Abstract
Arterial blood pressure and heart rate responses to static contraction of the hindlimb muscles are greater in rats whose femoral arteries were previously ligated than in control rats. Also, the prior findings demonstrate that nerve growth factor (NGF) is increased in sensory neurons-dorsal root ganglion (DRG) neurons of occluded rats. However, the role for endogenous NGF in engagement of the augmented sympathetic and pressor responses to stimulation of mechanically and/or metabolically sensitive muscle afferent nerves during static contraction after femoral artery ligation has not been specifically determined. In the present study, both afferent nerves and either of them were activated by muscle contraction, passive tendon stretch, and arterial injection of lactic acid into the hindlimb muscles. Data showed that femoral occlusion-augmented blood pressure response to contraction was significantly attenuated by a prior administration of the NGF antibody (NGF-Ab) into the hindlimb muscles. The effects of NGF neutralization were not seen when the sympathetic nerve and pressor responses were evoked by stimulation of mechanically sensitive muscle afferent nerves with tendon stretch in occluded rats. In addition, chemically sensitive muscle afferent nerves were stimulated by lactic acid injected into arterial blood supply of the hindlimb muscles after the prior NGF-Ab, demonstrating that the reflex muscle responses to lactic acid were significantly attenuated. The results of this study further showed that NGF-Ab attenuated an increase in acid-sensing ion channel subtype 3 (ASIC3) of DRG in occluded rats. Moreover, immunohistochemistry was employed to examine the number of C-fiber and A-fiber DRG neurons. The data showed that distribution of DRG neurons with different thin fiber phenotypes was not notably altered when NGF was infused into the hindlimb muscles. However, NGF increased expression of ASIC3 in DRG neurons with C-fiber but not A-fiber. Overall, these data suggest that 1) NGF is amplified in sensory nerves of occluded rats and contributes to augmented reflex sympathetic and blood pressure responses evoked by stimulation of chemically, but not mechanically, sensitive muscle afferent nerves and 2) NGF likely plays a role in modulating the muscle metaboreflex via enhancement of ASIC3 expression in C-fiber of DRG neurons.
Keywords: nerve growth factor, muscle pressor reflex, ASIC3, peripheral arterial disease, hindlimb ischemia
during exercise, an increase in sympathetic nervous activity (SNA) leads to rises in blood pressure (BP) and heart rate (HR), myocardial contractility, and peripheral vasoconstriction (46, 53). A basic mechanism termed the “exercise pressor reflex” (4, 32, 35, 36) is thought to contribute to sympathetic engagement during exercise. This autonomic reflex is initiated as afferents arising from contracting skeletal muscle are engaged (18, 32, 35). This system responds to mechanical deformation of the muscle afferents receptive field as well as to muscle by-products (18). Group III afferents are predominantly mechanically sensitive (mechanoreceptor) and Group IV afferents are predominantly metabosensitive (metaboreceptor) (20). When these receptors are stimulated, thin fiber muscle afferent nerves are engaged and cardiovascular nuclei in the brainstem are activated to increase SNA, BP, and HR (35). In addition, the sympathetic and cardiovascular responses to exercise are modulated by the “central command” (12, 57) and the arterial baroreflex (9, 41).
These reflex mechanisms found in healthy individuals are altered in cardiovascular diseases in the processing of muscle afferent signals via afferent nerve receptors (10, 22, 25, 26, 47, 49, 51, 60). For example, when the exercise pressor reflex is activated in patients with peripheral artery disease (PAD), increases in SNA, BP, and HR are exaggerated (1, 2). As noted, PAD, caused by a restriction of the blood vessels in the lower limbs, is typically common in older adults (5, 37, 38). The most common symptom of this disease is intermittent claudication, which frequently occurs during physical activity but is relieved promptly by rest (43). Thus a rat model of femoral artery ligation that impairs reserve capacity of limb blood flow during exercise has been employed to study PAD in humans (58). Using this rat model, a number of prior studies have further demonstrated that the SNA and pressor responses to static muscle contraction and stimulation of muscle metabolite receptors, i.e., acid sensing ion channels (ASICs), purinergic P2X, and capsaicin-sensitive TRPV1 are amplified in occluded rats compared with control rats (25, 26, 51, 60). Nevertheless, the underlying mechanisms by which femoral occlusion augments responsiveness of SNA and BP to activation of muscle mechanosensitive and metabosensitive afferents remain to be determined.
Prior studies demonstrated that femoral artery occlusion elevates the levels of nerve growth factor (NGF) in the hindlimb muscles and DRG neurons of rats (8, 62). NGF can induce expression of ASIC3, P2X3, and TRPV1 receptors in the DRG neurons (31, 42). Those receptors have been reported to respond to muscle metabolites (i.e., lactic acid and ATP) (13, 21, 23). Specifically, the prior studies suggest that ASIC3 and P2X are engaged in the muscle metaboreflex (15, 23, 34). In addition, NGF can change the neuronal phenotype such as capsaicin-insensitive sensory neurons (17), which possibly alters afferent mediated response in the processing of sensory signals. Thus the first hypothesis of this study was that NGF contributes to augmented reflex sympathetic and blood pressure responses evoked by stimulation of metabolically sensitive muscle afferent nerves.
Among ASICs, ASIC3 is found predominantly on sensory neurons and maintains functional channels in response to proton concentration fluctuation (24, 54–56). The pH range required to activate ASIC3 is approximately 6.5–7.0 (6, 7), which is close to what is observed in exercising muscle and/or moderately ischemic tissues (28, 29, 45, 63). Additionally, it is well established that lactic acid stimulates ASIC3 receptor and that femoral artery occlusion (i.e., 24–72 h) increases expression of ASIC3 receptor and its responsiveness in the DRG neurons (25, 61). However, the role for NGF in regulating ASIC3 expression was not previously determined. Furthermore, whether NGF can selectively increase expression of ASIC3 in DRG neurons that project C-fiber/A-fiber afferents needed to be examined. Thus the second hypothesis was that NGF plays a role in exaggeration of the muscle metaboreflex via enhancement of ASIC3 expression in C-fiber afferent neurons.
METHODS
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State College of Medicine and complied with the National Institutes of Health (NIH) guidelines.
Ligation of femoral artery.
The surgical procedures were performed on male Sprague-Dawley rats (5–7 wk old) as previously described (25, 60, 62). Under inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen), the rat's femoral artery on one limb was surgically exposed, dissected, and ligated ∼3 mm distal to the inguinal ligament. The same procedures were performed on the other limb of some rats except that a suture was placed below the femoral artery but was not tied; this served as the control. Then, 6, 24, and 72 h were allowed for recovery before the experiments began.
