Abstract
Acute intermittent hypoxia (AIH) elicits a form of spinal respiratory plasticity known as phrenic long-term facilitation (pLTF). pLTF requires spinal serotonin receptor-2 activation, the synthesis of new brain-derived neurotrophic factor (BDNF), and the activation of its high-affinity receptor tyrosine kinase, TrkB. Spinal adenosine 2A receptor activation elicits a distinct pathway to phrenic motor facilitation (pMF); this BDNF synthesis-independent pathway instead requires new synthesis of an immature TrkB isoform. Since hypoxia increases extracellular adenosine levels, we tested the hypothesis that new synthesis of TrkB and BDNF contribute to AIH-induced pLTF. Furthermore, given that signaling mechanisms “downstream” from TrkB are unknown in either mechanism, we tested the hypothesis that pLTF requires MEK/ERK and/or phosphatidylinositol 3-kinase (PI3K)/Akt activation. In anesthetized Sprague-Dawley rats, an intrathecal catheter at cervical level 4 was used to deliver drugs near the phrenic motor nucleus. Since pLTF was blocked by spinal injections of small interfering RNAs targeting BDNF mRNA but not TrkB mRNA, only new BDNF synthesis is required for AIH-induced pLTF. Pretreatment with a MEK inhibitor (U0126) blocked pLTF, whereas a PI3K inhibitor (PI-828) had no effect. Thus, AIH-induced pLTF requires MEK/ERK (not PI3K/AKT) signaling pathways. When U0126 was injected post-AIH, pLTF development was halted but not reversed, suggesting that ERK is critical for the development but not maintenance of pLTF. Thus, there are clear mechanistic distinctions between AIH-induced pLTF (i.e., BDNF synthesis and MEK/ERK dependent) versus adenosine 2A receptor-induced pMF (i.e., TrkB synthesis and PI3K/Akt dependent).
Keywords: respiratory plasticity, brain-derived neurotrophic factor, TrkB, extracellular signal-regulated kinase, respiratory control
the neural system controlling breathing exhibits considerable plasticity in response to environmental and physiological perturbations (13). One frequently studied model of respiratory plasticity is observed after exposure to acute intermittent hypoxia (AIH). AIH elicits long-lasting facilitation of phrenic motor output, an effect known as phrenic long-term facilitation (pLTF) (13). A hallmark of pLTF is that it requires episodic versus continuous hypoxia (1, 39). In our working model of pLTF, AIH repeatedly activates medullary raphe serotonergic neurons (42), releasing serotonin (5-HT) in the phrenic motor nucleus (23), where it activates 5-HT2 receptors on or near phrenic motor neurons (23). Indeed, spinal 5-HT receptor activation is both necessary (4) and sufficient (30) for pLTF. Episodic 5-HT receptor activation initiates new synthesis of brain-derived neurotrophic factor (BDNF), subsequently activating its high-affinity receptor, TrkB (2). The signaling pathways downstream from TrkB are unknown.
A distinct mechanism of long-lasting phrenic motor facilitation (pMF) results from the activation of spinal metabotropic receptors coupled to Gs proteins, such as the adenosine 2A (A2A) receptor (26) or 5-HT7 receptors (29). A2A receptor agonist-induced pMF is independent of 5-HT receptor activation and new BDNF synthesis (16); instead, it requires new synthesis of an immature TrkB isoform believed to auto-activate and signal from within phrenic motor neurons (16, 20, 28). Because adenosine released into the extracellular space during hypoxia (18) may activate A2A receptors, we wondered whether immature TrkB signaling contributes to AIH-induced pLTF.
Once activated, TrkB signals via multiple cascades, including Ras/MAPK, phosphatidylinositol 3-kinase (PI3K), and phospholipase C-γ (49). In cultured spinal motor neurons, TrkB receptors signal predominantly via ERK and/or PI3K/PKB [phospho-Akt (25)]. ERK and PI3K/Akt signaling have been implicated in respiratory neural plasticity. For example, AIH increases phosphorylation and activation of ERK in the ventral cervical spinal cord encompassing the phrenic motor nucleus (54). Furthermore, repetitive AIH exposure upregulates ERK and phospho-ERK protein levels within presumptive phrenic motor neurons (47). On the other hand, spinal A2A receptor activation increases Akt phosphorylation in the ventral cervical spinal cord (16), an effect attributed to increased immature TrkB signaling (16, 53). Thus, we wondered whether Akt and/or ERK signaling contribute to AIH-induced pLTF.
Here, we tested the hypotheses that new synthesis of immature TrkB and TrkB signaling via both ERK and Akt contribute to AIH-induced pLTF. Whereas we confirmed that new BDNF synthesis is necessary for AIH-induced pLTF, no evidence was found that new TrkB synthesis plays a role. Furthermore, whereas inhibition of cervical spinal ERK activation blocked AIH-induced pLTF, inhibition of Akt activation had no effect. Finally, whereas ERK activation was necessary for pLTF initiation and development, continued ERK activation was not necessary for pLTF maintenance.
METHODS
Animals.
All experiments were performed on adult (3–6 mo, n = 110) male Sprague-Dawley rats (280–450 g, colonies 217 and 218a, Harlan, Indianapolis, IN). In the first experimental series, small interfering (si)RNAs were spinally injected targeting BDNF (siBDNF) or TrkB (siTrkB) mRNA to prevent the translation and new synthesis of BDNF or TrkB protein, respectively; these injections enabled us to test the hypothesis that AIH-induced pLTF requires new TrkB synthesis in anesthetized rats. In the second series of experiments, spinal injections of selective inhibitors for MEK/ERK (U0126, IC50: ∼0.065 μM) (10) and PI3K/Akt (PI-828, IC50: ∼0.098 μM) (15) were used to test their involvement in AIH-induced pLTF. Since pretreatment with the MEK/ERK inhibitor U0126 but not the PI3K/Akt inhibitor PI-828 blocked pLTF, we tested whether continued MEK/ERK activation is necessary for pLTF maintenance by spinally injecting U0126 after the final hypoxic episode in a third series of experiments.
All procedures were approved by the Institutional Animal Care and Use Committee of the School of Veterinary Medicine of theUniversity of Wisconsin (Madison, WI) and are in compliance with the policies and regulations outlined by the American Physiological Society.
Surgical preparation.
