic activity, has not been established.
SYMPATHETIC ACTIVITY IN RODENT MODELS OF CIH
Several factors besides CIH can potentially contribute to sympathetic activation in OSA subjects, including chronic intermittent hypercapnia, sleep fragmentation, changes in thoracic pressure, and the ensuing hemodynamic changes during obstruction of the upper airway. However, a major advance in the field of apnea research was the demonstration that exposing rodents to CIH alone is sufficient to elicit sympathetic activation (15). Rats exposed to alternating cycles of hypoxia (30 s of hypoxia and 30 s of normoxia), 8 h/day for 30 days, showed elevated cervical sympathetic nerve activity (20). Other investigators reported similar increases in renal (27), splanchnic (13), thoracic (82), and lumbar (37) sympathetic nerve activities in CIH-exposed rats, albeit using different intermittent hypoxia (IH) paradigms and duration of CIH exposures. Dick et al. (13) reported that the increased sympathetic nerve activity by acute IH is reflected in the late expiratory phase of respiration. This finding was subsequently confirmed in juvenile rats exposed to CIH for 10 days (82), suggesting that CIH alters the coupling of sympathetic-respiratory outputs at the CNS. A recent study by Silva and Schreihofer (65) showed that sympathetic activation evoked by stimulation of the sciatic nerve and the nasal mucosa was more pronounced in rats exposed to 2 wk of CIH than in controls, indicating hypersensitivity of the sympathetic nervous system to a variety of stimuli. Taken together, these studies demonstrate that rats exposed to CIH exhibit sympathetic activation similar to those reported in human subjects with recurrent apnea.
Effects of CIH on arterial chemoreflex and carotid chemoreceptor activity.
Arterial chemoreflex is a major regulator of the sympathetic activity. The following lines of evidence suggest that the arterial chemoreflex is augmented in OSA subjects: 1) brief hyperoxic exposure, which inhibits chemoreceptor activity, reduces blood pressure in OSA patients but not in control subjects (44); 2) hypoxic ventilatory response (HVR), a hallmark response of the chemoreflex, is augmented in OSA subjects compared with controls (23); and 3) activation of muscle sympathetic nerve activity by apneas is more pronounced in OSA subjects compared with controls (66).
Arterial chemoreflex is also augmented in experimental animals exposed to CIH, as evidenced by augmented HVR in cats exposed to 4 days of CIH (61) and in mice exposed to CIH for 10 days (54). Rats exposed to CIH exhibit exaggerated sympathetic nerve responses to hypoxia (3, 27, 37, 82), and this effect was abolished after chronic bilateral sectioning of sinus nerves (15, 56, 57).
The effect of CIH on the carotid body, the primary sensory organ, which initiates the chemoreflex, was examined initially in rats and mice. CIH produces two major effects on the carotid body: 1) enhances the response to acute hypoxia and 2) progressively increases baseline sensory activity following repetitive hypoxia, a phenomenon termed as sensory long-term facilitation (sLTF) (48, 49, 51–53). Subsequent studies in cats exposed to several days of CIH also showed a similar enhanced response of the carotid body to hypoxia (61), suggesting no apparent species differences. Characterization of the carotid body responses further revealed that the effects of CIH develop over time without any morphological changes in the glomus tissue and can be reversed by cessation of CIH (51). The effects of CIH were seen in ex vivo carotid body preparations, suggesting that the effects of CIH are due to a direct action on the chemoreceptor tissue per se and not secondary to cardiovascular changes caused by CIH (51).
Recent studies have shown further that CIH-induced hypersensitivity to hypoxia and sLTF of the carotid body involve distinct neurotransmitters/modulators. For instance, endothelin-1 (ET-1), which is up-regulated by CIH in glomus cells via activation of the ET-1A receptor, mediates the augmented hypoxic sensitivity (47, 61) but not the sLTF induced by CIH (unpublished observations). On the other hand, recent studies suggest that 5-hydroxytryptamine (49) and angiotensin II (53) contribute to CIH-induced sLTF. It has been proposed that an exaggerated carotid body response to hypoxia may account for the pronounced sympathetic activation induced by apnea (66), whereas the sLTF may mediate the daytime increase in sympathetic activity reported in OSA subjects.
