Abstract
We tested the hypothesis that elevated sympathetic tone is responsible for lower peak vasodilation after single muscle contractions in older adults. Young (n = 13, 7 men and 6 women, age: 27 ± 1 yr) and older (n = 13, 7 men and 6 women, age: 69 ± 2 yr) adults performed single forearm contractions at 10%, 20%, and 40% of maximum during 1) control, 2) sympathetic activation via lower body negative pressure (LBNP; −20 mmHg), and 3) intra-arterial infusion of phentolamine (α-adrenergic antagonist). Brachial artery diameter and velocities were measured via Doppler ultrasound, and forearm vascular conductance (FVC; in ml·min−1·100 mmHg−1) was calculated from blood flow (in ml/min) and blood pressure (in mmHg). Peak vasodilator responses [change in (Δ) FVC from baseline] were attenuated in older adults at 20% and 40% of maximum (P < 0.05). LBNP reduced peak ΔFVC at 10% (98 ± 17 vs. 70 ± 12 ml·min−1·100 mmHg−1), 20% (144 ± 12 vs. 98 ± 3 ml·min−1·100 mmHg−1), and 40% (209 ± 20 vs. 161 ± 21 ml·min−1·100 mmHg−1, P < 0.01 vs. control) in younger adults but not in older adults (71 ± 11 vs. 68 ± 11, 107 ± 13 vs. 106 ± 16, and 161 ± 22 vs. 144 ± 22 ml·min−1·100 mmHg−1, respectively, P = 0.22–0.99). With phentolamine, peak ΔFVC was enhanced in older adults at each contraction intensity (100 ± 14, 147 ± 22, and 200 ± 26 ml·min−1·100 mmHg−1, respectively, P < 0.01 vs. control) but not in younger adults (94 ± 13, 153 ± 13, and 224 ± 27 ml·min−1·100 mmHg−1, respectively, P = 0.30–0.81 vs. control). Our data indicate that α-adrenergic vasoconstriction and/or blunted functional sympatholysis might contribute to the age-related decreases in skeletal muscle contraction-induced rapid vasodilation in humans.
Keywords: aging, vasodilation, α-adrenergic receptors, muscle contraction
aging is associated with a variety of adaptations within the cardiovascular system that can compromise muscle blood flow or alter its regulation during dynamic exercise. Some of these adaptations include reduced cardiac pump capacity, structural alterations in the vasculature, alterations in local vascular control mechanisms, and increased muscle sympathetic neural outflow (43). Collectively these changes contribute to a decline in the capacity to augment skeletal muscle blood flow, which may play a role in the age-related reductions in maximal O2 consumption and physical function (23, 41). Indeed, numerous human studies (29, 40, 42, 44) have demonstrated that the control of blood flow to dynamically contracting skeletal muscle is altered with normal aging during submaximal exercise. However, a majority of studies concerned with how aging alters vascular control during exercise have focused primarily on blood flow responses in contracting limb muscles during steady-state submaximal exercise. In this context, the mechanisms influencing vascular tone at the onset of exercise may be different than those involved in the control during steady-state exercise (6).
In young adults, muscle blood flow increases within the first 1–2 s after the completion of a single muscle contraction and the magnitude of this response is graded with contraction intensity (4, 5, 9, 26, 50). Evidence from both humans (5, 26) and experimental animals (1, 2, 24) suggests that rapid vasodilation and increases in blood flow after a muscle contraction are attenuated with aging. However, the mechanisms for the age-related discrepancies in contraction-induced rapid vasodilation in humans are unknown. It is well documented that there is a progressive increase in basal (resting) muscle sympathetic nerve activity (MSNA) in aging humans (36, 48). Moreover, norepinephrine concentrations obtained from a catheter placed in a deep vein draining forearm muscles are significantly elevated with age (13). These elevations in MSNA and norepinephrine evoke vascular smooth muscle contraction and, thus, vasoconstriction. Interestingly, a previous study (13) has suggested that tonic sympathetic vasoconstriction is reduced in the forearm of older men under resting conditions, despite the greater MSNA and circulating levels of norepinephrine often observed with aging. Important to the present study, alterations in basal vasoconstrictor tone may contribute to the blunted vasodilator response to a single muscle contraction. Along these lines, recent evidence in animals suggests that aging blunts rapid vasodilation and restricts blood flow to skeletal muscle through subtle activation of α-adrenergic receptors in microvascular resistance networks (24).
Blunting of sympathetic vasoconstriction (i.e., functional sympatholysis) in the vascular beds of contracting skeletal muscle can occur early in exercise (49) and might contribute to the rapid vasodilator response after a single contraction in young adults (11). Aging is associated with impaired functional sympatholysis during exercise under steady-state conditions (14). Taken together, the attenuated contraction-induced rapid vasodilation in older adults might be related to less blunting of sympathetic α-adrenergic vasoconstriction.
Under resting conditions, sex-related differences in the vasoconstrictor responsiveness to sympathetic stimulation and norepinephrine-induced vasoconstriction have been observed in young humans (18, 20, 27) but may be less apparent in older adults (20). Moreover, sex-related differences in muscle blood flow during exercise have been observed in young and older adults (43); however, these findings are not consistent (30). Important to the present study, the degree of functional sympatholysis during moderate-intensity exercise appears to be similar between young men and women (30). Despite evidence for sex-specific differences in α-adrenergic restraint of rapid vasodilation in older mice (24), it is unclear if this occurs in humans.
With this information as background, we tested the hypothesis that elevated sympathetic tone and/or impaired functional sympatholysis is responsible for lower peak vasodilation after single muscle contractions in older adults. Specifically, we examined whether 1) local α-adrenergic blockade augments the rapid vasodilator response after a single forearm contraction in healthy older adults and 2) sympathetic activation reduces the rapid vasodilator response to a single contraction. Additionally, we examined whether sex-related differences in the rapid vasodilator response existed within and between age groups.
METHODS
Subjects
A total of 13 young (7 men and 6 women, age: 20–37 yr) and 13 older (7 men and 6 women, age: 61–81 yr) healthy subjects volunteered to participate in the study. Subjects completed written informed consent and underwent a standard screening. Subjects were healthy, nonobese (body mass index: ≤30 kg/m2), nonsmokers, not taking any vasoactive medications, and sedentary to moderately active. Four older subjects were taking omeprazole (proton pump inhibitor) to treat gastroesophageal reflux (withheld for a minimum of 3 days before study), one older subject was taking aspirin (withheld for 1 wk before study), and two older subjects were taking synthroid to treat hypothyroidism (withheld for 3 days before study). Five older subjects reported taking a daily vitamin. Studies were performed after an overnight fast and refraining from exercise and caffeine for at least 24 h. Young female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives (33). All older female subjects were postmenopausal and were not taking any form of hormone replacement therapy. All study protocols were approved by our Institutional Review Board and were performed according to the Declaration of Helsinki.
Arterial Catheterization
A 20-gauge, 5-cm catheter (model RA-04020, Arrow, Reading, PA) was placed in the brachial artery of the experimental arm under aseptic conditions after local anesthesia (2% lidocaine) for the administration of study drugs. The catheter was connected to a three-port connector in series, as previously described in detail (12). One port was linked to a pressure transducer positioned at heart level (model PX600F, Edwards Lifescience, Irvine, CA) to allow the measurement of arterial pressure and was continuously flushed (3 ml/h) with saline with a stop-cock system to enable arterial blood sampling. The remaining two ports allowed arterial drug administration.
Heart Rate and Systemic Blood Pressure
Heart rate (HR) was recorded via continuous three-lead ECG. A pressure transducer connected to the arterial catheter measured beat-to-beat blood pressure (Cardiocap/5, Datex-Ohmeda, Louisville, CO).
Forearm Blood Flow
Brachial artery mean blood velocity and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole at rest (before contraction) and 45 s postcontraction. Forearm blood flow (FBF) was calculated as the product of mean blood velocity (in cm/s) and brachial artery cross-sectional area (in cm2) and expressed as milliliters per minute.
