Abstract
Titer improvement is a constant requirement in the fermentation industry. The traditional method of “random mutation and screening” has been very effective despite the considerable amount of time and resources it demands. Rational metabolic engineering, with the use of recombinant DNA technology, provides a novel, alternative strategy for titer improvement that complements the empirical method used in industry. Manipulation of the specific regulatory systems that govern secondary metabolite production is an important aspect of metabolic engineering that can efficiently improve fermentation titers. In this review, we use examples from Streptomyces secondary metabolism, the most prolific source of clinically used drugs, to demonstrate the power and utility of exploiting natural regulatory networks, in particular pathway-specific regulators, for titer improvement. Efforts to improve the titers of fredericamycin, C-1027, platensimycin, and platencin in our lab are highlighted.
Keywords: Titer improvement, Streptomyces, Secondary metabolite, Regulator, Biosynthesis
Introduction
Microorganisms are a prolific source of many commercially valuable natural products including clinically important antibiotic, anticancer, and antiviral compounds along with agents utilized in agricultural, veterinary, and food industries. Strain improvement is indispensable during the commercialization process of these natural products, as the low titer of wild-type strains can never meet the requirements of industrial development and sales (Parekh et al. 2000). The strategy traditionally used in the industrial setting relies upon iterative rounds of random mutagenesis and empirical screening to achieve titer improvements (Demain 2006). This strategy of “random mutation and screening” has proven itself many times during the past century making it the best option for strain improvement today and in the foreseeable future. However, the considerable amount of time and effort required for such an approach is a significant shortcoming. New strategies that can complement the traditional method to increase the overall efficiency will lower both the costs and duration of the commercialization process.
The development of molecular microbiology and recombinant DNA technology has led to a number of strategies for rational strain improvement known collectively as “metabolic engineering” (Nielsen 2001; Koffas et al. 1999). The success of any metabolic engineering proposal depends heavily on (1) knowledge and understanding of the biosynthesis of a targeted molecule and its related metabolic fluxes, and (2) a set of tools and methodologies to efficiently carry out the desired genetic manipulations in the producers. Along these lines, the advances in our understanding of the biosynthetic machineries and regulatory networks of secondary metabolite production in the genus Streptomyces made over the past few decades (Borodina et al. 2005; Koglin and Walsh 2009; Hertweck 2009) and the development of new strategies for genetic manipulation in both native producers, e.g. PCR-targeting system (Gust et al. 2003), and heterologous hosts (Galm and Shen 2006) has led to many successful examples of metabolic engineering for titer improvement of secondary metabolites (Adrio and Demain 2006; Cropp et al. 2001; Olano et al. 2008).
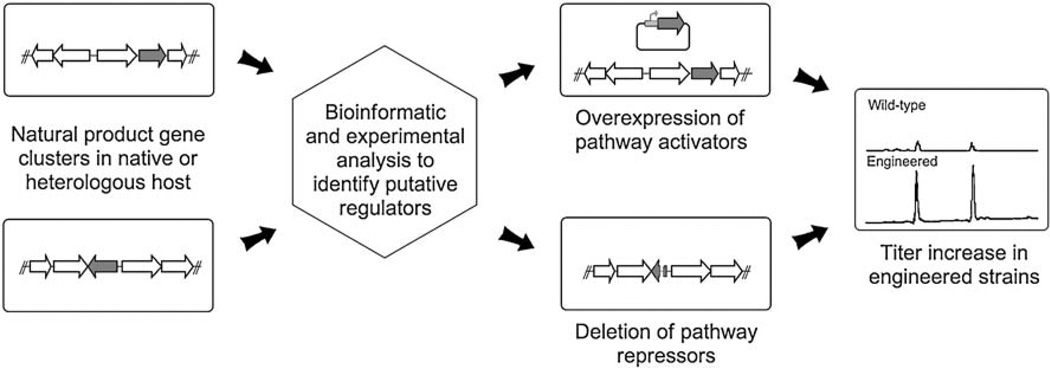
In this mini-review, we will focus on one strategy of metabolic engineering—the manipulation of pathway specific regulatory elements—that has been exploited to improve Streptomyces secondary metabolite production in both native producers and heterologous hosts (Fig. 1). After highlighting the advancements in the understanding of Streptomyces secondary metabolism regulation that have enabled metabolic engineering on regulatory elements to become an important option for titer improvement efforts, we will share examples from our lab that illustrate a variety of strategies to quickly achieve titer improvements of Streptomyces natural products. Readers are referred to several other excellent reviews in the recent literature for a comprehensive overview (Adrio and Demain 2006; Demain 2006; Cropp et al. 2001; Olano et al. 2008; Parekh et al. 2000).
