Abstract
Tauopathies are characterized by progressive neurodegeneration caused by intracellular accumulation of hyperphosphorylated tau protein aggregates in the brain. The present study was designed to test whether a grape seed polyphenolic extract (GSPE) previously shown to inhibit tau protein aggregation in vitro could benefit tau-mediated neuropathology and behavior deficits in JNPL3 transgenic mice expressing a human tau protein containing the P301L mutation. Nine months old JNPL3 mice were treated with GSPE delivered through their drinking water for six months. We found that GSPE treatment significantly reduced the number of motor neurons immunoreactive for hyperphosphorylated and conformationally-modified tau in the ventral horns of the spinal cord identified using AT100, PHF-1, AT8 and Alz50 tau antibodies. This coincided with a drastically reduced level of hyperphosphorylated and sarcosyl-insoluble tau in spinal cord fractions. Furthermore, the reduction of tau pathology was accompanied by an improvement in the motor function assessed by a wire hang test. Collectively, our results suggest that GSPE can interfere with tau-mediated neurodegenerative mechanisms and ameliorate neurodegenerative phenotype in an animal model of tauopathy. Our studies support further evaluation of GSPE for preventing and/or treating of tauopathies in humans.
Introduction
Misfolding and aberrant aggregation of the microtubule associated protein tau are key neuropathologic features shared by different neurodegenerative disorders that are collectively known as tauopathies (Lee et al., 2001; Hernandez and Avila, 2007). Under physiological conditions, the majority of tau is associated with microtubules, stabilizing the microtubule network within axons and facilitating axonal transport of trophic factors, neurotransmitters and other cellular constituents (Drubin et al., 1985; Congdon et al., 2008). The binding of tau to microtubules is controlled predominantly by the phosphorylation state of tau that modulates the affinity of tau protein to the microtubules (Mazanetz and Fischer, 2007). Several protein kinases have been shown to phosphorylate tau (Hanger DP, ; Hanger et al., 1998; Reynolds et al., 2000). Among them, extracellular-signal-regulated kinases 1/2 (ERK1/2) , glycogen synthase kinase 3 (GSK-3) and cyclin-dependent kinase 5 (CDK5) have been proposed to be the most relevant kinases responsible for abnormal tau phosphorylation in tauopathies (Mazanetz and Fischer, 2007). Under pathological conditions hyperphosphorylated tau disengages from the microtubules and is prone to misfolding (Alonso et al., 1994; Ballatore et al., 2007). Formation of characteristic structures, such as neurofibrillary tangles (NFTs) and neuropil threads from misfolded tau, constitutes the diagnostic signature of different tauopathies. The loss of the normal microtubule-stabilizing function of tau (Ballatore et al., 2007) contributes to axonal transport deficits and neuropathology.
Agents capable of reducing abnormal phosphorylation and self-assembly of tau offer attractive strategies for the prevention and/or treatment of tau-mediated neurodegenerative disorders (Brunden et al., 2009; Brunden et al., 2010). Currently, different studies have identified several inhibitors of fibrillogenesis in vitro using a variety of tau assembly assays (Wischik et al., 1996; Chirita et al., 2004; Taniguchi et al., 2005; Brunden et al., 2009; Crowe et al., 2009; Li et al., 2009; Bulic et al., 2010). With the exception of methylene blue, which has already progressed to human clinical trials, none of these compounds have been assessed for efficacy in vivo. Grape seed polyphenolic extract (GSPE) is enriched in natural polyphenolic compounds comprised of proanthocyanidins, which are the most abundant and complex class of grape polyphenols (Shi et al., 2003; Yadav et al., 2009). Our previous evidence indicates that GSPE may interfere with aberrant aggregation of tau and promote disassembly of tau aggregates. For example, we found that GSPE significantly inhibits self-aggregation of a synthetic tau peptide containing the 306VQIVYK311 nucleation motif and promotes its dissociation from already assembled filaments (Ho et al., 2009). Moreover, GSPE potently disrupts and destabilizes the ultrastructure of paired helical filaments (PHFs) isolated from Alzheimer's disease (AD) brains (Ksiezak-Reding et al., 2010). In addition to modulating tau aggregation, we recently found that GSPE may also attenuate phosphorylation of tau through mechanisms that influence the activation of ERK1/2 pathway in the mouse brain (Wang et al., 2010).
In the present studies we examined the effect of GSPE on tau pathology and motor disturbances in a transgenic mouse model of tauopathy (JNPL3 mice) expressing a human tau protein containing the P301L mutation (Lewis et al., 2000). JNPL3 mice are characterized by increasing hyperphosphorylation and aggregation of tau that lead to the formation of NFTs in the spinal cord (Lewis et al., 2000). They also develop progressive motor disturbances. Besides a number of acknowledged anatomical and biochemical differences, the JNPL3 mouse model replicates selected neurofibrillary features as well as motor and movement abnormalities associated with a number of tauopathies (Lewis et al., 2000; Sahara et al., 2002). Due to neuropathological and behavioral characteristics, JNPL3 mice are considered a valuable animal model for tau-directed drug discovery studies (Lin et al., 2003; Lin et al., 2005; Radde et al., 2008).
