Abstract
Skeletal muscle represents an attractive target tissue for adenoviral gene therapy to treat muscle disorders and as a production platform for systemic expression of therapeutic proteins. However, adenovirus serotype 5 vectors do not efficiently transduce adult muscle tissue. Here we evaluated whether capsid modifications on adenoviral vectors could improve transduction in mature murine muscle tissue. First-generation and helper-dependent serotype 5 adenoviral vectors featuring the serotype 3 knob (5/3) showed significantly increased transduction of skeletal muscle after intramuscular injection in adult mice. Furthermore, we showed that full-length dystrophin could be more efficiently transferred to muscles of mdx mice using a 5/3-modified helper-dependent adenoviral vector. In contrast to first-generation vectors, helper-dependent adenoviral vectors mediated stable marker gene expression for at least 1 year after intramuscular injection. In conclusion, 5/3 capsid-modified helper-dependent adenoviral vectors show enhanced transduction in adult murine muscle tissue and mediate long-term gene expression, suggesting the suitability of these vectors for muscle-directed gene therapy.
Guse and colleagues evaluate whether capsid modification of adenoviral vectors can improve transduction in mature murine muscle tissue. They show that full-length dystrophin can be efficiently transferred to muscles of mdx mice, using a serotype 5 adenoviral vector featuring the serotype 3 knob (5/3 vector); moreover, they show that this vector can mediate stable gene expression of luciferase in B6 mice for at least 1 year.
Introduction
Skeletal muscle-directed gene transfer holds promise for the treatment of inherited skeletal muscle disorders, as well as for systemic gene therapy approaches where muscle is used as a protein production platform. Skeletal muscle is an attractive gene therapy target tissue, because it can efficiently secrete biologically active proteins (Arruda et al., 2001), skeletal myofibers have a long half-life time, thereby representing a stable platform for gene expression, and the intramuscular injection procedure is minimally invasive. Moreover, intramuscular injection of viral vectors is not associated with acute toxicity, as opposed to intravenous administration of certain vector types (Schnell et al., 2001; Raper et al., 2003). Adeno-associated viruses (AAVs) have been extensively used for local and systemic muscle-directed gene therapy studies, e.g., for Duchenne's muscular dystrophy (Foster et al., 2006) and hemophilia B (Hasbrouck and High, 2008). The transduction efficiency of AAV vectors has generally been at levels that produce sufficient amounts of therapeutic protein. However, as AAVs cannot carry transgenes larger than 5 kb (Wu et al., 2010), it is unsuitable for the transfer of genes such as full-length dystrophin (14 kb), full-length utrophin (13 kb), etc. Furthermore, the packaging capacity of AAVs limits the use of large upstream regulatory elements for tissue-specific expression and on/off regulatory systems. Adenoviral vectors (Ads) and, in particular, helper-dependent Ads (HDAds) can, however, accommodate genes, combinations of genes, and regulatory elements of up to a total length of 36 kb (Kochanek, 1999). HDAds lack all viral genes, in contrast to the widely used first-generation Ads (FGAds) that only have E1 and E3 genes deleted. Consequently, HDAds are less cytotoxic and immunogenic, leading to long-term gene expression in nondividing tissues such as the liver (Brunetti-Pierri et al., 2009). However, the major drawback of Ad vectors based on serotype 5 is their low transduction efficiency in adult skeletal muscle tissue due to low expression of the primary attachment receptor coxsackie adenovirus receptor (CAR) (Nalbantoglu et al., 1999). Retargeting Ads to receptors with higher expression levels in mature skeletal might, therefore, represent a means of improving transduction efficiency.
Several different strategies have been used to target Ads to specific tissues. Examples include genetic modification of fiber, knob, or capsid proteins, as well as adapter-molecule–mediated attachment of targeting moieties to the knob (Bouri et al., 1999; Bramson et al., 2004; Glasgow et al., 2006). In this study, we evaluated the transduction efficiency in adult muscle tissue using genetically modified Ads that have the serotype 5 knob replaced with a serotype 3 knob (5/3 serotype chimerism) and vectors that have an arginine-glycine-aspartic acid (RGD) motif inserted in the HI loop. Unlike serotype 5 Ads, which primarily bind to CAR, 5/3-modified vectors bind to desmoglein 2 (DSG2) (Wang et al., 2011), and the RGD modification leads to attachment to αvβ cell-surface integrins (Dmitriev et al., 1998). Interestingly, DSG2 seems to be expressed to higher levels than CAR in mouse skeletal tissue (www.biogps.org, NCBI accession nos. 13511 and 13052).
