Abstract
Midthoracic spinal cord injury (SCI) is associated with enhanced sympathetic support of heart rate as well as myocardial damage related to calcium overload. The myocardial damage may elicit an enhanced sympathetic support of contractility to maintain ventricular function. In contrast, the level of inotropic drive may be reduced to match the lower afterload that results from the injury-induced reduction in arterial pressure. Accordingly, the inotropic response to midthoracic SCI may be increased or decreased but has not been investigated and therefore remains unknown. Furthermore, the altered ventricular function may be associated with anatomical changes in cardiac sympathetic innervation. To determine the inotropic drive following midthoracic SCI, a telemetry device was used for repeated measurements of left ventricular (LV) function, with and without beta-adrenergic receptor blockade, in rats before and after midthoracic SCI or sham SCI. In addition, NGF content (ELISA) and dendritic arborization (cholera toxin B immunohistochemistry and Sholl analysis) of cardiac-projecting sympathetic postganglionic neurons in the stellate ganglia were determined. Midthoracic SCI was associated with an enhanced sympathetic support of heart rate, dP/dt+, and dP/dt−. Importantly, cardiac function was lower following blockade of the sympathetic nervous system in rats with midthoracic SCI compared with sham-operated rats. Finally, these functional neuroplastic changes were associated with an increased NGF content and structural neuroplasticity within the stellate ganglia. Results document impaired LV function with codirectional changes in chronotropic and inotropic responses following midthoracic SCI. These functional changes were associated with a dynamic interaction between the heart and its sympathetic innervation.
Keywords: autonomic nervous system, cardiac parameters, paraplegia
sympathetic function is elevated above the lesion in individuals with midthoracic spinal cord injury (SCI), and the regulation of heart rate and cardiac function is abnormal (5). This may be due, in part, to a baroreflex-mediated increase in cardiac sympathetic drive following hypotension due to loss of sympathetic vasoconstrictor tone below the site of the lesion.
The old concept that sympathetic nerve activity (SNA) is either increased or decreased in different physiological and pathophysiological states is no longer valid. It is now accepted that sympathetic activity to individual targets, such as the heart, kidneys, splanchnic organs, and skeletal muscle, is independently and differentially controlled such that SNA to one target may increase, whereas SNA to another may be decreased or unchanged (35).
Similarly, evidence suggests that different cardiac functions (e.g., chronotropy, inotropy, and dromotropy) are independently and differentially controlled by the sympathetic nervous system (40). Specifically, there is a degree of independent and differential control of heart rate and contractility with either left or right stellate ganglion (14) or spinal cord (10, 11) stimulation. Thus, although chronotropic, dromotropic, and inotropic responses often change concordantly, for example, during the early stages of exercise, there can be independent and differential regulation under specific conditions, for example, the Bainbridge reflex and perhaps the later stages of exercise (19, 40, 41).
Midthoracic SCI may present a condition when independent and differential regulation of cardiac chronotropy, inotropy, and dromotropy promotes maximal efficiency. Specifically, as mentioned, midthoracic SCI is associated with enhanced sympathetic support of heart rate as well as myocardial damage related to calcium overload (25, 26). Importantly, elevated heart rate, myocardial damage, and left ventricular (LV) dysfunction are associated with adverse cardiovascular outcomes. The myocardial damage may elicit an enhanced sympathetic support of contractility to maintain ventricular function, thus matching the enhanced sympathetic support of heart rate. However, this would be an inefficient way to raise cardiac output since afterload is reduced following SCI (13). In contrast, the level of inotropic drive may be reduced to match the lower afterload that results from the injury-induced reduction in arterial pressure (40). This would be an efficient way to raise cardiac output. Accordingly, the inotropic drive following midthoracic SCI may be increased or decreased but has not been investigated and therefore remains unknown.
A dynamic interaction between a target tissue and its innervation is required for optimal functioning (38, 48). Changes in target tissue function are associated with NGF induced neuroplasticity in autonomic pathways regulating target organ function (6, 45). For example, bladder enlargement and smooth muscle hypertrophy produced by anatomical or functional obstruction of the bladder outlet are associated with NGF-induced morphological and physiological neuroplasticity in the pathways regulating micturition (6, 45). Accordingly, SCI-induced changes in cardiac function may be associated with increased stellate ganglion NGF content and sympathetic postganglionic neuron morphology since the majority of cardiac sympathetic axons originate in the stellate ganglia (37, 52).
Therefore the effects of midthoracic SCI on LV function, the sympathetic support of chronotropic and inotropic drive, stellate ganglia NGF content, and cardiac sympathetic postganglionic neuron morphology were determined. The hypothesis that T5 spinal cord transection (T5X) is associated with impaired LV function, codirectional changes in chronotropic and inotropic drive, increased stellate ganglia NGF content, and increased cardiac sympathetic postganglionic neuron dendritic arborization was tested. To test this hypothesis, a telemetry device for repeated measurements of LV function, with and without beta-adrenergic receptor blockade, was used in rats before (weeks 1 and 2) and after (days 1, 3, 7, 14, and 21) midthoracic SCI or sham SCI. In addition, NGF content (ELISA) was determined in the stellate ganglia. Finally, cholera toxin B subunit (CTB) immunohistochemistry procedures and Sholl analysis were used to examine the dendritic branching pattern of cardiac-projecting sympathetic postganglionic neurons within the stellate ganglion. Specifically, CTB was injected into the pericardial space to retrogradely label sympathetic postganglionic neurons projecting to the heart. This is an important procedure because only cardiac-projecting sympathetic postganglionic neurons were examined. Results document impaired LV function with codirectional changes in chronotropic and inotropic responses following midthoracic SCI. These functional changes were associated with increased stellate ganglia NGF content and structural neuroplasticity within the stellate ganglia.
