Abstract
An antitumor T-cell response can lead to tumor control without clearing all tumor cells. As long as residual tumor cells remain, there is a constant risk of escape from that T-cell response. We previously showed that adoptive transfer of anti-ova OT-I T cells into B16ova-bearing mice led to tumor regression followed by escape of tumors that had lost the ova gene, rendering the OT-I T cells ineffective. In this study, we hypothesized that simultaneous transfer of cytotoxic T lymphocytes targeted against two independent antigens would reduce selection for single-antigen-loss cells, thereby limiting tumor escape. Using OT-I and Pmel T cells to treat B16ova tumors, we found that early cotransfer could prevent tumor emergence in most mice, whereas neither T-cell specificity alone was able to do so. When combined with total body irradiation for the treatment of larger 7-day tumors, cotransfer was also better at limiting tumor recurrence, and the tumors that did escape combination therapy continued to express both target antigens. As adoptively transferred T cells also persisted in vivo, even in mice with recurrent tumors, we hypothesized that restimulation of these antitumor T cells would prolong survival of mice with recurrent tumors. Consistent with this hypothesis, administration of a low-dose regimen of cyclophosphamide following tumor escape slowed tumor growth in mice that had previously received T-cell therapy, but not in control-treated mice, an effect that was associated with increased activation of T cells in vitro by low- but not high-dose cyclophosphamide.
Kaluza and colleagues demonstrate that the simultaneous transfer of cytotoxic T lymphocytes targeted against two independent antigens (OT-1 and Pmel) has significant added value in tumor-bearing mice compared with similar therapies targeting a single antigen. The authors suggest that such combinations will be most beneficial when administered as early as possible during tumor development and should be give in approximately equal proportions in order to preserve expression of the target antigens in tumor cells.
Introduction
Adoptive T-cell therapy for melanoma can result in tumor regression and apparent cures. However, more often, transient responses are followed by tumor recurrence. We have previously shown that adoptive transfer of OT-I T cells into mice with B16ova tumors induced tumor regression, transient control, and eventual tumor recurrence. In this model, tumor recurrence was associated with loss of the gene for the target tumor antigen, ova (Kaluza et al., 2011). Mice with recurrent tumors maintained persistent populations of OT-I T cells, but loss of the target antigen rendered those cells ineffective. In the present study, we hypothesized that combining OT-I transfer with transfer of T cells targeting a second antigen would improve overall survival by necessitating the loss of expression of more than a single antigen in order for tumor cells to escape the T-cell immune-selective pressure.
Several recent clinical trials have used protocols involving the transfer of either a single T-cell specificity (Khammari et al., 2009) or polyclonal tumor-infiltrating lymphocytes with multiple specificities (Rosenberg et al., 2008). The frequent observation of antigen loss from recurrent tumors (Yee et al., 2002; Dudley et al., 2005) suggests that transfer of T cells against only a single antigen may not be ideal (Yee, 2010). Although it has been shown that cotransfer of antitumor cytotoxic T lymphocytes (CTL) together with antitumor Th1 cells can improve therapy over CTL alone (Shafer-Weaver et al., 2009) to overcome T-cell tolerance, most murine models of adoptive therapy use T-cell receptor (TCR)–transgenic T cells with just a single specificity. Here, we show that control of B16ova tumors was significantly improved by cotransferring CTL targeting two different antigens (OT-I against ova and Pmel against gp100), and that, in contrast to reproducible loss of the ova antigen in response to OT-I therapy alone (Kaluza et al., 2011), combination therapy was associated with maintenance of antigen expression in escape tumors.
In addition to immediate tumor control, a goal of adoptive T-cell therapies is to produce memory T-cell populations that can protect against recurrent tumors. Once such populations have been established in vivo, it should be possible to derive further benefit from the cells even after recurrent tumors are detected. In this respect, cyclophosphamide (CPA) is often used as an adjunct to adoptive T-cell transfer because of its multiple, pleiotropic effects, including activation of endogenous antigen-presenting cells (Salem et al., 2010), depletion of regulatory T cells (Zhao et al., 2010), and induction of immunogenic tumor cell death (Schiavoni et al., 2011)—all of which can enhance the function of the adoptively transferred cells (Bracci et al., 2007). Therefore, we tested whether CPA could rescue therapy following tumor escape in situations where both populations of adoptively transferred T cells persisted in vivo and in which the combination T-cell therapy had preserved antigen expression in the escape tumors. Significantly, the combination of T-cell transfer and CPA generated better survival than either T-cell transfer or CPA alone, and the best overall survival was achieved in mice that received both OT-I and Pmel CTL as well as subsequent CPA. Our results demonstrate that the benefits of adoptive T-cell therapy can be improved, at several levels, by targeting multiple antigens, with important implications for the development of future clinical approaches using either patient-derived or chimeric immune receptor–engineered T cells (Kochenderfer and Rosenberg, 2011; Porter et al., 2011; Rosenberg, 2011).
Materials and Methods
Mice, cell lines, antibodies, and reagents
Six- to 8-week-old female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). OT-I mice and Pmel mice have been described previously (Hogquist et al., 1994; Overwijk et al., 2003) and were bred at the Mayo Clinic. The B16ova cell line was derived from a B16.F1 clone transfected with a pcDNA3.1ova plasmid. B16ova cells were grown in Dulbecco's modified Eagle's medium (HyClone, Logan, UT)+10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA)+5 mg/mL G418 (Mediatech, Manassas, VA). The following antibodies were used for flow cytometry: from BD Biosciences (San Jose, CA): CD45-PerCP (clone 30-F11), CD3e-PerCP (clone 145-2C11), H-2Db-FITC (clone KH95), CD8β.2-PE (clone 53-5.8), Vβ5-PE (clone MR9-4), Vα2-FITC (clone B20.1), and anti-mouse IgG1-PE (clone A85-1); from eBioscience (San Diego, CA): CD3ɛ-FITC (clone 145-2C11), Thy1.1-PE (clone HIS51), and Thy1.2-PE (clone 53-2.1); and from Dako (Carpinteria, CA): anti-gp100 (clone HMB45). For detection of intracellular gp100, cells were permeabilized with the eBioscience Foxp3 staining buffer set.