Materials and Methods
Mice
C57BL/6 and mdx mice were purchased from Jackson Laboratories (Bar Harbor, ME) and kept and treated in accordance with the institutional guidelines for animal care. Mice were injected under isoflurane anesthesia with 109 virus particles of the respective virus suspended in 20 μL of PBS directly into the hamstring or tibialis anterior muscles at 4 weeks of age.
Viruses
The FGAds Ad5luc1 and Ad5/3luc1 and their production and purification have been described before (Krasnykh et al., 2001; Kanerva et al., 2002). A helper virus based on serotype 5 featuring a serotype 3 knob was constructed by homologous recombination of the genomic helper virus plasmid pNG163-R2 and the plasmid pNEB.PK.F5/3, which has been described before (Krasnykh et al., 1996). The recombined plasmid pNG163-5/3 was used to rescue the fiber-modified helper virus Ad5/3-NG163 as described before (Palmer and Ng, 2003). The helper viruses Ad5/3-NG163 and AdNG163-R2 (Palmer and Ng, 2003) were used to generate helper-dependent vectors HDAd5/3-luc and HDAd5-luc. All FGAd-luc and HDAd-luc vectors express the luciferase gene under the control of the cytomegalovirus promoter. HDAd5/3-Dys and HDAd5-Dys were rescued by transfection of a plasmid containing the full-length dystrophin gene driven by the 6.5-kb muscle creatine kinase promoter (kindly provided by Dr. Jeffrey Chamberlain) and subsequent infection with Ad5/3-NG163 or AdNG163-R2. All vectors were amplified and purified as described before (Suzuki et al., 2010). Physical titers of the vector preparations were determined by standard OD 260 nm assay. Infectious titers of the first Ads were determined by standard tissue culture infectious dose 50 (TCID50) assay, and the virus particle to infectious units ratios were found to be similar (Ad5luc1, 26.3; Ad5/3luc1, 33.8).
In vivo bioluminescence imaging
Mice were imaged with an IVIS 200 series imaging system (Caliper Life Sciences, Hopkinton, MA) while under isoflurane anesthesia. Luciferase expression was quantified using Living Image version 2.5 software.
Ex vivo luciferase measurement
Muscle tissue was excised from mice, homogenized using an Ultra Turrax homogenizer (IKA, Staufen, Germany), and lysed in Cell Culture Lysis Buffer (Promega, Madison, WI). Following addition of the substrate D-luciferin (Promega), relative light units were measured using a DCR-1 Luminometer (Digene Diagnostics, Gaithersburg MD).
Histology/western blot
Frozen mouse tibialis anterior sections of 10-μm thickness were stained with primary antibodies against DSG2 (Santa Cruz Biotechnology, Santa Cruz, CA) or dystrophin (Mandra1 clone 7A10; Developmental Studies Hybridoma Bank, Iowa City, IA), and secondary anti-IgG antibodies conjugated to Alexa Fluor 488 or 594 (Invitrogen, Carlsbad, CA) according to the manufacturers' instructions. For western blots, the anti-dystrophin antibody (Mandra1 clone) and anti-GAPDH antibody (Invitrogen) were used as primary antibodies, and red and green IRDye antibodies (LI-COR, Lincoln, NE) as secondary antibodies. Western blots were visualized with an Odyssey Infrared Imaging System (LI-COR.)
Quantitative real-time PCR
Total DNA was extracted from muscles using the QIAamp DNA extraction kit (Qiagen, Valencia, CA). Quantitative real-time PCR was performed using a LightCycler V2 and FastStart DNA Master SYBR Green (Roche, Indianapolis, IN) with HDAd-specific primers (5′-TCT GAA TAA TTT TGT GTT ACT CAT AGC GCG-3′ and 5′-CCC ATA AGC TCC TTT TAA CTT TTA AAG TC-3′).