Fig. 1.
Strategies to increase secondary metabolite titers by manipulating pathway regulations
Titer improvement by manipulating regulators in native producers
The regulators controlling secondary metabolite production have been considered as important targets for metabolic engineering efforts, the success of which is largely based on our current understanding of these regulatory systems. Production of secondary metabolites in Streptomyces typically coincides with aerial mycelium formation in a growth-phase dependent manner (Olano et al. 2008). The activation process involves complicated signal transmissions, which are not yet fully understood. Signals from pleiotropic regulators, which control both morphological differentiation and secondary metabolite production or influence several secondary metabolic pathways, are ultimately transmitted to the pathway-specific regulators that switch on the transcription of the biosynthesis-related genes (Bibb 2005). The majority of secondary metabolite gene clusters that have been sequenced thus far contain one or more of these pathway specific regulators.
The hierarchical structure of secondary metabolite regulation offers two distinct strategies for engineering: (1) manipulating global regulators to increase production of many secondary metabolites versus (2) targeting pathway specific regulators for titer increase of a particular compound of interest. The former strategy has the advantage that a single manipulation could affect many aspects of secondary metabolite production, including precursor supply and biosynthetic enzyme expression; global regulators may also function across different producers. While finding a universal master regulator of antibiotic production remains an elusive goal, recent findings showcase the potential of this strategy to facilitate titer improvement and drug discovery (Scherlach and Hertweck 2009). Pleiotropic regulators such as DasR, PhoP, and ArpA control secondary metabolism in multiple organisms, allowing them to be targeted in cases where knowledge of the pathway specific regulators is lacking. On the other end of the regulatory spectrum, the intrinsic relationship between pathway specific regulators and the expression level of biosynthetic genes makes them obvious targets for metabolic engineering to improve titers of specific metabolites.
The enthusiasm in secondary metabolite gene cluster cloning and sequencing in the past two decades has resulted in an explosion in the number of pathway-specific regulators from a variety of regulator families that have been identified and characterized (Parakar et al. 2003). Regulators from some families preferentially act as transcriptional activators, such as those from the SARP (Streptomyces antibiotic regulatory proteins) (Bruheim et al. 2002; Wietzorrek and Bibb 1997) or AraC/XylS families (Gallegos et al. 1997). Regulators from other families, such as the GntR (Haydon and Guest 1991) and TetR families (Ramos et al., 2005), usually function as transcriptional repressors. Although exceptions exist in these activator or repressor families, prediction of the functions of some regulators based on the families to which they belong can save significant time and effort in metabolic engineering. On the other hand, some regulator families contain both positive and negative regulation members, like the LysR (Maddocks and Oyston 2008) and MarR families (Oh et al. 2007). Thus, a careful analysis of the regulatory proteins in these families is a prerequisite to their successful utilization in metabolic engineering.
Positive regulation
Most of the secondary metabolite pathways involve specific activator genes, and a commonly used strategy to improve natural product production is to overexpress these pathway-specific activators (Fig. 1). Two examples from our lab show the effectiveness of this strategy: the case of fredericamycin (FDM) exemplifies the typical approach to study and utilize a pathway-specific activator; titer improvement of C-1027 exhibits a simplified process based on bioinformatics analysis of undefined regulators.