In vivo experiments
All in vivo studies were approved by the Mayo Institutional Animal Care and Use Committee. Mice were challenged subcutaneously with 5×105 B16ova cells in 100 μL of PBS (HyClone). Tumors were measured three times per week, and mice were euthanized when tumors reached 10-mm diameter. For adoptive therapy experiments, 1×107 in vitro activated T cells were injected intravenously in 100 μL of PBS on day 3 or 7. In some experiments, mice were subjected to 5 Gy total body irradiation (TBI) on day 6 using a 137Cs irradiator. In some experiments, mice were injected intraperitoneally with 3 mg of cyclophosphamide (CPA; Baxter, Deerfield, IL). The first CPA injection was administered when tumors reached 0.3-mm diameter. Mice received two more injections 7 and 14 days after the first. In vivo data were analyzed using GraphPad Prism software (GraphPad Software, La Jolla, CA). To monitor OT-I and Pmel T-cell populations, tumors and spleens were harvested from mice with 10-mm tumors. Tissues were manually dissociated, and red blood cells were lysed with ACK lysis buffer. Cells were then stained for OT-I or Pmel T cells as indicated in the figure legends. Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Two to 3 months following tumor challenge, experiments were ended and surviving mice were inspected for visible tumor burden at the challenge site. Black tumor specks and the associated fat pad were fixed in 10% formalin for histological analysis.
In vitro T-cell activation
Spleens and peripheral lymph nodes were harvested from naive OT-I or Pmel mice and dissociated to obtain a single-cell suspension. Red blood cells were lysed with ACK lysis buffer. Cells were resuspended at 1×106 cells/mL in Iscove's modified Dulbecco's medium (GIBCO, Grand Island, NY)+5% FBS+1% penicillin/streptomycin+40 μM 2-mercaptoethanol. Medium was supplemented with SIINFEKL or gp100 peptide at 1 μg/mL and human interleukin-2 (hIL-2) at 50 U/mL. After 2 days, cells were split into new medium supplemented with IL-2. Cells were used for adoptive transfer or in vitro assays following 4 days of activation.
51Cr cytotoxicity assay
B16ova target cells were treated overnight with 500 U/mL murine interferon-γ (IFNγ; Peprotech, Rocky Hill, NJ). They were simultaneously loaded with 51Cr and peptide for 90 min at 37°C, washed, and then plated at 1×104 cells/well in 96-well V-bottom plates (Nunc, Roskilde, Denmark). Effectors were plated at varying concentrations to obtain a range of effector cell to target cell (E:T) ratios. Wells containing targets without effectors were used to determine spontaneous release. Wells containing targets and 0.1% Triton X-100 were used to determine maximum release. Plates were incubated at 37°C for 4 hr and then spun down. Supernatant (40 μL/well) was transferred to LumaPlates (PerkinElmer, Shelton, CT) and allowed to dry overnight before being read on a PerkinElmer TopCount machine. Percent specific lysis was defined as (sample cpm – spontaneous cpm)/(maximum cpm – spontaneous cpm)×100.
ELISA
Activated OT-I or Pmel T cells (2×106) were cultured for 24 hr in 2 mL of medium with 10 μg of peptide or 4×105 B16ova cells. Supernatants were collected and analyzed for IFNγ content by ELISA according to the manufacturer's instructions (BD OptEIA Mouse IFNγ ELISA Set; BD Biosciences Pharmingen, San Diego, CA).
PCR
RNA was isolated using the RNeasy kit (Qiagen, Germantown, MD). cDNA was made from 1 μg of RNA using the First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN). One tenth of this cDNA was used in each PCR reaction. The following primers were used: ova sense, CACAAGCAATGCCTTTCAGA; ova antisense, TACCACCTCTCTGCCTGCTT; gp100 sense, CCAGCCCATTGCTGCCCACA; gp100 antisense, CCCGCCTTGGCAGGACACAG; β-actin sense, GGCGGACTGTTACTGAGCTGCG; β-actin antisense, CCAGCCCTGGCTGCCTCAAC. Genomic DNA was isolated using the DNeasy kit (Qiagen). PCR was performed using the above ova primers and GAPDH primers: sense, TCATGACCACAGTCCATGCC; and antisense, TCAGCTCTGGGATGACCTTG.
Statistics
Overall survival, days to tumor, and days with tumor were analyzed by the log-rank test. All other data were analyzed by the two-tailed t test. Statistical significance was set at p<0.05 for all experiments.