Effects of CIH on arterial baroreflex and carotid baroreceptor activity.
Arterial baroreflex exerts tonic inhibitory influence on sympathetic activity. Lai et al. (35) were the first to report that CIH attenuates baroreflex function in conscious rats, as evidenced by spectral analysis of heart-rate responses. Recently, Peng et al. (50) examined the effects of CIH on arterial baroreflex function and carotid baroreceptor activity in adult rats. They found that CIH decreases baroreflex control of sympathetic nerve activity, as evidenced by reduced inhibition of splanchnic nerve responses to phenylephrine (PE). These investigators reported further that CIH markedly attenuates carotid baroreceptor activity in response to increased carotid sinus pressure. The attenuated carotid baroreceptor activity by CIH was attributed to upregulation of ET-1 in endothelial cells of the carotid sinus region and activation of ETA receptors (50). However, Gu et al. (21) reported no significant alteration in aortic baroreceptor activity in Fischer 344 rats exposed to CIH.
In contrast to the effects of CIH on baroreflex regulation of splanchnic nerve activity, cervical sympathetic nerve response to PE was unaffected in adult rats exposed to 35 days of CIH (20). Likewise, Machado and his coworkers (80), with the use of a working-heart brain stem preparation, reported an increase in baroreflex function in CIH-exposed juvenile rats. These findings suggest that the effects of CIH on baroreflex regulation of sympathetic activity differ between splanchnic and cervical sympathetic nerves as well as anesthetized vs. decerebrate working-heart brain stem preparations.
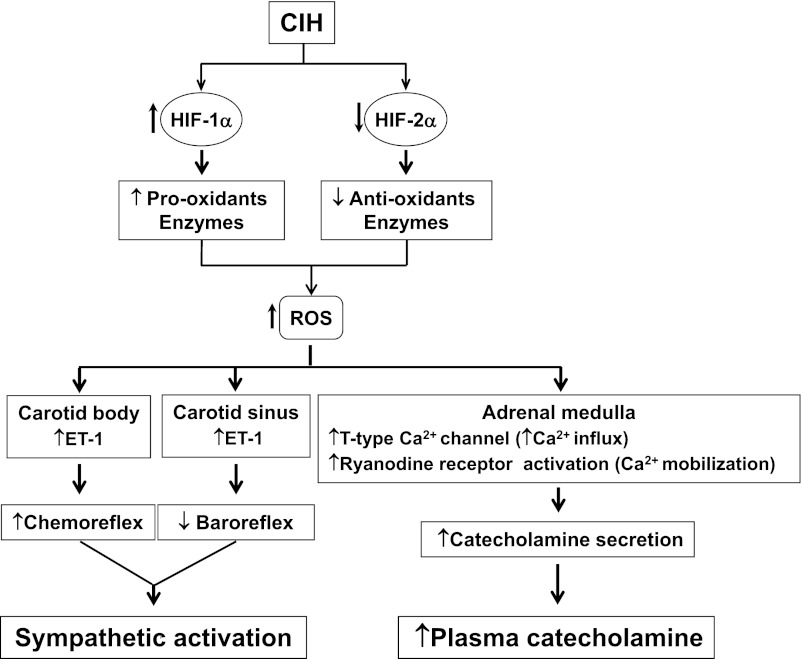
Unlike the studies with rodent models of CIH, patients with OSA exhibit downregulation of baroreflex function, as evidenced by attenuated heart rate and vascular responses to baroreceptor activation (2, 10, 39), and CPAP treatment restores baroreflex function (2). Thus it seems reasonable to conclude that CIH attenuates arterial baroreflex function, which is, in part, due to reduced carotid baroreceptor activity. It is interesting to note that upregulation of ET-1 by CIH on one hand depresses baroreceptor responses to increased sinus pressures, whereas on the other hand, it mediates the hypersensitivity of the carotid body to hypoxia. The strikingly opposite effects of CIH on the chemo- and baroreflex functions and their consequences on the sympathetic activity are shown in Fig. 1.