MATERIALS AND METHODS
Surgical Procedures
Experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wayne State University and complied with the American Physiological Society's Guiding Principles in the Care and Use of Animals. Ten adult Sprague-Dawley male rats were studied to determine the sympathetic support of heart rate and LV function before and after T5X or sham T5X. Specifically, following the experimental procedures in the intact condition, 6 of the 10 rats were subjected to T5 spinal cord transection, and the remaining 4 rats were subjected to sham T5X. Thirty-two additional adult Sprague-Dawley male rats [n = 16: T5X and n = 16: intact (sham T5X)] were studied to determine stellate ganglia NGF content 3 days (n = 4/group), 7 days (n = 6/group), and 28 days (n = 6/group) post spinal cord transection or sham transection. Finally, 10 additional adult Sprague-Dawley male rats [n = 5: T5X and n = 5: intact (sham T5X)] were studied to determine stellate ganglion neuron morphology (CTB immunohistochemistry and Sholl analysis) 28 days post spinal cord transection or sham transection.
All surgical procedures were performed using aseptic surgical techniques. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), atropinized (0.05 mg/kg ip), intubated, and prepared for aseptic surgery by removing the fur over the surgical site and cleansing the skin with a povidone-iodine solution. Subsequently, the rats were mechanically ventilated and placed on a feedback-based temperature control system (model no. 40–90-8; FHC, Bowdoin, ME) for monitoring and maintaining body temperature within the physiological range. Supplemental doses of pentobarbital sodium (10–20 mg/kg ip) were administered if the rats regained the blink reflex or responded during the surgical procedures.
Radiotelemetry Implantation
After anesthesia was induced, 10 rats were positioned in a right lateral decubitus position, and the hearts were approached via a left thoracotomy through the fourth intercostal space. Subsequently, the catheter of a telemetry device (Data Sciences International, TA11PA-C40) was inserted into the left ventricle through an apical stab wound for continuous, nontethered recording of pulsatile LV pressure and calculation of its derivative, dP/dt, [calculation of velocity of myocardial contraction (dP/dt+) and velocity of myocardial relaxation (dP/dt−); (27)]. The transmitter body and a catheter (for the infusion of fluids and drugs) were placed in the intraperitoneal space through a ventral abdominal approach. The intraperitoneal catheter was exteriorized on the dorsal aspect of the neck. The diets of all rats were supplemented postsurgery with palatable, nutritious treats (Bio-Serv, Frenchtown, NJ). No other dietary interventions were necessary. A minimum of 1 wk was allowed for recovery and for the animals to regain their presurgical weight. During the recovery period, the rats were handled, weighed, and acclimatized to the laboratory and investigators.
Experimental Procedures (Sympathetic Support of HR and LV Parameters)
Following recovery, the subjects were brought to the laboratory and allowed to adapt to the environment for ∼1 h to ensure stable hemodynamic conditions. After the stabilization period, beat-by-beat, steady-state hemodynamic variables were recorded over 10–15 s. Subsequently, the recordings were repeated with cardiac β1-adrenergic receptor blockade. Cardiac β1-adrenergic receptor blockade was achieved by infusion of the specific β1-adrenergic receptor antagonist, metoprolol (10 mg/kg), into the intraperitoneal catheter. Fifteen minutes after metoprolol administration, beat-by-beat, steady-state hemodynamic variables were recorded. These procedures were repeated each week for 2 wk.
Spinal Cord Transection
Following the experimental procedures in the intact condition, 6 of the 10 rats were subjected to T5 spinal cord transection, and the remaining 4 rats were subjected to sham T5X. Specifically, rats were anesthetized as described above, intubated, and positioned prone over a thoracic roll that slightly flexed the trunk. The fourth thoracic vertebra was exposed via a midline dorsal incision, and the spinous process and laminae were removed. Because the spinal cord is shorter than the vertebral column, spinal cord segment T5 lies at the level of the T4 vertebral body. Two ligatures (6-0 silk) were tightened around the underlying spinal cord between the fifth and sixth thoracic segments, and the spinal cord was completely transected by cutting between the ligatures with scissors (25, 26). In this way there was minimal bleeding. Identical procedures were followed for the four sham T5X rats, except the spinal cord was not tied or transected. Sympathetic innervation to the heart is derived from preganglionic fibers that exit the spinal cord at the first through fourth thoracic levels (46). Transection between the fifth and sixth thoracic levels of the spinal cord preserves supraspinal control of cardiac sympathetic activity. The completeness of the transection was confirmed by visual inspection of the lesion site. The diets of all rats were supplemented postsurgery with palatable, nutritious treats. No other dietary interventions were necessary.
Experimental Procedures (Sympathetic Support of HR and LV Parameters)
The experimental procedures described above were repeated on days 1, 3, 7, 14, and 21 after spinal cord transection or sham spinal cord transection. Furthermore, on day 7 posttransection, the rats received a motor activity score using criteria described previously (47). The motor activity score was assessed by placing the animal on a paper-covered table and observing spontaneous motor activity for 1 min. Motor scores ranged from 0 to 5. A motor score of 5 indicates normal walking, whereas a score of 0 indicates no weight-bearing or spontaneous voluntary movement in the hind limbs. All rats had a motor score of 0, which indicates no weight bearing. Upon completion of the studies, the site of the spinal cord transection was confirmed by autopsy.
Intrapericardial Sac Injections
Ten additional adult Sprague-Dawley male rats [n = 5: T5X and n = 5: intact (sham T5X)] were studied to determine stellate neuron dendritic arborization and morphology. To reconstruct sympathetic postganglionic neurons located within the stellate ganglia, the neuronal tracer CTB was injected into the pericardial space by inserting a pipette tip through the thymus gland 21–25 days after spinal cord transection or sham transection. Specifically, the animals were anesthetized as described above, and the heart was approached via a thoracotomy through the second or third intercostal space. Ten microliters of 1% CTB was mixed with 1 μl of 3% Evans blue dye. The Evans blue dye was used to visualize the injectate because CTB is colorless. This assured localization within the pericardial sac. All injections were confined within the pericardial sac. Five to seven days were allowed for CTB to be picked up at synaptic endings and transported in a retrograde fashion back to the cell bodies of neurons located within the stellate ganglia. Subsequently, the animals were deeply anesthetized and perfused transcardially, and the heart and stellate ganglia were preserved as previously described (28).