FDM A and E (Fig. 2) are aromatic pentadecaketides with antitumor activity produced by Streptomyces griseus ATCC 49344 (Chen et al. 2008a). The FDM biosynthetic gene cluster spans 26-kb and contains 28 genes (Wendt-Pienkowski et al. 2005). Three genes, fdmR, fdmR1, and fdmR2, encode deduced regulators with one, FdmR1, belonging to the SARP family. The SARP regulators are prominent among pathway-specific activators in Streptomyces, and are characterized by an N-terminal winged helix-turn-helix motif. At least some of these SARP regulators recognize heptameric direct repeats “TCGAGXX” located precisely eight on the upstream of the −10 region (Bibb 2005; Tang et al. 1996). Nine of such heptameric repeat loci were identified in the promoter regions of the fdm cluster.
Fig. 2.
Structures of C-1027, fredericamycins (FDMs), neocarzinostatin (NCS), platensimycin, and platencin, whose titers have been improved by engineering their pathway specific regulators in either the native producers, heterologous hosts, or both
The abolishment of FDM production in the ΔfdmR1 mutant confirmed its annotation as a pathway activator. Complementation of the ΔfdmR1 mutant with a medium-copy-number pHJL401-derived plasmid with fdmR1 under the control of ErmE* promoter resulted in a 2-fold improvement of FDM production. RT-PCR analyses revealed that 26 of the total 28 fdm genes were controlled directly or indirectly by FdmR1, including all the structure genes. Finally, the FDM titer was increased 5.6-fold to 1.36 g/L by overexpressing fdmR1 under ErmE* control in a high-copy-number pWHM3-derived plasmid (Chen et al. 2008b), which well exemplified manipulation of pathway specific regulators as a powerful and time-saving strain improvement approach.
C-1027 (Fig. 2) belongs to the enediyne family that is best known for their extreme cytotoxicities. Two members of this family, neocarzinostatin and calicheamicin, have already been developed into clinically used anticancer drugs (Horsman et al. 2009). While C-1027 has entered phase II clinical trials recently in China (Shao and Zhen 2008), the low titer of C-1027 has hindered drug development efforts. The C-1027 biosynthetic gene cluster has been cloned from Streptomyces globisporus, and three putative regulatory genes, sgcR1, sgcR2, and sgcR3, were found in this 80-kb gene cluster (Liu et al. 2002). Previous work showed that the TylR-like regulator SgcR3 was an activator, and C-1027 production was increased 30–40% when sgcR3 was overexpressed (Wang et al. 2009). Bioinformatics analysis revealed that SgcR2 was a member of the AraC/XylS activator family (Gallegos et al. 1997) and SgcR1 was a StrR-like protein. The StrR-like regulators have not been extensively studied. Two characterized StrR-like regulators, StrR and NovG, are both activators (Retzlaff and Distler 1995; Eustáquio et al. 2005), suggesting that SgcR1 acts as a positive regulator of C-1027 production.
We overexpressed sgcR1 and sgcR2 separately in S. globisporus. In both cases, C-1027 was produced earlier in the engineered strains than in the wild type. The C-1027 titer in the sgcR1-overexpressing strain was improved by 2- to 3-fold relative to wild type, and a noticeable increase in C-1027 production was also observed upon overexpression of sgcR2. These results support predictions that both SgcR1 and SgcR2 are activators. Given that the three C-1027 regulators, i.e., sgcR1, sgcR2, and sgcR3, all act positively, we tried to overexpress them together to further improve C-1027 production. However, placing the three activator genes in tandem on a high-copy-number plasmid under the control of ErmE* promoter and overexpressing them together in S. globisporus failed to further increase the production of C-1027 (Chen et al., unpublished data), a finding that underscores the challenges in manipulating multiple regulators synergistically for titer improvement.
Negative regulation
In cases where antibiotic production is regulated by a pathway specific negative regulator, knocking that gene out is usually sufficient to achieve titer improvements (Fig. 1). This is exemplified by our efforts to increase the titers of platensimycin and platencin in the native producers, Streptomyces platensis MA7327 and S. platensis MA7339, respectively.