Examination of muscle pressor reflex.
A total of 93 control and occluded rats were anesthetized with a mixture of 2–5% isoflurane and oxygen and ventilated as previously described (25, 60). The right jugular vein and common carotid artery were cannulated to deliver fluids and to connect a pressure transducer for measurement of arterial blood pressure, respectively. A catheter (PE10) was then inserted into the femoral artery and the tip of the catheter was placed in the popliteal artery for injection of drugs into the arterial blood supply of the hindlimb muscles. During the experiments, baseline BP and fluid balance were maintained with a continuous infusion of saline. Body temperature was continuously monitored and maintained at 37.5–38.5°C with a heating pad and external heating lamps.
A laminectomy was performed to expose the lower lumbar and upper sacral portions of the spinal cord after the rats were placed in a spinal unit (Kopf Instruments). The spinal roots were exposed and the right L4 and L5 ventral roots were visually identified with assistance of an anatomical microscope (Cooper Surgical). The peripheral ends of the transected L4 and L5 ventral roots were then placed on bipolar platinum stimulating electrodes. A pool was formed by using the skin and muscle on the back and the exposed spinal region was filled with warmed (37°C) mineral oil.
In a subset group of experiments, a bundle of the renal nerves on the left side was carefully dissected from other connective tissues. A piece of laboratory film was placed under the isolated nerves, and two tips of a bipolar electrode used to record neural activity were placed between the nerves and the film; these were embedded in a silicone gel. Once the gel hardened, the silicone rubber was fixed to the surrounding tissue with a glue containing α-cyanoacrylate. The skin on the back was used to form a pool that was filled with warm (37°C) mineral oil. The renal SNA (RSNA) signal was amplified with an amplifier (P511, Grass Instruments) with a band-pass filter of 300 Hz in low-cut frequency and of 3 kHz in high-cut frequency and recorded as previously described (25, 60).
Decerebration was performed as previously described (10, 25, 60) to avoid the confounding effects of anesthesia on the reflex pressor response. A transverse section was made anterior to the superior colliculus and extending ventrally to the mammillary bodies. All brain tissues from rostral to the section were removed. Following this procedure, the anesthesia was withdrawn from the rats. The calcaneal bone of right hindlimb was cut and its tendon was attached to a force transducer (Grass FT10); the knee joints were then secured by clamping the patellar tendon to a spinal unit. A recovery period of 60 min was allowed before the experiment.
In the first group of experiments, to evoke both mechanocomponents and metabocomponents of the exercise pressor reflex, static muscle contractions were induced by electrical stimulation of the L4 and L5 ventral roots (30 s, 3-times motor threshold with a period of 0.1 ms at 40 Hz) in control rats and occluded rats (n = 5 in each group). The reflex BP and HR responses to contraction were examined. In an additional group, the femoral occlusion was performed and 10 μg of NGF-Ab (Abcam) was administered into the hindlimb muscles (the superficial white portion of the gastrocnemius muscle) to neutralize effects of NGF 24 h prior to experiments in five rats. Then, static muscle contraction was induced by electrical stimulation of the L4 and L5 ventral roots. This dose of NGF-Ab was selected on the basis of the previous data demonstrating that 10 μg of NGF-Ab effectively attenuated the increases of NGF in the DRG induced by 24 h of femoral artery ligation.
In the second group of experiments, passive tendon stretch was performed in control rats (n = 8 in each group, a total of 24 rats) and rats whose femoral artery was ligated for 6, 24, and 72 h (n = 6, 10, and 8, respectively) before the experiments. Passive tendon stretch (250 and 500 g tension) was produced manually over ∼5 s by using a rack and pinion attached to the Achilles' tendon of the decerebrated rats. Each bout of muscle stretch was maintained for 30 s after 250 g/500 g tension was achieved. Muscle stretch was used to activate the mechanoreceptor component of the exercise pressor reflex. The purpose of this study was to examine if vascular insufficiency seen in occluded rats altered sympathetic and pressor responses evoked by stimulation of mechanically sensitive muscle afferent nerves alone. Also, in order to look at effects of NGF induced by vascular insufficiency, 10 μg of NGF-Ab was administered into the hindlimb muscles with femoral occlusion 24 h prior to experiments in six rats. Next, the SNA and BP responses to muscle stretch were examined.
In the third group of experiments, 4 μmol/kg of lactic acid was administered into the femoral artery of control limb and occluded limb in eight rats to examine the effects of femoral artery ligation on the reflex sympathetic and BP responses to stimulation of chemically sensitive muscle afferent nerves. In this group, femoral occlusion was performed on one leg and sham procedure was performed on the opposite leg as the control. Also, 10 μg of NGF-Ab was administered into the hindlimb muscles of the occluded legs of eight rats 24 h prior to experiments. Then, 4 μmol/kg of lactic acid was arterially injected to examine effects of blocking NGF on SNA and pressor responses to stimulation of ASICs. In addition, the same dose of lactic acid was injected into the arterial line of five rats after cutting the femoral and sciatic nerves to confirm that the SNA and pressor responses were due to stimulation of afferents within the hindlimb. The injection volume was adjusted to 0.1–0.2 ml according to rat's body weight. The duration of the injection was 1 min.
All measured data of RSNA, BP, and HR were continuously recorded and stored on a computer with PowerLab system (AD Instruments, Castle Hill, New South Wales, Australia). Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. HR was calculated on a basis of beat to beat from the arterial pressure pulse. The peak responses of MAP and HR were determined by the peak change from the control value. RSNA signals were transformed into absolute values, integrated over 1 s interval, and subtracted by 1 s of integrated background noise. To quantify RSNA response to muscle stretch and lactic acid injection, baseline values were obtained by taking the mean value for the 30 s immediately before each injection and by ascribing the mean value of 100%, and relative change from baseline during the injection was then evaluated.
Administration of NGF.
The micro-osmotic pump (Alzet model 1003D, 3-day delivery; length 1.5 cm and diameter 0.6 cm) containing NGF or saline was implanted subcutaneously in the hindlimbs of 12 healthy rats under anesthesia (26, 62). The pumps were placed in the femoral triangle region and outlet of the pump was 2–3 mm distal to the inguinal ligament. Then, NGF was delivered at a rate of 0.25 μg/h to one leg. A total of 18 μg NGF was delivered over 72 h. Saline in the same volume was delivered at the same infusion rate on the opposite leg as the control. Previous experiments have shown that NGF delivered in this manner can effectively elevate the levels of NGF in DRG and increase responsiveness and protein expression of sensory nerve receptors (26, 62). Therefore, the levels of ASIC3 protein in DRG neurons were examined after infusion of NGF into hindlimb muscles using the Western blot methods in the present study. In addition, the dual immunofluorescence technique was employed to examine if NGF can alter distribution of DRG neurons with the two fiber phenotypes: C-fiber and A-fiber.