Materials and Methods
Composition of GSPE
GSPE was obtained from Polyphenolics Inc. (Madera, CA). GSPE studied in the present paper was the same bioactive material (Lot #25952501-30) as described in our previous reports (Ho et al., 2009; Wang et al., 2008; Wang et al., 2010). HPLC analysis determined that GSPE was composed of catechins and epicatechins in monomeric, dimeric, oligomeric, and polymeric forms (available at www.jneurosci.org as supplemental material Wang et al., 2008, supplemental Fig. 1A). Typically, GSPE contains 8% monomers, 75% oligomers, and ∼17% polymers. We arbitrarily used the molecular weight of catechin and epicatechin dimer (which is the most abundant form of oligomers in GSPE) to calculate the molarity for GSPE (MW 500) in the present studies.
Animals and GSPE Treatment
Hemizygous male transgenic JNPL3 mice (Taconic Farms, Inc, Germantown, NY, USA) (Lewis, et al., 2000) were maintained at the Mount Sinai School of Medicine Center for Comparative Medicine and Surgery. Seven male JNPL3 mice were treated with GSPE delivered through their drinking water starting at approximately 9 month of age and continued for 6 months until 15 months of age. In the present study, we used the GSPE dosage of 150 mg/Kg-BW/day. Nine age- and gender (males) matched non-treated JNPL3 mice received drinking water without GSPE. Motor functions were assessed using a wire hang test (see below for behavior testing). At the end of the study, animals were sacrificed and brains and spinal cords were removed. Half of the brain was frozen in liquid nitrogen for biochemical analysis and the other half was fixed with 4% paraformaldehyde and paraffin-embedded for histochemical examination. Procedures involving animals and their care were approved by the Animal Care and Use Committee in compliance with National Institute of Health guidelines and policies.
Brain and spinal cord tissue extraction
Forebrain and spinal cord extracts were prepared from frozen tissue according to previously published protocol with some modifications (Sahara et al., 2002; Xu et al., 2010). Briefly, forebrains and spinal cords were homogenized on ice using 10 volumes (w/v) of extraction buffer containing 20 mM HEPES, pH 7.4, 100 mM NaCl, 20 mM NaF, 1% Triton X-100, 1 mM sodium orthovanadate, 5 mM EDTA with protease inhibitors (2 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin). Homogenates were sonicated (Fisher Scientific Sonic Dismembrator 500, amplitude 24% for 5 seconds) and centrifuged at 3000g at 4°C for 5 min. The supernatant designated as crude tau extract was aliquoted and stored at −80°C until used. Preparation of sarcosyl-soluble and insoluble fractions was carried out as described previously (Takahashi et al., 2002). Briefly, Sarcosyl powder was added to crude tau extracts to the final concentration of 1% (w/v), followed by 1h incubation at 25°C with stirring and 2h centrifugation at 100,000×g. The resulting supernatant and the pellet were considered as Sarcosyl-soluble and Sarcosyl-insoluble tau fractions, respectively.
Antibodies
Primary antibodies against conformational (Alz50) and phosphorylated (PHF-1) tau proteins were generous gifts of Dr. Peter Davies (Albert Einstein College of Medicine, Bronx, NY). Antibodies recognizing phospho-epitopes of tau (AT8, AT100, AT180, and AT270) and total human tau (HT7) were purchased from Thermo Scientific (Rockford, IL). Antibody against pS214tau was purchased from Calbiochem (EMD Chemicals, Inc. Gibbstown, NJ). Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was purchased from Chemicon (Millipore, USA).
Western blot analysis
Samples were prepared by adding Laemmli sample buffer and boiling for 5 min. Proteins were separated by electrophoresis on 10% sodium dodecyl sulfate–polyacrylamide gels (SDS-PAGE) and electro-transferred onto nitrocellulose membranes (Bio-Rad Labs, Hercules, CA). The membranes were blocked with a solution containing 5% nonfat dried milk (Sigma) in Tris-buffered saline (TBS), then incubated with primary tau antibodies as specified, followed by incubation with secondary antibodies conjugated to horse radish peroxidase (Santa Cruz Biotechnology Inc., CA). Immunoreactive protein signals were detected using the enhanced chemiluminescence system (ECL plus; PerkinElmer LAS, Inc., Boston, MA) and X-ray film. Signals were quantified by densitometry using The Discovery Series Imaging Software version 4.1.1. (BioRad Labs). Relative protein levels were normalized using GAPDH as internal protein standard.
Immunogold electron microscopy
Samples were adsorbed for 5 min on carbon/formvar-coated 200 mesh copper grids (EM Sciences, Fort Washington, PA). The grids were processed using tau antibodies and 10-nm gold-conjugated secondary antibodies as described previously (Takahashi et al., 2002). Grids were stained with 2% uranyl acetate for 5 min and viewed using Hitachi H7000 (Japan) electron microscope operated at 75 kV. Electron microscope was equipped with an AMT Advantage HS digital camera (Danvers, MA) and micrographs were recorded digitally.
Immunohistochemistry
Brain and spinal cord paraffin embedded sections (5 microns thick) were deparaffinized in Histo-Clear II (National Diagnostics, GA) and processed for immunohistochemistry using anti-mouse tau antibodies according to manufacturer's protocol for mouse brain sections (MOM kit; Vector Labs, Cat # PK-2200) with some modifications. A 30 min incubation with 3% H2O2/10% methanol/0.25% Triton X-100 was used to block endogenous peroxidase activity. 3,3′-Diaminobenzidine was used as a peroxidase substrate (Vector DAB Substrate Kit for Peroxidase, Cat # SK-4100). Tissue sections were counterstained with hematoxylin and mounted using Cytoseal 60 (Thermo Scientific, Cat # 8310-16). Sections were viewed using Nikon Eclipse E600 brightfield microscope (Nikon, Japan) and images recorded digitally. For quantification of tau-positive neurons we used 5 slides per animal group and antibody. The number of tau-bearing neurons in the ventral horn region of the spinal cord was counted in a field of 20× objective lens.