Anesthesia was induced with isoflurane (2.5–3.5% in 50% O2-balance N2), and isoflurane was continued during surgical preparations. Once surgical procedures were completed, rats were converted to urethane anesthesia by slow intravenous injections over 15 min (1.8 μg/kg). Adequate anesthetic depth was tested by lack of blood pressure (pressor) or respiratory neural responses to toe pinch with a hemostat. After conversion to urethane anesthesia, a continuous infusion (4–6.5 ml·kg−1·h−1) of 6% Hetastarch (artificial colloid composed and dissolved in 0.9% normal saline) and lactated Ringer solution (1:4 mixture, respectively) was implemented to maintain appropriate blood volume, fluid balance, and acid-base status. A tracheal cannula was placed in the neck to enable artificial ventilation (Rodent Respirator, model 683, Harvard Apparatus, Holliston, MA, tidal volume: 2.5 ml, variable frequency). A rapidly responding flow-through CO2 analyzer (Capnogard, Novametrix, Wallingford, CT) was placed on the expired limb of a Y-tube connected to the tracheal cannula to enable measurements of end-tidal Pco2 (PetCO2). The vagus nerves were cut in the midcervical region to prevent entrainment of respiratory neural activity with the ventilator. During ventilation, rats were paralyzed with pancuronium bromide (2.5 mg/kg). A polyethylene (PE) catheter (PE-50, Intramedic) was placed in the right femoral artery, and blood pressure was monitored with a pressure transducer (P23ID, Gould). A three-way stop-cock, attached to the arterial catheter, was used to withdraw blood samples (0.2–0.4 ml) for blood gas analysis (ABL-500, Radiometer, Copenhagen, Denmark); during an experiment, blood gas determinations were made during baseline conditions, during the first hypoxic episode, and at 15, 30, and 60 min post-AIH (with an additional 90 min in the third experimental series). Body temperature was monitored with a rectal thermometer (Fischer Scientific) and maintained (37.5 ± 1°C) with a heated surgical table.
Peak integrated phrenic burst amplitude strongly correlates with tidal volume and respiratory muscle activity in spontaneously breathing animals (11). Therefore, we used this index to quantify changes in respiratory neural output. The left phrenic nerve was isolated using a dorsal approach, cut distally, desheathed, and placed on bipolar silver electrodes to record respiratory neural activity. Phrenic nerve signals were amplified (100,000×), bandpass filtered (300–10,000 Hz, model 1800, A-M Systems, Carlsborg, WA), and rectified and integrated (Paynter filter, time constant: 50 ms, CWE, MA-821, Ardmore, PA). The resulting integrated nerve bursts were digitized (8,000 Hz) and analyzed using a WINDAQ data-acquisition system (DATAQ Instruments, Akron, OH). After completion of the surgical preparation and conversion to urethane anesthesia, rats were allowed a minimum of 1 h to stabilize before an experimental protocol was begun.
An intrathecal catheter was placed in the cervical region to enable localized siRNA and drug delivery. The spinal column was exposed dorsally followed by a laminectomy at cervical level 2, where a small incision was made in the dura. A soft silicone catheter (2-Fr, Access Technologies) was inserted caudally through the incision until the tip was located at approximately cervical level 4 (C4). The catheter was attached to a 50-μl Hamilton syringe filled with drug solutions (see treatment groups below) to allow localized drug injections into the cervical spinal region.
Experimental protocol.
At least 1 h after the conversion to urethane anesthesia, apneic and recruitment thresholds were determined by increasing ventilation and lowering PetCO2 until rhythmic nerve bursts could no longer be detected (apneic threshold). After 1 min, the ventilator rate was slowly decreased and/or inspired CO2 was slowly increased until rhythmic nerve bursts resumed (i.e., recruitment threshold). Baseline conditions were then established by holding PetCO2 ∼ 2 mmHg above the recruitment threshold until neural activity had stabilized (≥15 min). An arterial blood sample was then taken to document baseline blood gas levels. Arterial Pco2 (PaCO2) was maintained isocapnic (±1.5 mmHg) with respect to baseline by manipulating inspired CO2 and/or the ventilation rate. Subsets of rats received intrathecal vehicle (siRNA universal buffer or saline-10% DMSO) or drug injections (see treatment groups below); injections were made slowly over 1 min before the first hypoxic episode (or equivalent time for control groups) and shortly after the final hypoxic episode in AIH-treated rats.
AIH consisted of three 5-min episodes of isocapnic (±1.5 mmHg) hypoxia [10% inspired O2, arterial Po2 (PaO2): 35–45 mmHg] separated by 5-min intervals of baseline O2 conditions (50% inspired O2, PaO2: ≥150 mmHg). After the third hypoxic episode, rats were returned to baseline inspired O2 levels and maintained for the duration of an experiment. To test the hypothesis that new spinal TrkB and/or BDNF synthesis are required for AIH-induced pLTF, siRNAs targeting BDNF and TrkB mRNA were injected via an intrathecal catheter over the cervical spinal cord (∼C4) to inhibit new BDNF and TrkB protein synthesis in the vicinity of the phrenic motor nucleus.
Rats receiving siRNAs were surgically prepared as described above. A pool of siRNAs targeting TrkB mRNA was used to determine the role of newly synthesized TrkB protein in AIH-induced pLTF. TrkB siRNAs were obtained as a pool of four 21-nucleotide duplexes (ON-TARGET plus, Dharmacon, Lafayette, CO gene, NTRK2, GenBank Accession No. NM 012731); this same pool has been shown to effectively block new TrkB synthesis in an earlier study (16) from our laboratory using the same experimental preparation. We also used a pool of siRNAs targeting BDNF to verify that AIH-induced pLTF required new BDNF synthesis and to provide an internal positive control for siRNAs; these same BDNF siRNA sequences have previously been shown to prevent new synthesis of spinal BDNF in the same experimental preparation (2). siRNAs were reconstituted with siRNA universal buffer (Dharmacon) and stored at −20°C. Stock TrkB siRNAs (4 μl of 5 μM solution) were combined with the transfection reagent, oligofectamine (16 μl, Invitrogen, Carlsbad, CA), and RNase-free water (180 μl, final concentration: 100 nM) and incubated at room temperature for 20 min. Stock BDNF siRNAs (1.25 μM) were also diluted in oligofectamine and RNase-free before injection (final concentration: 100 nM). siRNAs were slowly injected over the C4 spinal segment via an intrathecal catheter (2- and 10-μl injections separated by 10 min) 2 h before baseline measurements and AIH exposures were performed.
In the second and third experimental series, an intrathecal catheter was used to pretreat rats 20 min before AIH exposures with a PI3K inhibitor (PI-828, 100 μM, 12 μl, Tocris Bioscience) or a MEK inhibitor (U0126, 100 μM, 12 μl, Tocris Bioscience). In the final experimental series, rats received an intrathecal injection of U0126 (100 μM, 12 μl) immediately (<5 min) after the final hypoxic episode.
Data analysis.
Nerve recordings were analyzed using custom software (LabView 6.1, National Instruments, Austin, TX, courtesy of Dr. S. Mahamed). Statistical comparisons between treatment groups for mean arterial pressures (MAPs), PaCO2, and PaO2 were made using two-way ANOVA with a repeated-measures design. Integrated phrenic nerve burst amplitude and burst frequency were averaged over 1-min bins at each experimental time point (baseline and 15, 30, 60, and 90 min). Changes in nerve burst amplitude were normalized as a percentage of baseline. Nerve burst frequency was expressed as an absolute change from baseline (in bursts/min). Comparisons for nerve activity (amplitude and frequency) were made using two-way ANOVA with a repeated-measures design. Since no differences were detected between hypoxic exposures within groups (data not shown), comparisons were made using two-way ANOVA of phrenic burst amplitude during the fifth minute of hypoxic episodes averaged from all three episodes. All individual comparisons were made using the Student-Neuman-Keuls post hoc test (SigmaStat version 2.03, Jandel Scientific, St. Louis, MO). Differences between groups were considered significant if P < 0.05. All values are expressed as means ± SE.