Tissue Processing, Analysis, and Immunohistochemistry
Stellate ganglia.
Investigators have reported independent and differential control of heart rate, contractility, and ventricular arrhythmic events from either the left or right stellate ganglion (10, 11, 14, 40, 42). In this context, heart rate is predominately modulated by neurons in the right stellate ganglion while inotropism is controlled by neurons in both stellate ganglia (14). Accordingly, the right stellate ganglion was selected to include neurons controlling both chronotropy and inotropy. Specifically, the right stellate ganglia from five T5X and five intact (sham T5X) rats were sectioned horizontally at 30-μm intervals. Tissue sections were washed in 10 mM Tris, 0.9% NaCl, 0.05% thimerosal in 10 mM phosphate buffer (TPBS), pH 7.4, containing 0.3% Triton X-100 for 3 × 10 min then incubated in 10% heat-inactivated normal horse serum (NHS, Invitrogen) in TPBS-Triton for at least 1 h. The sections were then incubated in goat anti-CTB antiserum (1:25000, List Biologicals) in TPBS-Triton containing 10% NHS for 3 days at room temperature. After rinsing (TPBS, 3 × 10 min each), sections were incubated with biotinylated donkey anti-goat immunoglobulin (1:500, Jackson Laboratories) in TPBS-Triton with 1% NHS overnight at room temperature. Sections were rinsed again (TPBS, 3 × 10 min each) and incubated 4–6 h in 1:1500 ExtrAvidin-HRP (catalog no. E-2886; Sigma) in TPBS-Triton. Immunoreactive neurons were revealed with the nickel-intensified diaminobenzidine reaction (23).
Structural analysis.
Multiplanar photomicrographs were taken, and the images were stacked using MicroBrightfield Neurolucida software. Stacked images of CTB-labeled stellate neurons were examined on an Olympus BH-2 microscope outfitted with a motorized stage, Neurolucida imaging software, and a high-resolution digital camera. Selection criteria were similar to previous studies examining dendritic arborization in other regions (32, 49). Cell bodies and dendrites were reconstructed using the neuron tracing feature on the Neurolucida system, and dendritic branching was assessed in the NeuroExplorer 3D visualization and morphometric analysis program included with the Neurolucida system.
Morphological features of cardiac-projecting stellate neurons were analyzed as described by Iwamoto and colleagues (31–33). Specifically, each neuron was analyzed using the Sholl analysis (28, 44) of dendritic branching, which assumes that dendritic arborization is an indirect measure of available postsynaptic space. A series of concentric rings calibrated at 10-μm intervals was superimposed on each neuron and centered on the cell body. Intersections between dendrites and each concentric ring were then counted. The location and number of intersections were plotted (28) and used for statistical comparisons. In each animal, 9–10 sections were examined and 9–10 neurons per section were measured using Neurolucida software. Each cell's morphometric features were measured within one 30-μm-thick section. Only cells with clearly distinguishable perikarya and dendritic trees were assessed. Specifically, to be selected for analysis, CTB-labeled neurons satisfied the following criteria: 1) dark and consistent staining in the entire dendritic tree, 2) lack of truncated dendrites, and 3) relative separation from nearby stained neurons to avoid overlapping dendrites. The examined neurons were chosen randomly. Three additional morphological features were recorded: area of soma, overall length of all visible processes (maximum dendritic length), and number of intersections per animal. The morphological features were compared between T5X and sham-operated intact rats.
ELISA for Stellate Ganglia NGF Content
Thirty-two additional adult Sprague-Dawley male rats [n = 16: T5X and n = 16: intact (sham T5X)] were studied to determine stellate ganglia NGF content 3 days (n = 4/group), 7 days (n = 6/group), and 28 days (n = 6/group) post spinal cord transection or sham transection. The animals were anesthetized with pentobarbital (50 mg/kg ip), and the right stellate ganglion was removed. The tissue was flash frozen in liquid nitrogen and stored at −80°C until NGF extraction. On the day of the analysis, the ganglia were homogenized using a sonic dismembrator in 100 μl of lysis buffer containing 137 mM NaCl, 20 mM Tris-HCl (pH = 8.0), 1% NP40, 10% glycerol, protease inhibitors (cOmplete ULTRA Tablets, Mini, EDTA free; Roche Diagnostics), and phosphatase inhibitors (PhosSTOP; Roche Diagnostics). The samples were centrifuged at 10,000 g for 10 min at 4°C. The supernatant was diluted 1:5 in Dulbecco's PBS buffer (0.2 g KCl, 8.0 g NaCl, 0.2 g KH2PO4, 1.15 g Na2HPO4, 133 mg CaCl2·2H2O, and 100 mg MgCl2·6H2O per 1 liter ddH2O). The samples were acidified to approximately pH 2.0–3.0 for 15 min at room temperature and then neutralized by 1 N NaOH to pH 7.5–8.0 to promote dissociation of NGF from receptors or binding proteins. After acid treatment, the samples were stored at −80°C. The samples were assayed in an antigen capture ELISA using Promega's NGF Emax ImmunoAssay System (Promega, Madison, WI) according to the manufacturer's instructions with minor modifications. Briefly, Nunc MaxiSorp Plates (catalog no. 439454) were incubated with carbonate coating buffer containing polyclonal anti-NGF overnight at 4°C. The next day, the wells were washed and blocked overnight at 4°C. After blocking, the wells were washed and 100 μl of either sample or standard was added to each well and incubated for 6 h at room temperature. Subsequently, the wells were washed and then incubated with a secondary monoclonal anti-NGF overnight at 4°C. The next day, the wells were again washed and then incubated with anti-rat IgG conjugated to horseradish peroxidase for 2.5 h at room temperature. Between incubations, the plates were washed five times. A TMB One solution was used to develop color in the wells for 10 min at room temperature. The reaction was stopped with the addition of 100 μl/well 1 N HCl. The absorbance was read at A450 within 30 min using a Synergy H1 microplate reader (BioTek Instruments) and Gen5 Data Analysis Software.