Behavioral analysis by wire hang test
The wire hang test was conducted as described by Karl et al. (Karl et al., 2003) with some modifications. The animals were placed on the top of a wire cage lid. The lid was shaken lightly three times to prompt the animals to grip the wires and then the lid was turned upside down. The latency of animals to fall off the wire grid was measured in seconds (up to 100 seconds). Tests were performed for three consecutive days right before the end of the study and average values for each animal were used to make the group comparison.
Statistical analysis
All values are expressed as means ± standard deviation (SD) unless stated otherwise. Differences between means were analyzed with one- way ANOVA and two-tailed Student t-test using the Prism Stat program (GraphPad Software, Inc., San Diego CA) and Excel (Microsoft Inc.). To analyze data with non-parametric distribution, we used Mann Whitney t-test. In all analyses, the null hypothesis was rejected at the 0.05 level.
Results
GSPE treatment is well tolerated and attenuates motor function deficits in JNPL3 mice
JNPL3 male mice were either non-treated (9 mice) or treated (7 mice) with 150 mg/kg-BW/day GSPE starting at 9 months of age. Long-time GSPE treatment for 6 months was well tolerated as reflected by normal grooming behavior and steady body weight values (Fig. 1A). To explore whether GSPE treatment might modulate clinical tauopathy phenotype, we used a wire hang test to assess the motor behavioral function following 6 months of treatment. We found that GSPE treatment led to a significant improvement in motor function. Our results showed that 15 months old untreated JNPL3 mice were able to stay on the wire grid for about 6 seconds before falling off while the GSPE-treated JNPL3 mice stayed on the wire grid for an average of 26 seconds (Fig. 1B). Our results indicate that GSPE treatment might attenuate the neuromuscular deficiency in JNPL3 mice.
Figure 1. GSPE treatment reduces motor deficits in JNPL3 mice.
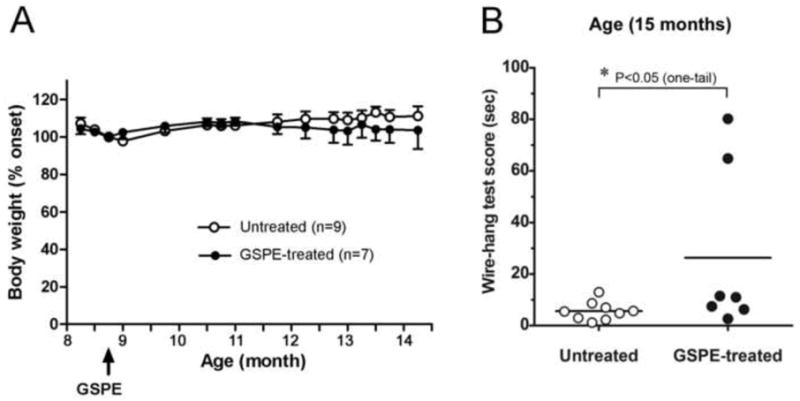
(A) Body weight in untreated (n=9) and GSPE-treated (n=7) JNPL3 mice following 6 months of treatment. Arrow indicates the initiation of GSPE treatment in JNPL3mice at approximately 9 months of age. Mean values ± SEM. One-way ANOVA showed no significant differences between the groups. (B) Wire hang test assessed in untreated (n=9) and 6-months GSPE treated (n=7) animals at 15 months of age. Results presented as scatter plots and means. GSPE-treatment improved the test scores from 6 sec to 26 sec at p= 0.0368 using one-tailed Mann Whitney t-test for non-parametric data distribution.
GSPE reduces tau hyperphosphorylation and conformational changes as well as the number of neurons with tau inclusions in the spinal cord
We performed histopathological examination of forebrain and spinal cord paraffin sections of five untreated and five GSPE-treated JNPL3 mice using a panel of antibodies raised against total and phosphorylated tau protein. We found that the majority of tau-positive inclusions were present in the spinal cord and that they were mostly located in motor neurons of the ventral horn region with fewer inclusions seen in the dorsal horns. Next, we compared the number of neurons displaying tau positive inclusions in five sections of ventral horns of untreated and GSPE-treated groups of animals. Using HT7 monoclonal antibody specific for total human tau, we found that GSPE treatment did not significantly effect the total number of human tau-positive neurons as compared to untreated JNPL3 mice. Although the number of HT7-positive neurons declined by 20% in GSPE-treated mice (Table 1), the decrease was not statistically significant. In comparison, the number of tau-positive neurons declined sharply by 62% using conformation-dependent Alz50 tau antibody and up to 90% (AT100) using phospho-dependent tau antibodies (Table 1). The results suggest that the GSPE treatment significantly reduced the levels of hyperphosphorylated as well as conformationally modified tau in spinal cord ventral horn neurons.
Table 1. Tau-positive inclusions in spinal cord of JNPL3 mice.
| Antibody | Number of neurons | ||
|---|---|---|---|
| Untreated JNPL3 mice | GSPE-treated JNPL3 mice | % Change GSPE-treated vs. untreated | |
| HT7 | 7.5±1.0 | 6.0±0.2 | 80±2.7 |
| Alz-50 | 7.0±0.5 | 1.0±0.5* | 38±5.4* |
| AT8 | 10.0±2.0 | 1.3±0.1* | 13±1.0* |
| AT100 | 6.0±2.2 | 0.6±0.2* | 10±3.3* |
| PHF-1 | 12±0.5 | 4.2±0.5* | 35±4.2* |
Means ± SD
p<0.05
n=5 per group (Student's t-test)
Quantitative analysis in ventral horns.