RESULTS
Blood gases and MAPs.
Measurements of PaCO2 and PaO2 during baseline, hypoxia, and 60 and 90 min post-AIH were similar in all experimental groups. Since arterial CO2 was actively maintained within 1.5 mmHg of baseline, isocapnia was observed at post-AIH time points (see Table 1). During hypoxic episodes, PaO2 decreased (∼40 mmHg) but returned to baseline levels (PaO2: ≥150 mmHg) for the duration of the experiments. MAP (in mmHg) was similar between treatment groups under baseline conditions (Table 1). During hypoxia, MAP decreased, as is usually observed in anesthetized rats; this decrease was similar between treatment groups, and MAP returned to near-baseline levels by the completion of an experiment (Table 1).
Table 1.
Measurements of PaCO2, PaO2, and MAP during baseline, hypoxia, and 60 min posthypoxia
| PaCO2, mmHg |
PaO2, mmHg |
MAP, mmHg |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment groups | Baseline | Hypoxia | 60 min Posthypoxia | Baseline | Hypoxia | 60 min Posthypoxia | Baseline | Hypoxia | 60 min Posthypoxia |
| Pre-AIH | |||||||||
| Vehicle | 42.2 ± 0.4 | 41.6 ± 0.6 | 41.9 ± 0.4 | 242 ± 22 | 37 ± 2‡* | 250 ± 10 | 120 ± 7 | 92 ± 11* | 102 ± 8 |
| Vehicle control | 45.8 ± 0.8† | 45.7 ± 1.3† | 45.7 ± 1.0† | 290 ± 11† | 287 ± 9 | 276 ± 6 | 109 ± 9 | 109 ± 11 | 109 ± 12 |
| siBDNF | 46.1 ± 0.7† | 46.3 ± 1.3† | 45.8 ± 0.9† | 285 ± 5 | 39 ± 3‡* | 277 ± 6 | 81 ± 11 | 60 ± 6 | 77 ± 9 |
| siTrkB | 46.9 ± 0.5 | 43.6 ± 1.4* | 47.4 ± 0.4† | 292 ± 7 | 38 ± 1‡* | 283 ± 4 | 85 ± 11 | 70 ± 8 | 81 ± 9 |
| U0126 | 46.6 ± 0.9† | 46.3 ± 0.9† | 47.0 ± 1.2† | 300 ± 10† | 42 ± 2‡* | 289 ± 15 | 111 ± 5 | 84 ± 10* | 106 ± 5 |
| PI-828 | 47.7 ± 1.4† | 46.5 ± 1.4† | 48.5 ± 1.2† | 290 ± 15† | 39 ± 1‡* | 265 ± 21 | 106 ± 6 | 88 ± 7 | 95 ± 4 |
| Baseline | Hypoxia | 60 min | Baseline | Hypoxia | 60 min | Baseline | Hypoxia | 60 min | |
|---|---|---|---|---|---|---|---|---|---|
| Post-AIH | |||||||||
| Vehicle | 46.9 ± 2.0 | 49.0 ± 3.0 | 46.7 ± 2.3 | 266 ± 18 | 36 ± 2* | 279 ± 12 | 109 ± 5 | 65 ± 13* | 90 ± 6 |
| U0126 | 48.2 ± 1.1 | 47.0 ± 1.7 | 48.3 ± 1.4 | 265 ± 13 | 68 ± 2* | 255 ± 7 | 118 ± 7 | 90 ± 7 | 84 ± 9 |
| Vehicle control | 47.5 ± 1.8 | 46.6 ± 1.6 | 46.3 ± 1.9 | 262 ± 19 | 261 ± 23 | 262 ± 16 | 106 ± 7 | 100 ± 9 | 99 ± 9 |
| U0126 control | 47.4 ± 2.0 | 46.3 ± 1.2 | 47.3 ± 1.4 | 296 ± 8 | 295 ± 11 | 294 ± 8 | 112 ± 6 | 102 ± 5 | 85 ± 8 |
Values are means ± SE. PaCO2, arterial Pco2; PaO2, arterial Po2; MAP, mean arterial pressure; AIH, acute intermittent hypoxia; siBDNF, small interfering (si)RNA targeting brain-derived neurotrophic factor; siTrkB, siRNA targeting TrkB.
Significantly different than baseline;
significantly different than vehicle (with AIH);
significantly different than vehicle (without AIH) (P < 0.05).
Short-term hypoxic phrenic responses.
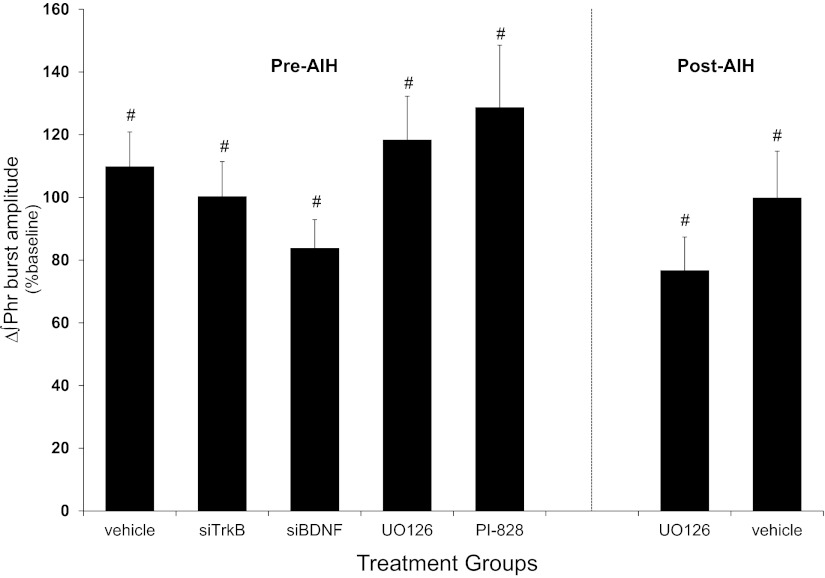
Baseline measurements of the frequency and amplitude of peak integrated inspiratory phrenic nerve bursts were similar for all treatment groups; therefore, normalization to baseline measurements was appropriate to quantify pLTF magnitude. In rats that received vehicle or drug injections before AIH, hypoxia elicited a rapid increase in phrenic burst amplitude that was significantly elevated from baseline (vehicle: 110 ± 11%, n = 7; siTrkB: 100 ± 11%, n = 8; siBDNF: 84 ± 9%, n = 9; U0126: 118 ± 14%, n = 9, and PI-828: 129 ± 20%, n = 6; change from baseline, P < 0.001; Fig. 1). Hypoxia also elicited a rapid increase in phrenic burst amplitude in rats that received vehicle or drug after AIH that was significantly different from baseline (vehicle: 100 ± 15%, n = 8; U0126: 77 ± 11%, n = 8; change from baseline, P < 0.001; Fig. 1). No between-group differences were detected in phrenic burst amplitude from either pre-AIH-treated rats (P = 0.121) or post-AIH-treated rats (P = 0.226).