Western blot analysis.
The six rats were used to examine expression of ASIC3 protein in lumbar (L4–L6) DRGs of control and limbs that were infused with NGF. Western blot methods were performed as previously described (25, 27). In brief, DRGs of the rats were removed. All DRG tissues from individual rats were sampled for Western blot analysis. Total protein was then extracted by homogenizing DRG sample in ice-cold radioimmunoprecipitation assay buffer containing 25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) with protease inhibitor cocktail kit (Sigma-Aldrich, St. Louis, MO). The lysates were centrifuged at 15,000 g for 15 min at 4°C; the supernatants were collected for measurements of protein concentrations using a bicinchoninic acid assay reagent kit (Pierce Biotechnology, Rockford, IL) and then stored in −80 °C for later use.
After being denatured by heating at 95°C for 5 min in an SDS sample buffer (Cell Signaling Technology, Danvers, MA), the supernatant samples containing 20 μg of protein were loaded onto 10–12% SDS-polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA) and then electrically transferred to a polyvinylidene fluoride membrane (GE Water and Process Tech, Trevose, PA). The membrane was blocked in 5% nonfat milk in 0.1% Tween-TBS buffer for 1 h and was then incubated overnight with primary antibody: rabbit anti-ASIC3 at 1:200 dilutions (Sigma-Aldrich).
After being fully washed, the membrane was incubated with horseradish peroxidase-linked anti-rabbit secondary antibody at 1:1,000 dilutions and visualized for immunoreactivity using an enhanced chemiluminescence system (Cell Signaling Technology). The membrane was stripped and incubated with mouse anti-β-actin (Sigma-Aldrich) to show equal loading of the protein in the Western blot analysis. The densities of ASIC3 and β-actin bands were determined using the NIH Scion Image Software.
Fluorescence immunohistochemistry.
The six rats were anesthetized by inhalation of an isoflurane-oxygen mixture and then transcardially perfused with 200 ml of ice-cold saline containing 1,000 units heparin followed by 500 ml of 4% freshly prepared ice-cold paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). L4–L6 DRGs of control limbs and limbs infused with NGF were immediately dissected out and immersed in the same fixative at 4°C for 2 h. The tissues were then stored in PBS containing 30% sucrose overnight. Then, a cryostat was used to obtain 10 μm of DRG sections.
DRG sections were fixed in 4% of paraformaldehyde in PBS for 10 min at room temperature. After being washed with PBS, the tissues were permeabilized, blocked in 0.3% Triton X-100 in PBS supplemented with 5% goat serum for 1 h, and then incubated with guinea pig polyclonal anti-ASIC3 (1:250, Neruomics) antibody overnight at 4°C. After being washed in PBS, the sections were incubated with goat anti-guinea pig fluorescein isothiocyanate (FITC) labeled secondary antibody (1:200, Neruomics) for 2 h at room temperature.
To examine localization of ASIC3 within C-fiber and A-fiber DRG neurons, the sections were incubated with the second primary antibody (mouse anti-peripherin at 1:200, Sigma; and anti-NF200 at 1:200 Abcam) overnight. Peripherin and NF200 are used to label neurons with C-fiber and A-fiber, respectively. Briefly, after incubation, the sections were washed and incubated for 1 h at room temperature with secondary antibody (Alexa Fluor-594 conjugated goat anti-mouse IgG, dilution: 1:200) for 2 h at room temperature. Then, the sections were washed in PBS and coverslipped.
FITC- and Alexa Fluor-594-labeled DRG neurons were examined using a Nikon Eclipse 80i microscope with appropriate filters, and the images were stored digitally on a computer. As described previously (25), at least five sections containing L4–L6 DRGs per rat were randomly chosen for analysis of FITC and Alexa Fluor-594 staining intensity. The sections were coded, and immunolabeling was examined in a blinded fashion. A threshold value of staining intensity was set according to the mean staining intensity of background using the Nis-Elements software (Nikon). Cells with >1.75 times of background intensity were considered to be positive. The number of total ASIC3 immunostaining and peripherin/NF200 positive neurons was counted in each section. Percentages of double (FITC and peripherin/NF200)-labeled neurons were calculated as:(total number of double-labeled cells × 100)/(total number of peripherin/NF200 positive cells). The majority of DRG neurons showed a clear nucleus and perimeter and were counted. To minimize the possibility of counting a single DRG neuron more than once, DRG sections were collected on five glass slides in series, and tissues from one of the slides were processed for immunocytochemical analysis.
Data statistical analysis.
All statistical analyses were performed using SPSS for Windows version 15.0 (SPSS Sci, Chicago, IL). Two-way repeated-measures ANOVA was used to compare variables for MAP and RSNA responses to tendon stretch (control vs. occlusion for two levels of tension). In other groups of the reflex experiments, comparisons of variables for MAP, HR, and RSNA were performed using one-way repeated-measures ANOVA, followed by Tukey post hoc test as appropriate. Comparisons of variables for ASIC3 optical density (Western blot analysis) and percentages of double-labeled neurons for ASIC3 and peripherin/NF200 (immunohistochemistry) were performed using one-way ANOVA. All values are presented as means ± SE. For all analyses, differences were considered significant at P < 0.05.
RESULTS
Effects of NGF on muscle pressor reflex.
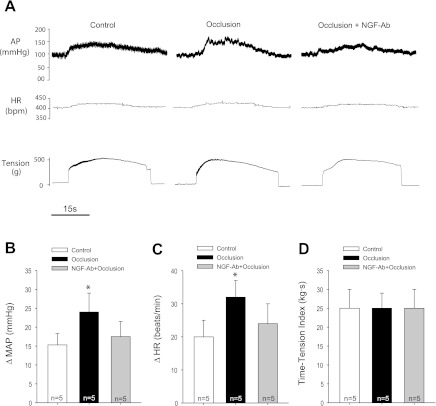
In the first group of experiments, in order to activate muscle mechanically and chemically sensitive sensory nerves, static muscle contractions were induced by electrical stimulation of the L4 and L5 ventral roots in control rats and occluded rats. The reflex BP, HR, and muscle tension responses to contraction were examined (Fig. 1, A–D). The raw data that represent cardiovascular responses to muscle contraction in control and occlusion are shown in Fig. 1A. Twenty-four hours of the femoral artery ligation augmented the responses of MAP and HR to contraction. Those responses were 15 ± 3 mmHg and 20 ± 5 beats/min (bpm) in control (n = 5), and 24 ± 5 mmHg (P < 0.05 vs. control) and 32 ± 5 bpm (P < 0.05 vs. control) in arterial occlusion (n = 5). Muscle tension development was similar in both groups (Fig. 1D). There were no significant differences in baseline MAP (90 ± 5 mmHg in control vs. 89 ± 5 mmHg in occlusion, P > 0.05) and HR (395 ± 10 bpm in control vs. 390 ± 8 bpm in occlusion, P > 0.05) in both groups. In addition, the previous injection of NGF-Ab into the hindlimb muscles attenuated enhancement of the pressor and HR responses induced by muscle contraction in occluded rats (Fig. 1, B and C). Also, the original traces in Fig. 1A show the raw data that represent cardiovascular responses to muscle contraction in an occluded rat infused with NGF-Ab.