Results and Discussion
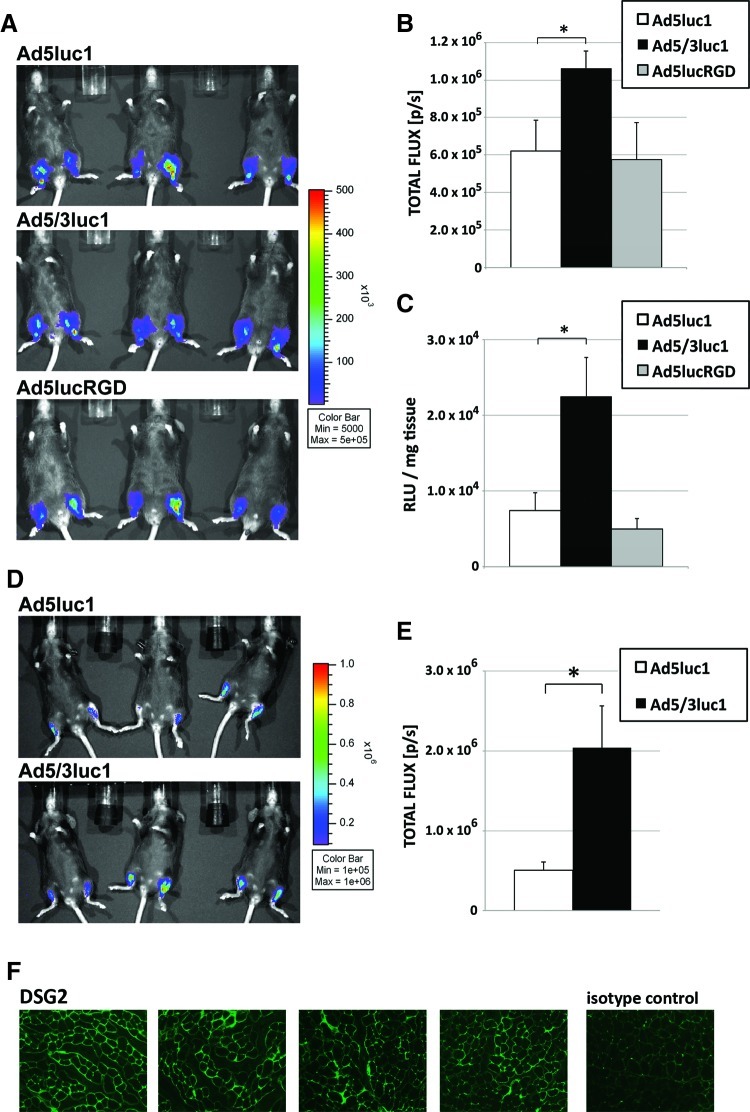
The hamstring muscles of C57 mice were injected with luciferase-expressing unmodified FGAd (Ad5luc1), 5/3-modified (Ad5/3luc1), or RGD-modified (Ad5lucRGD) vectors. Two days later, luciferase expression was assessed by in vivo bioluminescence imaging (Fig. 1A). Quantification of the luciferase imaging showed significantly higher bioluminescence light emission for Ad5/3luc1 compared with the unmodified control Ad5luc1, whereas Ad5lucRGD had values comparable to the control (Fig. 1B). To repeat the measurement ex vivo, mice from Fig. 1B were sacrificed the same day, muscles were excised and homogenized, and luciferase light emission was measured. Ex vivo analysis confirmed the result, although differences were even more pronounced, with Ad5/3luc1-injected muscles exhibiting threefold higher luciferase light emission compared with Ad5lucRGD and Ad5luc1 (Fig. 1C). These results indicate that the RGD modification does not improve in vivo muscle transduction, which is in line with previous studies with RGD-modified HDAds (Bramson et al., 2004). Therefore, we did not conduct further experiments with vectors bearing this modification. Next, we injected Ad5luc1 and Ad5/3luc1 into the tibialis anterior muscles of C57 mice and performed in vivo bioluminescence imaging 2 days later (Fig. 1D). Quantification of luciferase signals in this experiment showed threefold higher values for Ad5/3luc1 compared with Ad5luc1 (p<0.05; Fig. 1E). Immunofluorescence staining of tibialis anterior muscles confirmed expression of DSG2 (Fig. 1F), which was reported to be the primary attachment receptor of Ad5/3-modified vectors (Wang et al., 2011).