Platencimycin and platencin (Fig. 2) are members of a newly discovered class of antibiotics (Wang et al. 2006; Wang et al. 2007), and we have recently established that their production is encoded by a single gene cluster in S. platensis MA7327 (Smanski et al., unpublished data). The ptmR1 gene contains an N-terminal DNA-binding domain characteristic of the GntR family of transcriptional repressors (Haydon and Guest 1991) and a C-terminal effector-binding domain places it in the FadR subdivision (Rigali et al. 2002). GntR proteins are numerous and widespread although only a few of the 57 GntR-like proteins encoded by the Streptomyces coelicolor genome have been characterized (Hillerich and Westpheling 2006; Rigali et al. 2008). Based on of the location of ptmR1 within the cloned ptm gene cluster, we predicted that it played a role in regulating the biosynthesis of platensimycin and platencin.
Inactivation of ptmR1 in S. platensis MA7327 resulted in ΔptmR1 mutant strains that overproduce platensimycin and platencin by a factor of ~100-fold compared with the previously reported titers for the wild-type strain (Smanski et al. 2009). Not only was antibiotic production accelerated and increased in the standard fermentation medium, but both compounds were produced in nutrient rich media as well. Additionally, knocking out the ptmR1 gene in MA7327 and its homolog, ptnR1, in the closely related platencin producer S. platensis MA7339, has led to the isolation of many new congeners that were not detectable in wild-type producers (Smanski et al., unpublished data). While there is still much to learn about the mechanism(s) of how PtmR1 and PtnR1 regulate platensimycin and platencin production in S. platensis MA7327 or MA7339, these examples illustrate the effectiveness of applying metabolic engineering strategies for titer improvement.
Titer improvement by manipulating regulators in heterologous hosts
Heterologous hosts are increasingly being used to bypass difficult genetic manipulations in recalcitrant native producing strains and in mining silent gene clusters from sequenced genomes or meta-genomic DNA libraries. (Galm and Shen 2006; Wenzel and Müller 2005). As a metabolic engineering tool, heterologous expression has also shown its effectiveness by improving secondary metabolite titers. One impressive example involves increasing the titer of tetracenomycin (TCM) from 0.3 g/L in the wild-type Streptomyces glaucescens to about 5 g/L (0.6 g/L TCM C and 4.35 g/L TCM A2) by heterologously expressing its biosynthetic genes in an industrial monensin A producing strain, Streptomyces cinnamonensis C730.7 (Li et al. 2009). Besides testing different heterologous hosts, manipulation of regulatory elements of the target gene cluster has also proven to be a practical approach for improving metabolite production (Dangel et al. 2008; Jung et al. 2008).
We have recently cloned and characterized the iso-migrastatin gene cluster from S. platensis NRRL18993 (Lim et al. 2009) and successfully expressed the 50-kb mgs cluster in five genetically well characterized heterologous strains: Streptomyces albus J1074, Streptomyces lividans K4-114, S. coelicolor M512, Streptomyces avermitilis SUKA4 and S. avermitilis SUKA5 (Feng et al. 2009). The resultant recombinant strains all produced iso-migrastatin, demonstrating the flexibility of strain selection in heterologous expression. However, when we tried different hosts for heterologously expressing the fdm cluster, production of FDM was only achieved in S. albus J1074, but not in S. lividans K4-114. RT-PCR analyses revealed that the characterized SARP activator gene fdmR1 was not expressed in S. lividans. The copy number of fdmR1 in the recombinant S. lividans strain containing the whole fdm cluster was increased by introducing a pWHM3-derived high-copy-number plasmid expressing fdmR1 under its native promoter. The resultant fdmR1-expressing strain was capable of FDM production albeit in low titer (~0.5 mg/L). The titer of FDM in S. lividans was improved to ~1.4 mg/L by overexpressing fdmR1 under the control of the constitutive promoter ErmE*. The titer was further increased to ~17 mg/L by overexpressing the ketoreductase gene, fdmC, together with fdmR1 based on the information acquired from RT-PCR analyses (Chen et al. 2008b). This example is paralleled by our experience with the heterologous expression of the ncs gene cluster in S. lividans TK64 for the production of the enediyne antitumor antibiotic neocarzinostatin (NCS). The 63-kb ncs cluster was cloned from Streptotmyces carzinostaticus ATCC 15944 (Liu et al. 2005) and successfully mobilized into the heterologous host S. lividans TK64 although NCS production was not detected in the resultant recombinant strain. RT-PCR revealed that one of the seven ncs regulatory genes, ncsR7, was not expressed in the recombinant S. lividans TK64 strain (Nonaka et al., unpublished data). Subsequently, NcsR7 has been characterized as a TylR-like positive regulator, and its engineered overexpression in the recombinant S. lividans TK64 strain was sufficient to turn on NCS production (Nonaka et al., unpublished data).