Fig. 1.
Schematic presentation of molecular and cellular mechanisms associated with sympatho-adrenal activation by chronic intermittent hypoxia (CIH). HIF-1α and HIF-2α, hypoxia-inducible factor-1α and -2α, respectively; ROS, reactive oxygen species; ET-1, endothelin-1; Ca2+, calcium ion.
Effects of CIH on the CNS-regulating sympathetic activity.
Processing of sensory information from the arterial chemo- and baroreceptors at the CNS is critical for the translation of these inputs to appropriate reflex changes in the sympathetic motor output. The dorsal and medial subnuclei of the nucleus tractus solitarius (NTS), including the commissural part of the NTS (cNTS), receive inputs from the carotid sinus nerve (6, 79). The effects of CIH on neuronal activation in the brain stem regions associated with the sympathetic regulation were assessed by monitoring the protein expressions of c-fos and fosB. Rats exposed to short-term CIH (for 7 days) exhibited increases in FosB/ΔFosB expression in NTS and rostral ventrolateral medulla (RVLM) (32). On the other hand, c-fos expression was increased in the dorsal and medial subnuclei of the NTS following exposure to 30 days of CIH (64). These findings indicate that both short- and long-term exposures to CIH result in the activation of brain stem neurons associated with the regulation of spinal sympathetic preganglionic neuronal activity.
Neuronal activity in cNTS is regulated by various neurotransmitters, including glutamate (Glu), an excitatory amino acid transmitter, and dopamine (DA), an inhibitory biogenic amine. CIH upregulates N-methyl-d-aspartate receptor 1 (NMDA-R1) expression in the dorsocaudal brain stem (60), Glu receptor types 2/3 subunit expression in cNTS (11), and AMPA- and NMDA-mediated currents in NTS neurons (12). On the other hand, CIH downregulates tyrosine hydroxylase expression in dorsal medulla, the rate-limiting enzyme in DA synthesis (18). Since DA inhibits synaptic transmission at NTS (31), it is likely that CIH, by downregulating the synthesis of DA, enhances glutamatergic excitatory transmission in NTS (5). Studies by Kline et al. (30) showed that CIH not only increases postsynaptic cell activity in NTS but also attenuates synaptic transmission between sensory afferents and NTS second-order neurons. This effect seems to occur via reduced transmitter release involving calcium/calmodulin-dependent kinase II activation (30).
Neurons in NTS relay chemoafferent information to the hypothalamic paraventricular nucleus (PVN) and brain stem sympathoexcitatory sites located in the RVLM (24, 62). An earlier study by Greenberg et al. (20) showed that CIH alters neuronal activity of the ventrolateral medulla (A1 noradrenergic cells). Zoccal et al. (81) reported that CIH-induced sympathetic activation is mediated by enhanced purinergic but not glutamatergic transmission in RVLM of juvenile rats. On the other hand, Silva and Schreihofer (65) found that glutamatergic transmission in RVLM is critical for the augmented sympathetic activation by CIH in adult rats. These findings implicate that age is an important variable that determines the type of neurotransmitter(s) mediating the effects of CIH on sympathetic activation by RVLM. A recent study by Coleman et al. (8) reported that PVN neurons in CIH-exposed mice exhibit decreased NMDA-R-mediated currents, reduced NO production by NMDA, and downregulation of NMDA-NR1 receptors in neuronal nitric oxide synthase-positive neurons. Collectively, these studies indicate that the exaggerated chemoreflex-mediated sympathetic activation by CIH involves reconfiguration of neurotransmitter profiles in the CNS.