FIG. 1.
5/3 capsid-modified FGAd shows increased transduction in hamstring and tibialis anterior muscles. (A) Mice were injected with the FGAds Ad5luc1, Ad5/3luc1, or Ad5lucRGD into hamstring muscles, and in vivo luciferase imaging was performed 2 days later. (B) Quantification of the in vivo bioluminescence signals revealed significantly higher luciferase expression in Ad5/3luc1-injected muscle compared with Ad5luc1 and Ad5lucRGD. (C) Ex vivo luminescence analysis of the same muscles showed even greater (threefold) significant difference between Ad5/3luc1- and Ad5luc1-injected muscles. (D) In another experiment, mice were injected with Ad5luc1 or Ad5/3luc1 into tibialis anterior muscles and imaged 2 days later. (E) Also in this muscle group, Ad5/3luc1 led to significantly higher luciferase expression than Ad5luc1. (F) Tibialis anterior muscles were cryosectioned and stained for the adenovirus serotype 3 receptor DSG2 to confirm the presence of the primary attachment receptor for 5/3-based Ads. n=6 muscles for A–E; n=4 for F; error bars: standard error.
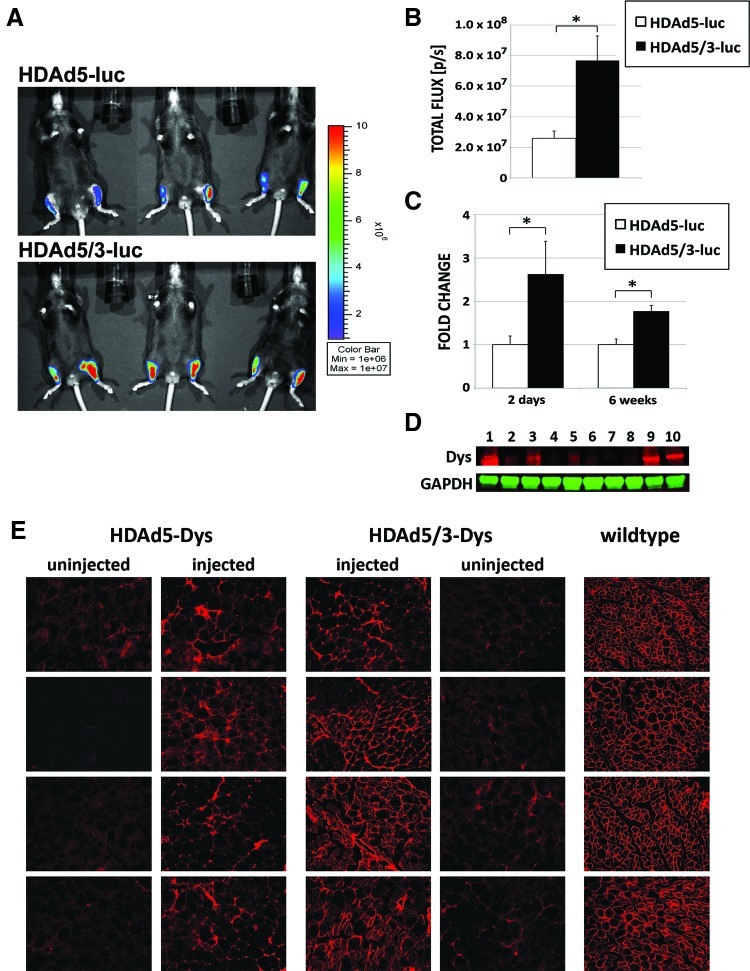
Based on the results with the FGAds, we generated an HDAd with the same genetic 5/3 serotype chimerism modification expressing luciferase (HDAd5/3-luc). HDAd5/3-luc and HDAd5-luc (serotype 5 capsid) were injected into tibialis anterior muscles of C57 mice. In vivo imaging 2 days after injection showed approximately threefold higher luciferase light emission for HDAd5/3-luc–injected muscles compared with HDAd5-luc (p=0.01; Fig. 2A and B). Mice were sacrificed the same day (2 days after injection) or in another experiment 6 weeks post injection, muscles were excised and homogenized, and DNA was extracted. Real-time PCR specific for HDAd genomes showed significantly higher vector copy numbers in HDAd5/3-luc–injected muscles compared with HDAd5-luc at both time points, with approximately 2.5- and 1.8-fold differences (Fig. 2C). This suggests that the increased luciferase expression is due, in fact, to improved transduction, although it cannot be excluded that virus particles attached to the cell surface but not internalized contributed to the quantitative PCR results. To evaluate whether these results would translate into