Fig. 1.
Phrenic hypoxic responses during episodes of hypoxia. Changes in phrenic burst amplitudes during 5-min of hypoxic exposures (average of 3 episodes) from rats that received spinal injections before acute intermittent hypoxia (AIH) or vehicle (siBuffer; n = 7), small interfering (si)RNAs targeting TrkB (siTrkB; n = 8), siRNA targetting brain-derived neurotrophic factor (siBDNF; n = 9), MEK inhibitor (U0126, n = 9), or phosphatidylinositol 3-kinase (PI3K) inhibitor (PI-828; n = 6) and rats that received post-AIH injections of vehicle (n = 8) and U0126 (n = 8) are shown. Phrenic burst amplitudes increased from baseline in all groups, although no between-group differences were detected. ∫Phr, integrated phrenic. Values are means ± SE. #Significantly ifferent from baseline (P < 0.05).
pLTF requires new BDNF but not TrkB synthesis.
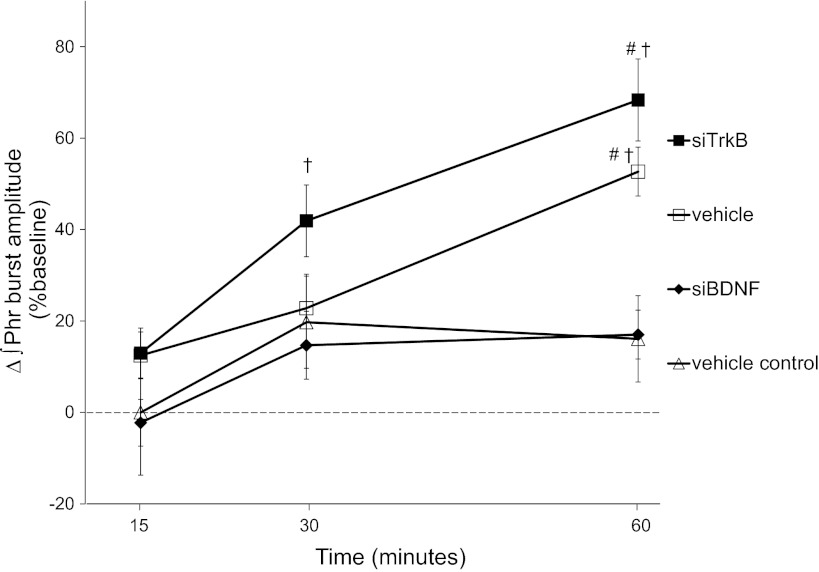
Typical integrated phrenic neurograms during experimental protocols (first series) are shown in Fig. 2. In vehicle (siRNA buffer, n = 7)-treated rats, phrenic nerve burst amplitude was increased over baseline at 30 min (23 ± 11%, P = 0.015) and remained elevated at least 60 min post-AIH (53 ± 9%, P < 0.001; Fig. 3). Phrenic burst amplitude was also significantly greater in vehicle-treated rats that received AIH versus those not exposed to AIH (i.e., time controls, n = 5) at 60 min post-AIH (17 ± 11%, P < 0.011; Fig. 3), thus confirming pLTF in these rats.
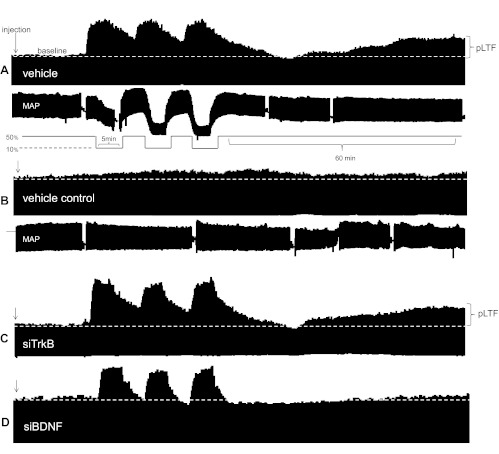
Fig. 2.
Representative phrenic neurograms depicting experimental protocols in rats treated before AIH. A: intrathecal vehicle [RNA inhibition (RNAi) buffer] before (∼2 h) AIH. B: intrathecal siTrkB before AIH. C: intrathecal siBDNF before AIH. D: time control rats that received intrathecal vehicle without AIH. Pretreatment with siTrkB did not inhibit phrenic long-term facilitation (pLTF) compared with vehicle-treated rats exposed to AIH. siBDNF inhibited pLTF versus vehicle, time control experiments. MAP, mean arterial pressure.
Fig. 3.
siTrkB RNA does not block BDNF synthesis-dependent pLTF. Comparisons were made for ∫Phr amplitudes from rats spinally injected with siTrkB RNA (n = 8) versus siBDNF RNA (n = 9). ∫Phr amplitudes from siTrkB-treated rats were not different from vehicle-treated rats (RNAi buffer, n = 7) at 60 min post-AIH, whereas siBDNF treatment significantly inhibited pLTF at 60 min post-AIH. A group of rats treated with vehicle did not receive AIH (i.e., time controls, n = 5). ∫Phr amplitude in vehicle-treated control rats was not different than baseline at any time point. Values are means ± SE. #Significantly different from vehicle control; †significantly different from siBDNF treatment (P < 0.05).
After pretreatment with siTrkB (n = 8, 2 h before AIH), phrenic amplitude was increased over baseline at 30 min and beyond (30 min: 42 ± 8% and 60 min: 68 ± 9%, P < 0.001) and was not different from control rats that received only AIH at 60 min (68 ± 9% vs. 53 ± 9%, respectively, P = 0.118; Fig. 3). Consistent with our previous report (2), phrenic burst amplitude from siBDNF-treated rats (n = 9) was significantly decreased at 60 min post-AIH compared with vehicle-treated rats (17 ± 5% vs. 53 ± 9%, P < 0.001) and siTrkB-treated rats (68 ± 9%, P < 0.001) but was not different from vehicle time control (AIH) rats at 60 min post-AIH (17 ± 9%, P = 0.968; Fig. 3). Thus, AIH-induced pLTF requires new BDNF synthesis but is independent of new TrkB synthesis.
Spinal inhibition of MEK/ERK activity blocks AIH-induced pLTF.