Five sections for each antibody.
To confirm our histopathological findings, we examined the phosphorylation status of tau protein by western blotting analysis of crude tau extracts. To differentiate between tau species of human origin (transgene product) and mouse origin (endogenous tau), we used extracts of spinal cord and forebrain tissue from JNPL3 and non-transgenic (wild-type) mice. Both spinal cord and forebrain of JNPL3 mice expressed human tau immunoreactive with HT7 antibody (Fig. 3, HT7). In the spinal cord, three species of human tau were detected at 50 kDa, 55 kDa and 64 kDa whereas in the forebrain the 64 kDa tau polypeptide was absent. From all three polypeptides of tau in the spinal cord only the 64 kDa polypeptide was immunoreactive with PHF-1 antibody suggesting that it may represent hyperphosphorylated tau species of human origin. PHF-1-positive tau species were absent in the wild-type spinal cord, confirming that the 64 kDa tau polypeptide was unlikely to derive from the endogenous mouse tau. Forebrain of JNPL3 mice also contained PHF-1 immunoreactive tau species (53 kDa and 55 kDa) (Fig. 3). The human origin of these tau species was uncertain, however, since similar PHF-1 immunoreactive tau polypeptides (53 kDa and 55 kDa) were found in the forebrain of wild-type animals. In addition, the 53 kDa band which reacted more strongly with PHF-1 was clearly HT7 negative (Fig. 3, Forebrain). In summary, our results showed that although both the spinal cord and the forebrain of JNPL3 mice expressed human tau, only the spinal cord expressed hyperphosphorylated human tau species of 64 kDa. Such expression pattern is consistent with previous findings in JNPL3 mice showing more abundant 64 kDa hyperphosphorylated tau species in subcortical and spinal cord regions than in cortico-limbic region (Lewis et al., 2000; Sahara et al., 2002).
Figure 3. Human and wild-type tau protein expression in the brain and spinal cord of JNPL3.
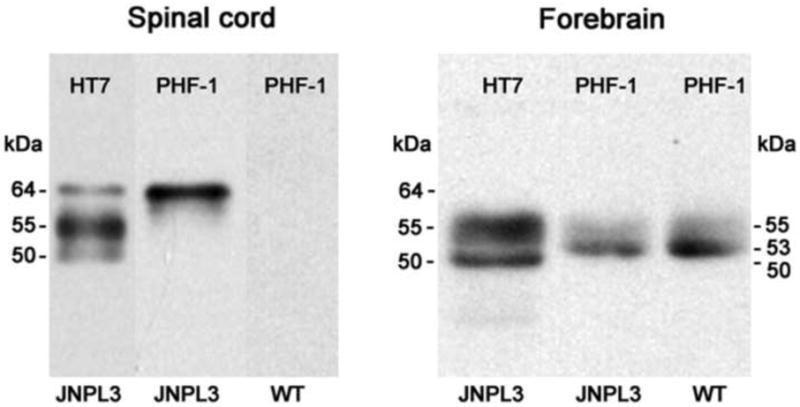
Western blotting of crude extracts from the spinal cord and the forebrain tissues in untreated JNPL3 mice and wild type (WT) mice using human-specific (HT7) and phosphorylated (PHF-1) tau antibodies. JNPL3 spinal cord but not wild-type spinal cord expressed 50 kD, 55 kD and 64 kD species of human tau. Only 64 kD tau species was phosphorylated at PHF-1 site and could correspond to hyperphosphorylated tau protein of 64 kD described in previous studies (Lewis et al., 2002; Sahara et al., 2002). JNPL3 forebrain expressed 50 kD and 55 kD human tau species and contained most likely endogenous 53 kD and 55 kD mouse tau species phosphorylated at PHF-1 site since they were also detected in wild-type forebrain.
Next, we evaluated effects of six months GSPE treatment on the content of phosphorylated tau species in the spinal cord of JNPL3 mice. Following GSPE treatment, total human tau content (HT7 immunoreactivity) was reduced by approximately 20%, most noticeable with the 64 kDa tau species (Fig. 4A). Consistent with the results of histochemical analysis (see Fig. 1, Table 1), the changes in total human tau content were not statistically significant. In comparison, Alz50 immunoreactivity was almost completely abolished following 6 months of GSPE treatment (Fig. 4B). Furthermore, there was a significant reduction in the level of phosphorylated tau species identified with antibodies AT8 (decrease by ∼87%), AT100 (by 90%), and PHF-1 (by 58%) (Fig. 4C-E). Very little change in phospho-tau immunoreactivity was observed using antibodies AT180 and AT270 (data not shown). GAPDH immunoreactivity remained unchanged confirming the selectivity of tau protein alterations (Fig. 4F). Collectively, our western blotting and immunohistochemical analyses suggest that GSPE treatment has a profound inhibitory effect on the phosphorylation and conformational changes of selected tau epitopes. Our data indicate that by inhibiting tau phosphorylation and interfering with tau conformational change, GSPE may significantly reduce the number of abnormal inclusions expressing phospho-tau in the spinal cord neurons of JNPL3 mice.
Figure 4. GSPE treatment reduces tau phosphorylation in the spinal cord of JNPL3 mice.