The overexpression of a pathway specific regulator not only can ‘turn on’ the gene cluster as the previous two examples indicated, but it also can ‘turn up’ the production of metabolites in heterologous hosts. The recombinant S. albus strain heterologously expressing the entire fdm gene cluster was used to study the functions of specific fdm genes. When fdmM was inactivated in the recombinant S. albus strain, FDM production was abolished and three new compounds, FDM M-1, FDM M-2, and FDM M-3, were produced albeit in very low titers. By overexpressing the activator gene fdmR1 in this ΔfdmM mutant strain, the production of all three FDM analogs was significantly increased more than 12-fold. Similarly, the titer of the accumulated FDM analog FDM M1-1 in the ΔfdmM1 mutant strain was improved about 25-fold upon overexpression of fdmR1, highlighting once again that manipulation of the well studied regulator can also be used to facilitate the investigation of the secondary metabolite biosynthetic genes in both the native producer and heterologous hosts. Titer improvement of all these FDM analogs in the heterologous mutant strains significantly simplified their purification and structural characterization. On the basis of the metabolites accumulated by the ΔfdmM and ΔfdmM1 mutant strains, FdmM and FdmM1 were finally defined as members of a novel family of oxygenases (Chen et al. 2009).
Conclusion and perspective
Bioinformatics-based predictions have alleviated a detailed investigation of the regulators in the ptm, ptn, and C-1207 gene clusters and allowed us to achieve significant titer improvements for platensimycin, platencin, and C-1027 very quickly. However, metabolic engineering as a field, has not yet reached the level of predictability characteristic of other engineering fields. As noted earlier, regulators in some protein families cannot be confidently assigned to positive or negative roles a priori, and exceptions exist for even the most common activator and repressor families. Also, establishing the natural role of a pathway specific regulator does not guarantee it can be exploited for titer improvement, as overexpression of some positive regulators can actually hinder overall production (Kitani et al. 2009). Achieving a higher level of predictability in metabolic engineering requires and warrants more basic and applied research relating to the regulatory networks that govern of antibiotic production.
It is also important to note that manipulation of pathway regulatory factors typically results in altered expression levels of the biosynthetic and resistance genes, but other aspects of secondary metabolite production, such as precursor supply, remain untouched. These other requirements can limit the extent of titer improvement efforts that rely solely on pathway specific regulators. A combination of different metabolic engineering approaches should be considered in such situations. On the other hand, the presence of genes providing the isoprene precursors for platensimycin and platencin production within the ptm and ptn clusters could account for the dramatic titer improvement with only a single mutation.
Although the empirical strain improvement approach used in industry remains undoubtedly the most powerful method to achieve high titers, metabolic engineering can complement and expedite these efforts. For example, metabolically engineered strains should be much better starter strains for random mutation and screening than the wild-type producers. Alternatively, metabolic engineering can benefit from the industrial strain improvement efforts, as high-yielding industrial strains are logical starting points for the heterologous production of secondary metabolites (Li et al. 2009).
Though not comprehensive, the examples above illustrate a range of strategies that can be employed to improve secondary metabolite production in both native producers and heterologous hosts. Achieving gram-per-liter titers by manipulating pathway specific regulators as with FDM (Chen et al. 2008b) is still rare but is a harbinger of things to come. The rapidly growing number of cloned secondary metabolite gene clusters and our ever-increasing understanding of their complex regulatory systems will open the door to new, more powerful strategies for titer improvement in the coming years.