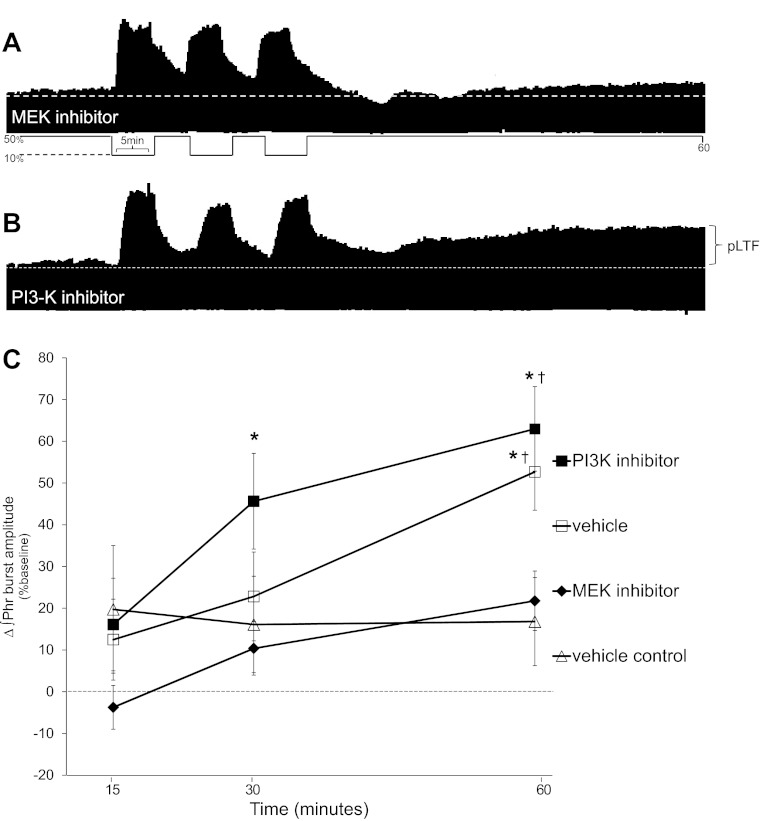
Since AIH-induced pLTF requires TrkB receptor activation (2), we wondered if “downstream” MEK/ERK and/or PI3K/Akt activity is necessary for pLTF. Typical integrated phrenic neurograms during the second experimental series are shown in Fig. 4, A and B. In rats pretreated with spinal injections of a MEK inhibitor (U0126; n = 9), pLTF was significantly attenuated 60 min post-AIH compared with vehicle-treated rats (22 ± 7% vs. 53 ± 9%, respectively, P = 0.004; Fig. 4, A and C). Thus, AIH-induced pLTF requires activation of the MEK/ERK pathway. On the other hand, spinal pretreatment with a selective PI3K/Akt inhibitor (PI-828; n = 6) had no effect on pLTF; at 60 min post-AIH, there were no significant differences between PI-828- and vehicle-treated rats (63 ± 10% and 53 ± 9%, respectively, P = 0.370; Fig. 4, B and C). A subset of rats received vehicle (control, saline, n = 5) without AIH; in these rats, phrenic burst amplitude was not different from baseline at any time and was not different from U0126-treated rats at the 60-min time point (17 ± 11% vs. 22 ± 7%, respectively, P = 0.664; Fig. 4). These data demonstrate that AIH-induced pLTF requires MEK/ERK but not PI3K/Akt activation.
Fig. 4.
Spinal MEK inhibition blocks pLTF. A: representative phrenic neurogram after intrathecal MEK inhibitor (U0126) before AIH. B: representative phrenic neurogram after intrathecal PI3K inhibitor (PI-828) before AIH. C: comparisons were made for ∫Phr burst amplitudes from spinally treated rats (expressed as the percent change from baseline). ∫Phr burst amplitudes from rats pretreated (20 min before) with a MEK inhibitor (U0126, n = 9) were decreased compared with vehicle treatment (n = 7) at 60 min post-AIH. In rats pretreated with PI3K inhibitor (PI-828, n = 6), ∫Phr burst amplitudes increased over baseline at 60 min post-AIH and were not different than vehicle treatment. Hence, MEK activity, not PI3K, is required for pLTF. Values are means ± SE. #Significantly different from vehicle control; †significantly different from MEK inhibitor (P < 0.05).
MEK/ERK activity is necessary for pLTF development.
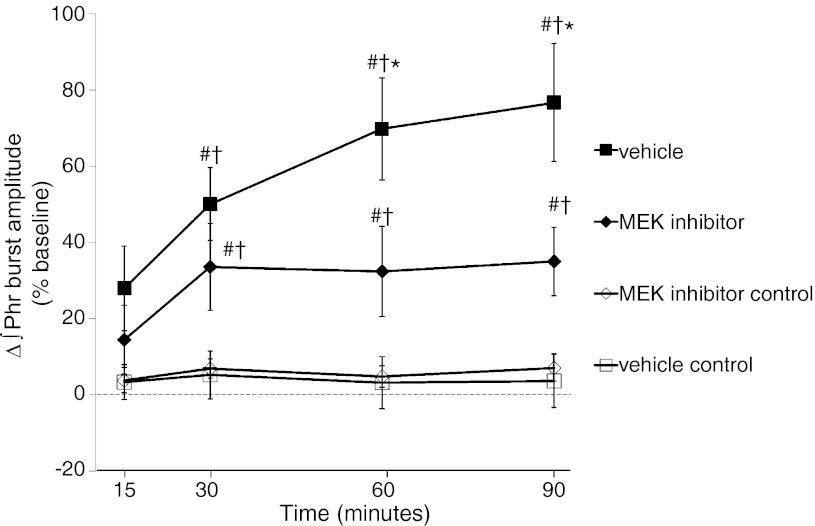
Rats that received spinal U0126 injections (100 μM) after AIH (<5 min) were monitored an 30 additional min (90-min time point) to provide adequate postdrug data. A subset of rats received vehicle (n = 8) post-AIH to serve as a control. Representative phrenic neurograms of the third experimental series are shown in Fig. 5. Phrenic burst amplitude in vehicle-treated rats increased from baseline at 30 min and beyond (Fig. 6), indicating progressive pLTF. In U0126 post-AIH-treated rats (n = 8), pLTF was evident but was significantly attenuated at 30 min versus vehicle-treated rats (34 ± 11% vs. 50 ± 10%, P = 0.004) and at 60 min (32 ± 12% vs. 70 ± 13%, P < 0.001) and 90 min post-AIH (35 ± 9% vs. 77 ± 15%, P < 0.001; Fig. 6). Phrenic burst amplitude in U0126 post-AIH-treated rats was increased compared with vehicle control rats (no hypoxia, n = 8) at 60 min post-AIH and beyond (60 min: 32 ± 12% vs. 3 ± 8%, P = 0.047; and 90 min: 35 ± 9% vs. 4 ± 8%, P = 0.034; Fig. 6), demonstrating residual pLTF. However, residual pLTF was unchanged between 30 and 90 min post-AIH, suggesting that further development of pLTF was effectively blocked.
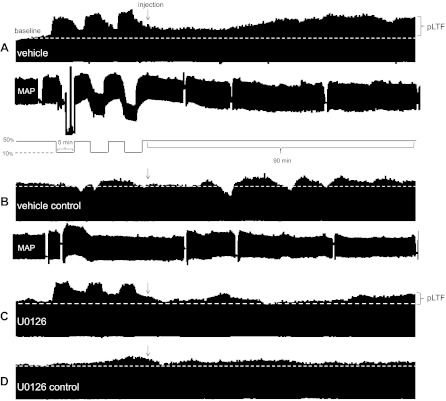
Fig. 5.