Results
Pmel T cells are weakly effective against B16ova
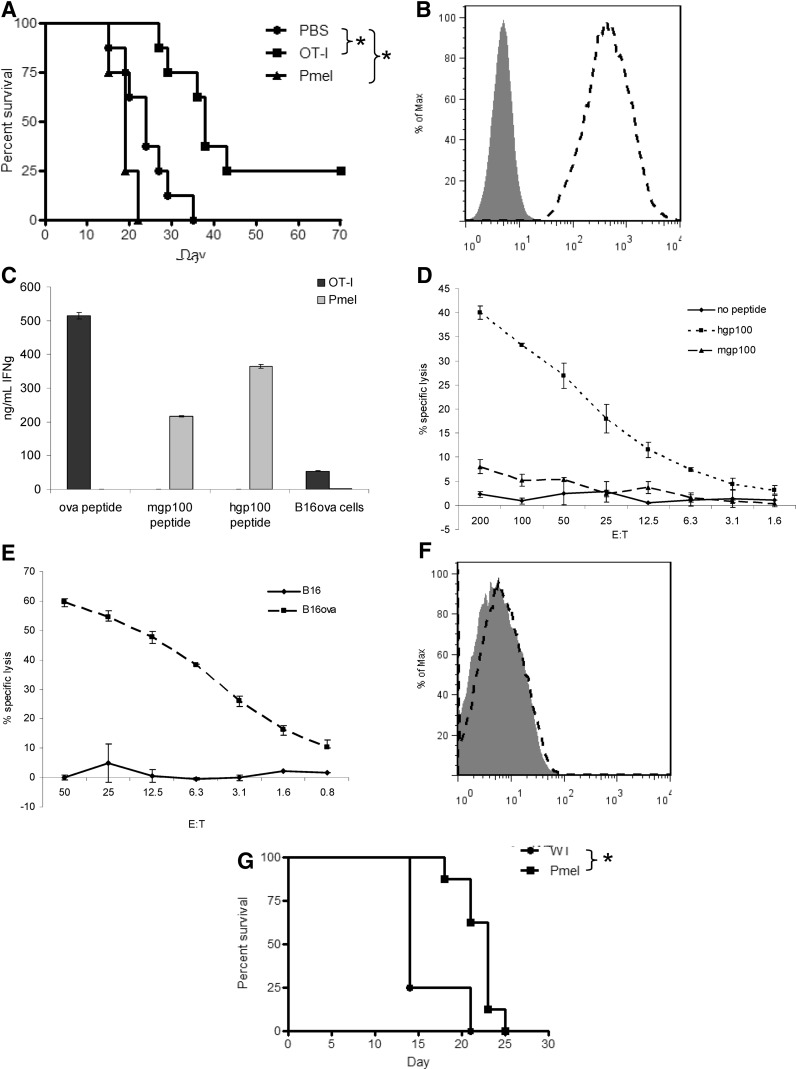
As we have shown previously, adoptive transfer of in vitro activated OT-I T cells into mice with B16ova tumors led to tumor regression and a significant prolongation of survival (Fig. 1A) (Kaluza et al., 2011). In contrast, adoptive transfer of Pmel T cells did not generate tumor regression or prolongation of survival (Fig. 1A), despite the fact that B16ova cells express high levels of the target antigen, gp100 (Fig. 1B). Although Pmel T cells were weakly responsive to B16ova in vitro as measured by IFNγ release (Fig. 1C) and cytotoxicity (Fig. 1D), their function was comparable to that of OT-I T cells when stimulated with cognate peptide [human gp100 (hgp100)] (Fig. 1C and D). This lack of recognition of B16ova by Pmel in vitro (Fig. 1C and D) was consistent over a range of coculture conditions, with both widely ranging E:T ratios and peptide concentrations (0.1, 1, or 10 μg). Consistent with the disparate results in vivo, OT-I T cells were more highly reactive against B16ova in vitro (Fig. 1C and E). Two possible explanations for the poor efficacy of Pmel T cells include their low responsiveness to the endogenous mouse gp100 (mgp100) peptide (as opposed to the hgp100 peptide) (Fig. 1C and D) and low expression of H-2Db by B16ova cells (Fig. 1F). Nonetheless, Pmel mice challenged with B16ova survived significantly longer than did untreated wild-type mice (Fig. 1G), suggesting that Pmel T cells can, under certain circumstances, have some antitumor activity against B16ova tumor cells. This discrepancy between in vitro and in vivo recognition of B16ova tumors may be due to the release of additional cytokines in vivo at the tumor, and/or in the lymph nodes, which potentiate T-cell activity, a hypothesis that we are currently testing using knockout mice.
FIG. 1.
Pmel T cells are weakly effective against B16ova. (A) C57BL/6 mice (8 mice/group) were challenged subcutaneously with 5×105 B16ova cells. PBS or 1×107 in vitro activated T cells were administered intravenously 7 days later. Data are representative of three independent experiments. (B) Intracellular staining for gp100 protein in B16ova cells (gp100, dashed histogram; secondary antibody alone, shaded histogram). (C) Activated OT-I or Pmel T cells were cultured with SIINFEKL peptide, mgp100 peptide, hgp100 peptide, or B16ova cells for 24 hr. IFNγ release was measured by ELISA of the supernatants. (D, E) Activated Pmel (D) or OT-I (E) T cells were used as effectors in a chromium release assay. Targets were B16ova cells with or without exogenous gp100 peptides, or B16 cells. (F) H-2Db expression on B16ova tumor cells (H-2Db, dashed histogram; isotype control, shaded histogram). (G) Wild-type C57BL/6 mice or TCR-transgenic Pmel mice (8 mice/group) were challenged with B16ova as in (A) and received no therapy. Data are representative of two experiments. *p<0.05 for all analyses.
Early transfer of a combination of T cells prevents tumor escape
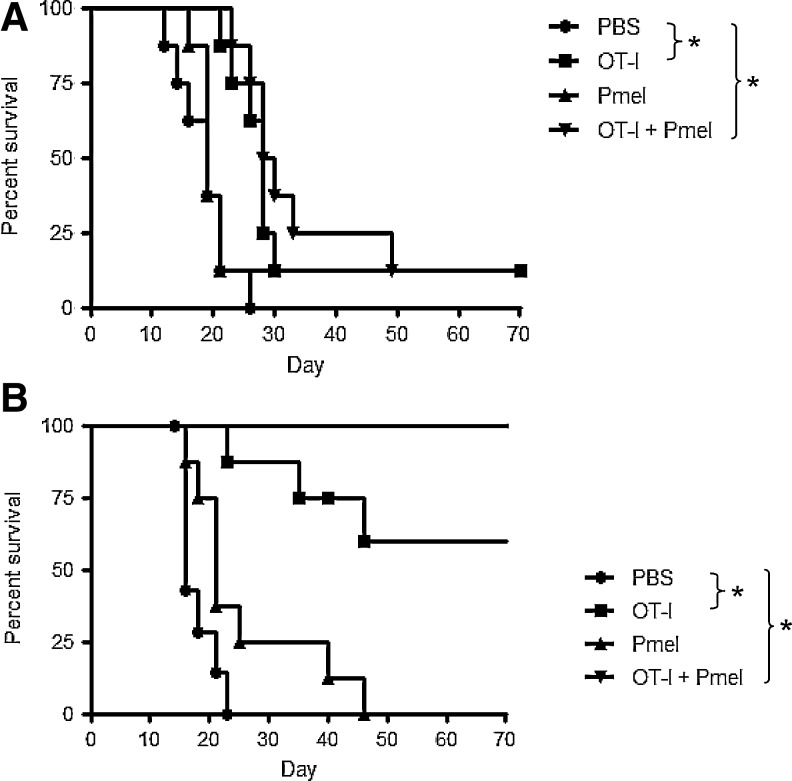
As OT-I T cells induced regression of B16ova tumors (Kaluza et al., 2011), we hypothesized that the ability of Pmel T cells to delay development of B16ova tumors in Pmel mice (Fig. 1G) would cooperate with OT-I therapy to inhibit tumor recurrence. Upon transfer of equal numbers of T cells (OT-I only, Pmel only, or a 1:1 ratio) into wild-type C57BL/6 mice with 7-day tumors, cotransfer of OT-I and Pmel T cells provided no benefit over OT-I T cells alone (Fig. 2A). Similarly, providing a second dose of T cells on day 11, or giving a full dose of OT-I T cells on day 7 followed by a full dose of Pmel T cells on day 11, showed no evidence of synergy between OT-I and Pmel T cells (data not shown).
FIG. 2.
Early combination T-cell transfer prevents tumor escape. Wild-type C57BL/6 mice (8 mice/group) were challenged subcutaneously with 5×105 B16ova cells. (A) PBS or 1×107 activated T cells were administered on day 7. Cotransfer consisted of OT-I and Pmel T cells (5×106 of each). (B) PBS or 1×107 activated T cells were administered on day 3. Data are representative of three independent experiments.