EFFECTS OF CIH ON THE ADRENAL MEDULLA
The adrenal medulla is one of the major sources of circulating catecholamines. Bao et al. (1) reported that adrenal demedullation prevents a CIH-induced increase in blood pressure and circulating catecholamine levels. Recent studies examined the effects of CIH on catecholamine secretion from the adrenal medulla in adult (33) and neonatal rats (69). Hypoxia-evoked catecholamine secretion from the adrenal medulla was markedly potentiated in adult and neonatal rats by CIH. Studies on mouse adrenal medullary chromaffin cells (AMC) showed that CIH increases the readily releasable pool of secretory vesicles via activation of PKC (34). Further analysis on neonatal rat AMC showed that the augmented catecholamine secretion by hypoxia by CIH involves facilitation of calcium ion (Ca2+) influx via low-threshold T-type Ca2+ channels, as well as mobilization of intracellular Ca2+ stores by ryanodine receptor (RyR) activation (70). These studies suggest that the adrenal medulla contributes to increased plasma catecholamines by CIH.
REACTIVE OXYGEN SPECIES—A MAJOR CELLULAR MECHANISM MEDIATING THE EFFECTS OF CIH
Studies on experimental models of CIH.
Reactive oxygen species (ROS) signaling is an important cellular mechanism mediating the systemic effects of CIH (55a). CIH increases ROS generation in the carotid body (51), adrenal medulla (33), brain stem (59, 63), and the carotid sinus region (50). Systemic administration of ROS scavengers—e.g., manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride, an O2˙− scavenger, and N-acetyl-cysteine, a precursor of glutathione—prevented CIH-induced: 1) hypoxic sensitivity and sLTF of the carotid body, as well as the augmented chemoreflex (51, 52), 2) decreases in carotid baroreceptor activity and baroreflex responses (50), and 3) increases in catecholamine secretion from the adrenal medulla (33, 69), as well as plasma catecholamine levels (33).
Studies on cell cultures and rodent models have identified two major sources of ROS generation by CIH: 1) NADPH oxidases (Nox), especially the Nox2 isoform (29, 47, 49, 70, 77, 78), and 2) inhibition of the electron transport chain at the mitochondrial complex I (29, 51, 75). A study by Khan et al. (29) showed that ROS generated by Nox2 inhibit the mitochondrial complex I via increased S-glutathionylation of the complex I subunits, resulting in increased mitochondrial ROS generation (i.e., ROS-induced ROS). In addition, CIH downregulated antioxidant enzymes in the carotid body and adrenal medulla (41). These studies demonstrate that both upregulation of pro-oxidant enzymes and downregulation of antioxidant enzymes contribute to increased ROS generation by CIH.
Cellular targets of CIH-induced ROS.
In the carotid body, ROS mediate the CIH-induced, enhanced hypoxic sensitivity by transcriptional upregulation of ET-1 (47), whereas in the carotid sinus region, ROS activate an ET-converting enzyme, resulting in increased ET-1 levels (50). In the adrenal medulla, ROS lead to transcriptional upregulation of T-type Ca2+ channels and facilitate Ca2+ influx as well as mobilize Ca2+ stores by activating RyRs via S-glutathionylation (70). These observations suggest that ROS mediate the effects of CIH via affecting multiple cellular targets.
Evidence for increased ROS in recurrent apnea subjects.
Recent clinical studies documented elevated levels of biomarkers of oxidative stress in recurrent apnea patients (7, 72). Dyugovskaya et al. (14) report increased ROS generation in CD11C-positive monocytes derived from OSA patients and implicated ROS in the upregulation of adhesion molecules (CD15 and CD11C) and increased adhesion to endothelial cells. These effects are reversed after nasal CPAP treatment. A recent study suggests that antioxidant therapy might be beneficial in restoring vascular function in recurrent apnea subjects. Grebe et al. (19) reported that patients with OSA exhibit reduced vasodilator response, and this effect was prevented by systemic administration of vitamin C, an antioxidant. However, another study reports a lack of oxidative stress in patients with recurrent apneas (40), which could be attributed, in part, to methodological difficulties in assessing ROS levels and gender differences. Nonetheless, the above studies suggest that increased generation of ROS and the resulting oxidative stress is one of the major cellular mechanism(s) mediating the systemic responses to CIH, including sympatho-adrenal activation.
