Abstract
Individuals with a history of non-specific low back pain (LBP) while in a quiescent pain period demonstrate altered automatic postural responses (APRs) characterized by reduced trunk torque contributions and increased co-activation of trunk musculature. However it is unknown whether these changes preceded or resulted from pain. To further delineate the relationship between cyclic pain recurrence and APRs, we quantified postural responses following multi-directional support surface translations, in individuals with non-specific LBP, following an active pain episode. Sixteen subjects with and 16 without LBP stood on two force plates that were translated unexpectedly in 12 directions. Net joint torques of the ankles, knees (sagittal only), hips and trunk, in the frontal and sagittal planes were quantified and activation of 12 muscles of the lower limb unilaterally and the dorsal and ventral trunk, bilaterally, were recorded using surface electromyography (EMG). Peaks and latencies to peak joint torques, rates of torque development (slopes) and integrated EMGs characterizing baseline and active muscle contributions were analysed for group by perturbation direction (torques) and group by perturbation by epoch interaction (EMG) effects. In general, the LBP cohort demonstrated APRs that were of similar torque magnitude and rate but peaked earlier compared to individuals without LBP. Individuals with LBP also demonstrated increased muscle activity following perturbation directions in which the muscle was acting as a prime mover and reduced muscle activity in opposing directions, proximally and distally, with some proximal asymmetries. These altered postural responses may reflect increased muscle spindle sensitivity. Given that these motor alterations are demonstrated proximally and distally, they likely reflect the influence of central nervous system processing in this cohort.
Keywords: postural control, automatic postural response, joint torque, surface electromyography, ankle strategy, hip strategy
Introduction
Despite the prevalence of low back pain (LBP) in our society, there is still no consensus on the etiology of the most common type of LBP, non-specific LBP (Von Korff 1994). Although individuals with non-specific LBP demonstrate altered movement patterns (Al-Obaidi et al. 2003; Henry et al. 2006; Lamoth et al. 2006; MacDonald et al. 2010), it is unknown whether these patterns developed prior to or subsequent to their first bout of pain. However, it is clear that patients with LBP exhibit altered movement patterns between recurrent episodes (Henry et al. 2006; MacDonald et al. 2010) and these altered patterns may put patients at risk for future exacerbations of LBP. Thus, an important step in clarifying the effects of LBP on motor coordination, in particular automatic postural control, is to determine if patients who are experiencing active pain episodes exert different movement patterns than those that persist between pain episodes.
Individuals with non-specific LBP in a quiescent period demonstrate altered anticipatory and automatic postural coordination. Following predictable and unpredictable trunk loading, people with LBP demonstrated earlier onsets of or reduced lumbar muscle activation (MacDonald et al. 2010), and following unexpected, multi-directional translation perturbations they demonstrated reduced trunk torques and co-activation of the trunk musculature (Henry et al. 2006; Jones et al. 2012). These alterations are notable as they likely represent detrimental impairments in motor coordination that persist between recurrent episodes of LBP. Postural responses altered in this manner may have consequences for stability for this population. Indeed, during a quiescent period, individuals with LBP also demonstrated reduced center of pressure and increased center of mass responses in the sagittal plane which results in a reduced margin of stability [center of pressure – center of mass; (Henry et al. 2006)]. This measure is thought to represent postural efficacy (Corriveau et al. 2001) and could indicate that individuals with LBP have a reduced driving force with which to respond to unexpected perturbations, even during a quiescent period of their pain.
During an active episode, individuals with LBP also demonstrated inadequate or inappropriate muscle activation (co-activation) in response to unexpected perturbation (Radebold et al. 2000; Stokes et al. 2006). These altered responses, observed during exacerbation of pain may be localized to the site of pain and influenced through segmental neural mechanisms, or the responses may be influenced via cognitive mechanisms due either to stress (Moseley et al. 2003) or the anticipation of pain (Moseley et al. 2004) through descending pathways. Previous studies reported only trunk muscle activation patterns in response to unexpected perturbation, thus limiting our ability to determine whether these responses are segmentally and/or centrally-mediated. Thus it is important that we characterize not only the differences in postural responses at different points in the recurrent pain cycle but also at sites both local to the site of pain and distal to it. Given that automatic postural responses (APRs) are not initiated by the motor cortex, peripheral and descending influences on postural coordination can be examined whilst minimizing the behavioural effects related to pain that could accompany volitional movements.