Fig. 1.
Effects of blocking nerve growth factor (NGF) on the muscle pressor reflex. Muscle contraction was evoked by electrical stimulation of the L4 and L5 ventral roots. Mean arterial pressure (MAP) and heart rate (HR) to static muscle contraction were examined in control rats and rats with 24 h of femoral artery ligation. A: raw data showing that arterial occlusion increased arterial blood pressure (AP) and HR responses to contraction. Prior injection of NGF antibody (10 μg) into the hindlimb muscles attenuated enhancement of the pressor response induced by contraction in occluded rats. B and C: average data for the responses of MAP and HR. *P < 0.05, vs. control and NGF-Ab plus occlusion. There was no significant difference in MAP and HR responses in control rats and occluded rats with NGF antibody injection. D: similar muscle tension development was seen in three groups.
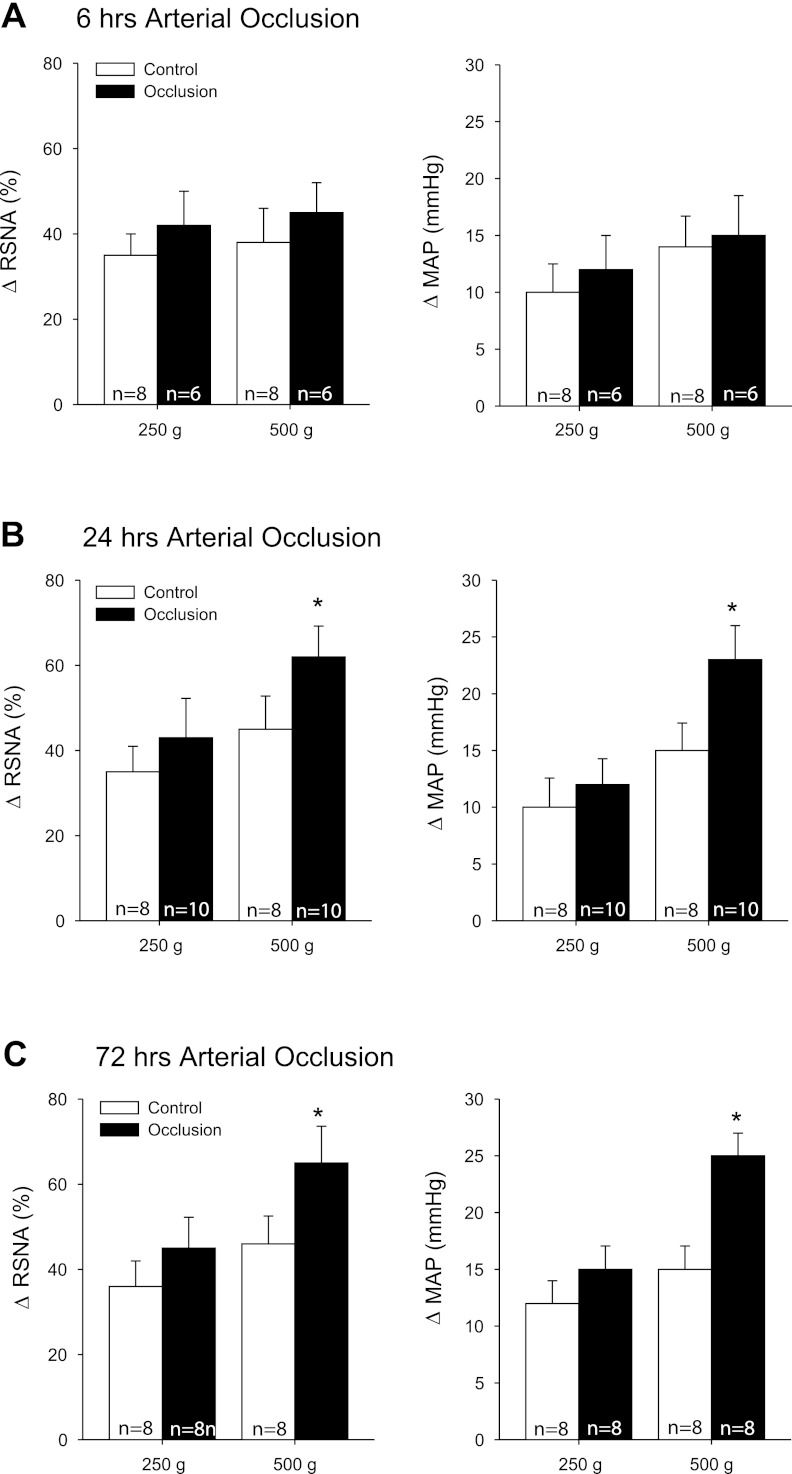
In the second group of experiments, passive tendon stretch was performed in control rats (n = 8 in each group) and rats whose femoral artery was ligated for 6 h (n = 6), 24 h (n = 10), and 72 h (n = 8) before the experiments (Fig. 2, A–C). Their basal MAPs were 95 ± 5, 97 ± 7, and 93 ± 5 mmHg in control rats and 99 ± 6, 90 ± 5, and 89 ± 5 mmHg in occluded rats (P > 0.05, vs. respective control). The basal HRs were 400 ± 12, 405 ± 15, and 398 ± 10 bpm in control and 396 ± 10, 400 ± 12, and 395 ± 20 bpm in occlusion (P > 0.05, vs. respective control). Treatment with 24 and 72 h of femoral occlusion significantly increased the responses of RSNA and MAP evoked by stretch with 500 g of muscle tension. No significant differences in HR response were seen between the two groups. Moreover, Fig. 3, A and B, shows typical traces and average data showing that 24 h of occlusion augmented the responses of RSNA and MAP; however, there were no significant differences in changes of RSNA and MAP in occluded rats with and without application of NGF-Ab into the hindlimb muscles as 500 g of tension was employed to induce tendon stretch.