MOLECULAR MECHANISMS CONTRIBUTING TO INCREASED ROS GENERATION BY CIH
The effects of CIH on sympathetic activation, the adrenal medulla, and chemo- and baroreflexes develop over time. The time-dependent effects of a given stimulus are generally attributed to activation of transcriptional mechanisms and the resulting changes in gene expression. Several studies showed that CIH affects a variety of transcription factors, including the hypoxia-inducible factors 1 and 2 (HIF-1 and HIF-2), activator protein 1, NF of activated T cells, and NF-κB [for a review, see Nanduri et al. (42)]. Recent studies suggest that transcriptional activators HIF-1 and HIF-2 are the major molecular mechanisms underlying increased ROS generation by CIH.
HIF-1 is the prototypical member of the HIF family of transcriptional activators and comprises an O2-regulated α-subunit and a constitutive β-subunit (74). Hypoxia increases HIF-1 transcriptional activity as a consequence of increased accumulation of HIF-1α protein via decreased O2-dependent proline hydroxylation, ubiquitination, and proteasomal degradation (9). HIF-2α (also known as endothelial Per-ARNT-Sim domain protein-1) is another member of the HIF family, which shares ∼60% sequence homology to HIF-1α and also interacts with HIF-1β (73).
Continuous hypoxia leads to accumulation of both HIF-1α and HIF-2α (25). In striking contrast, CIH increases HIF-1α and decreases HIF-2α protein in cell cultures as well as in rodents (41). These observations demonstrate strikingly opposite effects of continuous hypoxia and IH on HIF-1 and HIF-2 expression. Yuan et al. (77) reported that a CIH-evoked increase in HIF-1α protein is mediated by a dual mechanism involving activation of mammalian target of rapamycin and S6 kinase pathway and decreased hydroxylation of HIF-1α protein by activation of Ca2+ signaling by ROS. On other hand, downregulation of HIF-2α by CIH requires activation of Ca2+-dependent proteases, calpains (41).
Recent studies examined the functional consequences of CIH-induced changes in HIF-1 and HIF-2α. Yuan et al. (76) demonstrated that HIF-1 mediates the transcriptional upregulation of Nox2 by CIH. Hif1α+/− heterozygous mice, exposed to CIH, exhibit a striking absence of 1) elevated plasma catecholamines (an index of sympathetic activation), 2) an augmented chemoreflex, 3) increased ROS generation, and 4) Nox2 upregulation (54, 76). CIH-evoked downregulation of SOD-2 was prevented by overexpressing the transcriptionally active but not the inactive HIF-2α (41). In intact rats, systemic administration of N-acetyl-Leu-Leu-methional, a potent inhibitor of calpains, rescues CIH-induced degradation of HIF-2α in the carotid body and adrenal medulla, restores SOD-2 activity, and prevents the increased ROS levels (41). These observations suggest that increased degradation of HIF-2α contributes to a CIH-induced increase in ROS via insufficient transcription of antioxidative enzymes, such as SOD-2. Thus the imbalance between HIF-1 and HIF-2 transcriptional activators contributes to a CIH-induced increase in ROS generation via transcriptional regulation of pro- and antioxidant enzymes, and the resulting oxidative stress mediates the effects of CIH on sympatho-adrenal activation, as illustrated in Fig. 1.