Acknowledgements
Studies on natural product biosynthesis and engineered described from the Shen laboratories were supported in part by the National Institutes of Health (NIH) grants CA78747, CA106150, and CA113297. M.J.S. is supported in part by NIH grant T32 GM008347.
Contributor Information
Yihua Chen, Division of Pharmaceutical Sciences, University of Wisconsin-Madison, Madison, WI 53705-2222, USA.
Michael J. Smanski, Microbiology Doctoral Training Program, University of Wisconsin-Madison, Madison, WI 53705-2222, USA
Ben Shen, Division of Pharmaceutical Sciences, University of Wisconsin National Cooperative Drug Discovery Group, and Department of Chemistry, University of Wisconsin-Madison, 777 Highland Ave, Madison, WI 53705-2222, USA, bshen@pharmacy.wisc.edu.
References
- Adrio JL, Demain AL. Genetic improvement of process yielding microbial products. FEMS Microbiol Rev. 2006;30:187–214. doi: 10.1111/j.1574-6976.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- Bibb MJ. Regulation of secondary metabolism in streptomycetes. Curr Opin Microbiol. 2005;8:208–215. doi: 10.1016/j.mib.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Borodina I, Krabben P, Nielsen J. Genome-scale analysis of Streptomyces coelicolor A3(2) metabolism. Genome Res. 2005;15:820–829. doi: 10.1101/gr.3364705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruheim P, Sletta H, Bibb MJ, White J, Levine DW. High-yield actinorhodin production in fed-batch culture by a Streptomyces lividans strain overexpressing the pathway-specific activator gene actll-ORF4. J Ind Microbiol Biotech. 2002;28:103–111. doi: 10.1038/sj/jim/7000219. [DOI] [PubMed] [Google Scholar]
- Chen Y, Luo Y, Ju J, Wendt-Pienkowski E, Rajski SR, Shen B. Identification of fredericamycin E from Streptomyces griseus: insight into fredericamycin A biosynthesis highlighting carbaspirocycle formation. J Nat Prod. 2008a;71:431–437. doi: 10.1021/np070664n. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wendt-Pienkowski E, Shen B. Identification and utility of FdmR1 as a Streptomyces antibiotic regulatory protein activator for fredericamycin production in Streptomyces griseus ATCC 49344 and heterologous hosts. J Bacteriol. 2008b;190:5587–5596. doi: 10.1128/JB.00592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wendt-Pienkowski E, Rajski S, Shen B. In vivo investigation of the roles of FdmM and FdmM1 in fredericamycin biosynthesis unveiling a new family of oxygenases. J Biol Chem. 2009;284:24735–24743. doi: 10.1074/jbc.M109.014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropp A, Chen S, Liu H, Zhang W, Reynolds KA. Genetic approaches for controlling ratios of related polyketide products in fermentation processes. J Ind Microbiol Biotech. 2001;27:368–377. doi: 10.1038/sj.jim.7000206. [DOI] [PubMed] [Google Scholar]
- Dangel V, Eustáquio AS, Gust B, Heide L. novE and novG act as positive regulators of novobiocin biosynthesis. Arch Microbiol. 2008;190:509–519. doi: 10.1007/s00203-008-0396-0. [DOI] [PubMed] [Google Scholar]
- Demain AL. From natural products discovery to commercialization: a success story. J Ind Microbiol Biotech. 2006;33:486–495. doi: 10.1007/s10295-005-0076-x. [DOI] [PubMed] [Google Scholar]
- Eustáquio AS, Li SM, Heide L. NovG, a DNA-binding protein acting as a positive regulator of novobiocin biosynthesis. Microbiology. 2005;151:1946–1961. doi: 10.1099/mic.0.27669-0. [DOI] [PubMed] [Google Scholar]