improved gene transfer of a relevant large therapeutic gene, we injected the tibialis anterior muscles of dystrophin-deficient mice (mdx) with wild-type capsid and 5/3-modified HDAds expressing full-length dystrophin (HDAd5-Dys and HDAd5/3-Dys). Six weeks after injection, mice were sacrificed and tibialis anterior muscles were homogenized for western blot or sectioned for dystrophin immunofluorescence staining. Western blot analysis demonstrated stronger dystrophin in HDAd5/3-Dys–injected muscles compared with the uninjected contralateral control muscles (Fig. 2D, lanes 1 and 3 vs. 2 and 4). A slight increase in dystrophin expression was also seen in HDAd5-Dys–injected muscles compared with uninjected controls (Fig. 2D, lanes 5 and 7 vs. 6 and 8). However, HDAd5/3-Dys conferred stronger dystrophin expression compared with HDAd5-Dys, which was nevertheless weaker than in wild-type mice (Fig. 2D, lanes 9 and 10). Immunofluorescence staining demonstrated dystrophin expression in all injected muscles, which was weaker and patchier compared with that in wild-type muscles (Fig. 2E). However, immunofluorescence analysis also showed stronger dystrophin expression with HDAd5/3-Dys than with HDAd5-Dys.
FIG. 2.
5/3 capsid-modified HDAd demonstrates increased transduction in mouse tibialis anterior. (A) HDAds HDAd5-luc and HDAd5/3-luc were injected into tibialis anterior, and in vivo luciferase imaging was performed 2 days later. (B) HDAd5/3-luc demonstrated significantly higher transduction compared with wild-type capsid HDAd5-luc as shown by significantly increased in vivo luciferase light emission. (C) Vector cDNA copy number in HDAd5/3-luc–injected muscles was also significantly increased in mice sacrificed 2 days and 6 weeks after injection. mdx mice injected with dystrophin-expressing HDAd5/3-Dys showed stronger expression of dystrophin 6 weeks after injection compared with HDAd5-Dys as shown by western blot (D; lanes 1 and 3, HDAd5/3-Dys–injected; lanes 5 and 7, HDAd5-Dys–injected; lanes 2, 4, 6, and 8, contralateral uninjected muscle; lanes 9 and 10, uninjected wild-type C57 muscles) and immunofluorescences staining (E) of tibialis anterior muscles. n=6 muscles for A–C; n=4 for D and E; error bars: standard error.
