Abstract
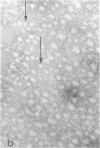
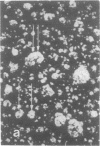
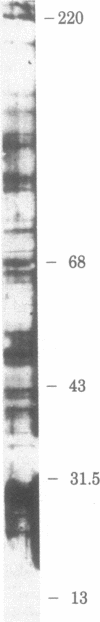
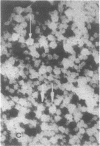
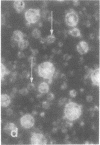
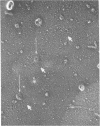
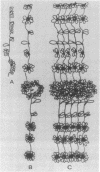
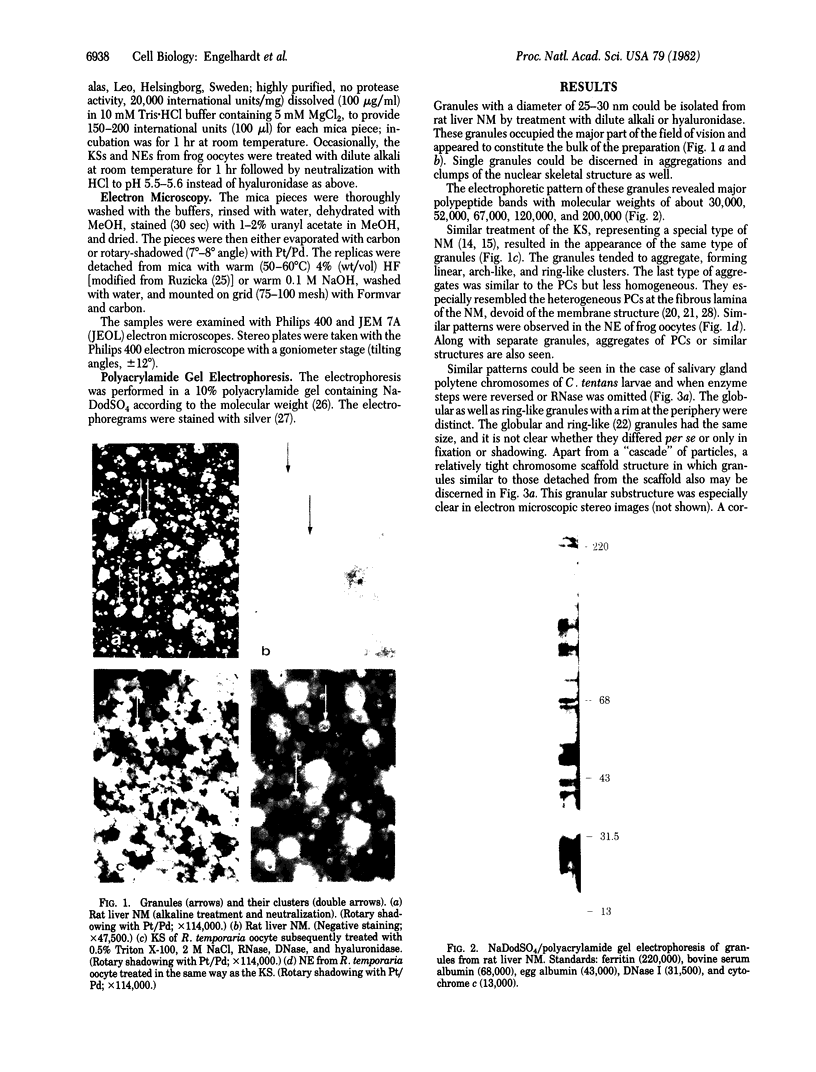
Rat liver nuclear matrix and similar structures derived from isolated Chironomus polytene chromosomes, nuclear envelopes, and intranuclear bodies of frog late oocytes (the karyospheres) were studied by electron microscopy with platinum shadowing and negative staining. We have shown that the treatment of whole nuclei, nuclear envelopes, polytene chromosomes, or karyospheres with nonionic detergent, high salt, and RNase and DNase followed by dilute alkali or hyaluronidase digestion reveals numerous rather uniform granules 25-30 nm in diameter. With omission of the nucleases the granules appear to be associated with DNA strands mostly organized in loops. Many granules form clusters and are arranged in linear or arch-like aggregates or cycles resembling the pore complexes. We suppose that these spherical bodies constitute a basic component of the nuclear matrix, chromosome scaffold, and nuclear envelope and are bound together by hyaluronic acid or some similar glycosaminoglycan.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph K. W., Cheng S. M., Laemmli U. K. Role of nonhistone proteins in metaphase chromosome structure. Cell. 1977 Nov;12(3):805–816. doi: 10.1016/0092-8674(77)90279-3. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. The nuclear protein matrix: isolation, structure, and functions. Adv Enzyme Regul. 1976;14:63–100. doi: 10.1016/0065-2571(76)90008-x. [DOI] [PubMed] [Google Scholar]
- Dwyer N., Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J Cell Biol. 1976 Sep;70(3):581–591. doi: 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt P., Pusa K. Nuclear pore complexes: "press-stud" elements of chromosomes in pairing and control. Nat New Biol. 1972 Dec 6;240(101):163–166. doi: 10.1038/newbio240163a0. [DOI] [PubMed] [Google Scholar]
- Fiil A., Moens P. B. The development, structure and function of modified synaptonemal complexes in mosquito oocytes. Chromosoma. 1973;41(1):37–62. doi: 10.1007/BF00284073. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzova M. N., Parfenov V. N. Ultrastructure of late oocyte nuclei in Rana temporaria. J Cell Sci. 1977 Dec;28:1–13. doi: 10.1242/jcs.28.1.1. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Ely S., D'Arcy A., Jost E. Localization of a nuclear envelope-associated protein by indirect immunofluorescence microscopy using antibodies against a major polypeptide from rat liver fractions enriched in nuclear envelope-associated material. Cytobiologie. 1978 Oct;18(1):22–38. [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Scheer U. The major polypeptides of the nuclear pore complex. Exp Cell Res. 1978 Oct 1;116(1):85–102. doi: 10.1016/0014-4827(78)90067-8. [DOI] [PubMed] [Google Scholar]
- Kuz'mina S. N., Virtanen I., Lekhto V. P., Bul'diaeva T. V. Immunologicheskaia kharakteristika belka iadernogo matriksa. Dokl Akad Nauk SSSR. 1982;262(5):1253–1256. [PubMed] [Google Scholar]