Single Muscle Contractions
For the experimental trials, brief forearm contractions were performed with a handgrip device at 10%, 20%, and 40% of the subject's maximal voluntary contraction (MVC), determined at the beginning of each experiment. The weight was lifted 4–5 cm over a pulley for a single, 1-s forearm muscle contraction. Subjects were instructed to contract and relax on a verbal command issued from the laboratory personnel. Each contraction was visually observed by laboratory personnel to ensure the proper timing of contraction. Two minutes of relaxation were given between each contraction to allow continuous measures of forearm hemodynamics postcontraction. Workload intensity was randomized within each condition [control, lower body negative pressure (LBNP), and phentolamine], and each contraction intensity was performed in duplicate to calculate the average response for each subject for a given condition. The average weight used for young subjects was 4.3 ± 0.3, 8.7 ± 0.7, and 17.2 ± 1.3 kg for 10%, 20%, and 40% MVC, respectively. The average weight used for older subjects was 3.8 ± 0.3, 7.6 ± 0.6, and 15.2 ± 1.1 kg for 10%, 20%, and 40% MVC, respectively (P = 0.20–0.25 compared with young subjects).
LBNP
LBNP was used to examine the influence of increased sympathetic outflow on the rapid vasodilator responses to single muscle contractions. While in the supine position, the lower body of each subject was sealed in an airtight box above the level of the iliac crests. The box was attached to a vacuum source, which allowed for rapid reductions in pressure. During the LBNP trials, the pressure was reduced to −20 mmHg. Application of low levels of LBNP (−20 mmHg) increase MSNA without concurrent changes in HR and arterial pressure (45). Moreover, this level of LBNP increases MSNA in young adults to similar levels observed in older adults at rest (i.e., without LBNP) (10).
Pharmacological Infusions
Phentolamine, a nonselective α-adrenergic receptor antagonist, was administered to the experimental forearm via brachial artery catheter as a loading dose (10 μg·dl forearm volume−1·min−1 for 5 min) followed by a continuous maintenance dose (25 μg/min). This dose of phentolamine has been shown to effectively inhibit α-adrenergic receptor vasoconstriction (16).
Experimental Protocol
Each subject completed a single study day, which consisted of single forearm contractions during control, sympathetic stimulation (via LBNP), and local α-adrenergic blockade (via intra-arterial infusion of phentolamine) conditions. Contraction intensity (10%, 20%, and 40% MVC) was performed in duplicate and randomized within each condition. Thus, each subject performed a total of 18 single forearm contraction trials during the study [6 contractions (2 at each intensity) × 3 conditions]. Each trial consisted of 2 min of rest followed by a single forearm contraction. Brachial artery velocity and hemodynamics were measured during the rest period and for 45-s postcontraction. Due to the long half-life of phentolamine, α-adrenergic blockade trials were always performed last. A rest period of 15 min was allowed between conditions.
Data Analysis and Statistics
Data were collected at 250 Hz, stored on a computer, and analyzed offline with signal processing software (WinDaq, DATAQ Instruments, Akron). Mean arterial pressure (MAP) was determined from the brachial artery pressure waveform, and HR was determined from the ECG. Baseline FBF and MAP represent an average of the last 30 s of the resting time period before each muscle contraction and were used to quantify the hyperemic response. Forearm vascular conductance (FVC) was calculated as FBF/MAP × 100 (and expressed as ml·min−1·100 mmHg−1). To account for baseline changes in FBF and FVC with LBNP and phentolamine, flow and vasodilator responses after muscle contraction were adjusted (i.e., postcontraction value − baseline value) and expressed as the change in (Δ) FBF and FVC. Of particular interest to this study, the immediate (first cardiac cycle postcontraction), peak, and total FBF and FVC responses were analyzed between conditions and age groups. Total FBF and FVC were defined as the area under the curve after respective baseline values were subtracted for a given flow or conductance curve (17, 32). To investigate potential sex-related differences in the hyperemic (ΔFBF) and vasodilator (ΔFVC) responses to sympathetic stimulation (LBNP) and α-adrenergic blockade (phentolamine), we compared the relative changes (in %) within and between age groups.
All values are expressed as means ± SE. ANOVA was used to analyze baseline differences between age groups. Baseline MAP, HR, FBF, FVC, and brachial artery diameter were compared via repeated-measures ANOVA to detect differences between age groups and across conditions. To determine the effect of age (group) and α-adrenergic vasoconstrictor tone (condition) on hyperemic and vasodilator responses to single forearm contractions, differences in immediate ΔFBF and ΔFVC, peak ΔFBF and ΔFVC, and total FBF and FVC at each contraction intensity (10%, 20%, and 40% MVC) were determined via repeated-measures ANOVA. Additional two-way repeated-measures ANOVAs were performed to examine whether sex-related differences existed within and between age groups. The appropriate post hoc analysis determined where statistical differences occurred. When significance was detected, Tukey's post hoc analysis was used to identify differences between groups. Statistical difference was set a priori at P < 0.05.
RESULTS
Subject characteristics are shown in Table 1. Young and older subjects were of similar height, weight, and body mass index (P > 0.05). Additionally, young and older subjects exhibited similar forearm volume, MVC, and brachial artery diameter size (P > 0.05 for all). A strong relation between forearm volume and MVC was observed in both young (r = 0.70, P < 0.01) and older (r = 0.78, P < 0.01) groups. Older subjects demonstrated greater MAP, total cholesterol, and low-density lipoprotein than their younger counterparts (P < 0.05).
Table 1.
Subject characteristics
| Variable | Young Adults | Older Adults |
|---|---|---|
| Age, years | 27 ± 1 | 69 ± 2* |
| Men/Women | 7/6 | 7/6 |
| Height, cm | 176 ± 2 | 170 ± 3 |
| Weight, kg | 78 ± 4 | 76 ± 4 |
| Body mass index, kg/m2 | 25.0 ± 0.8 | 26.1 ± 0.9 |
| Forearm volume, ml | 1010 ± 64 | 1009 ± 72 |
| MVC, kg | 43 ± 3 | 38 ± 3 |
| MAP, mmHg | 96 ± 2 | 103 ± 2* |
| Brachial artery diameter, cm | 0.38 ± 0.02 | 0.40 ± 0.02 |
| Total cholesterol, mmol/l | 3.7 ± 0.5 | 4.8 ± 0.2* |
| Low-density lipoprotein, mmol/l | 1.9 ± 0.1 | 2.9 ± 0.2* |
| High-density lipoprotein, mmol/l | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Triglycerides, mmol/l | 0.9 ± 0.3 | 1.0 ± 0.1 |
Values are means ± SE. MVC, maximal voluntary contraction; MAP, mean arterial pressure.
P < 0.05 vs. young adults.
Hemodynamic Responses to LBNP and Phentolamine at Baseline
Baseline (resting) hemodynamics under each condition are shown in Table 2. FBF, FVC, and HR at rest did not differ with age across all conditions. MAP was consistently higher across conditions in older subjects (P < 0.05). In young adults, LBNP resulted in an elevated HR during the 10% and 20% MVC trials in young subjects (P < 0.05). LBNP did not alter FBF, FVC, or MAP in young or older adults. Baseline FBF and FVC increased to a similar extent in young and older adults during the infusion of phentolamine (P < 0.01). MAP and HR were unchanged in both groups during phentolamine administration.
Hyperemic and Vasodilator Responses to Single Forearm Contractions in Young and Older Adults
Under control conditions, immediate (first cardiac cycle postcontraction) hyperemic (ΔFBF) and vasodilator (ΔFVC) responses to single forearm contractions at 10%, 20%, and 40% MVC were not different between young and older adults (Fig. 1). Both young and older adults achieved a peak vasodilator response at the fifth cardiac cycle postcontraction for each relative workload. However, older adults demonstrated reduced peak hyperemic and vasodilator responses at 20% and 40% MVC compared with their young counterparts (P < 0.05; Fig. 2). Moreover, total (area under the curve for 30 cardiac cycles postcontraction) hyperemic and vasodilator responses were attenuated in older adults at 40% MVC (P < 0.05; Fig. 3).