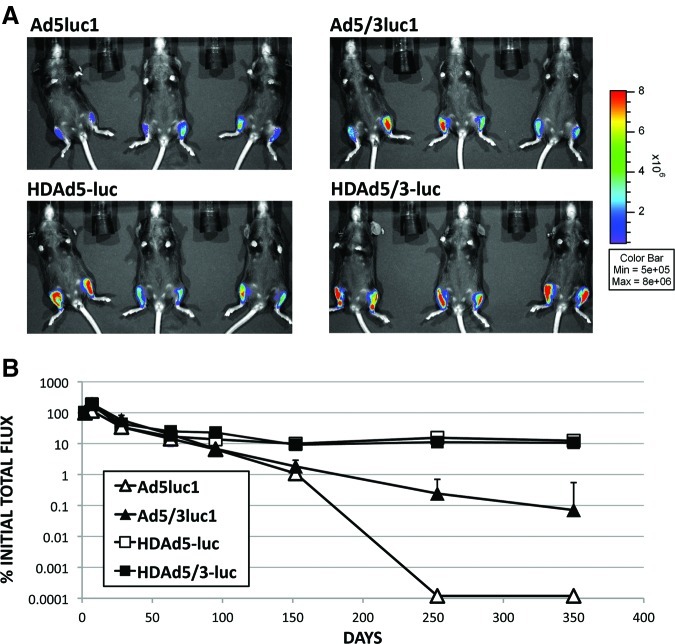
To evaluate long-term expression, C57BL/6 mice were injected into tibialis anterior muscles with FGAds (Ad5luc1, Ad5/3luc1) or HDAds (HDAd5-luc, HDAd5/3-luc), and luciferase expression was followed over time. Two days after injection, strong luciferase bioluminescence was detected in all injected muscles (Fig. 3A). Luciferase expression in muscles injected with Ad5luc1 decreased to undetectable levels at day 250, and Ad5/3luc1-mediated luciferase expression was below 0.1% of the initial expression 1 year after injection (Fig. 3B). In contrast, muscles injected with HDAd5-luc and HDAd5/3-luc demonstrated stable luciferase expression of around 10% of the initial expression 1 year after injection.
FIG. 3.
HDAds mediate long-term gene expression in skeletal muscle tissue. Mice were injected with the FGAds Ad5luc1 and Ad5/3luc1 or the HDAds HDAd5-luc and HDAd5/3luc. (A) Two days later, strong luciferase expression was seen in all mice, with the helper-dependent viruses showing generally higher levels. Both FGAds and HDAds with the 5/3 capsid modification demonstrated three- to fourfold higher luciferase expression compared with their wild-type capsid control vectors. (B) Over the course of almost 1 year, luciferase expression with FGAds decreased to less than 0.1% (Ad5/3luc1) and undetectable levels (Ad5luc1) relative to initial expression on day 2. HDAds maintained expression over this time period with about 10% of initial expression levels. n=6 muscles; error bars: standard error.
The major limitation of using Ads for adult skeletal muscle–directed gene therapy is inefficient transduction. This might be partly due to low expression of Ad's primary attachment receptor CAR, although other factors, such as the structure of the basal lamina and the presence of myoblasts, have been implicated (Cao et al., 2001). Retargeting Ads to receptors other than CAR has been shown to lead to increased transduction in previous studies. For example, FGAds and HDAds featuring a polylysine moiety at the C-terminus of the fiber knob (pK7) have shown 1.5–3-fold improvement in the transduction of adult skeletal muscle (Bouri et al., 1999; Cao et al., 2001). Furthermore, Goncalves and colleagues showed improved in vitro transduction of myogenic cells derived from Duchenne's muscular dystrophy patients with a serotype 5/50 chimeric HDAd (Gonçalves et al., 2006). In our study, we have shown that the 5/3 serotype chimerism capsid modification increases transduction of FGAds and HDAds in hamstring and tibialis anterior muscles of adult mice. In particular, we demonstrated that full-length dystrophin, as an example of a relevant therapeutic gene, can be more efficiently transferred to adult mdx muscle tissue using a 5/3-modified HDAd. This is of relevance because HDAds are, besides herpes simplex viruses, currently the only viral vectors that are capable of packaging full-length dystrophin cDNA. Several groups have studied dystrophin gene therapy using HDAds with encouraging results; however, complete functional correction of dystrophic tissue remains to be seen (DelloRusso et al., 2002; Gilbert et al., 2003). Another attribute that makes HDAds attractive for muscle gene therapy is their ability to achieve long-term expression, which has been demonstrated for numerous other tissues (Brunetti-Pierri and Ng, 2008), including skeletal muscle (Maione et al., 2001; Gilbert et al., 2003). Our study also demonstrated that HDAds had robust long-term gene expression over 1 year in skeletal muscle tissue. Taken together, our results suggest that 5/3-modified HDAd vectors could be a useful tool for skeletal muscle–directed gene therapy.
Acknowledgments
This work was supported by the German Research Foundation (DFG; Kilian Guse), NIH K99HL098692 (Masataka Suzuki), NIH R01DK067324 (Philip Ng), and NIH R01HL87836 (Brendan Lee). Akseli Hemminki is K. Albin Johansson Research Professor of the Foundation for the Finnish Cancer Institute.