Total protein concentrations for the samples were also determined with a protein assay kit (Micro BCA Protein Assay Kit; Pierce Biotechnology, Rockford, IL). Briefly, 150 μl of albumin standard or sample was pipetted into a microplate well, and 150 μl of the Micro BCA Working Reagent was added to each well and incubated at 37°C for 2 h. The absorbance was read at A562 using a Synergy H1 microplate reader and Gen5 Data Analysis Software. All tissue NGF values were standardized by tissue protein levels and expressed as picograms per micrograms of total protein.
Data Analysis
All physiological recordings were sampled at 2 kHz, and the data were expressed as means ± standard error. All data were the average of every beat during the last 10–15 s of the period. A two-factor ANOVA with repeated measures was used to compare LV function parameters and heart rate before and after beta-adrenergic receptor blockade in the sham-operated intact and T5X conditions. Post hoc Holm-Sidak analysis was used to document significant differences. Similarly, a two-factor ANOVA was used to compare stellate ganglia NGF content 3 days (n = 4/group), 7 days (n = 6/group), and 28 days (n = 6/group) post spinal cord transection or sham transection. Post hoc Holm-Sidak analysis was used to document significant differences.
The Sholl analysis was evaluated by a two-way repeated measure ANOVA applied to the dendritic intersections found at each concentric ring. Specifically, a two-way repeated measures ANOVA [group (sham T5X or T5X) × branching order] was applied to the numbers of dendrites according to their order of branching. Post hoc Holm-Sidak analysis was used to document a significant difference between the two groups for every point. Significance was set at P < 0.05. A Student's unpaired t-test was used to compare sympathetic postganglionic neuron soma size, maximum dendritic length, and number of intersections per animal (which represents the total dendritic field) between sham-operated intact and T5 spinal cord transected rats.
RESULTS
Figure 1 presents 1-s recordings of left ventricular pressure and its derivative, dP/dt [calculation of velocity of myocardial contraction (dP/dt+) and calculation of velocity of myocardial relaxation (dP/dt−)], before (Control) and in response to beta-adrenergic receptor blockade (β-x) in intact (Fig. 1, top) and T5X (Fig. 1, bottom) rats.
Fig. 1.
One-second recordings of left ventricular pressure and its derivative, dP/dt, [calculation of velocity of myocardial contraction (dP/dt+) and calculation of velocity of myocardial relaxation (dP/dt−)] before (Control) and in response to beta-adrenergic receptor blockade (β-x) in intact (top) and T5 spinal cord transection (T5X; bottom) rats.
Figure 2 presents the effects of midthoracic SCI on heart rate and the sympathetic support of heart rate (demonstrated by the action of beta-adrenergic receptor blockade) in 10 intact male rats (weeks 1 and 2). Following the experimental procedures in the intact condition, 6 of the 10 rats were subjected to T5 spinal cord transection, and the remaining 4 rats were subjected to sham T5X. Without beta blockade, heart rate was higher following SCI. Beta-adrenergic receptor blockade lowered HR more in T5X compared with sham-operated intact rats; however, heart rates remained higher in T5X rats, starting with day 3, compared with intact rats following beta-adrenergic receptor blockade.
Fig. 2.
Heart rate and the sympathetic support of heart rate in intact and T5X rats. Effects of midthoracic spinal cord injury on heart rate and the sympathetic support of heart rate (demonstrated by the action of beta-adrenergic receptor blockade) in 10 intact male rats. Following the experimental procedures in the intact condition (weeks 1 and 2), 6 of the 10 rats were subjected to T5X, and the remaining 4 rats were subjected to sham T5X (indicated by the arrow) and subsequently studied 1, 3, 7, 14 and 21 days postsurgery. Without beta blockade, heart rate was higher following spinal cord injury (SCI). Beta-adrenergic receptor blockade lowered heart rate more in T5X compared with sham-operated intact rats; however, heart rates remained higher in T5X rats, starting with day 3, compared with intact rats following beta-adrenergic receptor blockade. *P < 0.05, intact vs. T5X; #P < 0.05, no drug vs. β-x.
Figure 3 presents the effects of midthoracic SCI and beta-adrenergic receptor blockade on dP/dt+ (Fig. 3A), and dP/dt− (Fig. 3B) in 10 intact male rats (weeks 1 and 2). Following the experimental procedures in the intact condition, 6 of the 10 rats were subjected to T5 spinal cord transection, and the remaining 4 rats were subjected to sham T5X. Without beta blockade, dP/dt+ and dP/dt− were not different between sham-operated intact and T5X rats. Beta-adrenergic receptor blockade lowered dP/dt+ and dP/dt− in rats with SCI only, and importantly, dP/dt+ and dP/dt− were lower in rats with SCI compared with sham-operated intact rats after beta-adrenergic receptor blockade, documenting LV dysfunction.
Fig. 3.
Cardiac function and the sympathetic support of cardiac function in intact and T5X rats. Effects of midthoracic SCI and beta-adrenergic receptor blockade on dP/dt+ (A) and dP/dt− (B) in 10 intact male rats. Following the experimental procedures in the intact condition (weeks 1 and 2), 6 of the 10 rats were subjected to T5 spinal cord transection, and the remaining 4 rats were subjected to sham T5X (indicated by the arrow) and were subsequently studied 1, 3, 7, 14, and 21 days postsurgery. Without beta blockade, dP/dt+ and dP/dt− were not different between sham-operated intact and T5X rats. Beta-adrenergic receptor blockade lowered dP/dt+ and dP/dt− in rats with SCI only, and importantly, dP/dt+ and dP/dt− were lower in rats with SCI compared with sham-operated intact rats after beta-adrenergic receptor blockade, documenting LV dysfunction. *P < 0.05, intact vs. T5X; #P < 0.05, no drug vs. β-x.