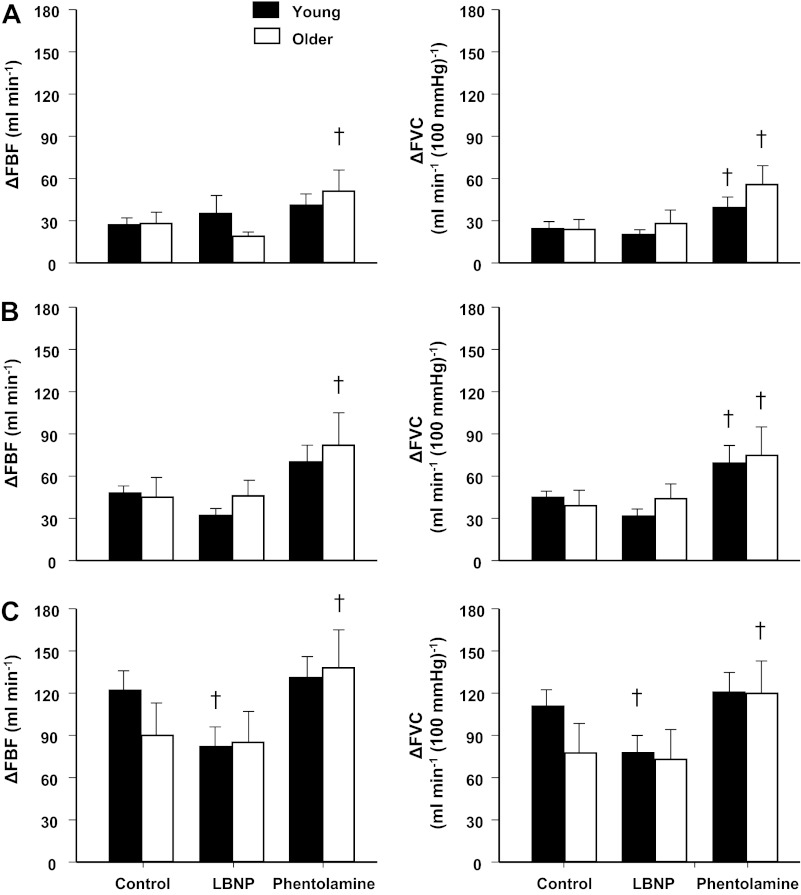
Fig. 1.
Immediate (first cardiac cycle postcontraction) hyperemic [change in forearm blood flow (ΔFBF)] and vasodilator [change in forearm vascular conductance (ΔFVC)] responses to single forearm contractions at 10% (A), 20% (B), and 40% (C) maximal voluntary contraction (MVC) across conditions [control, lower body negative pressure (LBNP), and phentolamine]. Immediate hyperemic and vasodilator responses were similar between young and older adults at all contraction intensities. α-Adrenergic blockade (via phentolamine) enhanced immediate vasodilator (ΔFVC) responses in young and older adults at 10% and 20% MVC and in older adults at 40% MVC. †P < 0.01 vs. control.
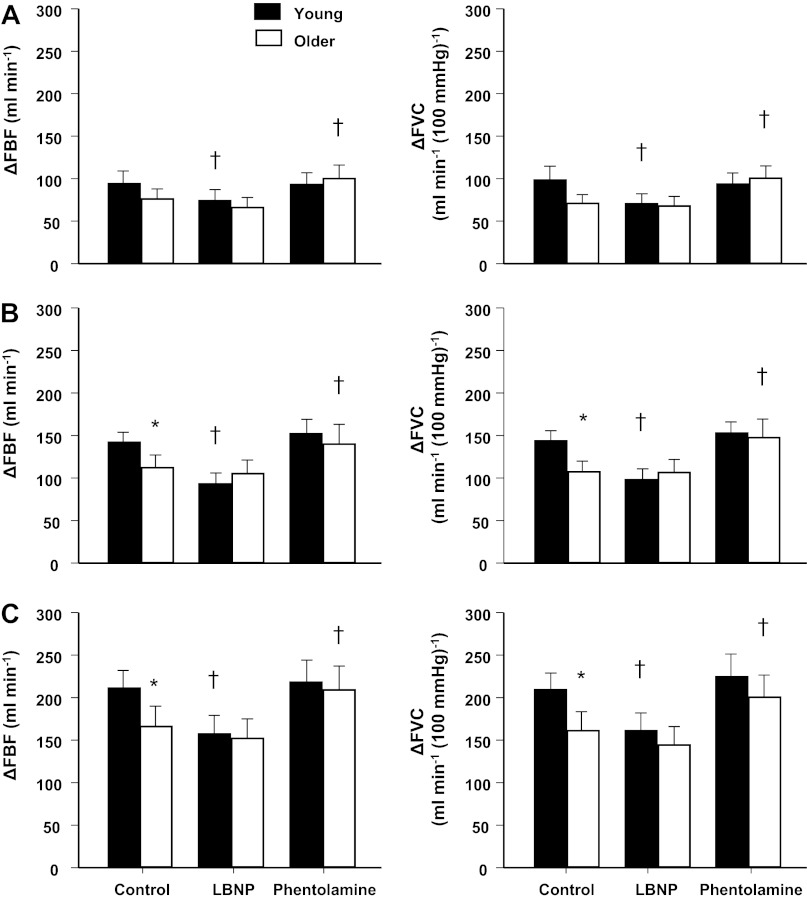
Fig. 2.
Peak hyperemic (ΔFBF) and vasodilator (ΔFVC) responses to single forearm contractions at 10% (A), 20% (B), and 40% (C) MVC across conditions (control, LBNP, and phentolamine). Under control conditions, peak hyperemic vasodilator responses were similar in young and older adults at 10% MVC. However, peak responses were attenuated in older adults at 20% and 40% MVC. Sympathetic stimulation (via LBNP) blunted peak hyperemic and vasodilator responses at all contraction intensities in young adults only. Conversely, α-adrenergic blockade (via phentolamine) augmented peak responses at all contraction intensities in older adults only. *P < 0.05 vs. young adults; †P < 0.01 vs. control.
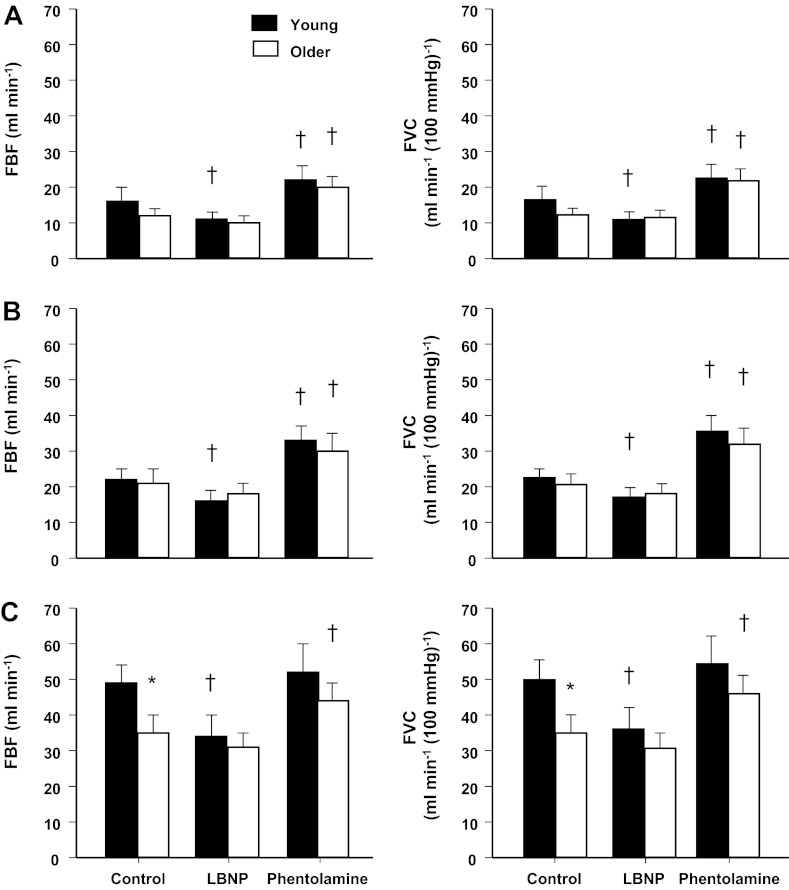
Fig. 3.