Representative phrenic neurograms depicting experimental protocols in rats treated post-AIH. Spinal injection post-AIH (<5 min) of vehicle (12 μl; A), vehicle control (no AIH; B), MEK inhibitor (U0126, 12 μl; C), and U0126 control (no AIH; D) treatments are shown. Post-AIH U0126 attenuated pLTF at 60 and 90 min compared with vehicle-treated rats exposed to AIH.
Fig. 6.
Spinal MEK inhibition post-AIH attenuates pLTF expression. Comparisons were made for ∫Phr burst amplitudes from spinally treated rats (expressed as the percent change from baseline). ∫Phr burst amplitudes from rats treated with vehicle (n = 8) immediately after AIH (<5 min) exhibited significant pLTF up to 90 min post-AIH. To test the hypothesis that pLTF maintanence requires MEK activity, rats were given a spinal injection of a selective inhibitor (U0126, 100 μM, n = 8) immediately after AIH. U0126 attenuated ∫Phr burst amplitudes at 60 min post-AIH and beyond, suggesting that continued MEK/ERK activity is required to maintain full pLTF expression. Control rats that received either vehicle (n = 8) or U0126 (n = 8) without AIH exhibited facilitation. Values are means ± SE. *Significantly different from MEK inhibitor; #significantly different from vehicle control; †significantly different from MEK inhibitor control (P < 0.05).
To address concerns that spinal MEK/ERK inhibition alone affected phrenic activity, a subset of control rats received U0126 without AIH (n = 8). In these rats, phrenic burst amplitude was not different than baseline at any time point (Fig. 6).
Respiratory frequency facilitation.
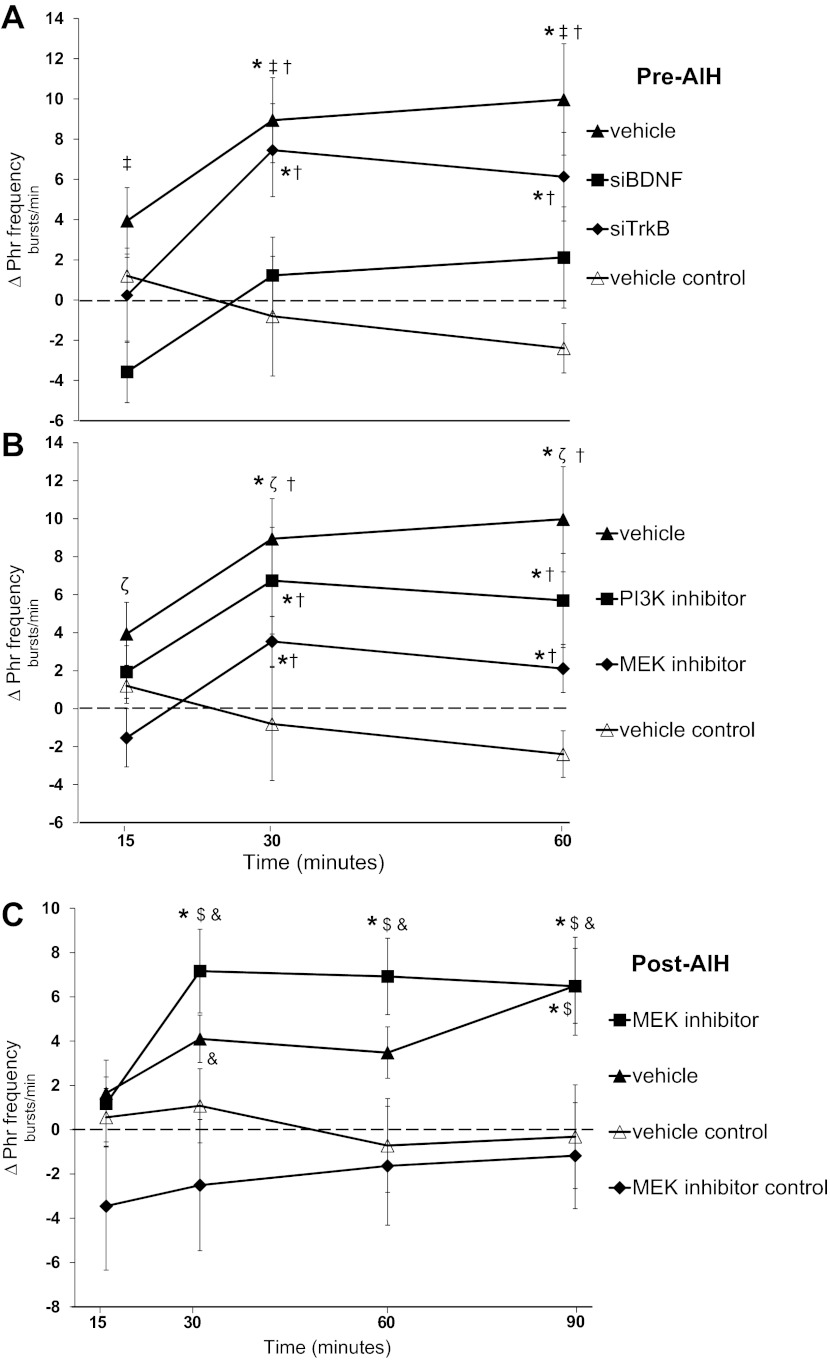
AIH-induced frequency LTF in anesthetized, vagotomized rats is generally small and inconsistent (3). Phrenic burst frequency increased over baseline in vehicle-treated ras (10 ± 3 bursts/min, P < 0.001) and siTrkB-pretreated rats (6 ± 2 bursts/min, P = 0.016) at 60 min post-AIH (Fig. 7A). Burst frequency differences were not detected between vehicle- and siTrkB-treated rats (P = 0.129). In rats pretreated with siBDNF, burst frequency was not increased at 60 min post-AIH (2 ± 3 bursts/min, P = 0.66) and was decreased relative to vehicle-pretreated rats (60 min, P = 0.003). In vehicle time control rats, phrenic burst frequency was not different than baseline at any time point (15 min: 1 ± 1 bursts/min, P = 0.66; 30 min: −1 ± 3 bursts/min, P = 0.77; and 60 min: −2 ± 1, P = 0.66)3 and was significantly less than in vehicle-treated rats (P < 0.001) and siTrkB-pretreated rats (P = 0.008) at 60 min post-AIH. Burst frequency responses in vehicle time control and siBDNF-treated rats did not differ 60 min post-AIH (P = 0.127; Fig. 7A).
Fig. 7.