To investigate the importance of tumor size on therapeutic efficacy, T cells were transferred into C57BL/6 mice 3 days following tumor challenge. OT-I transfer delayed the emergence of tumors in most mice, and a significant proportion of mice survived long-term (Fig. 2B). Pmel transfer led to a delay in tumor development, but did not result in any long-term survivors (Table 1; *p<0.05 versus PBS within each experiment). Unlike treatment of 7-day established tumors, the combination of OT-I and Pmel T cells on day 3 was significantly more effective than either clone individually (Fig. 2B and Table 1).
Table 1.
Early Combination T-Cell Transfer Prevents Tumor Escape
| Median days to measurable tumora | Long-term survivors/total mice treatedb | |
|---|---|---|
| PBS | 8, 8, 5 | 0/32 |
| OT-I | 23,c 49,c 22.5c | 12/32 |
| Pmel | 13,c 11,c 8 | 0/32 |
| OT-I+Pmel | und,c und,c undc | 28/32 |
Median time from tumor challenge until the detection of a measurable tumor (>2 mm diameter). Data for three independent experiments are shown. “und” (undetermined) indicates that the median was not reached by day 70.
Compiled data from three experiments involving a total of 32 mice per treatment. Each experiment was ended approximately 70 days following tumor challenge, at which time none of the survivors had palpable tumors.
p<0.05 versus PBS group from the same experiment.
Therapy in TCR-transgenic mice parallels that in C57BL/6 mice
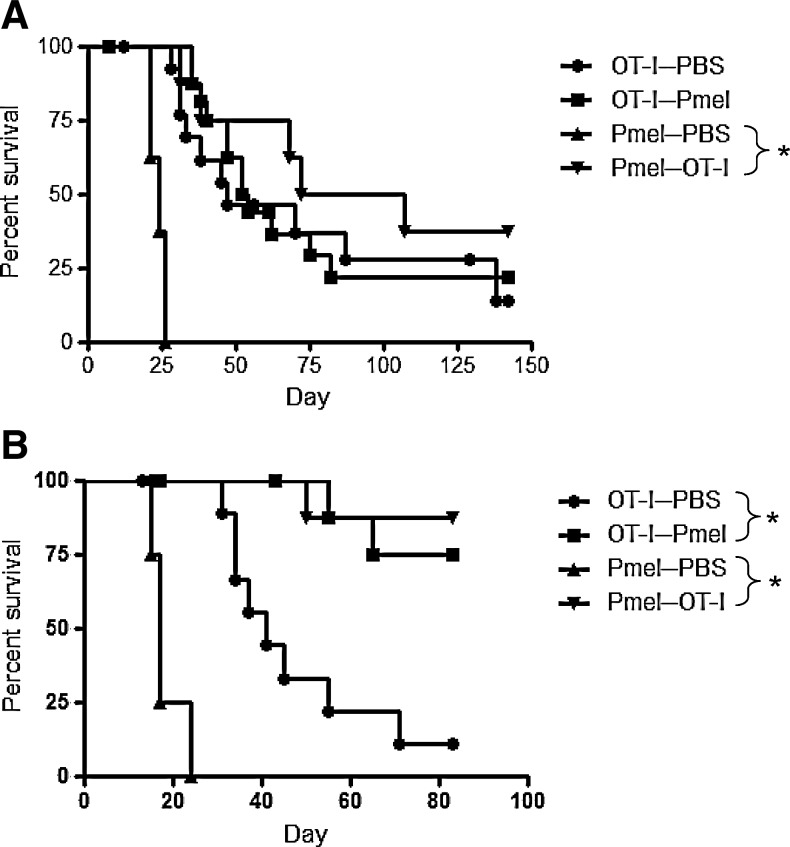
To investigate whether the number of adoptively transferred T cells limits the overall efficacy of therapy, adoptive transfers were performed between TCR-transgenic mice, in which large numbers of endogenous T cells with single antigen specificity were present prior to tumor challenge. Even where almost limitless numbers of T cells of one specificity were present, the effects of adoptive transfer of T cells with the second antigen specificity paralleled the results seen in C57BL/6 mice. Thus, OT-I mice that received Pmel T cells survived no longer than untreated OT-I mice (Fig. 3A). Although Pmel mice that received OT-I cells survived longer than untreated Pmel mice (Fig. 3A), this was not an improvement upon OT-I therapy alone in C57BL/6 mice (Fig. 1A).
FIG. 3.
Combination therapy in transgenic mice parallels that in C57BL/6 mice. OT-I and Pmel mice were challenged subcutaneously with 5×105 B16ova cells. (A) Activated T cells (1×107) were administered intravenously on day 7. One experiment representative of three is shown. Fifteen OT-I mice/group or 8 Pmel mice/group were used. (B) Activated T cells (1×107) were administered on day 3. Twelve OT-I mice/group or 8 Pmel mice/group were used. One experiment representative of two is shown.
Similarly, adoptive transfer on day 3 into TCR-transgenic mice paralleled the results in C57BL/6 mice, in that adoptive transfer of either T-cell population into the other strain was sufficient to prevent tumor growth in most animals (Fig. 3B). These data suggest that the numbers of adoptively transferred T cells, of either specificity, are not limiting in the outcome of therapy in either the 7- or 3-day established models.