Author Disclosure Statement
Akseli Hemminki is a shareholder of Oncos Therapeutics Ltd., Helsinki, Finland, a company involved in development of oncolytic viruses for cancer treatment. All other authors have no competing financial interest.
References
- Arruda V.R. Hagstrom J.N. Deitch J., et al. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001;97:130–138. doi: 10.1182/blood.v97.1.130. [DOI] [PubMed] [Google Scholar]
- Bouri K. Feero W.G. Myerburg M.M., et al. Polylysine modification of adenoviral fiber protein enhances muscle cell transduction. Hum. Gene Ther. 1999;10:1633–1640. doi: 10.1089/10430349950017635. [DOI] [PubMed] [Google Scholar]
- Bramson J.L. Grinshtein N. Meulenbroek R.A., et al. Helper-dependent adenoviral vectors containing modified fiber for improved transduction of developing and mature muscle cells. Hum. Gene Ther. 2004;15:179–188. doi: 10.1089/104303404772679986. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Ng P. Progress and prospects: gene therapy for genetic diseases with helper-dependent adenoviral vectors. Gene Ther. 2008;15:553–560. doi: 10.1038/gt.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Stapleton G.E. Law M., et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol. Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B. Pruchnic R. Ikezawa M., et al. The role of receptors in the maturation-dependent adenoviral transduction of myofibers. Gene Ther. 2001;8:627–637. doi: 10.1038/sj.gt.3301425. [DOI] [PubMed] [Google Scholar]
- DelloRusso C. Scott J.M. Hartigan-O'Connor D., et al. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12979–12984. doi: 10.1073/pnas.202300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev I. Krasnykh V. Miller C.R., et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. Foster H. Dickson J.G. Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene Ther. 2006;13:1677–1685. doi: 10.1038/sj.gt.3302877. [DOI] [PubMed] [Google Scholar]
- Gilbert R. Dudley R.W.R. Liu A.-B., et al. Prolonged dystrophin expression and functional correction of mdx mouse muscle following gene transfer with a helper-dependent (gutted) adenovirus-encoding murine dystrophin. Hum. Mol. Genet. 2003;12:1287–1299. doi: 10.1093/hmg/ddg141. [DOI] [PubMed] [Google Scholar]
- Glasgow J.N. Everts M. Curiel D.T. Transductional targeting of adenovirus vectors for gene therapy. Cancer Gene Ther. 2006;13:830–844. doi: 10.1038/sj.cgt.7700928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves M.A.F.V. Holkers M. Cudré-Mauroux C., et al. Transduction of myogenic cells by retargeted dual high-capacity hybrid viral vectors: robust dystrophin synthesis in Duchenne muscular dystrophy muscle cells. Mol. Ther. 2006;13:976–986. doi: 10.1016/j.ymthe.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Hasbrouck N.C. High K.A. AAV-mediated gene transfer for the treatment of hemophilia B: problems and prospects. Gene Ther. 2008;15:870–875. doi: 10.1038/gt.2008.71. [DOI] [PubMed] [Google Scholar]
- Kanerva A. Mikheeva G.V. Krasnykh V., et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 2002;8:275–280. [PubMed] [Google Scholar]
- Kochanek S. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum. Gene Ther. 1999;10:2451–2459. doi: 10.1089/10430349950016807. [DOI] [PubMed] [Google Scholar]
- Krasnykh V. Belousova N. Korokhov N., et al. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J. Virol. 2001;75:4176–4183. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnykh V.N. Mikheeva G.V. Douglas J.T. Curiel D.T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione D. Della Rocca C. Giannetti P., et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J. Pari G. Karpati G. Holland P.C. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum. Gene Ther. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- Palmer D. Ng P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Raper S.E. Chirmule N. Lee F.S., et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Schnell M.A. Zhang Y. Tazelaar J., et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Suzuki M. Cela R. Clarke C., et al. Large-scale production of high-quality helper-dependent adenoviral vectors using adherent cells in cell factories. Hum. Gene Ther. 2010;21:120–126. doi: 10.1089/hum.2009.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Li Z.-Y. Liu Y., et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. Yang H. Colosi P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]