SUMMARY AND PERSPECTIVE
Although clinical studies have shown that recurrent apnea patients exhibit sympathetic activation, the underlying mechanisms are not known. In recent years, studies using experimental models of IH, the hallmark of apnea, provided important insights into the mechanisms associated with sympatho-adrenal activation by chronic IH. There is a general agreement that augmented arterial chemoreflex contributes to the sympathetic activation by CIH. The CIH-induced chemoreflex activation is, in part, due to remodeling of the carotid body chemoreceptor function. In addition to the chemoreflex, evidence is emerging that CIH leads to attenuated baroreflex function, which is, in part, due to reduced carotid baroreceptor activity. Thus an imbalance between the chemo- and the baroreflex seems to be a major contributor to sympathetic activation by CIH. Evidence is emerging that CIH leads to reconfiguration of the central neuronal networks associated with sympathetic activation. A major advance in the field of apnea research is the identification of ROS as a major cellular mechanism mediating the adverse consequences of CIH on sympathetic function. However, the mechanism(s) underlying ROS generation by CIH, especially in the CNS, need further studies. Studies on cell cultures and rodents led to the identification of HIFs as one of the major molecular mechanisms contributing to sympatho-adrenal activation by CIH. Further studies are needed for identifying the role(s) of other transcriptional activators, interactions between the transcriptional activators, and identification of downstream target genes associated with autonomic dysfunction caused by CIH.
GRANTS
Support for this research was provided by Grants HL-76537, HL-90554, and HL-86493 from the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y-J.P. prepared figures; G.K.K. edited and revised manuscript; N.R.P. approved final version of manuscript.
REFERENCES
- 1. Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83: 95–101, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Bonsignore MR, Parati G, Insalaco G, Marrone O, Castiglioni P, Romano S, Di Rienzo M, Mancia G, Bonsignore G. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med 166: 279–286, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Braga VA, Soriano RN, Machado BH. Sympathoexcitatory response to peripheral chemoreflex activation is enhanced in juvenile rats exposed to chronic intermittent hypoxia. Exp Physiol 91: 1025–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Carlson JT, Hedner JA, Elam M, Ejnell H, Sellgren J, Wallin G. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103: 1763–1768, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Kombian SB, Zidichouski JA, Pittman QJ. Dopamine depresses glutamatergic synaptic transmission in the rat parabrachial nucleus in vitro. Neuroscience 90: 457–468, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol Regul Integr Comp Physiol 268: R851–R858, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath 7: 105–110, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 30: 12103–12112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coleman ML, Ratcliffe PJ. Oxygen sensing and hypoxia-induced responses. Essays Biochem 43: 1–15, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Cooper VL, Elliott MW, Pearson SB, Taylor CM, Mohammed MM, Hainsworth R. Daytime variability of baroreflex function in patients with obstructive sleep apnoea: implications for hypertension. Exp Physiol 92: 391–398, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Costa-Silva JH, Zoccal DB, Machado BH. Chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol 302: R785–R793, 2012 [DOI] [PubMed] [Google Scholar]
- 12. de Paula PM, Tolstykh G, Mifflin S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292: R2259–R2265, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Dick E, Hsieh YH, Wang N, Prabhakar NR. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 165: 934–939, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol 90: 1600–1605, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep 10: 35–44, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Rio F, Racionero MA, Pino JM, Martinez I, Ortuno F, Villasante C, Villamor J. Sleep apnea and hypertension. Chest 117: 1417–1425, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Gozal E, Shah ZA, Pequignot JM, Pequignot J, Sachleben LR, Czyzyk-Krzeska MF, Li RC, Guo SZ, Gozal D. Tyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol 99: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med 173: 897–901, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol 86: 298–305, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster RD, Chapleau MW, Ma X, Cheng ZJ. Selective impairment of central mediation of baroreflex in anesthetized young-adult Fischer 344 rats following chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 293: H2809–H2818, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hedner JA, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl 6: 5529–5531, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE. A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis 146: 1240–1245, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20: 78–84, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Påhlman S. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10: 413–423, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG. Sympathetic muscle nerve activity during sleep in man. Brain 114: 1281–1295, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y, Weiss JW. Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol 166: 102–106, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 131: 1406–1413, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14: 533–542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 88: 2736–2744, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuri BA, Khan SA, Chan SA, Prabhakar NR, Smith CB. Increased secretory capacity of mouse adrenal chromaffin cells by chronic intermittent hypoxia: involvement of protein kinase C. J Physiol 584: 313–319, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol 100: 1974–1982, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79: 581–588, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 171: 36–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest 103: 722–727, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol 574: 605–613, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Montgomery-Downs HE, Krishna J, Roberts LJ, 2nd, Gozal D. Urinary F2-isoprostane metabolite levels in children with sleep-disordered breathing. Sleep Breath 10: 211–215, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA 106: 1199–1204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol 164: 277–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens 15: 1613–1619, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 97: 943–945, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283: 1829–1836, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Okada H, Iwase S, Mano T, Sugiyama Y, Watanabe T. Changes in muscle sympathetic nerve activity during sleep in humans. Neurology 41: 1961–1966, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 296: R735–R742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol 104: 1287–1294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29: 4903–4910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, Kumar GK, Prabhakar NR. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol 112: 187–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96: 1236–1242, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Peng YJ, Raghuraman G, Khan SA, Kumar GK, Prabhakar NR. Angiotensin-II evokes sensory long-term facilitation of the carotid body via NADPH oxidase. J Appl Physiol 111: 964–970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate 65: 211–219, 1994 [DOI] [PubMed] [Google Scholar]
- 55a. Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol 90: 1986–1994, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol 92: 39–44, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol 32: 447–449, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Raghuraman G, Rai V, Peng YJ, Prabhakar NR, Kumar GK. Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases. Antioxid Redox Signal 11: 1777–1789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Reeves SR, Gozal E, Guo SZ, Sachleben LR, Jr, Brittian KR, Lipton AJ, Gozal D. Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J Appl Physiol 95: 1767–1774, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol 560: 577–586, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovács KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res 107: 201–222, 1996 [DOI] [PubMed] [Google Scholar]
- 63. Sharma SD, Raghuraman G, Lee MS, Prabhakar NR, Kumar GK. Intermittent hypoxia activates peptidylglycine α-amidating monooxygenase in rat brain stem via reactive oxygen species-mediated proteolytic processing. J Appl Physiol 106: 12–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol 121: 173–184, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 589: 1463–1476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst 56: 184–190, 1996 [DOI] [PubMed] [Google Scholar]
- 67. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307, 1993 [DOI] [PubMed] [Google Scholar]
- 69. Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol 101: 2837–2846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia treated neonatal rat chromaffin cells. J Neurosci 30: 10763–10772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Suzuki M, Otsuka K, Guilleminault C. Long-term nasal continuous positive airway pressure administration can normalize hypertension in obstructive sleep apnea patients. Sleep 16: 545–549, 1993 [DOI] [PubMed] [Google Scholar]
- 72. Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med 40: 1683–1692, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11: 72–82, 1997 [DOI] [PubMed] [Google Scholar]
- 74. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol 557: 773–783, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226: 2925–2933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 172: 921–929, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang W, Mifflin SW. Excitatory amino acid receptors within NTS mediate arterial chemoreceptor reflexes in rats. Am J Physiol Heart Circ Physiol 265: H770–H773, 1993 [DOI] [PubMed] [Google Scholar]
- 80. Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol 94: 972–983, 2009 [DOI] [PubMed] [Google Scholar]
- 81. Zoccal DB, Huidobro-Toro JP, Machado BH. Chronic intermittent hypoxia augments sympatho-excitatory response to ATP but not to l-glutamate in the RVLM of rats. Auton Neurosci 165: 156–162, 2011 [DOI] [PubMed] [Google Scholar]
- 82. Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586: 3253–3265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]