Therefore, the purpose of this study was to characterize the movement patterns of individuals with and without LBP, by comparing their APRs elicited by multi-directional support surface translations. To characterize their APRs, we computed net joint torques at the trunk, hips, knees (sagittal only) and ankles in the sagittal and frontal planes, as well as the myoelectric responses of the lower limb and trunk. We have previously reported that individuals with LBP during a quiescent period demonstrated a decreased trunk torque contribution and increased co-activation of trunk musculature elicited by unexpected support surface translations (Jacobs et al. 2011; Jones et al. 2012), likely reflecting an attempt to stiffen the trunk. Thus, for people with an exacerbation of LBP (i.e., during an active episode, ALBP), for which they were seeking treatment, we hypothesized these individuals, compared to individuals with no history of LBP, would adopt a similar strategy of stiffening at the trunk. Stiffening in this manner would appear as an effective reduction of trunk torque resulting from the greater equivalence of opposing joint torques (e.g., flexion vs. extension torques).Thus, we also hypothesized individuals with ALBP would reduce the amount of trunk torque used to respond to unexpected balance perturbations, given the potential inability or reluctance of these individuals to move and/or create force at the trunk (McGill 2002).
Methods
Subjects
All subjects were between the ages of 21-55 years and were currently employed at the time of testing or participating fully in their usual role (e.g. full-time students, homemaker). Subjects with non-specific LBP (ALBP) were recruited from local physical therapy clinics after an initial evaluation determined that they met the inclusion/exclusion criteria. ALBP subjects were included if they were experiencing an exacerbation of their LBP symptoms severe enough for them to seek treatment. Exclusion criteria for subjects with ALBP were pain below the knee consistent with a disc herniation, presence of any neurological signs, serious spinal complications (e.g. vertebral fracture, tumour or infection), spinal stenosis, previous spinal surgery, systemic infection, balance or cardiovascular disorders, current pregnancy, history of any surgery in the 3 months prior to testing, uncorrected vision problems, a severe musculoskeletal deformity (scoliosis or kyphosis) or injury to the lower extremity that would interfere with testing. Subjects were also excluded if they were receiving worker’s or disability compensation for their LBP, or had ongoing litigation because of their LBP problem. At the time of determination for inclusion/exclusion, subjects were in a recurrence of their LBP and seeking treatment for their pain.
Subjects with no history of LBP (NLBP) were recruited from the local community through posted advertisements and word of mouth. NLBP subjects were excluded if they had a neurological disease or balance disorder, uncorrected vision problems, cardiovascular disorders, severe musculoskeletal injuries, history of recurrent and/or chronic LBP or any back pain during the prior 12 months that required medical attention or resulted in missed work.
All subjects signed an informed consent document in accordance with University of Vermont Institutional Review Board policy and the rights of the subjects were protected.
Equipment
Two force plates (AMTI, Watertown, MA, USA (sampled at 1000 Hz with a 1050 Hz low pass, hardware filter) were mounted within the moveable platform that was driven by electromechanical motors (Compumotor, Parker Hannifin Corp., Rohnert Park, CA, USA). A 3-camera, passive marker system (BTS, Milan, Italy) was used to collect 3-dimensional body kinematic data (sampled at 50 Hz, dual pass, 2nd order, Butterworth low pass filter with cut-off frequencies ranging from 2-5 Hz). Surface electromyographs (EMG) of trunk and lower limb muscles were collected using silver-silver chloride surface electrodes (Norotrode 20 bipolar, fixed 2 cm inter-electrode distance, Myotronics, Kent, WA, USA). EMG signals were sampled at 1000 Hz in synchrony with the force plate signals, amplified (2000–10000x), band-pass filtered from 35-200 Hz and full-wave rectified.