Fig. 2.
Effects of femoral artery occlusion on the sympathetic and pressor responses evoked by stimulation of mechanically sensitive muscle afferent nerves. Responses of renal sympathetic nerve activity (RSNA) and MAP to muscle stretch were observed in control rats and rats whose femoral artery was ligated for 6 (A), 24 (B), and 72 h (C) before the experiments. Here 250 and 500 g of muscle tension was loaded to evoke passive tendon stretch. *P < 0.05, vs. control.
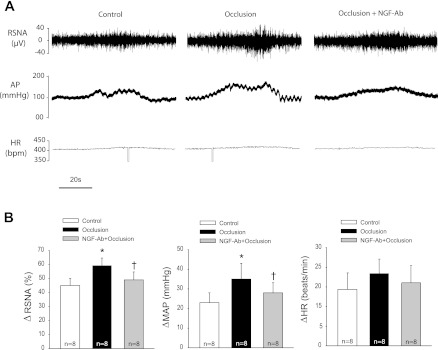
Fig. 3.
Effects of neutralizing NGF on sympathetic and pressor responses evoked by activation of the muscle mechanoreflex. A (original traces) and B (averaged data): RSNA, AP, and HR were examined when muscle stretch was induced by 500 g of tension in control rats and in rats with 24 h of femoral artery occlusion. Significant differences were observed in RSNA and MAP responses to muscle stretch between control and occluded groups. In addition, 24 h of femoral artery ligation was performed on six rats who received NGF-Ab injection. NGF-Ab failed to attenuate occlusion-enhanced responses. Similar time-tension index (TTI) was seen in three groups.
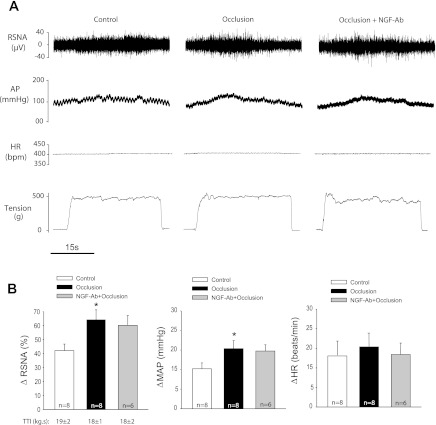
In the third group of experiments, 4 μmol/kg of lactic acid was administered into the femoral artery of control limb and occluded limb with 24 h of femoral artery ligation to examine the effects of femoral artery ligation on the reflex sympathetic and BP responses to stimulation of ASICs. Femoral artery ligation significantly enhanced RSNA and BP responses evoked by lactic acid (Fig. 4, A and B). The RSNA and MAP responses to arterial injection of lactic acid were increased by 45 ± 5% and 23 ± 5 mmHg (from baseline of 102 ± 8 mmHg) in eight control limbs and by 59 ± 5% and 35 ± 8 mmHg (from baseline of 105 ± 10 mmHg) in eight occluded limbs (P < 0.05, occlusion vs. control for both RSNA and MAP responses), respectively. Also, NGF-Ab was administered into the hindlimb muscles of the occluded leg 24 h prior to experiments. Then, 4 μmol/kg of lactic acid was arterially injected to examine the effects of blocking NGF on RSNA and pressor responses to stimulation of chemically sensitive afferent nerves. The results showed that NGF-Ab attenuated enhancement of sympathetic and pressor responses induced by arterial injection of lactic acid (Fig. 4, A and B). After a pretreatment of NGF-Ab, the RSNA and MAP responses to lactic acid were 47 ± 5% and 27 ± 6 mmHg (from baseline of 98 ± 5 mmHg). In addition, there were no differences in basal MAP before injection of lactic acid among three groups. No significant differences in baseline HRs were observed among three groups (395 ± 8, 399 ± 15, and 405 ± 16 bpm, P > 0.05 among groups), and femoral occlusion did not significantly alter HR response evoked by injection of lactic acid.
Fig. 4.
Effects of neutralizing NGF on sympathetic and pressor responses evoked by stimulation of muscle chemically sensitive afferent nerves. A (original traces) and B (averaged data) show changes in RSNA and MAP in response to arterial injection of lactic acid (4 μmol/kg) in control limbs and occluded limbs (24 h) with and without NGF-Ab administered previously into the hindlimb muscles. *P < 0.05 vs. control. †P < 0.05 vs. occlusion without NGF-Ab. There were no significant differences in RSNA and MAP responses in control and occlusion plus NGF-Ab.
Furthermore, sections of the sciatic and femoral nerves attenuated lactic acid-induced reflex responses (ΔRSNA 42 ± 3% and ΔMAP: 27 ± 3 mm Hg in control; ΔRSNA: 2±1% and ΔMAP 3 ± 1 mmHg after nerve section, respectively; n = 5, P < 0.05, control vs. after nerve section for both ΔRSNA and ΔMAP).
Effects of NGF on protein levels of ASIC3 in DRG.
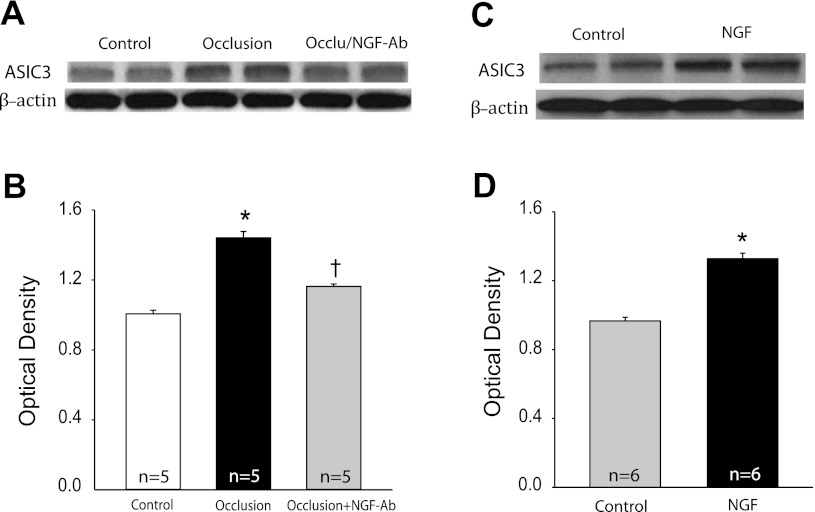
To better determine the role of NGF in regulating afferent nerve ASIC3 expression, Western blot analysis was used to examine the levels of ASIC3 protein in DRG tissues of occluded legs infused with saline and NGF-Ab (Fig. 5, A and B). Figure 5B shows blocking NGF with NGF-Ab significantly attenuated occlusion-augmented expression of ASIC3 in DRG. The intensity of the signal in lumbar DRG neurons was 1.01 ± 0.02 in control limbs and 1.44 ± 0.03 without NGF-Ab and 1.16 ± 0.01 with NGF-Ab in occluded limbs (P < 0.05 vs. without NGF; n = 5 in each group). Similarly, the levels of ASIC3 protein in DRG tissues of control legs infused with saline and legs infused with NGF were also examined (Fig. 5, C and D). Figure 5D further shows that NGF infused into the hindlimb muscles significantly increased expression of ASIC3 in DRG neurons compared with controls. The intensity of the signal in lumbar DRG neurons in NGF-infused limbs was ∼1.37-fold greater than that in control limbs (optical density, 1.33 ± 0.03 with NGF infusion vs. 0.97 ± 0.02 in control, P < 0.05; n = 6 in each group).