- Feng Z, Wang L, Rajski SR, Xu Z, Coeffet-LeGal MF, Shen B. Engineered production of iso-migrastatin in heterologous Streptomyces hosts. Bioorg Med Chem. 2009;17:2147–2153. doi: 10.1016/j.bmc.2008.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. AraC/Xyls family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galm U, Shen B. Expression of biosynthetic gene clusters in heterologous hosts for natural product production and combinatorial biosynthesis. Exp Opin Drug Discov. 2006;1:409–437. doi: 10.1517/17460441.1.5.409. [DOI] [PubMed] [Google Scholar]
- Gust B, Challis GL, Fowler K, Kieser T, Chater KF. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon DJ, Guest JR. A new family of bacterial regulatory proteins. FEMS Microbiol Lett. 1991;63:291–295. doi: 10.1016/0378-1097(91)90101-f. [DOI] [PubMed] [Google Scholar]
- Hillerich B, Westpheling J. A new GntR family transcriptional regulator in Streptomyces coelicolor is required for morphogenesis and antibiotic production and controls transcription of an ABC transporter in response to carbon source. J Bacteriol. 2006;188:7477. doi: 10.1128/JB.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertweck C. The biosynthetic logic of polyketide diversity. Angew Chem Int Ed. 2009;48:4688–4716. doi: 10.1002/anie.200806121. [DOI] [PubMed] [Google Scholar]
- Horsman GP, Van Lanen SG, Shen B. Iterative type I polyketide synthases for enediyne core biosynthesis. Methods Enzymol. 2009;459:97–112. doi: 10.1016/S0076-6879(09)04605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WS, Jeong SJ, Park SR, Choi CY, Park BC, Park JW, Yoon YJ. Enhanced heterologous production of desosaminyl macrolides and their hydroxylated derivatives by overexpression of the pikD regulatory gene in Streptomyces venezuelae. Appl Environ Microbiol. 2008;74:1972–1979. doi: 10.1128/AEM.02296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani S, Ikeda H, Sakamoto T, Noguchi S, Nihira T. Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis. Appl Microbiol Biotechnol. 2009;82:1089–1096. doi: 10.1007/s00253-008-1850-2. [DOI] [PubMed] [Google Scholar]
- Koffas M, Roberge C, Lee K, Stephanopoulos G. Metabolic engineering. Annu Rev Biomed Eng. 1999;1:535–557. doi: 10.1146/annurev.bioeng.1.1.535. [DOI] [PubMed] [Google Scholar]
- Koglin A, Walsh CT. Structural insights into nonribosomal peptide enzymatic assembly lines. Nat Prod Rep. 2009;26:987–1000. doi: 10.1039/b904543k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hazzard C, Florova G, Reynolds KA. High titer production of tetracenomycins by heterologous expression of the pathway in a Streptomyces cinnamonensis industrial monensin producer strain. Metab Eng. 2009 doi: 10.1016/j.ymben.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Lim SK, Ju J, Zazopoulos E, Jiang H, Seo JW, Chen Y, Feng Z, Rajski SR, Farnet CM, Shen B. iso-Migrastatin, migrastatin, and dorrigocin production in Streptomyces platensis NRRL 18993 is governed by a single biosynthetic machinery featuring an acyltransferase-less type I polyketide synthase. J Biol Chem. 2009;284:29746–29756. doi: 10.1074/jbc.M109.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Christenson SD, Standage S, Shen B. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science. 2002;297:1170–1173. doi: 10.1126/science.1072110. [DOI] [PubMed] [Google Scholar]
- Liu W, Nonaka K, Nie L, Zhang J, Christenson SD, Bae J, Van Lanen SG, Zazopoulos E, Farnet CM, Yang CF, Shen B. The neocarzinostatin biosynthetic gene cluster from Streptomyces carzinostaticus ATCC 15944 involving two iterative type I polyketide synthases. Chem Biol. 2005;12:293–302. doi: 10.1016/j.chembiol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Maddocks SE, Oyston PC. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiol. 2008;154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- Nielsen J. Metabolic engineering. Appl Microbiol Botechnol. 2001;55:263–283. doi: 10.1007/s002530000511. [DOI] [PubMed] [Google Scholar]