Changes in phrenic (ΔPhr) burst frequency. A–C: ΔPhr nerve burst frequency from baseline (in bursts/min) in rats that received a pre-AIH spinal injection of vehicle (siBuffer), siTrkB, siBDNF, and vehicle time control (A). B: comparisons from spinal MEK inhibitor (U0126) and PI3K inhibitor (PI-828). C: comparisons from groups that received post-AIH injections of vehicle, U0126, and time control treatments (vehicle and U0126). Values are means ± SE. #Significantly different than baseline; ‡significantly different than siBDNF; †significantly different than vehicle (pre-AIH treatment); ζsignificantly different than MEK inhibitor (pre-AIH); $significantly different than vehicle control (post-AIH); &significantly different than MEK inhibitor control (post-AIH) (P < 0.05).
Phrenic burst frequency in rats pretreated with the PI3K inhibitor was increased over baseline at 60 min post-AIH (6 ± 2 bursts/min, P = 0.005; Fig. 7B). However, phrenic burst frequency after MEK inhibition was not different from baseline at the 60-min time point (2 ± 1 bursts/min, P = 0.144) and was significantly decreased from vehicle-treated rats at 60 min post-AIH (P = 0.048; Fig. 7B). In contrast, phrenic burst frequency in rats that received spinal MEK inhibition post-AIH was increased over baseline at 60 min (7 ± 2 bursts/min, P < 0.001) and 90 min (6 ± 2 bursts/min, P = 0.001; Fig. 7C). In rats that received vehicle post-AIH, phrenic burst frequency was increased over baseline at 90 min (6 ± 2 bursts/min, P = 0.003). Phrenic burst frequency in control rats that received either vehicle alone or U0126 alone was not increased above baseline at any time point (Fig. 7C).
DISCUSSION
Here, we demonstrate that neither new synthesis of spinal TrkB nor PI3K activity is required for AIH-induced pLTF. These conclusions are supported by findings demonstrating that spinal siTrkB had no effect, whereas siBDNF blocked pLTF (Fig. 3). The requirement for new BDNF synthesis confirms our earlier report (2). In both cases, the specific siRNA sequences and doses used were validated to have the intended effect when used in this same experimental preparation (2, 16). On the other hand, tyrosine kinase activity is necessary for both AIH-induced pLTF (2) and A2A receptor-induced pMF (16). However, to date, there are no clearly identified signaling mechanisms downstream from TrkB that underlie pLTF expression and/or development.
Here, we provide the first direct evidence that activation of the MEK/ERK signaling pathway is necessary for AIH-induced pLTF, whereas no clear role for PI3K/Akt signaling was found. MEK/ERK activity appears to be involved in a distinct phase of pLTF since 1) pretreatment with U0126 blocked pLTF (Fig. 3), whereas 2) U0126 administered after AIH halted the development of further pLTF but did not block initial pLTF expression (Fig. 6).
Distinct mechanisms of pMF.
These results provide evidence showing that AIH-induced pLTF and pMF induced by Gs protein-coupled metabotropic receptors arise from distinct cellular mechanisms. AIH-induced pLTF is induced by the activation of Gq protein-coupled metabotropic receptors (i.e., 5-HT2) and requires new BDNF synthesis (2), mature TrkB activation, and subsequent activation of the MEK/ERK pathway. We refer to this mechanism as the “Q pathway” to pMF since it is initiated by Gq protein-coupled receptors (7). In contrast, the “S pathway” to pMF is induced by the activation of Gs protein-coupled metabotropic receptors [i.e., A2A and 5-HT7 receptors (16, 19, 21)], requires new synthesis of an immature TrkB isoform, is independent of new BDNF synthesis, and requires downstream signaling via the PI3K/Akt pathway (7). Future studies are necessary to reveal the respective roles of these distinct mechanisms in different physiological conditions and the nature of the interactions between them (43).
New protein synthesis is critical for phrenic motor plasticity.
Translational regulation of new protein synthesis is fundamental to many forms of synaptic plasticity (36, 48, 51). For example, new BDNF synthesis is necessary for AIH-induced pLTF (2), whereas synthesis of an immature TrkB isoform is necessary for the S pathway (7, 16). Although the specific trigger for increased translation of BDNF mRNA has not been identified, we confirm here that impaired translation of spinal BDNF mRNA with siRNAs targeting BDNF mRNA blocks pLTF (2).
A2A receptor activation constrains pLTF expression (19), suggesting that the S pathway is marginally activated by AIH but, in fact, inhibits the Q pathway (7). Recently, we (20) demonstrated that other spinal Gs protein-coupled receptors also elicit pMF, such as spinal 5-HT7 receptor activation. Hence, we wondered whether signaling via the S pathway “downstream” of Gs protein-coupled receptor activation (i.e., immature TrkB) contributes to AIH-induced pLTF. However, the RNA inhibition experiments performed here provide contrary evidence since new TrkB is not necessary for AIH-induced pLTF. Instead, the TrkB activation (critical for AIH-induced pLTF) likely results from ligand interactions (i.e., BDNF binding) with the mature TrkB isoform (2).
Spinal MEK/ERK signaling is required for pLTF expression.
BDNF activates ERK1/2 in multiple neuronal cell types (17, 46, 55), including spinal motor neurons (25). ERK1/2 activation is required for multiple forms of synaptic plasticity (50), including hippocampal long-term potentiation (12) and intermediate-term facilitation of the sensorimotor synapse in Aplysia (37). Activated (phosphorylated) ERK1/2 may contribute to pLTF by controlling the synthesis of “proplasticity” proteins via translational regulation (22).
We propose that AIH triggers new BDNF protein synthesis, which, once released from phrenic motor neurons, acts pre- and/or postsynaptically by activating TrkB receptors and, subsequently, ERK1/2. To date, little direct experimental evidence supports the involvement of ERK1/2 in AIH-induced pLTF. However, AIH increases ERK1/2 phosphorylation in the ventral spinal regions that encompass the phrenic motor nucleus (54). Furthermore, repetitive AIH exposure increases ventral spinal ERK1/2 expression and its phosphorylation state (54), an effect localized near synapses onto presumptive phrenic motor neurons (47). Conversely, episodic spinal A2A receptor activation increases Akt (not ERK) phosphorylation in cervical spinal regions encompassing the phrenic motor nucleus (16). Thus, indirect evidence supports a role for both kinases in different forms of phrenic motor plasticity (7). Here, we provide the first direct evidence that MEK/ERK (not PI3K/Akt) activation is critical for AIH-induced pLTF.
Multiple pathways to pMF.
Many lines of evidence are emerging suggesting that multiple distinct cellular mechanisms give rise to long-lasting pMF (7). For example, pMF results from episodic spinal administration of 1) 5-HT (33), 2) 5-HT2 receptor agonists (31), 3) BDNF (2), 4) A2A receptor agonists (16), 5) 5-HT7 receptor agonists (20), 6) VEGF (9), and 7) erythropoetin (8). We propose that each molecule ultimately activates a limited number of downstream signaling pathways that converge on TrkB receptor signaling via either MEK/ERK and/or PI3K/Akt to elicit pMF. The two pathways investigated in this paper (the Q and S pathways) elicit pMF via distinct mechanisms characterized by distinct G protein-coupled metabotropic receptors (Gq vs. Gs), their requirements for BDNF versus TrkB synthesis, and MEK/ERK versus PI3K/Akt activation.