Fig. 5.
Effects of NGF on expression of ASIC3. A: dual representative bands of ASIC3 expression for control limbs and occluded limbs without and with NGF-Ab infusion. Beta-actin was used as control for equal loading of protein. B: average data. The optical density was expressed in arbitrary units normalized against a control sample. The number of limbs = 5 for each group. *P < 0.05 vs. control. †P < 0.05 vs. without NGF-Ab. C and D: dual representative bands of ASIC3 expression and average data showing that ASIC3 was increased in NGF infusion experiment compared with control. The number of limbs = 6 for each group. *P < 0.05 vs. control. One-way ANOVA was performed to compare variables for ASIC3 optical density in those experiments (B and D).
Effects of NGF on expression of ASIC3 within DRG neurons with C- and A-fibers.
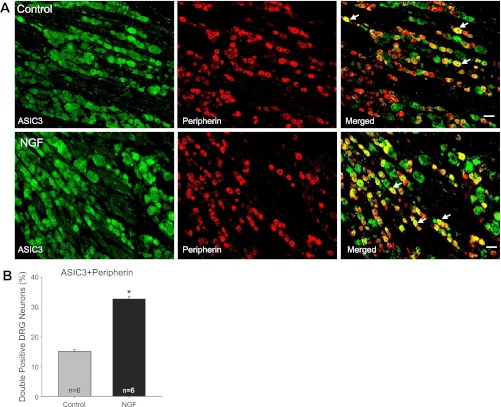
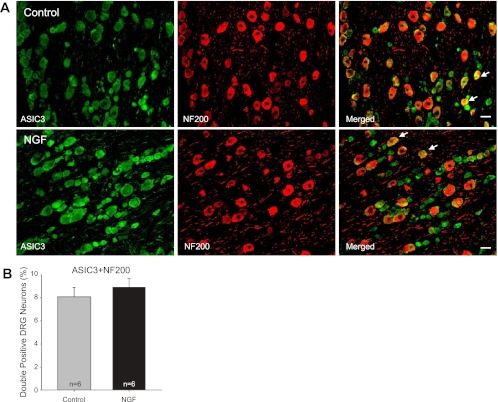
Fluorescence immunohistochemistry was employed to examine the labeling of peripherin and NF200. Figures 6 and 7 show that no differences were seen in the number of peripherin and NF200 positive DRG neurons in control and NGF infusion groups (n = 6 in each group), respectively. The positive DRG neurons for peripherin are 65 ± 2% in control and 67 ± 3% after NGF; the positive DRG neurons for NF200 are 34 ± 1% in control and 36 ± 2% after NGF. This result suggests that NGF has no distinct effects on the distribution of DRG neurons of the two different fiber phenotypes.
Fig. 6.
Colocalization of ASIC3 and peripherin. Fluorescence immunohistochemistry was employed to examine double labeling of ASIC3 and peripherin. In this experiment, peripherin was used to label DRG neurons that project C-fiber. A: representative photomicrographs show ASIC3 and peripherin staining in DRG neurons of a control limb (top panel) and a limb with NGF infusion (bottom panel). Arrows indicate representative cells positive for both ASIC3 and peripherin after they were merged. The number of double labeling DRG neurons is greater in infused limbs than that in control limbs. Scale bar = 50 μm. B: average data for colocalization of ASIC3 and peripherin, showing that the percentage of double labeling DRG neurons for ASIC3 and peripherin is greater in NGF infusion limbs than that in control limbs. *P < 0.05 vs. control (one-way ANOVA). No differences were observed in the number of peripherin positive DRG neurons in control and NGF infusion groups.
Fig. 7.
Colocalization of ASIC3 and NF200. Dual fluorescence immunohistochemistry was employed to examine labeling of ASIC3 and NF200. Note that NF200 was used to identify A-fiber of DRG neurons. A: photomicrographs are representative to illustrate staining of ASIC3 and NF200 in DRG neurons of a control limb (top panel) and a limb infused with NGF (bottom panel). Arrows indicate examples for merged ASIC3 and NF200 positive cells. There were no differences in the number of double staining of ASIC3 and NF200 in DRG neurons of control and infused groups. Scale bar = 50 μm. B: average data for colocalization of ASIC3 and NF-200, showing that there are no differences observed in the number of double staining for ASIC3 and NF200 in DRG neurons of control and NGF infusion groups. Note that no differences were observed in the number of NF200 positive DRG neurons in control and NGF infusion groups. One-way ANOVA was performed to compare variables for percentages of double-labeled neurons for ASIC3 and NF200.
In this experiment, the existence of ASIC3 within DRG neurons that project C- and A-fiber afferents was further determined. The double immunofluorescence technique was used to examine colocalization of fluorescent ASIC3 and peripherin (Fig. 6)/NF200 (Fig. 7) immunoreactivity in the DRG neurons of control limbs and limbs that were infused with NGF. The appearance of ASIC3 and peripherin/NF200 within DRG neurons was characterized by fluorescent green and red color, respectively (Fig. 6 and 7). The representative photomicrographs show that ASIC3 staining appears in C- and A-fiber of DRG neurons in both control group and group with NGF infusion (Fig. 6 and 7).
Figure 6B shows that the percentage of double-labeled DRG neurons with ASIC3 and peripherin was significantly greater in the NGF infused limbs than in that of controls. They were 15 ± 1% in controls and 32 ± 1% in the NGF infused groups (n = 6 in each group, P < 0.05 vs. control). Also, representative photomicrographs in Fig. 6A illustrate that ASIC3 was observed in more DRG neurons with C-fiber projection in NGF infused limbs than in control limbs.