Total hyperemic (FBF) and vasodilator (FVC) responses (area under curve) to single forearm contractions at 10% (A), 20% (B), and 40% (C) MVC across conditions (control, LBNP, and phentolamine). Under control conditions, total hyperemic and vasodilator responses were similar in young and older adults at 10% and 20% MVC. However, total hyperemic and vasodilator responses were attenuated in older adults at 40% MVC. Sympathetic stimulation (via LBNP) blunted total hyperemic and vasodilator responses at all contraction intensities in young adults only. α-Adrenergic blockade (via phentolamine) enhanced total responses in young and older adults at 10% and 20% MVC and in older adults at 40% MVC. *P < 0.05 vs. young adults; †P < 0.01 vs. control.
Although there were no significant age-related reductions in MVC or differences in the weight used for each relative intensity between groups, we examined whether the attenuated hyperemic and vasodilator responses to single muscle contractions observed in older adults persisted after correction for workload (i.e., flow and/or conductance per 100 g of weight lifted). Use of this approach revealed similar results as those reported above. Older adults still demonstrated reduced peak hyperemic (1.40 ± 0.09 vs. 1.66 ± 0.10 and 1.04 ± 0.08 vs. 1.24 ± 0.09 ml·min−1·100 g−1) and vasodilator (1.42 ± 0.07 vs. 1.68 ± 0.10 and 1.05 ± 0.07 vs. 1.24 ± 0.09 ml·min−1·100 mmHg−1·100 g −1) responses at 20% and 40% MVC, respectively, compared with their young counterparts (P < 0.05). Additionally, total hyperemic (0.23 ± 0.02 vs. 0.29 ± 0.02 ml·min−1·100 g−1) and vasodilator (0.23 ± 0.02 vs. 0.30 ± 0.03 ml·min−1·100 mmHg−1·100 g−1) responses remained lower in older adults compared with young adults at 40% MVC after correction for workload (P < 0.05).
Effect of Sympathetic Stimulation (via LBNP) on Hyperemic and Vasodilator Responses to Single Forearm Contractions
During the LBNP trials, immediate hyperemic and vasodilator responses were reduced at 40% MVC in the young adults (P < 0.01 vs. control; Fig. 1). Similar to control trials, both young and older adults achieved a peak vasodilator response at the fifth cardiac cycle postcontraction for each relative workload during the LBNP trials. However, LBNP reduced peak and total hyperemic and vasodilator responses at all contraction intensities in young adults (P < 0.01 vs. control; Figs. 2 and 3). Immediate, peak, and total hyperemic and vasodilator responses were unchanged during the LBNP trials in older adults (Figs. 1–3). The age-associated differences observed under control conditions for peak (20% and 40% MVC) and total (40% MVC) responses were not present during the LBNP trials.
Effect of α-Adrenergic Blockade (via Phentolamine) on Hyperemic and Vasodilator Responses to Single Forearm Contractions
Immediate hyperemic responses (ΔFBF) tended to be higher during the intra-arterial infusion of phentolamine compared with control conditions at 10% and 20% MVC (P = 0.06 for both) in young adults (Fig. 1). However, immediate vasodilator responses (ΔFVC) were enhanced at 10% and 20% MVC during the phentolamine trials (P < 0.01; Fig. 1). The time to reach a peak vasodilator response was achieved at the fourth cardiac cycle postcontraction in both young and older adults for each relative workload. α-Adrenergic blockade via phentolamine did not alter peak hyperemic and vasodilator responses in young adults (Fig. 2). Total hyperemic and vasodilator responses were increased during phentolamine at 10% and 20% MVC in the young adults (Fig. 3). Immediate, peak, and total hyperemic and vasodilator responses were all enhanced during the phentolamine trials in the older adults (Figs. 1–3). The age-associated differences observed under control conditions for peak (20% and 40% MVC) and total (40% MVC) responses were not present during the phentolamine trials (Fig. 4).
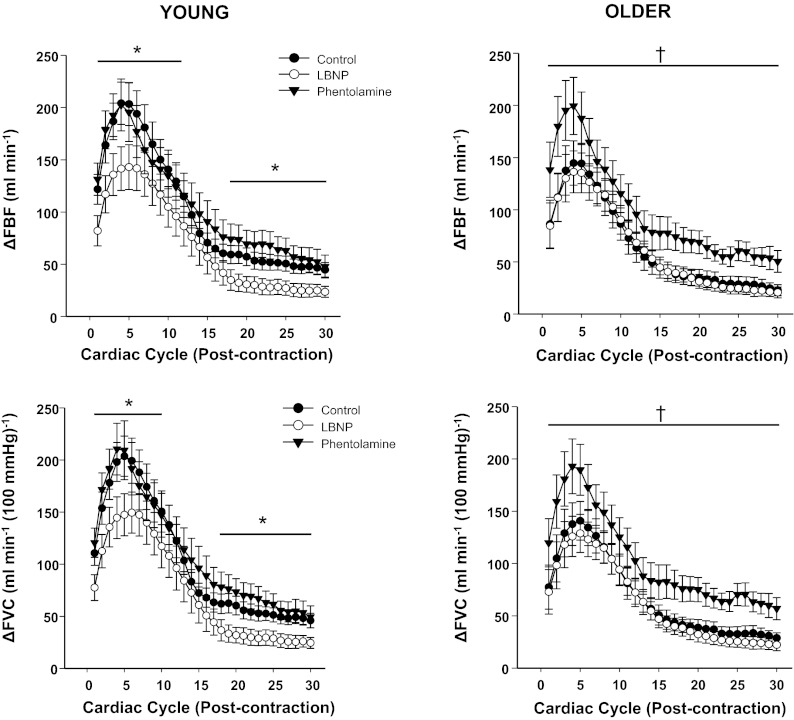
Fig. 4.
Rapid hyperemic (ΔFBF) and vasodilator (ΔFVC) responses over 30 cardiac cycles after single forearm contractions at 40% MVC. Sympathetic stimulation (via LBNP) blunted immediate, peak, and total hyperemic and vasodilator responses in young adults. α-Adrenergic blockade (via phentolamine) enhanced hyperemic and vasodilator responses for every cardiac cycle postcontraction in older adults. *P < 0.05 control vs. LBNP; †P < 0.05 control vs. phentolamine.
Sex-Related Differences in Hyperemic and Vasodilator Responses to Single Forearm Contractions
Within groups.
In young adults, immediate hyperemic (ΔFBF) and vasodilator (ΔFVC) responses did not differ between men and women at any contraction intensity (P > 0.05). Men demonstrated greater peak hyperemic (121 ± 24 vs. 63 ± 6, 174 ± 18 vs. 107 ± 10, and 254 ± 26 vs. 166 ± 16 ml/min) and vasodilator (127 ± 27 vs. 65 ± 6, 176 ± 17 vs. 109 ± 10, and 254 ± 24 vs. 164 ± 13 ml·min−1·100 mmHg−1) responses compared with women at 10%, 20%, and 40% MVC, respectively (P < 0.05). Total hyperemic (21 ± 6 vs. 10 ± 1 and 27 ± 4 vs. 17 ± 2 ml/min, P < 0.05) and vasodilator (22 ± 7 vs. 10 ± 1 and 27 ± 4 vs. 18 ± 1 ml·min−1·100 mmHg−1, P < 0.05) responses were greater in men at 10% and 20% MVC but not at 40% MVC (FBF: 55 ± 8 vs. 41 ± 4 ml/min, P = 0.08, and FVC = 56 ± 8 vs. 43 ± 4 ml·min−1·100 mmHg−1, P = 0.11).
In older adults, immediate hyperemic and vasodilator responses were greater in men compared with women at all intensities (P < 0.05). Older men also demonstrated greater peak hyperemic (102 ± 17 vs. 45 ± 7, 144 ± 12 vs. 72 ± 5, and 211 ± 17 vs. 106 ± 9 ml/min) and vasodilator (95 ± 17 vs. 46 ± 6, 140 ± 11 vs. 73 ± 5, and 201 ± 16 vs. 105 ± 7 ml·min−1·100 mmHg−1) responses compared with women at 10%, 20%, and 40% MVC, respectively (P < 0.05). Total hyperemic (29 ± 5 vs. 13 ± 2 and 41 ± 6 vs. 24 ± 3 ml/min−1, P < 0.05) and vasodilator (27 ± 4 vs. 14 ± 2 and 41 ± 6 vs. 25 ± 2 ml·min−1·100 mmHg−1, P < 0.05) responses were greater in men at 20% and 40% MVC but not at 10% MVC (FBF: 15 ± 3 vs. 9 ± 2 ml/min, P = 0.09, and FVC: 14 ± 3 vs. 10 ± 2 ml·min−1·100 mmHg−1, P = 0.12).