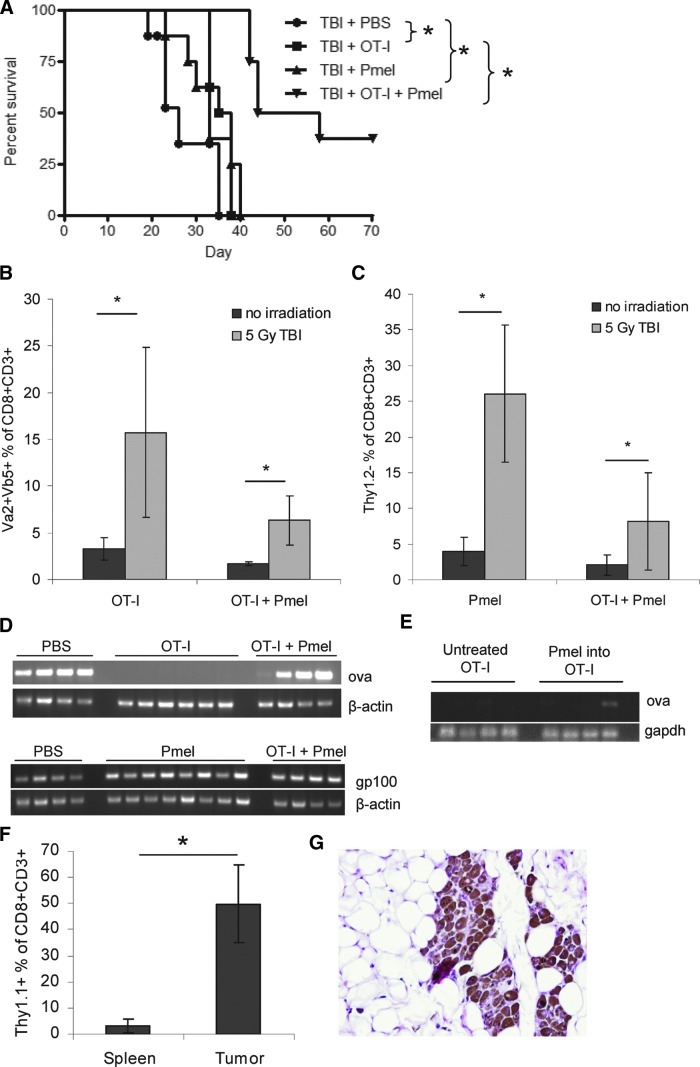
Combination transfer enhances therapy of 7-day tumors following host conditioning
Lymphodepleting TBI, prior to T-cell transfer, has been shown to improve Pmel therapy against B16 tumors (Paulos et al., 2007). Therefore, to improve combination therapy against 7-day tumors in C57BL/6 mice, mice were treated with TBI on day 6, 1 day prior to T-cell transfer. Although TBI had little effect on the overall survival of mice administered T cells of a single antigen specificity, TBI prior to cotransfer led to fewer tumors recurring and later recurrence of those that did (Fig. 4A). However, escape tumors still developed despite the persistence of OT-I (Fig. 4B) and Pmel (Fig. 4C) T-cell populations in the spleens, and the size of these populations was significantly increased in mice that had been irradiated. Consistent with our previous studies (Kaluza et al., 2011), escape from mono T-cell therapy with OT-I T cells alone involved loss of ova expression (Fig. 4D). However, most of the tumors that escaped combination OT-I/Pmel therapy retained ova expression (Fig. 4D). Escape tumors also continued to express gp100 mRNA (Fig. 4D). To assess whether Pmel T cells could inhibit loss of ova expression even in the presence of an abundant endogenous OT-I T-cell population, ova expression in tumors recovered from OT-I mice that had received Pmel transfer was measured. Although a minority of tumors retained a faint ova signal (Fig. 4E), Pmel transfer generally did not prevent ova loss, even though about 50% of all CD8+ T cells in the tumors were Pmel and persistent Pmel was detected in the spleens (Fig. 4F).
FIG. 4.
Combination T-cell transfer can enhance therapy of established 7-day tumors in combination with TBI. (A–D) C57BL/6 mice were challenged with 5×105 B16ova cells and administered 5 Gy TBI on day 6. Activated T cells (1×107) were administered on day 7. (A) Mice were monitored for tumor growth. One experiment representative of four is shown. (B, C) Spleens were harvested from mice with 10-mm tumors. Total splenocytes were analyzed by FACS for OT-I T cells (B; Vα2+Vβ5+) and Pmel T cells (C; Thy1.2−). Graphs represent percentage of total splenic CD8+CD3+ cells staining as each clone. (D) RNA was isolated from 10-mm tumors. RT-PCR was performed for ova and gp100. (E) OT-I mice were treated as in Fig. 3A. DNA was isolated from 10-mm escape tumors and tested for ova gene content by PCR. (F) Tumors and spleens were harvested from OT-I mice with 10-mm tumors. Pmel T cells were identified by FACS of single-cell suspensions. The graph indicates the percentage of CD8+CD3+ cells staining positive for Thy1.1. (G) Surviving mice were inspected after 2–3 months for persistent tumor burden. Black masses at the tumor challenge site were analyzed by histology. One representative picture is shown; it is from a wild-type C57BL/6 mouse treated with 5 Gy TBI followed by OT-I and Pmel cotransfer. Original magnification was 20×. Color images available online at www.liebertonline.com/hum
It is interesting that, although cotransfer of OT-I and Pmel T cells generated long-term survivors (Fig. 4A), histology showed a residual tumor burden of melanoma cells at the site of initial tumor challenge in most mice (Fig. 4G).
CPA delays tumor escape
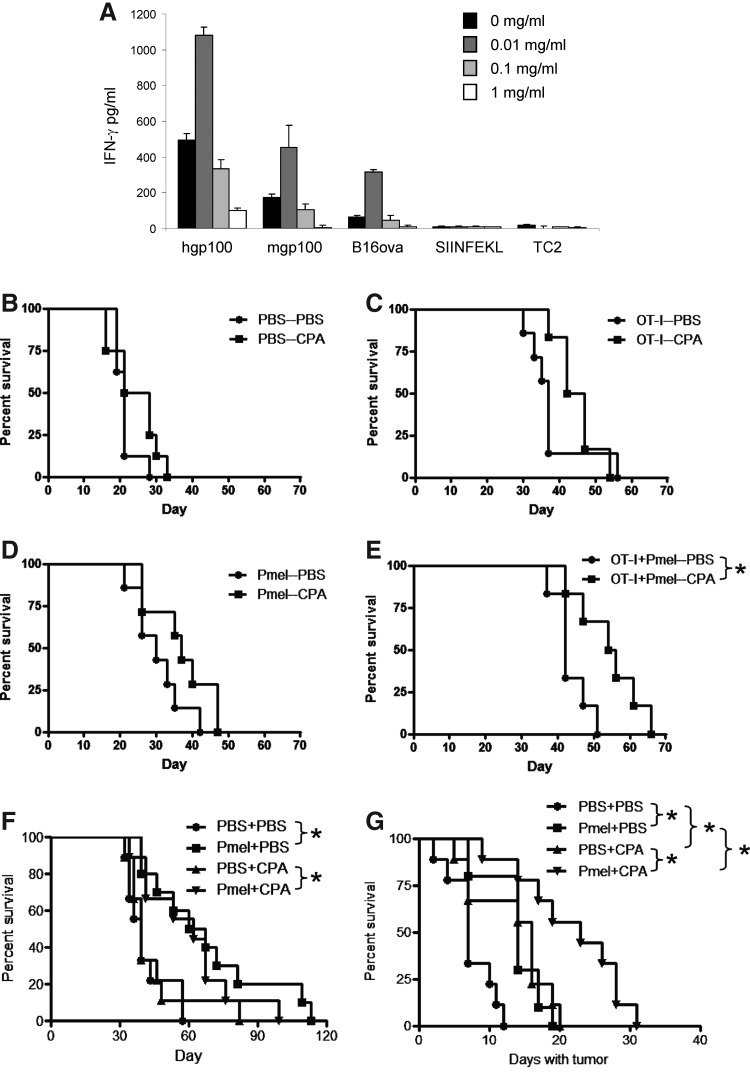
Although cotransfer of OT-I and Pmel T cells still led to escape tumors, both populations of T cells persisted in vivo, and expression of both ova and gp100 was preserved in escape tumors. Therefore, we investigated whether the antitumor activity of the persistent T-cell population could be reactivated in vivo against the retained antigens. Our previous in vivo studies have shown that a single dose of 150 mg/kg (3 mg/mouse) is immune stimulatory in that it both inhibits immune suppressors, such as regulatory T cells and myeloid-derived suppressor cells, and activates antigen-specific antitumor T cells and natural killer (NK) cells. This is in contrast to a higher dose regimen of three consecutive daily injections of 3 mg/mouse/day, which is highly immune suppressive (Qiao et al., 2008; Kottke et al., 2009; Willmon et al., 2011). Consistent with these in vivo findings, we observed a clear bimodal response of T-cell activation to CPA in vitro. Thus, at low concentration (0.01 mg/mL), CPA significantly enhanced activation of Pmel in response to both its cognate peptide from hgp100, the murine homologue from mgp100, and B16ova tumor cells, but not to the irrelevant SIINFEKL peptide or to TC2 tumor cells not expressing gp100 (Fig. 5A). In contrast, at higher concentrations (0.1 or 1.0 mg/mL), CPA inhibited IFNγ expression from Pmel cells in response to hgp100, mgp100, or B16ova cells compared with controls with