- Kuzmina S., Buldyaeva T., Troitskaya L., Zbarsky I. Characterization and fractionation of rat liver nuclear matrix. Eur J Cell Biol. 1981 Oct;25(2):225–232. [PubMed] [Google Scholar]
- Laemmli U. K., Cheng S. M., Adolph K. W., Paulson J. R., Brown J. A., Baumbach W. R. Metaphase chromosome structure: the role of nonhistone proteins. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):351–360. doi: 10.1101/sqb.1978.042.01.036. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mirkhamidova P., Kuz'mina S. N., Troitskaia L. P., Bul'diaeva T. V., Zbarskii I. B. Deitsvie rentgenovskogo oblucheniia na iadernuiu obolochku kletok pecheni krysy. Tsitologiia. 1979 Jul;21(7):768–774. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Okada T. A., Comings D. E. A search for protein cores in chromosomes: is the scaffold an artifact? Am J Hum Genet. 1980 Nov;32(6):814–832. [PMC free article] [PubMed] [Google Scholar]
- Patel G., Patel V., Wang T. Y., Zobel C. R. Studies of the nuclear residual proteins. Arch Biochem Biophys. 1968 Dec;128(3):654–662. doi: 10.1016/0003-9861(68)90075-1. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Plagens U. Effect of salt-treatment on manually isolated polytene chromosomes from Chironomus tentans. Chromosoma. 1978 Aug 21;68(1):1–19. doi: 10.1007/BF00330369. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Chernokhvostov V. V., Roodyn A. V., Zbarsky I. B., Georgiev G. P. Proteins tightly bound to DNA in the regions of DNA attachment to the skeletal structures of interphase nuclei and metaphase chromosomes. Cell. 1981 Nov;27(1 Pt 2):65–73. doi: 10.1016/0092-8674(81)90361-5. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Mantieva V. L., Georgiev G. P. DNA adjacent to attachment points of deoxyribonucleoprotein fibril to chromosomal axial structure is enriched in reiterated base sequences. Nucleic Acids Res. 1978 Dec;5(12):4737–4751. doi: 10.1093/nar/5.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka F. Eine Methode zur Darstellung bestimmter Mitosen im Lichtund Elektronenmikroskop--ein Zielpräparationsverfahren. Mikroskopie. 1973 May;29(3):109–115. [PubMed] [Google Scholar]
- Shaper J. H., Pardoll D. M., Kaufmann S. H., Barrack E. R., Vogelstein B., Coffey D. S. The relationship of the nuclear matrix to cellular structure and function. Adv Enzyme Regul. 1978;17:213–248. doi: 10.1016/0065-2571(79)90015-3. [DOI] [PubMed] [Google Scholar]
- Tsanev R., Avramova Z. Nonprotamine nucleoprotein ultrastructures in mature ram sperm nuclei. Eur J Cell Biol. 1981 Apr;24(1):139–145. [PubMed] [Google Scholar]
- ZBARSKY I. B., GEORGIEV G. P. Cytological characteristics of protein and nucleoprotein fractions of cell nuclei. Biochim Biophys Acta. 1959 Mar;32(1):301–302. doi: 10.1016/0006-3002(59)90600-6. [DOI] [PubMed] [Google Scholar]
- Zbarsky I. B. Nuclear skeleton structures in some normal and tumor cells. Mol Biol Rep. 1981 May 22;7(1-3):139–148. doi: 10.1007/BF00778745. [DOI] [PubMed] [Google Scholar]
- Zvarskii I. B., Filatova L. S. Issledovanie kariosfery ootsitov travianoi liagushki Rana temporaria. Ontogenez. 1979;10(5):502–506. [PubMed] [Google Scholar]