Between groups.
There were no differences in immediate hyperemic and vasodilator responses between young and older men at any of the contraction intensities (P > 0.05). There was a trend for lower peak hyperemic responses (ΔFBF) at 20% and 40% MVC in older men (P = 0.09 for both). However, peak vasodilator responses (ΔFVC) were reduced at both 20% and 40% MVC in older men compared with young men (P < 0.05 for both). Total hyperemic and vasodilator responses were not different at any intensity between young and older men; however, there was a trend for a blunted response in older men at 40% MVC (P = 0.10 for FBF and P = 0.09 for FVC). Older women demonstrated reduced immediate and peak hyperemic and vasodilator responses at all contraction intensities compared with young women (P < 0.05 for all). Additionally, total hyperemic and vasodilator responses tended to be lower in older females (P = 0.06 for both) at 20% MVC and were substantially lower at 40% MVC (P < 0.01 for both).
Influence of Sympathetic Activation on Rapid Vasodilation: Potential Sex Differences?
Within groups.
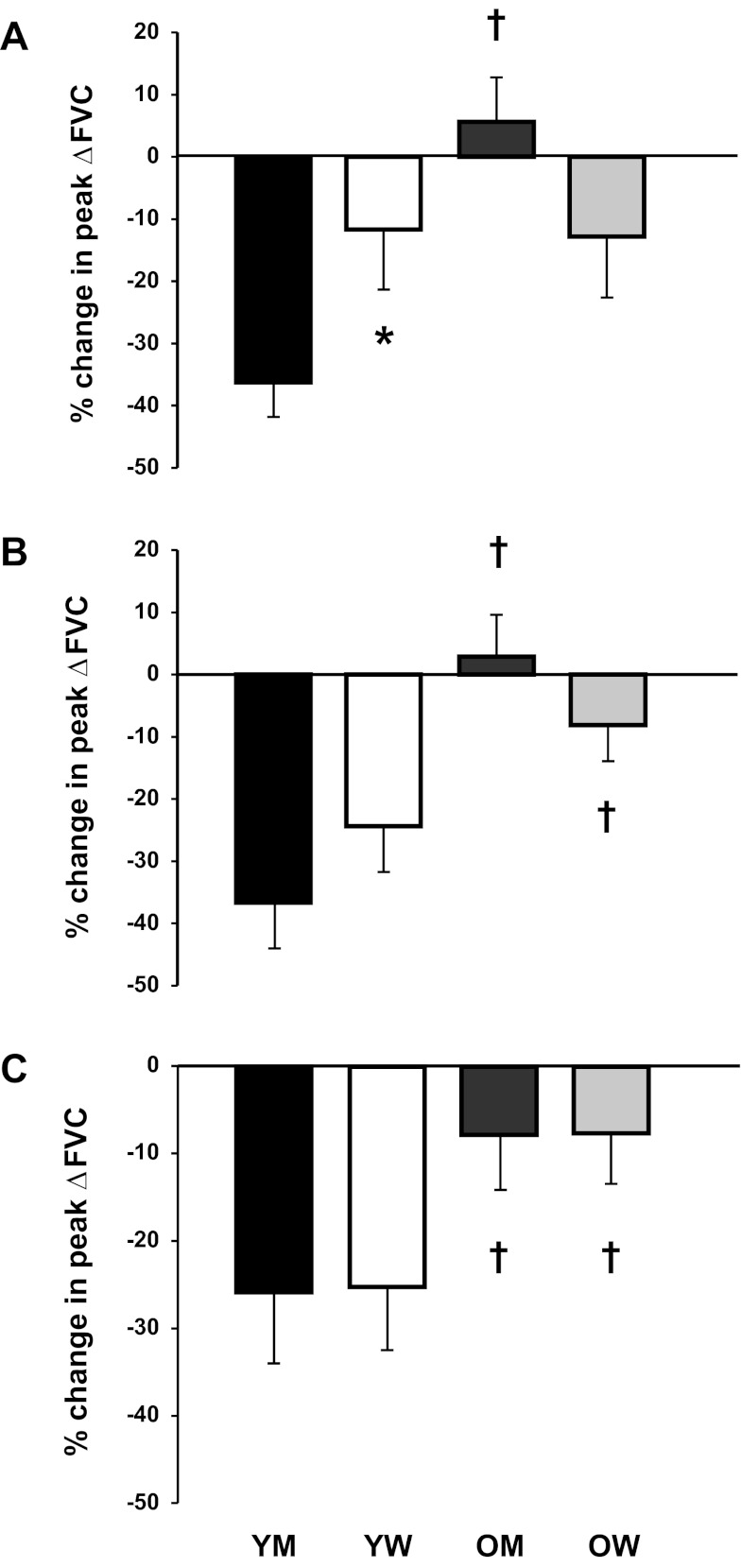
There were no significant sex-related differences in immediate hyperemic and vasodilator responses within or between groups during the LBNP trials compared with control conditions. Young men demonstrated a greater reduction in peak ΔFBF during LBNP trials at 10% MVC compared with young women (P < 0.05 for both). However, there were no sex-related differences in peak ΔFBF at 20% and 40% MVC (P > 0.05 for both). Differences in the reductions in total FBF were observed between sexes at 10% MVC (P < 0.05) but not at 20% and 40% MVC (P >0.05) within the young group. Similar results were observed for the reductions in ΔFVC across contraction intensities. There were no significant sex-related differences in peak and total hyperemic and vasodilator responses between older men and women when the changes in ΔFBF and ΔFVC during the LBNP trials compared with control conditions were examined. The relative changes in peak ΔFVC are shown in Fig. 5.
Fig. 5.
Relative change (in %) in peak vasodilator (ΔFVC) responses to single forearm contractions at 10% (A), 20% (B), and 40% (C) MVC during sympathetic stimulation via LBNP compared with control conditions in young men (YM) and young women (YW) and older men (OM) and older women (OW). At 10% MVC, the reduction in peak vasodilator responses during sympathetic stimulation was greater in YM compared with YW. Sympathetic stimulation blunted peak vasodilator responses to a greater extent in YM (at 10%, 20%, and 40% MVC) and YW (at 20% and 40% MVC) compared with their older counterparts. *P < 0.05 vs. men within same age group; †P < 0.05, age-related difference within the same sex.
Between groups.
There was no effect of age on immediate hyperemic and vasodilator responses during the LBNP trials for men or women at all intensities (P > 0.05). The reductions in peak and total hyperemic and vasodilator responses during LBNP were substantially greater in young men compared with older men at 10%, 20%, and 40% MVC (P < 0.05). Young women also had greater reductions in peak hyperemic and vasodilator responses at 20% and 40% MVC (P < 0.05). The changes in total hyperemic and vasodilator responses did not differ between young and older women across intensities.
Influence of α-Adrenergic Blockade on Rapid Vasodilation: Potential Sex Differences?
Within groups.