no added CPA (Fig. 5A). Therefore, we translated these results in vivo based on our definition of the dosing regimen of CPA (150 mg/kg) that leads to activation of antitumor effectors, including antigen-specific T cells and NK cells (Qiao et al., 2008; Kottke et al., 2009; Wongthida et al., 2011). Although treatment with CPA alone, upon the detection of B16ova tumors, provided no direct antitumor effect in the C57BL/6 model (Fig. 5B), CPA delayed tumor growth in mice that had received adoptive transfer (Table 2). The greatest benefit was observed in mice that had received both OT-I and Pmel T cells, as only in this group was CPA able to significantly prolong the overall survival of mice bearing escape tumors (Fig. 5C–E).
FIG. 5.
CPA therapy prolongs survival of mice with escape tumors. (A) Spleens and peripheral lymph nodes from naive Pmel mice were cultured with hgp100 peptide, mgp100 peptide, B16ova cells, SIINFEKL peptide, or TC2 murine prostate cells (gp100-ve) and hIL-2 (50 U/mL). Cultures were supplemented with CPA at 0, 0.01, 0.1, or 1.0 mg/mL. Two days later, cells were split into new medium supplemented with IL-2 and CPA. Forty-eight hours later, IFNγ release was measured by ELISA. (B–E) C57BL/6 mice (16 mice/group) were treated as in Fig. 4A. As tumors emerged, mice were split between PBS and CPA treatment groups on an alternating basis. CPA (3 mg) was administered intraperitoneally when tumors escaped (escape was considered to have occurred for either a progressively growing tumor that had never gone away or a recurrent tumor on the first day it measured >2 mm diameter). Two additional doses of CPA were given 7 and 14 days later. Graphs show overall survival of only those mice in which tumors escaped. Data are representative of two independent experiments. (F, G) OT-I mice were treated as in Fig. 3A. PBS or CPA treatment was initiated when tumors escaped. Graphs include only those mice in which tumors escaped. (F) Overall survival of mice in which tumors escaped. (G) Survival in days from the time of tumor escape until the tumor reached 10 mm. Data are representative of two independent experiments.
Table 2.
Median Survival (in Days) Following Tumor Emergence
Median number of days from the first CPA or PBS injection, administered when a growing tumor was detected, until mice were euthanized due to large tumor burden (>10 mm). Data include only those mice in which tumors escaped (8 mice/group for PBS, 6–7 mice/group for OT-I, 7 mice/group for Pmel, and 6 mice/group for OT-I+Pmel, out of 8 mice possible per group.
p<0.05 versus no CPA.
Similarly, when Pmel T cells were adoptively transferred into B16ova tumor-bearing OT-I transgenic mice, CPA treatment alone, upon emergence of tumors, did not significantly improve overall survival compared with no CPA (Fig. 5F). As before, adoptive transfer of Pmel T cells alone improved overall survival, but this was not enhanced by CPA treatment when tumors started to grow (Fig. 5F). However, when we analyzed the time taken for tumors to grow from being first detectable to 10 mm in diameter, it was clear that both adoptive transfer of Pmel alone (no CPA upon emergence of tumor) and treatment with CPA alone once tumors emerged significantly delayed tumor growth once they had appeared (Fig. 5G). Significantly, the adoptive transfer of Pmel into OT-I B16ova tumor-bearing mice, combined with CPA treatment as tumors became detectable, slowed the growth of these tumors significantly more than either treatment alone (Fig. 5G), indicating that CPA could positively impact tumor growth in the context of escape from initial T-cell therapy.
Discussion
We show here that adoptive transfer of two CTL populations, targeting different antigens, produced significantly better therapy than either alone. Cotransfer led to the escape of fewer tumors, delayed appearance of recurrent tumors, and improved antigen retention in tumors that did escape.
Notably, all of these benefits were achieved despite the dilution of the potent mono T-cell therapy with OT-I T cells by a T-cell population that alone provided minimal therapy (Pmel). The poor efficacy of Pmel T cells in our model is consistent with reports from other groups using other B16 sublines (Overwijk et al., 2003; Vo et al., 2009). Importantly, this was due neither to the absence of target antigen (Fig. 1B) nor to a lack of ability to activate the T cells (Fig. 1C and D). The disparity between OT-I and Pmel T cells in this model more likely reflected a low avidity of Pmel T cells for B16ova due to low expression of the relevant major histocompatibility complex molecule (Fig. 1F) and relatively low responsiveness to the endogenous mouse (as opposed to human) gp100 peptide (Fig. 1C and D) (Overwijk et al., 2003).