Furthermore, Fig. 7B represents average data showing that the percentage of double-labeled neurons with ASIC3 and NF200 was similar in both experimental groups. They were 8 ± 1% in control group and 9 ±1% in the NGF infused group (n = 6 in each group, P > 0.05 vs. control). In addition, photomicrographs in Fig. 7A demonstrate that A-fiber of DRG neurons that includes ASIC3 staining was similar in both control and NGF infused groups.
DISCUSSION
The data of this study demonstrate that NGF neutralization significantly attenuated femoral occlusion-augmented reflex sympathetic and BP responses evoked by static contraction and lactic acid but not by muscle stretch. Given that administration of NGF-Ab into the hindlimb muscles significantly attenuates occlusion-enhanced protein levels of ASIC3 in DRG tissues, these results suggest that NGF increased in sensory nerves of occluded limbs contributes to augmented reflex SNA and BP responses to stimulation of chemically, but not mechanically, sensitive muscle afferent nerves. In addition, NGF infused into the hindlimb muscles significantly increased the protein levels of ASIC3 in DRG and selectively increased expression of ASIC3 in DRG neurons that project C-fiber afferents. Thus the data support the hypothesis that NGF plays a role in exaggeration of the muscle metaboreflex via enhancement of ASIC3 expression in C-fiber afferent neurons.
Intermittent claudication is commonly seen in patients with PAD. The exercise pressor reflex is exaggerated in this disease. Previously, a rat model of femoral artery occlusion has been well established to study PAD, and a recent study using this model has illustrated that the pressor response to static muscle contraction is increased in rats with femoral occlusion (51). Furthermore, the upregulation of muscle metabolite receptors in sensory nerves is considered a mechanism that contributes to the augmented responses following femoral occlusion (25, 26, 60, 61). New findings provided in the present study suggest that NGF plays an important role in regulating the exaggerated exercise pressor reflex in PAD by increasing metabolic ASIC3 in afferent nerves.
NGF is synthesized in various cells including normal skeletal muscle tissue (59). Previous studies indicate that hindlimb ischemia induces an increase in endogenous NGF production in skeletal muscle (8). NGF is taken up by nerve terminals and retrogradely transported to the cell bodies once it is released into the muscle interstitium. Generally, the accumulated neurotrophin in the cell bodies is regarded as a requirement for physiological or pathological responsiveness in neurons (3). In addition, measurement of NGF in DRG tissue of rats with femoral artery occlusion revealed that 24 and 48 h of occlusion significantly increases NGF levels in DRG sensory neurons (62). Similarly, 24 to 72 h of ligation increases ASIC3 expression and function in DRG neurons (25, 61), suggesting that ASIC3 protein expression in DRG innervating the hindlimb muscles is likely to be regulated by NGF. Other studies have suggested that NGF is responsible for basal ASIC3 expression of DRG neurons via a TrkA activated phospholipase C/protein kinase C pathway involved in processing inflammation-induced sensitization to pain (30, 31). The results of the present study demonstrate that NGF infused into the hindlimb muscles specifically increases expression of ASIC3 in DRG neurons that project C-fiber afferent nerves, which are considered to be engaged in the metabolic component of the exercise pressor reflex (19, 20).
The TrkA receptors have been found in the DRG of rats (50) and are involved in NGF-induced upregulation of substance P (SP) expression (48). It is known that SP is an important neurotransmitter released into the dorsal horn from group III and IV muscle afferents in response to muscle contraction. Consequently, in the current study, there is a potential role for the TrkA receptors in contribution to the arterial ligation-enhanced sympathetic and pressor responses evoked by static contraction through NGF-induced upregulation of SP.
The prior studies have also suggested that lactic acid plays an important role in mediating the exercise pressor reflex (44, 45). Blocking ASICs receptors using amiloride and the more selective antagonist A-317567 has been reported to attenuate the pressor response evoked by static exercise and by arterial injection of lactic acid into the hindlimb muscles (14, 16). Accordingly, acid sensing is considered to be an important function of sensory neurons with thin fiber afferents and contributes to the reflex cardiovascular responses to muscle contraction. By recording discharges of the thin fiber muscle afferent nerves in cats, past studies further suggest that ASICs participate in the metabolic component but not the mechanoreceptor component of the exercise pressor reflex (33, 34).
Furthermore, a prior study has shown that arterial injection of a specific ASIC3 blocker significantly attenuates the reflex pressor response to muscle contraction in the rats with a ligated femoral artery but has only modest effects in the rats with freely perfused hindlimbs (52). Expression of ASIC3 is upregulated in DRG neurons innervating the hindlimb muscles with the occluded femoral artery (25). Also, lactic acid injected into the arterial blood supply of the hindlimb muscles to stimulate ASIC3 of muscle afferent nerves increases SNA and BP to a greater degree in occluded rats (25). In the current experiment, administration of NGF-Ab into the hindlimb muscles significantly attenuates occlusion-enhanced protein levels of ASIC3 in DRG tissues, which thereby attenuates SNA and BP responses to stimulation of the muscle metaboreflex. This suggests NGF plays a role in the exaggerated exercise pressor reflex in PAD.
Using the whole cell patch-clamp methods, the results of a recent study demonstrated that the peak current amplitude of DRG neuron response induced by stimulation of ASIC3 is greater in occluded limbs than in that of control limbs (61). This result suggests that the femoral artery occlusion is likely to augment ASIC3 function on thin fiber muscle afferent nerves, thereby leading to the exaggerated exercise pressor reflex. In addition, results of the immunohistochemical experiment show that ASIC3 appears in both C- and A-fiber of DRG neurons, and femoral artery occlusion mainly increases expression of ASIC3 in DRG neurons that project C-fiber afferents (61). However, the role for NGF in regulating ASIC3 expression in C-fiber and/or A-fiber of DRG neurons was not specifically determined in the previous studies. New findings provided here show that NGF infused into the hindlimb muscles selectively enhances expression of ASIC3 in DRG neurons that project C-fiber afferents. On the other hand, the present results show that distribution of DRG neurons with the two thin fiber phenotypes is not notably altered after infusion of NGF into the hindlimb muscles.
In addition to ASIC3, NGF plays a role in regulating a number of ligand-gated ion channels including TRPV1, P2X3, and bradykinin B2 receptor (11, 40). These receptors are responsive to ischemia (26, 60). Also, NGF infused into the hindlimb muscles has been shown to increase responses of DRG neurons with stimulation of TRPV1, as well as expression and responsiveness of P2X3 (26, 62). Among these receptors, P2X3 and bradykinin B2 have been reported to modulate the exercise pressor reflex (10, 22, 39). Thus it needs to be noted that elevation of NGF is likely to affect multiple afferent nerves' receptors in modulating the exercise pressor reflex after femoral occlusion.