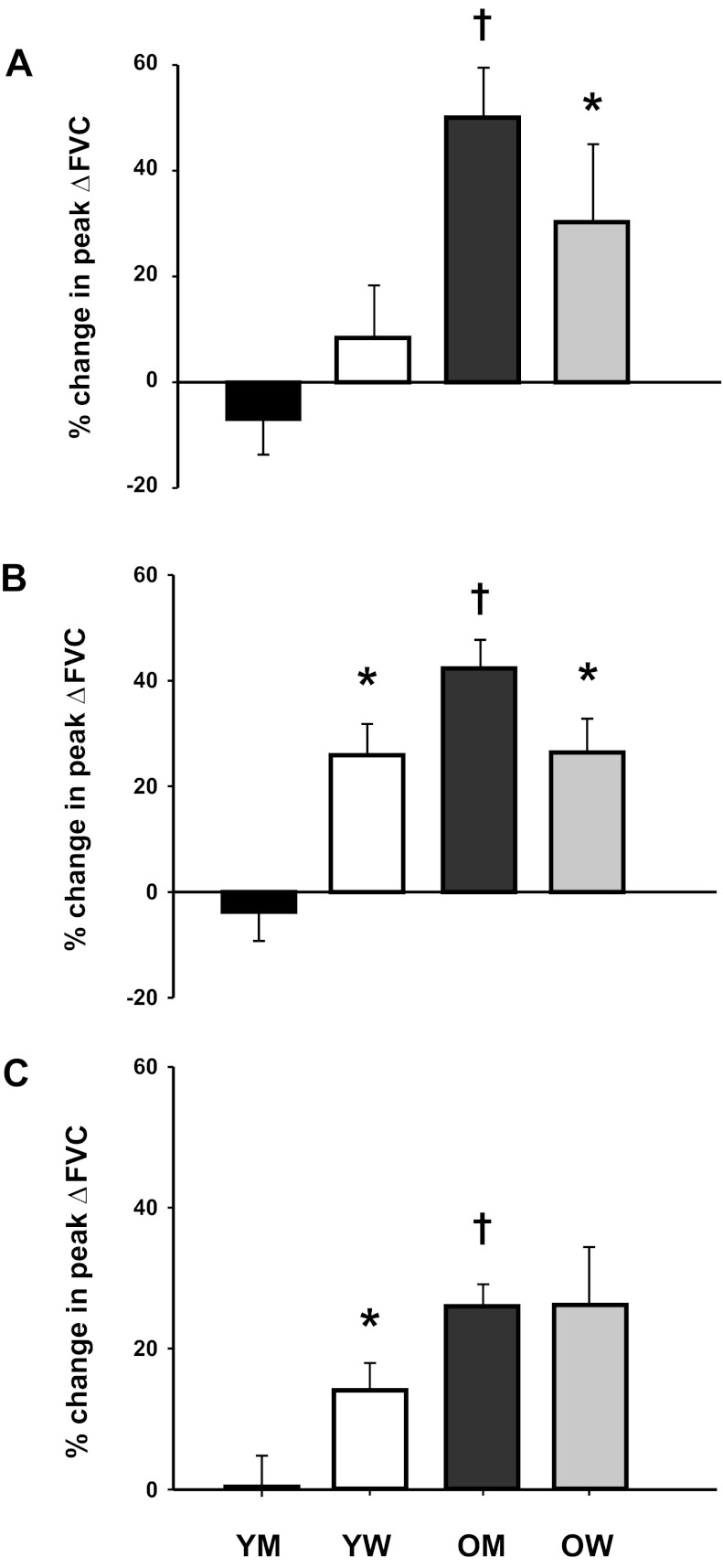
Sex-related differences were not observed in immediate and total hyperemic and vasodilator responses during phentolamine infusion in young or older groups at any contraction intensity. Young women demonstrated a greater increase in peak hyperemic and vasodilator responses at 20% and 40% MVC during phentolamine compared with young men (P < 0.05). Conversely, older men demonstrated a greater increase in peak hyperemic and vasodilator responses at 10% and 20% MVC during phentolamine compared with older women (P < 0.05; Fig. 6). The relative changes in peak vasodilator responses during phentolamine are shown in Fig. 6.
Fig. 6.
Relative change (in %) in peak vasodilator (ΔFVC) responses to single forearm contractions at 10% (A), 20% (B), and 40% (C) MVC during α-adrenergic blockade via phentolamine compared with control conditions in YM, YW, OM, and OW. α-Adrenergic blockade substantially increased peak vasodilator responses in OM compared with YM at all contraction intensities. Moreover, the increases in peak vasodilator responses during α-adrenergic blockade were greater in OM compared with OW at 10% and 20% MVC. Conversely, YW had a greater increase in peak vasodilator responses during α-adrenergic blockade than YM at 20% and 40% MVC. *P < 0.05 vs. men within same age group; †P < 0.05, age-related difference within the same sex.
Between groups.
There was no effect of age on immediate hyperemic and vasodilator responses during the phentolamine trials for men or women at all intensities (P > 0.05). The changes in peak and total hyperemic and vasodilator responses were substantially greater during the phentolamine trials in older men compared with young men at all intensities (P < 0.05). The changes in peak hyperemic and vasodilator responses during phentolamine were not different in women at all intensities (P > 0.05). However, the changes in total hyperemic and vasodilator responses were greater during the phentolamine trials in older women compared with young women at 40% MVC (P < 0.05).
DISCUSSION
Previous studies (5, 26) have clearly demonstrated age-related reductions in rapid contraction-induced rapid vasodilation. Recent evidence in experimental animals suggests that activation of α-adrenergic receptors in microvascular resistance networks contributes to the blunted rapid vasodilation in response to muscle contractions with aging (24). Our present study aimed to translate these findings to humans. The primary novel findings of the present study are 1) α-adrenergic blockade augments hyperemic and vasodilator responses after a single muscle contraction in older adults and 2) sympathetic activation reduces rapid hyperemic and vasodilator responses to a single muscle contraction in young adults. The profound effects of sympathetic stimulation and α-adrenergic blockade in young and older adults are clearly shown in Fig. 4.
Aging is associated with a number of changes in arterial function and structure that are thought to compromise muscle blood flow or alter its regulation during dynamic exercise (43). Numerous studies have demonstrated age-related decreases in skeletal muscle blood flow during dynamic submaximal exercise under steady-state conditions in both experimental animals (8, 19, 21, 35) and humans (15, 26, 28, 40, 42, 44). More recent evidence suggests that rapid hyperemic and vasodilator responses to single muscle contractions are also attenuated in older adults (5, 26). Carlson et al. (5) demonstrated that peak and total vasodilator responses after mild to moderate (10%, 20%, and 40% MVC) contraction intensities in the forearm were substantially blunted in older healthy adults. In general, our present results are in agreement with those reported by Carlson and colleagues (5). However, age-related impairments in contraction-induced rapid vasodilation were only observed at 20% and 40% MVC for the peak response and 40% MVC for the total response in the present study.
Role of Sympathetic Vasoconstriction on Rapid Vasodilation in Young and Older Adults
Our initial hypothesis was that elevated basal sympathetic tone is responsible for lower peak vasodilation after single muscle contractions in older adults. That is, a greater overlying α-adrenergic vasoconstrictor tone might restrain rapid vasodilation in older adults to a greater extent than their young counterparts. However, in the present study, baseline (resting) FBF and FVC were not different between age groups, thus raising the concern that sympathetic α-adrenergic vasoconstrictor tone at rest may not have been greater in older adults. Interestingly, the results from the phentolamine trials demonstrate that elimination of α-adrenergic vasoconstrictor tone via phentolamine enhanced the immediate, peak, and total vasodilator responses in older adults at 10%, 20%, and 40% MVC (Figs. 1–3). These findings are in agreement with recent findings in experimental animals (24), which demonstrated that aging blunts rapid onset vasodilation in response to a single tetanic contraction and restricts blood flow to skeletal muscle of older male mice through subtle activation of α-adrenergic receptors in microvascular resistance networks. In contrast to older adults, administration of phentolamine did not enhance the contraction-induced peak vasodilation in young humans (Fig. 2).
An alternative explanation for our present findings might be related to the idea that older adults have impairments in functional sympatholysis in the vascular beds of contracting skeletal muscle during dynamic exercise (14), although this idea has been recently challenged (52). Evidence in young adults suggests that functional sympatholysis occurs after a single muscle contraction (11). Therefore, impairments in rapid functional sympatholysis with aging could attenuate the hyperemic and vasodilator responses observed in the present study. The fact that elimination of α-adrenergic vasoconstrictor tone in older adults substantially enhanced hyperemic and vasodilator responses to a single muscle contraction supports this idea. However, the rapid vasodilator responses observed during LBNP in older adults indicate complete sympatholysis and likely suggest no impairment in the ability to inhibit sympathetic vasoconstriction at the onset of exercise with aging. Although speculative, another explanation might be related to shifts along the vascular smooth muscle length-tension curve of older adults during α-adrenergic blockade, which could result in a greater relaxation for the same stimulus (i.e., contraction intensity).