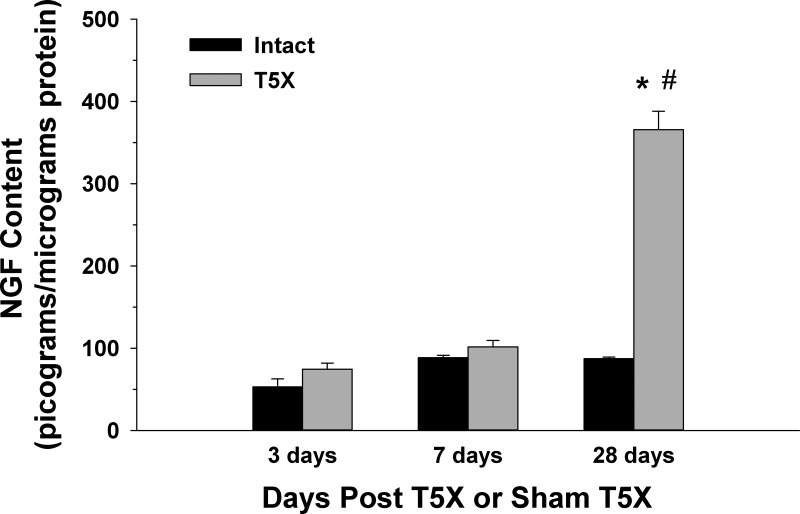
Figure 4 presents right stellate ganglia NGF content 3 days (n = 4/group), 7 days (n = 6/group), and 28 days (n = 6/group) post spinal cord transection or sham transection. NGF content was significantly higher in T5X rats compared with sham-operated intact rats only 28 days after SCI. In addition, NGF content measured 28 days post spinal cord transection was higher than values measured at 3 and 7 days post spinal cord transection or sham transection.
Fig. 4.
Right stellate ganglia NGF content 3 days (n = 4/group), 7 days (n = 6/group), and 28 days (n = 6/group) post T5 spinal cord transection (T5X) or sham T5X (Intact). NGF content was significantly higher in T5X rats compared with intact rats only 28 days after T5X. In addition, NGF content 28 days after T5X was higher compared with values measured at 3 and 7 days post spinal cord transection or sham transection. *P < 0.05, intact vs. T5X; #P < 0.05, 3 or 7 days vs. 28 days.
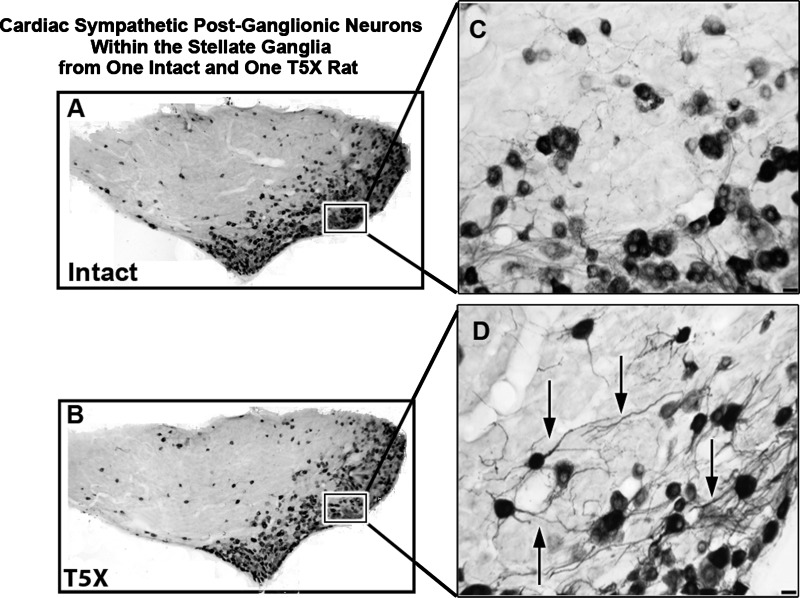
Figure 5 presents a photomicrograph of a 30-μm horizontal section through the right stellate ganglion processed for CTB immunoreactivity from one sham operated intact rat and one T5X rat. CTB was injected into the pericardial sac in 10 male rats [n = 5: T5X and n = 5: intact (sham T5X)] 21–25 days post spinal cord transection or sham transection to retrogradely label sympathetic postganglionic neurons projecting to the heart. CTB immunoreactivity was revealed with Ni-diaminobenzidine.
Fig. 5.
Cardiac sympathetic postganglionic neurons within the stellate ganglia from one intact and one T5X rat. Photomicrographs of a 30-μm horizontal section through the right stellate ganglia processed for cholera toxin B subunit immunoreactivity (black) from one sham-operated intact rat (A) and one T5X rat (B). CTB was injected into the pericardial sac to retrogradely label sympathetic postganglionic neurons projecting to the heart. The higher-power photomicrographs on the right (C and D) are of the boxed areas from A and B, showing details of CTB-labeled neurons and dendritic branching. Note the extensive dendritic branching (arrows) in the T5X rat compared with the intact rat. Bottom right scale bar = 25 μm in C and D.
Figure 6 presents the dendritic branching pattern of cardiac-projecting sympathetic postganglionic neurons within the stellate ganglia from five intact and five T5X rats 28 days after spinal cord transection or sham transection. To identify sympathetic postganglionic neurons, CTB was injected into the pericardial space 21–25 days after spinal cord transection or sham transection to retrogradely label sympathetic postganglionic neurons projecting to the heart. Five to seven days later the ganglia were harvested. This is an important procedure because only cardiac-projecting sympathetic postganglionic neurons were examined. Specifically, Fig. 6 presents the mean number of intersections, per CTB-labeled cardiac-projecting sympathetic postganglionic neuron, between each ring in a series of concentric rings. Sympathetic postganglionic neuron dendritic arborization was increased in T5X rats compared with sham-operated intact rats (Fig. 6). Specifically, the mean number of intersections per neuron at each point (with the exception of the tails) was significantly increased.