Moreover, 24–48 h of femoral occlusion significantly increases the levels of NGF in the DRG (62). Application of NGF-Ab (10 μg) attenuates occlusion-elevated NGF in the DRG (26). This prior study evidently demonstrates the effectiveness of NGF-Ab injected into the hindlimb muscles. Thus the same dosage of NGF-Ab was selected to neutralize NGF in the present study. Likewise, the levels of NGF in the DRG of control limbs and limbs infused with NGF (18 μg) were previously examined to determine effectiveness of NGF infusion. NGF was significantly increased in the DRG of the infused leg after it was infused into the muscles. The level of elevated NGF in the DRG is close to that seen under 24 h of ischemia. However, infusion of NGF did not significantly alter the levels of NGF in the DRG of the control leg infused with saline in the same rats, indicating that NGF infusion has negligible systemic effects. Thus the dosage of NGF infused into the hindlimb muscles is effective and rational in the present experiment.
Finally, it should be acknowledged that ASIC3 expression in the DRG may not be reflective of protein expression for these receptors at the afferent nerve terminals in muscles. However, given that DRG cells are the primary sensory projections to group III and IV fibers afferent nerves, expression and characteristics of sensory receptors (i.e., ASIC3) in DRG neurons are generally examined to study receptor physiology (6, 7, 30, 31). The receptors in question are found in both the peripheral terminals and cell bodies of sensory DRG neurons. Receptor activity and characteristics of the DRG cell body have been used to reflect activity and characteristics of the receptors located at the nerve endings (6, 7, 30, 31).
In summary, the data of the present study show that the femoral artery occlusion significantly augments SNA and BP responses to static muscle contraction, tendon stretch, and stimulation of chemically sensitive ASICs receptors. When NGF-Ab is previously administered into the hindlimb muscles to effectively block NGF induced by femoral occlusion, the reflex responses to muscle contraction and activation of ASICs are significantly attenuated. In contrast, NGF-Ab does not attenuate the reflex responses evoked by tendon stretch. Additional data demonstrate that NGF infused into the hindlimb muscles increases expression of ASIC3 within C-fiber of DRG neurons, a group of sensory cells that represent thin fiber afferent nerves involved in the muscle metaboreflex. Overall, these data suggest that 1) amplified NGF in sensory nerves of occluded limbs contributes to augmented reflex SNA and BP responses evoked by stimulation of chemically, but not mechanically, sensitive muscle afferent nerves and 2) NGF is likely to play a role in regulating muscle metaboreflex via enhancement of ASIC3 expression in C-fiber afferent nerves.
GRANTS
This study was supported by NIH Grant R01-HL-090720, American Heart Association Established Investigator Award 0840130N, and NIH Grant P01-HL-096570.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J. Lu, J.X., and J. Li conception and design of research; J. Lu and J.X. performed experiments; J. Lu and J.X. analyzed data; J. Lu, J.X., and J. Li interpreted results of experiments; J. Lu and J.X. prepared figures; J. Lu, J.X., and J. Li drafted manuscript; J. Li edited and revised manuscript; J. Li approved final version of manuscript.
ACKNOWLEDGMENTS
We express gratitude to Chunying Yang for technical assistance.
REFERENCES
- 1. Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Campenot RB, MacInnis BL. Retrograde transport of neurotrophins: fact and function. J Neurobiol 58: 217–229, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Coote JH, Hilton SM, Pérez-González JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 290: 86–97, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Deval E, Noël J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J Neurosci 31: 6059–6066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deval E, Noël J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 19: 3047–3055, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106: 2257–2262, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 280: H1383–H1390, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol 292: H939–H945, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol 37: 83–90, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna RL, Hayes SG, Kaufman M. ab-Methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol 93: 834–841, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–1282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayes SG, McCord JL, Kaufman MP. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol 104: 538–541, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1720–H1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 161: 1985–1990, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. New York: Oxford University Press, 1996, sect. 12, chapt. 10, p. 381–447 [Google Scholar]
- 19. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol 97: 1709–1714, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–H2643, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TROVE. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Li J, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Mao W, Ding B, Liang CS. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomytes. Am J Physiol Heart Circ Physiol 295: H1956–H1965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000 [DOI] [PubMed] [Google Scholar]
- 29. MacLean DA, LaNoue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitial metabolite responses in the cat. J Appl Physiol 85: 1583–1592, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 278: 48907–48913, 2003 [DOI] [PubMed] [Google Scholar]
- 32. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCord JL, Hayes SG, Kaufman MP. Acid-sensing ion and epithelial sodium channels do not contribute to the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1017–H1024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCord JL, Tsuchimochi H, Kaufman MP. Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 297: H443–H449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanism, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 36. Mitchell JH, Reardon WC, McCloskey DI. Reflex effects on circulation and respiration from contracting skeletal muscle. Am J Physiol Heart Circ Physiol 233: H374–H378, 1977 [DOI] [PubMed] [Google Scholar]
- 37. Muir RL. Peripheral arterial disease: Pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs 27: 26–30, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Ouriel K. Peripheral arterial disease. Lancet 358: 1257–1264, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol 75: 2061–2068, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Potts JT, Li J. Interaction between carotid baroreflex and exercise pressor reflex depends on baroreceptor afferent input. Am J Physiol Heart Circ Physiol 274: H1841–H1847, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem 77: 864–875, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Rejeski WJ, Tian L, Liao Y, McDermott MM. Social cognitive constructs and the promotion of physical activity in patients with peripheral artery disease. J Cardiopulm Rehabil Prev 28: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 45. Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67: 256–263, 1989 [DOI] [PubMed] [Google Scholar]
- 46. Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol 66: 429–436, 1989 [DOI] [PubMed] [Google Scholar]
- 47. Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol 197: 430–436, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Sugiura A, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Norimoto M, Orita S, Eguchi Y, Takahashi Y, Watanabe TS, Ochiai N, Takaso M, Takahashi K. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Phila Pa 1976) 33: 2047–2051, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol 589: 6173–6189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Victor RG, Bertocci L, Pryor S, Nunnally R. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997 [DOI] [PubMed] [Google Scholar]
- 55. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H(+)-gated cation channels. Ann NY Acad Sci 868: 67–76, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. New York: Oxford University Press, 1996, sect. 12, chapt. 9, p. 333–380 [Google Scholar]
- 58. Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology 110: 140–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TROVE-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xing J, Lu J, Li J. ASIC3 function and immunolabeling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol 590: 1261–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TROVE responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99: 501–509, 2006 [DOI] [PubMed] [Google Scholar]