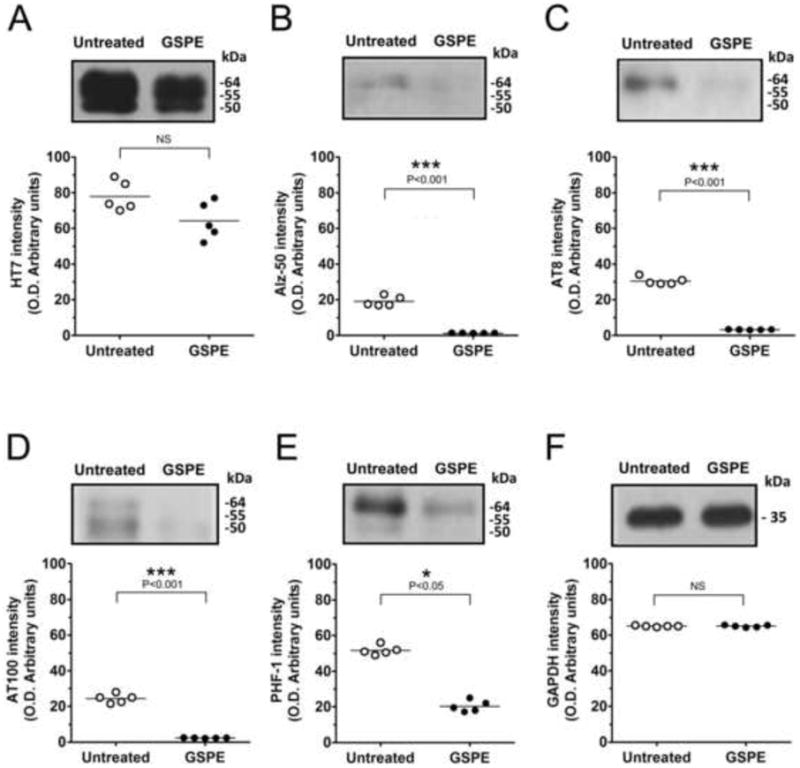
Quantitative western blotting analysis of tau protein content in crude spinal cord extracts using various antibodies (A) HT7, (B) Alz-50, (C) AT8, (D) AT100, (E) PHF-1, and (D) GAPDH as loading control with typical immunoblotting patterns shown. Densitometric analysis shows phosphorylated tau levels significantly reduced and total human tau unchanged. Results presented as scatter plots and means (n=5). ***P<0.001 for Alz50, AT8, and AT100; *P<0.05 for PHF-1 or NS (P>0.05) using Student's t-test.
GSPE reduces the content of sarcosyl-insoluble tau in the spinal cord of JNPL3 mice
Sarcosyl-soluble and Sarcosyl–insoluble fractions represent normal non-aggregated and abnormally aggregated tau, respectively. To investigate whether GSPE treatment might affect the solubility of tau in the spinal cord, we fractionated crude tau extracts from five JNPL3 mice using Sarcosyl. Consistent with previous findings (Lewis et al., 2000), HT7 immunoreactive human tau species were present in both Sarcosyl-soluble and Sarcosyl-insoluble fractions of non-treated JNPL3 mice (Fig. 5A, left panel, white circles). Sarcosyl-soluble human tau species were composed of three polypeptides with molecular sizes of 50 kDa, 55 kDa and 64 kDa. Sarcosyl-insoluble species contained only two polypeptides of tau, 55 kDa and 64 kDa, with the 64 kDa polypeptide showing a predominant expression. None of the two polypeptides were immunoreactive with pSer214tau and PHF-1 antibodies in Sarcosyl-soluble tau fraction whereas both tau polypeptides were immunoreactive with pSer214tau and PHF-1 in Sarcosyl-insoluble fraction (Fig. 5A, middle and right panels, white circles). The studies of untreated JNPL3 mice confirmed that Sarcosyl extraction effectively separated tau protein into less phosphorylated (Sarcosyl-soluble) and hyperphosphorylated (Sarcosyl-insoluble) tau species. In the spinal cord of five JNPL3 mice treated with GSPE for six months, we observed no significant changes in total human tau in the Sarcosyl-soluble fraction, whereas the Sarcosyl-insoluble total tau protein underwent a severe reduction by ∼82% (Fig. 5A, left panel, black circles). Phosphorylation status analysis with PHF-1 and pSer214tau antibodies showed that the content of hyperphosphorylated tau species in Sarcosyl-insoluble fractions was largely depleted in GSPE-treated samples (Fig. 5A, middle and left panels, black circles). These results indicate that GSPE preferentially reduces the content and phosphorylation status of the Sarcosyl-insoluble tau fraction and strongly suggest that GSPE treatment diminishes the aggregation of tau in spinal cord of JNPL3 mice.
Figure 5. GSPE treatment reduces the content of hyperphosphorylated tau in sarcosyl-insoluble fractions and the number of fibrillary aggregates in the spinal cord of JNPL3 mice.