Fig. 6.
Structural analysis of cardiac sympathetic postganglionic neurons in intact and T5X rats. Dendritic branching patterns of cardiac sympathetic postganglionic neurons in the stellate ganglia from five intact (sham operated) and five T5X rats were analyzed 28 days after spinal cord transection or sham transection, using the Sholl analysis of dendritic branching. A series of concentric rings calibrated at 10-μm intervals was superimposed on each neuron and centered on the cell body. The number of dendritic intersections within each concentric ring was counted, and the dendritic length was measured. The mean number of intersections per neuron at each point (with the exception of the tails) was significantly increased. *P < 0.05, intact vs. T5X.
Figure 7 presents cardiac sympathetic postganglionic neuron soma area (Fig. 7A), maximum dendritic length (Fig. 7B), and number of intersections/animal (Fig. 7C). Cardiac sympathetic postganglionic soma area (Fig. 7A) was not different between sham-operated intact and T5X rats. In contrast, the maximum dendritic length (Fig. 7B) and the total dendritic field (Fig. 7C) were increased in T5X rats.
Fig. 7.
Morphological features of cardiac sympathetic postganglionic neurons in the stellate ganglia from five intact (sham operated) and five T5X rats were analyzed using the Neurolucida software. Soma area (A), the maximum dendritic length (B), and the number of intersections/animal (C) of sympathetic postganglionic neurons projecting to the heart are presented. Maximum dendritic length and the total dendritic field were increased in T5X rats. *P < 0.05, intact vs. T5X.
DISCUSSION
In this study, the effects of midthoracic SCI on heart rate, cardiac contractility, sympathetic support of inotropic and chronotropic drive, stellate ganglia NGF content, and cardiac sympathetic postganglionic neuron dendritic arborization and morphology were determined. For the evaluation of heart rate, cardiac contractility, and the sympathetic support of inotropic and chronotropic drive, the same animals (n = 10) were studied before (weeks 1 and 2) and after (days 1, 3, 7, 14, and 21) spinal cord transection (n = 6) or sham spinal cord transection (n = 4). T5X was associated with an enhanced sympathetic support of heart rate, dP/dt+, and dP/dt−. Importantly, cardiac function (dP/dt+ and dP/dt−) was lower in rats with midthoracic SCI compared with sham-operated intact rats following blockade of the sympathetic nervous system, documenting LV dysfunction.
For the evaluation of stellate ganglia NGF content, sham-operated intact rats were compared with T5X rats (1, 7, and 28 days post T5 spinal cord transection or sham transection). Following T5 spinal cord transection, there was a significant increase in stellate ganglia NGF content only 28 days posttransection (Fig. 4). For the evaluation of cardiac sympathetic postganglionic neuron dendritic arborization, sham-operated intact rats were compared with T5X rats (4 wk post T5 spinal cord transection). Following T5 spinal cord transection, there was a significant increase in cardiac sympathetic postganglionic neuron dendritic arborization (Fig. 6) and morphology (Fig. 7).
The results from this study suggest a dynamic interaction between the target tissue (heart) and its innervation [sympathetic postganglionic neurons within the stellate ganglion (38, 48)]. This interaction between the target organ and its nerve supply may be mediated by NGF (18). Specifically, NGF is produced in the heart and is transported back to the stellate ganglia, where NGF promotes an increase in cardiac sympathetic innervation density (25, 28). On the basis of the observation that the sympathetic support of heart rate and ventricular function increased as early as 1 to 3 days after the injury but NGF content within the stellate ganglia did not increase until 28 days posttransection (or sometime between days 7 and 28 postinjury), it is hypothesized that the neuroplastic changes in cardiac innervation are a consequence of the enhanced sympathetic drive and cardiac function (i.e., form followed function). However, it is important to note that additional work is required before it can be concluded whether the structural neuroplasticity is a cause or a consequence of the enhanced cardiac function. Nevertheless, a similar dynamic interaction between the target organ and its innervation has been well documented for the urinary bladder. Specifically, bladder enlargement produced by obstruction of the bladder outlet is associated with NGF-induced neuroplasticity in the pathways regulating micturition (6, 45).
The enhanced sympathetic support of cardiac function, increased stellate ganglia NGF content, and structural neuroplasticity within the stellate ganglia are consistent with recent findings that T5X increased cardiac sympathetic tonus (25), altered cardiac electrophysiology (5, 39), and increased cardiac sympathetic preganglionic neuron dendritic arborization (25, 28). T5X also increased the susceptibility to ischemia-reperfusion-induced as well as ischemia-induced sustained ventricular tachycardia (25, 26). The increased susceptibility to the life-threatening arrhythmia was prevented with cardiac β1-adrenergic receptor blockade, documenting that increased sympathetic activity mediates, in part, the increased risk (26). These are important considerations because cardiovascular disease is now a leading cause of death and morbidity for individuals with SCI (4, 8, 22, 50), and cardiac sympathetic hyperactivity is responsible for and/or contributes to the morbidity and mortality associated with cardiovascular disease.
Consistency of the spinal level and completeness of the spinal cord injury, as well as the homogeneity of the subjects, are important factors for SCI research because these factors determine the extent of the injury as well as the variability in outcome measures. In this context, although LV function has been measured in three separate studies for individuals with SCI, two of the three studies investigated individuals with cervical lesions, and the third study combined results from individuals with tetra and paraplegia (7, 9, 29). Even within these groups, the specific level of the lesion, the time since the injury, the completeness of the injury, and the use of medications varied markedly within and across subjects. Accordingly, spared descending systems, demographic variables, and extent of the imposed inactivity also varied markedly within and across subjects. Specifically, lesions above thoracic level 1 completely eliminate inputs from descending bulbospinal pathways to sympathetic preganglionic neurons controlling the heart and upper body vasculature. In contrast, when the spinal cord is injured at T5, the heart and blood vessels innervated by upper thoracic segments remain under brainstem control, whereas the vasculature of the lower body does not. Thus combining results from individuals with different sites or level of lesions may introduce confounding variables. Animal models avoid these confounding variables.