Although we were somewhat surprised that removal of sympathetic vasoconstrictor tone did not augment rapid vasodilation in young adults in the present study, similar results have been reported in animals (24). Jackson and colleagues (24) did not observe an increase in the rapid onset vasodilation of young mice during α-adrenergic blockade compared with control conditions. Furthermore, the idea that removal of overlying sympathetic vasoconstrictor tone does not increase contraction-induced rapid vasodilation in young adults is supported by the observation that cervical sympathectomy did not lead to greater blood flow responses after single muscle contractions (9). Consistent with these findings, the change (from rest to exercise) in muscle blood flow and vasodilation during steady-state exercise in young adults is not enhanced by α-adrenergic blockade compared with control (saline) conditions (51).
It is well established that older adults demonstrate elevated MSNA compared with their younger counterparts (25). In the present study, we experimentally used a sympathetic stimulus via LBNP to determine if increases in the overlying sympathetic nervous system activity would blunt rapid vasodilation. Indeed, peak and total hyperemic and vasodilator responses in younger adults were reduced substantially during LBNP compared with control conditions (Figs. 2 and 3), and these results are in agreement with a previous study (11) using −40 mmHg of LBNP. Moreover, the magnitude of rapid vasodilation observed during sympathetic stimulation via LBNP in younger adults mirrored that of older subjects during the control trial (Fig. 4). In agreement with our present findings, direct stimulation of α-adrenergic receptors via topical administration of norepinephrine reduces the rapid onset vasodilation during a single tetanic contraction in young mice (24).
Interestingly, vasodilator responses to single muscle contractions in older adults were unaltered during LBNP compared with control trials in the present study. Since LBNP causes similar increases in MSNA in older and younger humans (10), the lack of change in rapid vasodilation during the LBNP trials in older adults might be related to decreased α-adrenergic responsiveness. Along these lines, aging is associated with a reduction in forearm postjunctional α-adrenergic responsiveness to exogenously administered norepinephrine (22) as well as endogenous norepinephrine release (13). However, the similar changes in baseline (resting) FVC during the LBNP trials between age groups (Table 2) would argue against the idea that α-adrenergic responsiveness was decreased in older adults. It is also possible that there might be an upward limit on the effects of sympathetic activity on rapid vasodilation in older subjects. However, we remain puzzled by these findings.
Table 2.
Baseline (resting) hemodynamics under each condition
| Young Adults |
Older Adults |
|||||||
|---|---|---|---|---|---|---|---|---|
| FBF, ml/min | FVC, ml/min | MAP, mmHg | HR, beats/min | FBF, ml/min | FVC, ml/min | MAP, mmHg | HR, beats/min | |
| Control | ||||||||
| 10% MVC | 50 ± 7 | 52 ± 7 | 97 ± 2 | 60 ± 2 | 67 ± 8 | 63 ± 7 | 105 ± 2* | 57 ± 2 |
| 20% MVC | 49 ± 7 | 51 ± 7 | 97 ± 2 | 60 ± 2 | 72 ± 9 | 68 ± 8 | 104 ± 3* | 57 ± 2 |
| 40% MVC | 50 ± 6 | 51 ± 6 | 98 ± 2 | 62 ± 2 | 71 ± 9 | 67 ± 8 | 104 ± 2* | 57 ± 2 |
| Lower body negative pressure | ||||||||
| 10% MVC | 42 ± 6 | 44 ± 6 | 96 ± 2 | 65 ± 2† | 57 ± 7 | 57 ± 8 | 102 ± 2* | 58 ± 2 |
| 20% MVC | 48 ± 7 | 50 ± 7 | 96 ± 1 | 65 ± 2† | 57 ± 7 | 55 ± 7 | 103 ± 1* | 59 ± 2 |
| 40% MVC | 50 ± 8 | 52 ± 9 | 96 ± 1 | 65 ± 2 | 63 ± 10 | 61 ± 9 | 103 ± 2* | 59 ± 2 |
| Phentolamine | ||||||||
| 10% MVC | 135 ± 21† | 136 ± 20† | 98 ± 2 | 62 ± 3 | 156 ± 25† | 147 ± 22† | 104 ± 2* | 56 ± 2 |
| 20% MVC | 130 ± 19† | 132 ± 18† | 98 ± 1 | 62 ± 3 | 148 ± 23† | 139 ± 20† | 104 ± 2* | 56 ± 2 |
| 40% MVC | 134 ± 21† | 134 ± 20† | 99 ± 2 | 63 ± 3 | 159 ± 23† | 150 ± 20† | 105 ± 2* | 56 ± 2 |
Values are means ± SE. FBF, forearm blood flow; FVC, forearm vascular conductance; HR, heart rate.
P < 0.05 vs. young adults;
P < 0.05 vs. control at the respective MVC.
Endothelium-dependent vasodilation is another potential local control mechanism that undergoes age-related impairment and contributes to the attenuated contraction-induced vasodilation in older adults. Recent data have suggested that the time course of endothelium-dependent vasodilation in isolated arterioles is significantly slower in skeletal muscle of old rats (3). In young humans, nitric oxide synthase inhibition reduces hyperemic responses to a single muscle contraction (4). Although nitric oxide has been suggested to explain the lower exercise hyperemia and vasodilation during steady-state exercise in older humans (46), its role has not been directly assessed in the age-related rapid vasodilation after single muscle contractions. However, an acute improvement in endothelial-dependent vasodilation (via ascorbic acid administration) does not reverse the age-related impairment in rapid hyperemic responses to a single muscle contraction (26). Future studies will be required to directly assess the role of nitric oxide and other potential vasodilators in the blunted rapid onset vasodilation in older adults.
Sex-Related Differences in Rapid Vasodilation
Since sex-differences exist in neurovascular control at rest (18, 20, 27) and potentially during exercise (43), we examined whether the effects of sympathetic stimulation and/or α-adrenergic blockade on rapid vasodilator responses differed between men and women within and between age groups. Sympathetic stimulation via LBNP resulted in a substantially greater reduction in the rapid vasodilator response during low-intensity (10% MVC) contraction in young men compared with women (Fig. 5). However, these sex differences in young adults did not persist with increasing contraction intensity (20% and 40% MVC). These findings suggest that similar to resting conditions (18, 20, 27), sympathetic vasoconstrictor responsiveness is greater in young men compared with women at lower intensity contractions but becomes less apparent with higher workloads. Conversely, α-adrenergic blockade via phentolamine revealed greater vasodilator responses in young women at moderate- to high-intensity muscle contraction (20% and 40% MVC) but not at low-intensity contraction (10% MVC; Fig. 6). The greater vasodilator response observed during α-adrenergic blockade in young women might suggest less functional sympatholysis than young men at moderate- to high-intensity contractions. However, recent data have demonstrated that the degree of functional sympatholysis of α1- and α2-adrenergic-mediated vasoconstriction is similar in young men and women (30). An alternative explanation might be an enhanced β-adrenergic vasodilation in young women (20, 27), especially when studied with a background of α-adrenergic blockade.
Older men and women demonstrated similar changes in rapid vasodilation during LBNP. However, sex-related differences in rapid vasodilator responses were observed at light to moderate contraction intensities during α-adrenergic blockade (Fig. 6). The greater increase in peak vasodilation in older men might be related to less functional sympatholysis compared with older women. Evidence has demonstrated reduced functional sympatholysis in older men (14) and women (38); however, to our knowledge, potential sex differences in the degree of functional sympatholysis in older adults are unknown.
When the changes in rapid vasodilator responses to LBNP between age groups were examined, both young men and women demonstrated a greater reduction during LBNP than their older counterparts (Fig. 5). Conversely, age-related differences in rapid vasodilator responses during α-adrenergic blockade across contraction intensities were only observed in men (Fig. 6). That is, older men demonstrated a substantially greater change in rapid vasodilation during phentolamine than young men. This difference between men might be explained by either overlying sympathetic vasoconstrictor tone and/or a blunted functional sympatholysis. Interestingly, the change in rapid vasodilation with phentolamine was similar between young and older women across contraction intensities. The lack of difference between the female age groups is surprising since older women have elevated MSNA (20, 36), reduced functional sympatholysis (38), and less β-adrenergic-mediated vasodilation (20) than young women.