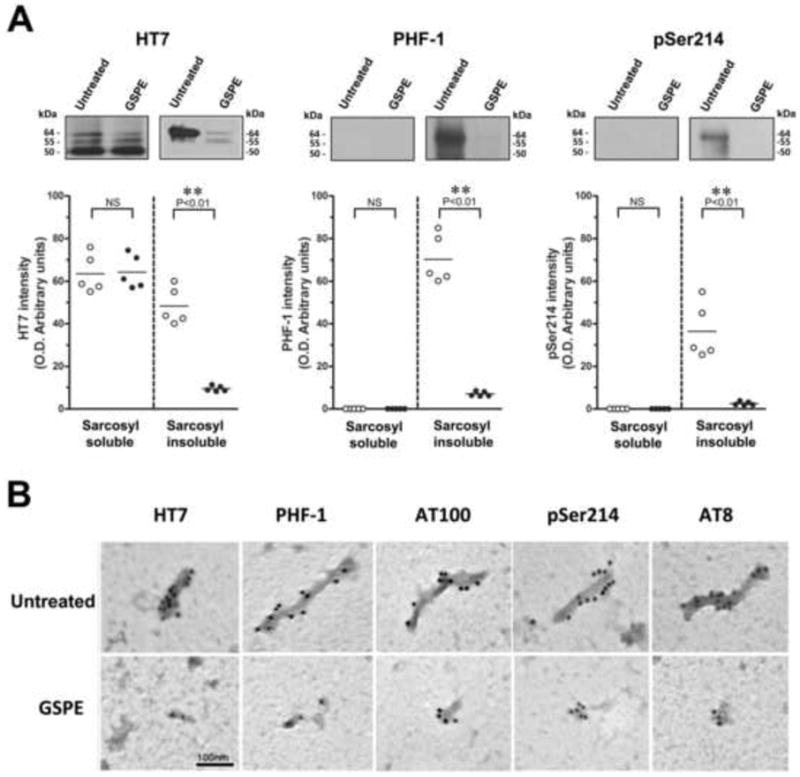
(A) Quantitative analysis of samples from Sarcosyl-soluble and Sarcosyl-insoluble fractions examined by western blotting using HT7, PHF-1 and pSer214tau antibodies. Inset: representative western blotting images. Results presented as scatter plots and means (n=5). **P<0.01 for HT7, PHF-1 and pSer214 or NS (P>0.05) using Student's t-test. (B) Immunogold electron microscopy of sarcosyl-insoluble fractions using 10-nm immunogold labeling with HT7, PHF-1, AT100, pSer214 tau and AT8 antibodies. Note well-defined PHF-like fibrils in untreated JNPL3 mice and smaller tau aggregates in GSPE treated mice. Scale bar = 100 nm.
To confirm the results of our biochemical analysis, we performed ultrastructural studies using immunogold electron microscopy. We found that Sarcosyl-insoluble tau fractions isolated from spinal cord of untreated JNPL3 mice contained fibrillary aggregates resembling PHFs of AD. These PHF-like aggregates were heavily labeled with 10-nm gold particles using tau antibodies including HT7, PHF-1, AT100, pS214tau and AT8 (Figure 5B, top panels). In comparison, Sarcosyl-insoluble tau fractions from GSPE treated mice showed a significant reduction in both the number and the size of immunogold-labeled tau aggregates (Fig. 5B, bottom panels). Most of the aggregates from GSPE-treated mice were shorter in length and the width as compared to untreated mice suggesting that PHF-like conformation of tau fibrils has been compromised by the GSPE treatment. The results are consistent with our previous studies showing that GSPE destabilizes ultrastructure of PHFs isolated from AD brains (Ksiezak-Reding et al., 2010) and assembled tau peptide fibrils in vitro (Ho et al., 2009).
In comparison to the spinal cord, neurons with tau-positive inclusions were rarely observed in the forebrain of JNPL3 mice at 15 months of age (data not shown) in spite of the fact that both the forebrain and spinal cord tissues expressed human tau (Fig. 3). Furthermore, we found that GSPE treatment did not have a significant impact on total (HT7) and phospho-tau (PHF-1) content in the forebrain of JNPL3 mice (Suppl. Fig. 1A). By western blotting and immunogold labeling, we observed very little Sarcosyl-insoluble tau material in the forebrain of untreated and GSPE-treated JNPL3 mice with the majority of tau protein present in Sarcosyl-soluble fraction (Suppl. Fig. 1B and C). These results were consistent with previous findings in JNPL3 mice, which showed that cortical regions display less severe tau pathology than spinal cord (Lewis et al., 2000; Sahara et al., 2002).
Discussion
The JNPL3 mouse model is characterized by age-dependent spinal cord neuropathology with progressive hyperphosphorylation and aggregation of tau that correlate with progressive impairment of motor function (Lewis et al., 2000; Sahara et al., 2002);(Lewis et al., 2000; Le Corre S. et al., 2006). In our studies, we found that JNPL3 mice at 15 months of age presented well-developed tau neuropathology. The extent of tau pathology was largely confined to the spinal cord with a minimal involvement of the forebrain. Furthermore, the ventral horn region of the spinal cord, containing motor neurons, was more affected than the dorsal horns, containing sensory neurons. Our observations were in a good agreement with prior neuropathogical and behavioral studies of JNPL3 mouse model of tauopathy.
The distribution of fibrillary tau pathology across the spectrum of neurodegenerative disorders including AD, progressive supranuclear palsy, corticobasal degeneration disease, and frontotemporal dementia with Parkinsonism linked to Chromosome 17 is wide spread, appearing in both neurons and glia and throughout all cortical regions, cerebellum and brainstem (Ballatore et al., 2007; Dickson, 2009). In the present studies, tau pathology was reflected by the presence of a significant number of human 4R tau-positive neurons primarily in the spinal cord. The accumulation of hyperphosphorylated and aggregated tau in JNPL3 spinal cord neurons was detected histochemically with conformation- and phosphorylation-dependent antibodies Alz50, AT8, AT100 and PHF-1. The aggregation and hyperphosphorylated status of tau protein in the spinal cord was further confirmed by biochemical examination and immunogold labeling of Sarcosyl-insoluble tau aggregates. Sarcosyl-insoluble fractions contained easily detectable fibrillary tau material which ultrastructurally resembled PHFs of AD and other tauopathies. Accordingly, some fibrils were approximately 20nm wide and displayed periodical twisting. Straight filaments were also noted. Our present studies affirmed that JNPL3 mice at 15 months of age harbored neuropathological attributes of tauopathies.