Since new BDNF synthesis and ERK activation are required, we propose the predominant mechanism of AIH-induced pLTF is the Q pathway to pMF. It is not yet known when and how Gs protein-coupled receptors interact with mechanisms of AIH-induced pLTF, although spinal application of A2A receptor antagonists amplify AIH-induced pLTF (19, 43). Thus, activation of the S pathway to pMF restrains (rather than augments) the Q pathway via “cross-talk inhibition.” On the other hand, 5-HT released during AIH activates multiple receptor subtypes coupled both to Gq (e.g., 5-HT2 receptors) and Gs proteins [e.g., 5-HT7 receptors (6)]. Thus, AIH-induced pLTF via 5-HT2 receptor activation may normally be limited (constrained) by coincident activation of Gs protein-coupled serotonin receptors.
Significance.
Although our understanding of the detailed cellular/synaptic mechanisms giving rise to pLTF remains incomplete, considerable progress has been made in recent years (3, 32, 34, 41). A detailed understanding of these cellular mechanisms is necessary to fully appreciate the biological significance of pLTF. For example, hypoxic episodes simulating apneas (<25 s) induce pLTF and hypoglossal LTF (35, 44), suggesting that realistic apneas are sufficient to elicit this mechanism. Facilitation of upper airway motor neurons, which preserve upper airway patency (e.g., hypoglossal LTF), may play a compensatory role, stabilizing breathing during sleep (5, 34, 45). On the other hand, one study (38) has suggested that ventilatory LTF destabilizes breathing via increased chemoreflex loop gain. The net balance of stabilizing/destabilizing influences may depend on the specific physiological (or pathological) condition in which LTF is induced.
From a clinical perspective, an understanding of the cellular/molecular events that underlie pLTF may aid in the development of strategies to “harness” respiratory plasticity for the treatment of ventilatory insufficiency (41). For instance, defects in 5-HT- and/or BDNF-dependent plasticity may contribute to severe respiratory control disorders, including obstructive sleep apnea (27, 52), apnea of prematurity (14), sudden infant death syndrome (13, 24), and respiratory instability in patients with Rett syndrome (40). We have only begun to develop an understanding of the basic mechanisms underlying pLTF (or pMF) and to appreciate its potential significance in the treatment of important clinical disorders.
GRANTS
Research support was provided by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-080209. M. S. Hoffman was supported by NHLBI Predoctoral Fellowship HL-092785. N. L. Nichols was supported by NHLBI Postdoctoral Fellowship T32-HL-007654. P. M. MacFarlane was supported by the Francis Families Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S.H., N.L.N., P.M.M., and G.S.M. conception and design of research; M.S.H., N.L.N., and P.M.M. performed experiments; M.S.H. and N.L.N. analyzed data; M.S.H., N.L.N., P.M.M., and G.S.M. interpreted results of experiments; M.S.H. prepared figures; M.S.H. drafted manuscript; M.S.H., N.L.N., P.M.M., and G.S.M. edited and revised manuscript; M.S.H., N.L.N., P.M.M., and G.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kalen Nichols for assistance with blood gas analysis.
REFERENCES
- 1. Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol 131: 65–77, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res 326: 553–572, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 32: 5973–5983, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31: 7682–7690, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duncia JV, Santella JB, 3rd, Higley CA, Pitts WJ, Wityak J, Frietze WE, Rankin FW, Sun JH, Earl RA, Tabaka AC, Teleha CA, Blom KF, Favata MF, Manos EJ, Daulerio AJ, Stradley DA, Horiuchi K, Copeland RA, Scherle PA, Trzaskos JM, Magolda RL, Trainor GL, Wexler RR, Hobbs RW, Olson RE. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett 8: 2839–2844, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Eldridge FL. Quantification of electrical activity in the phrenic nerve in the study of ventilatory control. Chest 70, Suppl 1: 154–157, 1976 [DOI] [PubMed] [Google Scholar]
- 12. English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem 272: 19103–19106, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, and chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the Apnea-of-Prematurity Group. Pediatrics 117: S47–S51, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, Waterfield MD. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J 404: 15–21, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gooney M, Lynch MA. Long-term potentiation in the dentate gyrus of the rat hippocampus is accompanied by brain-derived neurotrophic factor-induced activation of TrkB. J Neurochem 77: 1198–1207, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci 25: 1211–1218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine 2A receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 44: 59–73, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol 130: 207–218, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol 4: 517–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kishino A, Nakayama C. Enhancement of BDNF and activated-ERK immunoreactivity in spinal motor neurons after peripheral administration of BDNF. Brain Res 964: 56–66, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol 362: 382–391, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Kraiczi H, Hedner J, Dahlof P, Ejnell H, Carlson J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep 22: 61–67, 1999 [PubMed] [Google Scholar]
- 28. Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA 98: 3555–3560, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lovenberg TW, Baron BM, Lecea Lde Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11: 449–458, 1993 [DOI] [PubMed] [Google Scholar]
- 30. MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587: 5469–5481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacFarlane PM, Mitchell GS. NADPH oxidase activity is necessary for phrenic motor facilitation induced by 5HT2B receptor activation (Abstract). FASEB J 22: 1232.7, 2008 [Google Scholar]
- 32. Macfarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164: 263–271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacFarlane PM, Mitchell GS. Serotonin-induced phrenic long-term facilitation requires reactive oxygen species signaling via the NADPH oxidase complex (Abstract). Soc Neurosci Abst: 520.15, 2007 [Google Scholar]
- 34. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Mahamed S, Mitchell GS. Simulated apneas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol 586: 2171–2181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol 10: 587–592, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron 18: 899–912, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980 [DOI] [PubMed] [Google Scholar]
- 40. Mironov SL, Skorova E, Hartfelt N, Mironova LA, Hasan MT, Kugler S. Remodelling of the respiratory network in a mouse model of Rett syndrome depends on brain-derived neurotrophic factor regulated slow calcium buffering. J Physiol 587: 2473–2485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York: Springer, 2007, p. 291–306 [Google Scholar]
- 42. Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol 532: 483–497, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol 112: 1678–1688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behaviour elicited by chronic intermittent hypoxia. J Appl Physiol 94: 2342–2349, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol 160: 259–266, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron 37: 473–484, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Satriotomo I, Dale EA, Mitchell GS. Thrice weekly intermittent hypoxia increases expression of key proteins necessary for phrenic long-term facilitation: a possible mechanism of respiratory metaplasticity (Abstract)? FASEB J 21: A1292, 2007 [Google Scholar]
- 48. Schuman EM, Dynes JL, Steward O. Synaptic regulation of translation of dendritic mRNAs. J Neurosci 26: 7143–7146, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci 26: 299–330, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem 276: 37585–37593, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Veasey Serotonin SC. Culprit or promising therapy for obstructive sleep apnea? Am J Respir Crit Care Med 163: 1045–1047, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV, Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci USA 104: 17210–17215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 22: 1532–1540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]