- Oh SY, Shin JH, Roe JH. Dual role of OhrR as a repressor and an activator in response to organic hydroperoxides in Streptomyces coelicolor. J Bacteriol. 2007;189:6284–6292. doi: 10.1128/JB.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano C, Lombó F, Méndez C, Salas JA. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab Eng. 2008;10:281–292. doi: 10.1016/j.ymben.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Parekh S, Vinci VA, Strobel RJ. Improvement of microbial strains and fermentation process. Appl Microbiol Biotechnol. 2000;54:287–301. doi: 10.1007/s002530000403. [DOI] [PubMed] [Google Scholar]
- Parakar A, Trefzer A, Chakraburtty R, Stassi D. Stretomyces genetics: a genomic perspective. Crit Rev Biotechnol. 2003;23:1–27. doi: 10.1080/713609296. [DOI] [PubMed] [Google Scholar]
- Ramos JL, Martínez-Bueno M, Molina-Henares AJ, Terán W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzlaff L, Distler J. The regulator of streptomycin gene expression, StrR, of Streptomyces griseus is a DNA binding activator protein with multiple recognition sites. Mol Microbiol. 1995;18:151–162. doi: 10.1111/j.1365-2958.1995.mmi_18010151.x. [DOI] [PubMed] [Google Scholar]
- Rigali S, Derouaux A, Giannotta F, Dusart J. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J Biol Chem. 2002;277:12507–12515. doi: 10.1074/jbc.M110968200. [DOI] [PubMed] [Google Scholar]
- Rigali S, Titgemeyer F, Barends S, Mulder S, Thomae AW, Hopwood DA, Van Wezel GP. Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep. 2008;9:670–675. doi: 10.1038/embor.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherlach K, Hertweck C. Triggering cryptic natural product biosynthesis in microorganisms. Org Biomol Chem. 2009;7:1753–1760. doi: 10.1039/b821578b. [DOI] [PubMed] [Google Scholar]
- Smanski MJ, Peterson RM, Rajski SR, Shen B. Engineered Streptomyces platensis strains that overproduce antibiotics platensimycin and platencin. Antimicrob Agents Chemother. 2009;53:1299–1304. doi: 10.1128/AAC.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao RG, Zhen YS. Enediyne anticancer antibiotic lidamycin: chemistry, biology and pharmacology. Anticancer Agents Med Chem. 2008;8:123–131. doi: 10.2174/187152008783497055. [DOI] [PubMed] [Google Scholar]
- Tang L, Grimm A, Zhang YX, Hutchinson CR. Purification and characterization of the DNA-binding protein DnrI, a transcriptional factor of daunorubicin biosynthesis in Streptomyces peucetius. Mol Microbiol. 1996;22:801–813. doi: 10.1046/j.1365-2958.1996.01528.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, González I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci USA. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hu Y, Zhang Y, Wang S, Cui Z, Bao Y, Jiang W, Hong B. Role of sgcR3 in positive regulation of enediyne antibiotic C-1027 production of Streptomyces globisporus C-1027. BMC Microbiol. 2009;22:9–14. doi: 10.1186/1471-2180-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, Kwon H, Hutchinson CR, Shen B. Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces griseus. J Am Chem Soc. 2005;127:16442–16452. doi: 10.1021/ja054376u. [DOI] [PubMed] [Google Scholar]
- Wenzel SC, Müller R. Recent developments towards the heterologous expression of complex bacterial natural product biosynthetic pathways. Curr Opin Biotechnol. 2005;16:594–606. doi: 10.1016/j.copbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Wietzorrek A, Bibb M. A novel family of proteins that regulates antibiotic production in streptomycetes appears to contain an OmpR-like DNA-binding fold. Mol Microbiol. 1997;25:1177–1184. doi: 10.1046/j.1365-2958.1997.5421903.x. [DOI] [PubMed] [Google Scholar]