Six months of GSPE treatment resulted in a dramatic up to 90% reduction in the number of spinal cord neurons positive for hyperphosphorylated tau. In comparison, the number of spinal cord neurons expressing total human tau was not significantly decreased. A similar up to 95% reduction in hyperphosphorylated tau content with insignificant changes in the total tau was also noted at the biochemical level. Our results strongly suggest that GSPE treatment has a profound inhibitory effect on phosphorylation status of tau protein. Furthermore, GSPE treatment has a major inhibitory influence on the conformation and aggregation status of hyperphosphorylated tau. Alz50 immunoreactivity as well as the content of Sarcosyl-insoluble fibrillary tau aggregates was significantly diminished in GSPE-treated JNPL3 mice. Our studies strongly indicate that GSPE treatment reduces phosphorylation of tau and accumulation of fibrillary tau aggregates. The results let us to conclude that GSPE may exert its disease modifying activities by blocking or reversing the tau aggregation process through two different but not mutually exclusive mechanisms. There is evidence to support each of the mechanisms. Mechanism 1: GSPE may modulate phosphorylation and aggregation status of tau either by activating tau protein phosphatases or reducing activity of tau protein kinases. This may prevent formation of abnormal tau aggregates since it is widely acknowledged that a reduced tau phosphorylation could lead to a reduced tau aggregation in vivo (Perez et al., 2003; Karl et al., 2003; Le Corre S. et al., 2006). Our present studies have shown that GSPE treatment significantly reduced tau phosphorylation at the epitopes known to be substrates for specific tau kinases. Reduction in specific kinase activities is consistent with our previous observations that GSPE treatment inhibits ERK1/2 but not GSK3 or Akt activities in the brain of TMHT mice, a model of tauopathy expressing a human tau containing two missense V337M and R406W tau mutations (Wang et al., 2010). TMHT mice treated with GSPE for two months showed a reduced level of phosphorylation at Thr181 (AT270 antibody) and Ser396/400/404 (PHF-1/AD2 antibodies), sites identified as substrates for ERK1/2. Mechanism 2: GSPE may prevent tau aggregation and/or promote dissociation of preformed hyperphosphorylated tau aggregates. Such scenario may lead to proteolytic tau protein disposal. Our previous studies in vitro showed that GSPE could directly interfere with spontaneous aggregation of the synthetic tau peptide 306VQIVYK311 and promote the dissociation of pre-aggregated filaments (Ho et al., 2009; Wang et al., 2010). More recently, GSPE was found to significantly alter the ultrastructure of PHFs isolated from AD brain and partially disintegrate the filaments (Ksiezak-Reding et al., 2010).
The present studies demonstrated the efficacy of GSPE treatment in diminishing not only tau-related neuropathology but also clinical phenotype in the JNPL3 mouse model of tauopathy. We showed that GSPE treatment significantly attenuated mutant-tau mediated motor impairment in JNPL3 mice by improving wire hang test scores from 6 seconds to 26 seconds. Since we used a relatively small cohort of JNPL3 mice, further studies with a larger groups of animals are needed to confirm our findings. Beneficial effects of GSPE treatment on motor deficits directly correlated with a major decline (up to 90%) in a number of neurons with phospho-tau inclusions in the ventral horns of spinal cord. They also correlated with >80% reduced content of Sarcosyl-insoluble hyperphosphorylated tau. Such correlation is consistent with the notion that misfolding of hyperphosphorylated tau leading to the formation of insoluble tau aggregates in neurons may induce neurodegeneration either by a loss of the normal function or gain of toxic function of tau (Ballatore et al., 2007; Winklhofer et al., 2008). Therefore, a reduction in hyperphosphorylated and conformationally modified tau protein by GSPE treatment could be neuroprotective.
In summary, the demonstrated efficacy of GSPE to reduce tau neuropathology and to ameliorate motor deficits in JNPL3 mice provides impetus for the developing GSPE as tau-focus therapeutic intervention in human tauopathies, especially for progressive supranuclear palsy, corticobasal degeneration disease, and frontotemporal dementia with Parkinsonism linked to Chromosome 17 that are associated with motor abnormalities.
Supplementary Material
Figure 2. GSPE treatment reduces the number of neurons with inclusions in the spinal cord of JNPL3 mice.