There have been many attempts to identify the optimal phenotype of, and culture conditions for, adoptively transferred T cells (Zeh et al., 1999; Klebanoff et al., 2005; Janicki et al., 2008; Tran et al., 2008; Hinrichs et al., 2009; Caserta et al., 2010; Garcia-Hernandez et al., 2010; Pouw et al., 2010). Our results here suggest that therapy may also be significantly enhanced by combining multiple populations, with distinct characteristics, including the targeting of different antigens. Previously, we demonstrated almost complete loss of ova from all B16ova tumors that escaped OT-I T-cell therapy (Kaluza et al., 2011). In contrast, in our current studies, following a combination of T-cell pressure applied through both OT-I and Pmel T-cell therapy, even this nonessential, and nontolerized, protein was lost from escaping tumors far less frequently (1 of 4 in Fig. 4D). Continued expression of a highly immunogenic antigen, in a high proportion of tumors, is significant because it opens new avenues for the design of rational second-line therapies to target escape tumors still expressing the antigens that were initially present in the primary tumor. This may be especially relevant to trials of adoptive T-cell therapy using T cells redirected with novel, designer, high-potency receptors that, typically, have so far targeted single antigens (Morgan et al., 2006; Chinnasamy et al., 2010; Kochenderfer et al., 2010; Lo et al., 2010; Zhao et al., 2010; Kochenderfer and Rosenberg, 2011; Porter et al., 2011; Robbins et al., 2011). Our data here suggest that combining several of these T-cell populations into the same protocol may increase the durability of responses achieved. Alternatively, such redirected T cells might also synergize with less potent endogenous tumor-infiltrating lymphocytes (Weber et al., 2011), analogous to the cooperation we observed here between potent OT-I T cells and individually ineffective Pmel T cells. Notably, the cooperative effects were significantly greater in mice with minimal disease burden than in mice with more progressively growing tumors. Significantly, we showed that, in C57BL/6 mice, the loss of expression of ova in escape tumors that is induced by OT-I T cells alone (Kaluza et al., 2011) was greatly reduced by the coadministration of an equal number of Pmel T cells. In contrast, ova expression was lost from most of the escape tumors in OT-I mice that had received Pmel T cells, where there was an ongoing huge excess of ova-specific T cells. Therefore, for cooperation between T cells targeting different antigens to be effective at decreasing antigen loss and resultant tumor escape, a carefully balanced ratio of the population sizes of each T-cell type will be required. Nonetheless, as tumor antigen loss is a commonly observed escape mechanism (Yee et al., 2002; Dudley et al., 2005), adding a selective pressure against additional antigens may improve many different types of T-cell, and other immuno-, therapies, through the promotion of a polyclonal T-cell response instead of focusing on a single dominant antigen.
We are currently investigating the mechanism by which Pmel T cells prevent ova loss from escape tumors in the presence of matched levels of OT-I T cells. Importantly, ova signals were fainter in tumors escaping combination therapy than in untreated tumors, suggesting that Pmel T cells prevented selection for ova-loss cells rather than preventing the generation of such cells. This is an important distinction because, in patients, widespread tumor burden would already include a highly heterogeneous population of tumor cells expressing antigens at varying levels. Therefore, therapies that prevent selection for the least immunogenic cells would, potentially, have considerable clinical value.
With adoptive transfer of OT-I and Pmel into C57BL/6 mice, of OT-I into Pmel transgenic mice, or of Pmel into OT-I transgenic mice, significantly improved therapy only occurred upon transfer with 3-day tumors. These data show that the combined activity of both T-cell populations is most effective at very early time points of tumor development, which may be associated with the access of adoptively transferred T cells to tumor cells before, during, or after the tumor stroma and vasculature become well established in vivo. Nonetheless, although transfer of Pmel T cells into OT-I mice on day 7 did not improve overall survival (Fig. 3A), it did slow tumor growth (Fig. 5G) enough to prolong the survival of mice in which escape occurred (Fig. 5F). These effects were associated with high levels of Pmel accumulation within the tumor (Fig. 4F) and improved preservation of expression of ova compared with untreated OT-I mice (Fig. 4E). Of particular note, even the long-term survivors of combined T-cell therapy retained histological evidence of minimal residual disease at the site of tumor injection (Fig. 4G). Our initial attempts to culture these tumor cells from treated mice have been unsuccessful, and so it is unclear as to whether these residual tumor cells remain viable in vivo and whether these surviving mice were genuinely cured or remained at risk for eventual recurrence. These residual tumor deposits were very rarely associated with detectable immune infiltrates, suggesting that they may have become largely invisible to circulating immune and/or adoptively transferred T cells. Nonetheless, it remains to be seen whether these cells exist in an ongoing equilibrium between tumor-cell proliferation and immune control, or whether tumor recurrence from such deposits (if it occurs in vivo) is triggered by systemic factors from an essentially dormant state.
Taken together, our data showed that, although cotransfer of OT-I and Pmel T cells still led to escape tumors in a proportion of mice, preconditioning with TBI allowed for the persistence of both populations of adoptively transferred T cells in vivo and for the preservation of expression of both target antigens (ova and gp100) in the escape tumors. These data, along with the detection of minimal residual disease even in apparently cured mice, led us to investigate whether it would be possible to reactivate the antitumor T-cell activity of the persistently engrafted adoptively transferred T cells. In this respect, it has been shown that the in vivo activity of Pmel T cells can be boosted very effectively, beyond the effects of irradiation alone, by a combination of vaccination and IL-2 (Gattinoni et al., 2005; Wang et al., 2005). In addition, CPA has often been used as an adjunct to adoptive T-cell transfer due to its multiple, pleiotropic effects (Bracci et al., 2007; Salem et al., 2010; Zhao et al., 2010; Schiavoni et al., 2011), and we have previously reported that low-dose CPA leads to activation of antitumor lymphocytes in vivo (Kottke et al., 2009). Therefore, we investigated whether low-dose CPA could be used as a rescue strategy at the point of detectable tumor escape from combination T-cell therapy. In vitro, whereas high-dose CPA was strongly inhibitory, low-dose CPA in cultures of Pmel T cells significantly enhanced antigen-specific T-cell activation (Fig. 5A). To translate these results in vivo, we treated mice with a low-dose CPA regimen, which we have previously shown activates antitumor lymphocyte activity (Kottke et al., 2009). When this regimen was initiated as tumors escaped from initial adoptive T-cell therapy, there was a clear, albeit modest, benefit in combination-treated mice, supporting the notion that reactivation of resident T-cell activities may be possible. Although CPA only slowed the growth of escape tumors in these current studies, we believe it may be possible to improve these effects by careful titration of CPA doses to determine an optimal T-cell activating concentration in vivo. In the current studies, we have sought to activate the combination of T cells adoptively transferred in vivo with CPA, which is clinically approved and could be translated relatively rapidly into clinical trials. In addition, other T-cell activating approaches are possible, such as concurrent vaccination with the cognate antigens recognized by the T cells, which we have shown can also be effective at further increasing antitumor efficacy afforded by the combination T-cell therapy alone (Rommelfanger et al., manuscript in preparation). In addition, our data lay the foundation for the exploration of more potent T-cell reactivating regimens (Hodi et al., 2010; Park and Fisher, 2010) at the point of tumor escape from adoptive T-cell transfer therapies, which have been designed to lead to the retention of target antigens in the escape tumors.