In this context, Eysmann et al. (9) reported similar LV parameters in 17 subjects with cervical SCI (cervical spine injury levels C3 to C8) and in 17 able-bodied individuals. Similarly, de Groot et al. (7) studied a comparable but smaller sample [seven tetraplegics (cervical spine injury levels C5 to C6) and seven able-bodied subjects] and found no differences in LV function. In contrast, Matos-Souza et al. (29) studied a larger sample [34 individuals with SCI (the authors combined the results from 18 tetraplegic and 16 paraplegic individuals) and 31 control individuals] and reported a small decrease in LV function following SCI. Thus, to date, studies investigating the effects of SCI on LV function have yielded equivocal and inconsistent results. Furthermore the influence of the sympathetic nervous system on ventricular function was not investigated. Thus results from the current study may clarify the finding in human subjects, at least in subjects with lesions at T5.
Limitations
Left ventricular pressure and its derivative, dP/dt, are complex functions that are markedly dependent on alterations in ventricular end-diastolic pressure (ventricular preload) and arterial diastolic pressure (ventricular afterload). Specifically, ventricular preload and afterload can alter left ventricular pressure and dP/dt independent of the inotropic state. Following spinal cord injury, venous return and arterial pressure are reduced, and changes in preload and afterload accompany alterations in the inotropic state. Accordingly, in this situation, it is important to consider these factors when interpreting the results from this study (13, 17).
Perspectives
Results from this study, as well as from a number of other studies (6, 45, 54), document a dynamic interaction between the target tissue and its innervation. Specifically, changes in cardiac function altered its sympathetic innervation. In this context, pathological neuroplasticity, following SCI, is associated with many lingering complications, such as chronic pain, spasticity, neurogenic bladder, and autonomic dysreflexia (1,2–3, 12, 15, 16, 20, 21, 24, 34, 36, 43, 51). In many situations, the deleterious structural and functional neuroplasticity is associated with growth-promoting neurotrophins such as NGF. Similarly, T5X increased LV NGF content, LV sympathetic innervation density, and cardiac sympathetic tonus. These structural and functional neuroplastic changes were associated with an increased susceptibility to life-threatening, sustained, ventricular arrhythmias (25, 26, 28).
Although novel insights have been made, critical questions remain, and a number of potential mechanisms must be identified. For example, T5X is also associated with a reduced cardiac parasympathetic drive (25). However, almost nothing more is known about resting or reflex parasympathetic tone or structural alterations (and, potentially, phenotypic alterations) in cardiac parasympathetic preganglionic neurons located within the nucleus ambiguus or postganglionic neurons located within the intracardiac ganglia following T5X. Similarly, further examination of neuroplasticity within distinct and diverse regions of the myocardium as well as in sensory neurons is expected to provide additional insights (30). In addition, to firmly establish NGF as a critical mediator of the stellate ganglia neuroplasticity, additional studies designed to block the expression of NGF are required (30). Finally, although it is clear that NGF can be transported from the heart to the stellate ganglia within 3 days after a myocardial infarction (53), at this point little is known about NGF transport from the heart to the stellate ganglia following spinal cord injury. Furthermore, other than increases in cardiac NGF content and sympathetic innervation density, little is known regarding the local effects of cardiac NGF following SCI. Specifically, studies examining neuronal and other proteins that synthesize, store, remove, and respond to norepinephrine merit further investigation. However, although clinically relevant and physiologically interesting questions remain, important new insights into the dynamic interaction between a target organ and its innervation as well as sympathetic neuroplastic changes that occur following midthoracic SCI are reported.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-088615.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.L.L., H.J., and S.E.D. performed experiments; H.L.L., H.J., and S.E.D. analyzed data; H.L.L., H.J., and S.E.D. interpreted results of experiments; H.L.L. and H.J. prepared figures; H.L.L. and S.E.D. drafted manuscript; H.L.L. and S.E.D. edited and revised manuscript; H.L.L., H.J., and S.E.D. approved final version of manuscript; S.E.D. conception and design of research.
REFERENCES
- 1. Arnold JM, Feng QP, Delaney GA, Teasell RW. Autonomic dysreflexia in tetraplegic patients: evidence for alpha-adrenoceptor hyper-responsiveness. Clin Auton Res 5: 267–270, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Brock JA, Yeoh M, McLachlan EM. Enhanced neurally evoked responses and inhibition of norepinephrine reuptake in rat mesenteric arteries after spinal transection. Am J Physiol Heart Circ Physiol 290: H398–H405, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Brown A, Weaver LC. The dark side of neuroplasticity. Exp Neurol 235: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control Cardiovascular-cardiopulmonary secondary disabilities. In: Proceedings of the First Colloquium on Preventing Secondary Disabilities Among People With Spinal Cord Injuries (CDC, Atlanta, Georgia, 1990), edited by Graitcer PL, Maynard FM. Washington, DC: US Dept of Health and Human Services, 1991, p. 47–54 [Google Scholar]
- 5. Collins HL, Rodenbaugh DW, DiCarlo SE. Spinal cord injury alters cardiac electrophysiology and increases the susceptibility to ventricular arrhythmias. Prog Brain Res 152: 275–288, 2006 [DOI] [PubMed] [Google Scholar]
- 6. de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst 30, Suppl: S71–S77, 1990 [DOI] [PubMed] [Google Scholar]
- 7. de Groot PC, van DA, Dijk E, Hopman MT. Preserved cardiac function after chronic spinal cord injury. Arch Phys Med Rehabil 87: 1195–1200, 2006 [DOI] [PubMed] [Google Scholar]
- 8. DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 74: 248–254, 1993 [PubMed] [Google Scholar]
- 9. Eysmann SB, Douglas PS, Katz SE, Sarkarati M, Wei JY. Left ventricular mass and diastolic filling patterns in quadriplegia and implications for effects of normal aging on the heart. Am J Cardiol 75: 201–203, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Faden AI, Jacobs T, Woods M. Cardioacceleratory sites in the zona intermedia of the cat spinal cord. Exp Neurol 61: 301–310, 1978 [DOI] [PubMed] [Google Scholar]
- 11. Faden AI, Jacobs TF. Cardiac contractility and the spinal sympathetic neuron. Neurol Res 1: 227–237, 1980 [DOI] [PubMed] [Google Scholar]