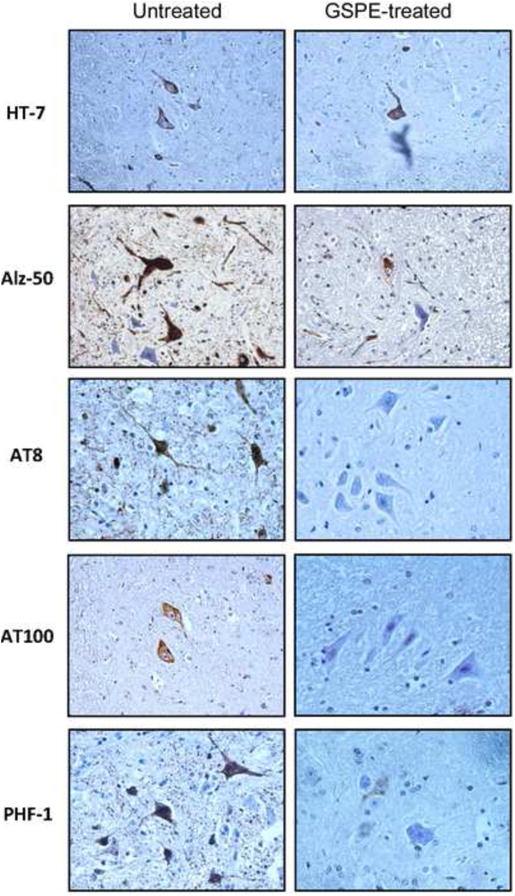
Spinal cord paraffin sections from ventral horns stained with a panel of antibodies against phosphorylated tau (AT8, AT100 and PHF-1), conformationally modified tau (Alz50) and human tau (HT7). Note a reduced number of phosphorylated tau-bearing neurons in the spinal cord of GSPE-treated mice as compared to untreated JNPL3 mice (see also Table 1). Six month GSPE treatment except for Alz50 (3-months).
List of abbreviations
- AD
Alzheimer's disease
- GSPE
grape seed polyphenolic extract
- NFTs
neurofibrillary tangles
- PHF
paired helical filament
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Ballatore C, Crowe A, Smith AB, III, Lee VM, Trojanowski JQ. Tau-directed drug discovery for Alzheimer's disease and related tauopathies: a focus on tau assembly inhibitors. Exp Neurol. 2010;223:304–310. doi: 10.1016/j.expneurol.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat Rev Drug Discov. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulic B, Pickhardt M, Mandelkow EM, Mandelkow E. Tau protein and tau aggregation inhibitors. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Chirita C, Necula M, Kuret J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. doi: 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- Congdon EE, Kim S, Bonchak J, Songrug T, Matzavinos A, Kuret J. Nucleation-dependent tau filament formation: the importance of dimerization and an estimation of elementary rate constants. J Biol Chem. 2008;283:13806–13816. doi: 10.1074/jbc.M800247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A, Huang W, Ballatore C, Johnson RL, Hogan AM, Huang R, Wichterman J, McCoy J, Huryn D, Auld DS, Smith AB, III, Inglese J, Trojanowski JQ, Austin CP, Brunden KR, Lee VM. Identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry. 2009;48:7732–7745. doi: 10.1021/bi9006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol. 2009;3:1–23. [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985;101:1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP. Tau phosphorylation sites. http://cnr.iop.kcl.ac.uk/hangerlab/tautable. Ref Type: Generic.
- Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Avila J. Tauopathies. Cell Mol Life Sci. 2007;64:2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Yemul S, Wang J, Pasinetti GM. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J Alzheimers Dis. 2009;16:433–439. doi: 10.3233/JAD-2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Pabst R, von HS. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp Toxicol Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Ho L, Santa-Maria I, Diaz-Ruiz C, Wang J, Pasinetti GM. Ultrastructural alterations of Alzheimer's disease paired helical filaments by grape seed-derived polyphenols. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci U S A. 2006;103:9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van SM, Gwinn-Hardy K, Paul MM, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Li W, Sperry JB, Crowe A, Trojanowski JQ, Smith AB, III, Lee VM. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J Neurochem. 2009;110:1339–1351. doi: 10.1111/j.1471-4159.2009.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2003;32:1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- Lin WL, Zehr C, Lewis J, Hutton M, Yen SH, Dickson DW. Progressive white matter pathology in the spinal cord of transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2005;34:397–410. doi: 10.1007/s11068-006-8726-0. [DOI] [PubMed] [Google Scholar]
- Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- Perez M, Hernandez F, Lim F, az-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis. 2003;5:301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- Radde R, Duma C, Goedert M, Jucker M. The value of incomplete mouse models of Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2008;35(1):S70–S74. doi: 10.1007/s00259-007-0704-y. [DOI] [PubMed] [Google Scholar]
- Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- Sahara N, Lewis J, DeTure M, McGowan E, Dickson DW, Hutton M, Yen SH. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–1508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6:291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Weidenheim KM, Dickson DW, Ksiezak-Reding H. Morphological and biochemical correlations of abnormal tau filaments in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2002;61:33–45. doi: 10.1093/jnen/61.1.33. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- Wang J, Santa-Maria I, Ho L, Ksiezak-Reding H, Pasinetti GM. Grape Derived Polyphenols Attenuate Tau Neuropathology in a Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 2010;22:653–61. doi: 10.3233/JAD-2010-101074. [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sato S, Okuyama S, Swan RJ, Jacobsen MT, Strunk E, Ikezu T. Tau-tubulin kinase 1 enhances prefibrillar tau aggregation and motor neuron degeneration in P301L FTDP-17 tau-mutant mice. FASEB J. 2010 doi: 10.1096/fj.09-150144. [DOI] [PubMed] [Google Scholar]
- Yadav M, Jain S, Bhardwaj A, Nagpal R, Puniya M, Tomar R, Singh V, Parkash O, Prasad GB, Marotta F, Yadav H. Biological and medicinal properties of grapes and their bioactive constituents: an update. J Med Food. 2009;12:473–484. doi: 10.1089/jmf.2008.0096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