In summary, our data here suggest that adoptive T-cell therapies targeted toward two or more antigens will have significant added value compared with similar therapies targeting a single antigen. However, such combinations will be most beneficial when administered as early as possible during development of the tumor and should be given in approximately equal proportions in order to preserve expression of the target antigens in the tumor cells. Combination T-cell therapies will be improved by host-cell conditioning to enhance long-term persistence of the therapeutic T cells in the host and, therefore, the ability to use additional therapies, such as CPA, to reactivate the T-cell response targeted to the retained targeted antigens. Finally, our data also suggest that even apparently curative T-cell therapies may establish a precarious equilibrium between dormant minimal residual disease and immune-based tumor control.
Acknowledgments
This work was supported by the Mayo Foundation and by National Institutes of Health grant R01 CA130878.
Author Disclosure Statement
No competing financial interests exist.
References
- Bracci L. Moschella F. Sestili P., et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin. Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- Caserta S. Kleczkowska J. Mondino A. Zamoyska R. Reduced functional avidity promotes central and effector memory CD4 T cell responses to tumor-associated antigens. J. Immunol. 2010;185:6545–6554. doi: 10.4049/jimmunol.1001867. [DOI] [PubMed] [Google Scholar]
- Chinnasamy D. Yu Z. Theoret M.R., et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J. Clin. Invest. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E. Wunderlich J.R. Yang J.C., et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez M.L. Hamada H. Reome J.B., et al. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J. Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L. Finkelstein S.E. Klebanoff C.A., et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C.S. Borman Z.A. Cassard L., et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S. O'Day S.J. McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K.A. Jameson S.C. Heath W.R., et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Janicki C.N. Jenkinson S.R. Williams N.A. Morgan D.J. Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res. 2008;68:2993–3000. doi: 10.1158/0008-5472.CAN-07-5008. [DOI] [PubMed] [Google Scholar]
- Kaluza K.M. Thompson J. Kottke T., et al. Adoptive T cell therapy promotes the emergence of genomically altered tumor escape variants. Int. J. Cancer. 2012;131:844–854. doi: 10.1002/ijc.26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khammari A. Labarrière N. Vignard V., et al. Treatment of metastatic melanoma with autologous Melan-A/MART-1-specific cytotoxic T lymphocyte clones. J. Invest. Dermatol. 2009;129:2835–2842. doi: 10.1038/jid.2009.144. [DOI] [PubMed] [Google Scholar]
- Klebanoff C.A. Gattinoni L. Torabi-Parizi P., et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J.N. Rosenberg S.A. Chimeric antigen receptor-modified T cells in CLL. N. Engl. J. Med. 2011;365:1937–1938. doi: 10.1056/NEJMc1111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J.N. Yu Z. Frasheri D., et al. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T. Thompson J. Diaz R.M., et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin. Cancer Res. 2009;15:561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A.S.Y. Ma Q. Liu D.L. Junghans R.P. Anti-GD3 chimeric sFv-CD28/T-cell receptor ζ designer T cells for treatment of metastatic melanoma and other neuroectodermal tumors. Clin. Cancer Res. 2010;16:2769–2780. doi: 10.1158/1078-0432.CCR-10-0043. [DOI] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk W.W. Theoret M.R. Finkelstein S.E., et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.M. Fisher D.E. Testimony from the bedside: from Coley's toxins to targeted immunotherapy. Cancer Cell. 2010;18:9–10. doi: 10.1016/j.ccr.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Paulos C.M. Wrzesinski C. Kaiser A., et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D.L. Levine B.L. Kalos M., et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouw N. Treffers-Westerlaken E. Kraan J., et al. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol. Immunother. 2010;59:921–931. doi: 10.1007/s00262-010-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J. Wang X. Kottke T., et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P.F. Morgan R.A. Feldman S.A., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. Cell transfer immunotherapy for metastatic solid cancer—what clinicians need to know. Nat. Rev. Clin. Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. Restifo N.P. Yang J.C., et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M.L. Al-Khami A.A. El-Naggar S.A., et al. Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells. J. Immunol. 2010;184:1737–1747. doi: 10.4049/jimmunol.0902309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni G. Sistigu A. Valentini M., et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–778. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- Shafer-Weaver K.A. Watkins S.K. Anderson M.J., et al. Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. Cancer Res. 2009;69:6256–6264. doi: 10.1158/0008-5472.CAN-08-4516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tran K.Q. Zhou J. Durflinger K.H., et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J. Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo D.D. Prins R.M. Begley J.L., et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-X. Li R. Yang G., et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.S. Atkins M. Hwu P., et al. Immunotherapy Task Force of the NCI Investigational Drug Steering Committee. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin. Cancer Res. 2011;17:1664–1673. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- Willmon C. Diaz R.M. Wongthida P., et al. Vesicular stomatitis virus-induced immune suppressor cells generate antagonism between intratumoral oncolytic virus and cyclophosphamide. Mol. Ther. 2011;19:140–149. doi: 10.1038/mt.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongthida P. Diaz R.M. Pulido C., et al. Activating systemic T-cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus. Hum. Gene Ther. 2011;22:1343–1353. doi: 10.1089/hum.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C. Adoptive therapy using antigen-specific T-cell clones. Cancer J. 2010;16:367–373. doi: 10.1097/PPO.0b013e3181eacba8. [DOI] [PubMed] [Google Scholar]
- Yee C. Thompson J.A. Byrd D.R., et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh H.J., 3rd Perry-Lalley D. Dudley M.E., et al. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- Zhao J. Cao Y. Lei Z., et al. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 2010;70:4850–4858. doi: 10.1158/0008-5472.CAN-10-0283. [DOI] [PubMed] [Google Scholar]