Experimental Considerations
LBNP was chosen for the model of sympathetic stimulation with the goal of increasing MSNA without concurrent changes in arterial pressure. While MSNA was not directly assessed in the present study, −20 mmHg of LBNP has been shown to significantly increase MSNA in young and older adults (37, 45). Important to this study, low levels of LBNP (i.e., −20 mmHg) increase MSNA in younger subjects to similar levels observed in older adults (10).
In contrast to previous studies (7, 10), baseline (resting) FBF was not significantly reduced during low-level LBNP in the present study (Table 2). The discrepant findings might be related to sex-related differences in peripheral vasoconstrictor responsiveness. In this context, increases in peripheral vasoconstriction in response to LBNP have mainly been reported in young male subjects, whereas our present study included both men and women. Recent evidence suggests that men demonstrate greater vasoconstrictor responses to LBNP than women (18). Moreover, young women compared with men have attenuated peripheral vasoconstrictor responses to increases in transmural pressure (31). Along these lines, the average LBNP-induced reduction in resting FBF across all exercise intensities was −6.2 ± 2.4 and −0.4 ± 2.3 ml/min for young men and women, respectively (P < 0.05).
Prior studies (5, 26) examining rapid vasodilator responses to single muscle contractions have expressed the magnitude of the response as a relative change (in %) from baseline. This analytic approach has demonstrated that aging is associated with a blunted rapid vasodilation. Our approach of expressing flow and vasodilator responses to a single muscle contraction as an absolute change above baseline (i.e., postcontraction value − baseline value) supports the findings that age-related decrements in rapid vasodilation exist. The novel finding that α-adrenergic blockade augments rapid flow and vasodilator responses after a single muscle contraction in older adults is also based on the absolute changes in FBF and FVC (Figs. 1–3). Because phentolamine increases baseline blood flow two- to threefold, expressing the vasodilator response as a relative change (in %) from baseline during the phentolamine trials would suggest that the rapid vasodilation is substantially reduced (nearly 3-fold) in young and older adults with α-adrenergic blockade compared with control trials. Important to the present study, it would also suggest that α-adrenergic blockade attenuates the rapid vasodilator response to single muscle contractions to a greater extent than sympathetic activation via LBNP in young adults. Therefore, we chose to report the absolute change in muscle blood flow and conductance from baseline and believe that these values most represent the rapid vasodilator responses to single muscle contractions. Along these lines, it has previously been shown that when baseline FBF was intentionally increased before handgrip exercise, the elevated baseline flow had no effect on the change in flow caused by exercise (39).
Whole leg blood flow has been shown to be related to leg muscle mass (34). Aging is often associated with decreases in skeletal muscle mass and strength (47), both of which might contribute to lower blood exercise blood flows in older adults. Lean muscle mass was not measured in this study, and we cannot be certain that potential differences in muscle mass contributed to the attenuated vasodilator responses in older adults. However, lean muscle mass in the forearm has been shown to be similar between young and older adults (5). Moreover, forearm volume and MVC were not different between the age groups in the present study (Table 1). Taken together, the age-related attenuation in contraction-induced rapid vasodilation is not likely explained by differences in forearm muscle mass.
Conclusions
This study has provided the first evidence in humans that pharmacological blockade of α-adrenergic receptors augments the contraction-induced rapid vasodilation in the forearm of older adults to the point that age-related differences were no longer apparent. Additionally, acute sympathetic stimulation in young adults results in an “older” rapid vasodilator phenotype. Taken together, our data suggest that an enhanced α-adrenergic vasoconstrictor tone and/or a blunted functional sympatholysis contribute to the age-related reductions in rapid vasodilation observed after a single muscle contraction. However, the lack of change in rapid vasodilation during acute sympathetic stimulation in older adults suggests that the ability to inhibit sympathetic vasoconstriction at the onset of exercise is not impaired with aging.
GRANTS
This work was supported by National Institutes of Health Grants HL-105467 (to D. P. Casey), HL-46493 (to M. J. Joyner), and RR-024150. The Caywood Professorship via the Mayo Foundation also supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P.C. and M.J.J. conception and design of research; D.P.C. and M.J.J. performed experiments; D.P.C. analyzed data; D.P.C. and M.J.J. interpreted results of experiments; D.P.C. prepared figures; D.P.C. drafted manuscript; D.P.C. and M.J.J. edited and revised manuscript; D.P.C. and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to the study volunteers for participation. The authors also thank Branton Walker, Essa Mohamed, Christopher Johnson, Pam Engrav, Shelly Roberts, and Sarah Wolhart for technical assistance.
REFERENCES
- 1. Bearden SE. Advancing age produces sex differences in vasomotor kinetics during and after skeletal muscle contraction. Am J Physiol Regul Integr Comp Physiol 293: R1274–R1279, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561: 535–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behnke BJ, Delp MD. Aging blunts the dynamics of vasodilation in isolated skeletal muscle resistance vessels. J Appl Physiol 108: 14–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 85: 2249–2254, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294: H1963–H1970, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Convertino VA, Doerr DF, Ludwig DA, Vernikos J. Effect of simulated microgravity on cardiopulmonary baroreflex control of forearm vascular resistance. Am J Physiol Regul Integr Comp Physiol 266: R1962–R1969, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Copp SW, Ferreira LF, Herspring KF, Musch TI, Poole DC. The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvasc Res 77: 113–119, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol 19: 142–146, 1964 [DOI] [PubMed] [Google Scholar]
- 10. Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32: 298–304, 1998 [DOI] [PubMed] [Google Scholar]
- 11. DeLorey DS, Wang SS, Shoemaker JK. Evidence for sympatholysis at the onset of forearm exercise. J Appl Physiol 93: 555–560, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Eklund B, Kaijser L. Effect of regional α- and β-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol 262: 39–50, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Hachiya T, Hashimoto I, Saito M, Blaber AP. Peripheral vascular responses of men and women to LBNP. Aviat Space Environ Med 83: 118–124, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Hammer LW, Boegehold MA. Functional hyperemia is reduced in skeletal muscle of aged rats. Microcirculation 12: 517–526, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirai DM, Copp SW, Hageman KS, Poole DC, Musch TI. Aging alters the contribution of nitric oxide to regional muscle hemodynamic control at rest and during exercise in rats. J Appl Physiol 111: 989–998, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Hogikyan RV, Supiano MA. Arterial α-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol Endocrinol Metab 266: E717–E724, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Holloszy JO, Kohrt WM. Exercise. In: Handbook of Physiology. Aging. Bethesda, MD: Am. Physiol. Soc., 1995, sect. 11, chapt. 24, p. 633–666 [Google Scholar]
- 24. Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol 588: 2269–2282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56: 10–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Lawrenson L, Hoff J, Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle V̇o2 max. Am J Physiol Heart Circ Physiol 286: H1565–H1572, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. α-Adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol 109: 1360–1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lott ME, Hogeman C, Herr M, Bhagat M, Sinoway LI. Sex differences in limb vasoconstriction responses to increases in transmural pressures. Am J Physiol Heart Circ Physiol 296: H186–H194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J 300: 230–235, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Miyachi M, Tanaka H, Kawano H, Okajima M, Tabata I. Lack of age-related decreases in basal whole leg blood flow in resistance-trained men. J Appl Physiol 99: 1384–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol 292: H1148–H1156, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Patterson GC, Shepherd JT. The effects of continuous infusions into the brachial artery of adenosine triphosphate, histamine and acetylcholine on the amount and rate of blood debt repayment following rhythmic exercise of the forearm muscles. Clin Sci (Lond) 13: 85–91, 1954 [PubMed] [Google Scholar]
- 40. Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of V̇o2 max in trained older subjects. J Appl Physiol 82: 1411–1415, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol 66: 2778–2781, 1989 [DOI] [PubMed] [Google Scholar]
- 46. Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Short KR, Nair KS. Muscle protein metabolism and the sarcopenia of aging. Int J Sport Nutr Exerc Metab Suppl 11: S119–S127, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tschakovsky ME, Hughson RL. Rapid blunting of sympathetic vasoconstriction in the human forearm at the onset of exercise. J Appl Physiol 94: 1785–1792, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96: 639–644, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of β-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wray DW, Nishiyama SK, Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]