- 12. Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Frankel HL, Michaelis LS, Golding DR, Beral V. The blood pressure in paraplegia. I. Paraplegia 10: 193–200, 1972 [DOI] [PubMed] [Google Scholar]
- 14. Gootman PM, Gandhi MR, Coren CV, Kaplan NM, Pisana FM, Buckley BJ, Armour JA, Gootman N. Cardiac responses elicited by stimulation of loci within stellate ganglia of developing swine. J Auton Nerv Syst 38: 191–200, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171: 153–169, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol 509: 382–399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobs PL, Mahoney ET, Robbins A, Nash M. Hypokinetic circulation in persons with paraplegia. Med Sci Sports Exerc 34: 1401–1407, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Johnson E, Ferguson I. Nerve growth factor. In: Nerve Growth Factors, edited by Rush R. New York: Wiley, 1989, p. 213–240 [Google Scholar]
- 19. Karim F, Kidd C, Malpus CM, Penna PE. The effects of stimulation of the left atrial receptors on sympathetic efferent nerve activity. J Physiol 227: 243–260, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krassioukov AV, Weaver LC. Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin Exp Hypertens 17: 361–373, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci 19: 7405–7414, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le CT, Price M. Survival from spinal cord injury. J Chronic Dis 35: 487–492, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Llewellyn-Smith IJ, DiCarlo SE, Collins HL, Keast JR. Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J Comp Neurol 488: 278–289, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J Comp Neurol 435: 226–240, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Lujan HL, Chen Y, DiCarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Lujan HL, Janbaih H, Feng HZ, Jin JP, DiCarlo SE. Ventricular function during exercise in mice and rats. Am J Physiol Regul Integr Comp Physiol 302: R68–R74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lujan HL, Palani G, DiCarlo SE. Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am J Physiol Regul Integr Comp Physiol 299: R985–R995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matos-Souza JR, Pithon KR, Oliveira RT, Teo FH, Blotta MH, Cliquet A, Jr, Nadruz W., Jr Altered left ventricular diastolic function in subjects with spinal cord injury. Spinal Cord 49: 65–69, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Moffitt JA. Editorial Focus: role for neural growth factor in autonomically driven arrhythmogenesis? Focus on: “Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization.” Am J Physiol Regul Integr Comp Physiol 299: R983–R984, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Nelson AJ, Iwamoto GA. Reversibility of exercise-induced dendritic attenuation in brain cardiorespiratory and locomotor areas following exercise detraining. J Appl Physiol 101: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Nelson AJ, Juraska JM, Musch TI, Iwamoto GA. Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas. J Appl Physiol 99: 2312–2322, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nelson AJ, Juraska JM, Ragan BG, Iwamoto GA. Effects of exercise training on dendritic morphology in the cardiorespiratory and locomotor centers of the mature rat brain. J Appl Physiol 108: 1582–1590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol 184: 373–380, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Osborn JW, Kuroki MT. Sympathetic signatures of cardiovascular disease: a blueprint for development of targeted sympathetic ablation therapies. Hypertension 59: 545–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pan B, Kim EJ, Schramm LP. Increased close appositions between corticospinal tract axons and spinal sympathetic neurons after spinal cord injury in rats. J Neurotrauma 22: 1399–1410, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Pardini BJ, Lund DD, Schmid PG. Organization of the sympathetic postganglionic innervation of the rat heart. J Auton Nerv Syst 28: 193–201, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Purves D, Snider WD, Voyvodic JT. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature 336: 123–128, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol 285: H2605–H2613, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Salo LM, Campos RR, McAllen RM. Differential control of cardiac functions by the brain. Clin Exp Pharmacol Physiol 33: 1255–1258, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Schmid PG, Dykstra RH, Mayer HE, Oda RP, Donnell JJ. Evidence of nonuniform sympathetic neural activity to heart regions in guinea pigs. Am J Physiol Heart Circ Physiol 237: H606–H611, 1979 [DOI] [PubMed] [Google Scholar]
- 42. Schwartz PJ, Stone HL. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ischemia in conscious dogs with anterior myocardial infarction. Circulation 62: 1256–1265, 1980 [DOI] [PubMed] [Google Scholar]
- 43. Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168: 2269–2274, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Sholl D. The quantification of neuronal connectivity. In: Organization of the Cerebral Cortex, edited by Sholl DA. New York: Wiley, 1956, p. 43–63 [Google Scholar]
- 45. Steers WD, Ciambotti J, Erdman S, de Groat WC. Morphological plasticity in efferent pathways to the urinary bladder of the rat following urethral obstruction. J Neurosci 10: 1943–1951, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Von Euler M, Akesson E, Samuelsson EB, Seiger A, Sundstrom E. Motor performance score: a new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp Neurol 137: 242–254, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Voyvodic JT. Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci 9: 1997–2010, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143: 387–393, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Whiteneck GG, Charlifue SW, Frankel HL, Fraser MH, Gardner BP, Gerhart KA, Krishnan KR, Menter RR, Nuseibeh I, Short DJ. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia 30: 617–630, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Yeoh M, McLachlan EM, Brock JA. Chronic decentralization potentiates neurovascular transmission in the isolated rat tail artery, mimicking the effects of spinal transection. J Physiol 561: 583–596, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoshimoto M, Wehrwein EA, Novotny M, Swain GM, Kreulen DL, Osborn JW. Effect of stellate ganglionectomy on basal cardiovascular function and responses to beta1-adrenoceptor blockade in the rat. Am J Physiol Heart Circ Physiol 295: H2447–H2454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95: 76–83, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin 8: 87–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]