Procedures
After signing the consent form, subjects completed the Numeric Pain Rating Scale (NPRS; (Stratford and Spadoni 2001)), McGill Pain Questionnaire (Melzack 1987) and the Modified Oswestry Disability Index (ALBP only; (Fritz and Irrgang 2001)) questionnaires prior to postural testing. To affix the EMG electrodes, the subject’s skin was shaved and cleaned with alcohol prior to electrode placement. Surface electrodes were then placed over the Tibialis Anterior (TA) over the muscle belly approximately 2.5 cm lateral to the tibia, Medial Gastrocnemius (GA) over the most prominent part of the muscle belly oriented rostral-caudal, Rectus Abdominus (LRA and RRA corresponding to left and right RA, respectively) 3 cm lateral to the umbilicus oriented rostral-caudal, Internal Oblique (LIO, RIO) 2.5 cm medial and rostral to the anterior-superior iliac spine, rotated 45° toward the midline , External Oblique (LEO, REO) equidistant between the iliac crest and the lower ribs along the mid-axillary line, rotated posteriorly 45°, Erector Spinae at the 3rd lumbar segment (LES3, RES3), and 1st lumbar segment (LES1, RES1) 3 cm lateral to the lumbar segments oriented rostral-caudal.
Next subjects were instructed to stand barefoot, a foot on each of two force plates, at self-selected stance width and toe-out angle with arms hanging comfortably at their sides while looking forward. Subjects were given 3 practice trials in two translation directions (forward and leftward), prior to which they were told the direction of impending platform movement. Following practice trials, linear translations of the support surface in the transverse plane were randomly presented in 12 directions of 30° increments (Figure 1) with four trials in each direction (velocity 43 cm/s; peak acceleration 127 cm/s2). Any trials in which the subjects stepped were discarded and repeated randomly at a later point in the protocol.
Fig. 1.
Experimental setup for support surface translations. Panel a depicts the kinematic marker setup, black dots represent reflective marker placement and the grey bars represent two force plates. Panel b depicts a displacement (cm) vs. time (ms) trace for platform movement during a rightward (0°) perturbation, as determined by the force plate kinematic markers. Panel c depicts the directions of platform perturbations with the induced body sway resulting from perturbations in the cardinal directions (i.e. left, forward, right and backward perturbations). Schematic stick figures are depicted with the subject facing to the right for the sagittal plane views and are viewed from the back for the frontal plane views.
Data processing
A detailed description of the methods used in this study have been published previously (Jones et al. 2008). Using the kinematic data an eight-segment, rigid link model was constructed for each subject (Zatsiorsky and Seluyanov 1983), representing the feet, shanks and thighs bilaterally as well as the pelvis and trunk segments. Force data were filtered using a 4th order Butterworth low pass filter, at a cut-off frequency of 10 Hz and downsampled to 50 Hz to match the sampling frequency of the kinematic data. Kinematics and force data were used to compute ankle, knee (sagittal only), hip and trunk (relative to pelvis) net joint torques in the frontal and sagittal planes (SD/Fast, Needham, MA, USA) through inverse dynamics techniques.
For each joint we computed (1) peak torques (relative to baseline prior to the perturbation), (2) rates of torque development (slopes) and (3) latencies to peak torques relative to perturbation onset using custom software (Matlab, Natick, MA, USA) to quantify the active torque responses. A single peak was measured for all torques except at the trunk, in both the sagittal and frontal planes, which consistently demonstrated a biphasic response within the time course of the other joint torque peaks; these two peaks were delineated, early and late based on their sequence. Rate of torque development was measured by the slope of the line of best fit from the deflection prior to peak torque (e.g., if the peak torque was a maximum, the slope was calculated from the minimum that occurred between onset of the perturbation and the peak) to the peak. All torque values were normalized to height (m) and weight (N). The absolute values of the peak torques were computed to analyze whether the torque magnitude varied with perturbation direction, regardless of whether the torque polarity was negative or positive. For sagittal plane torques, statistical comparisons of the peaks, rates and latencies to peak torques were computed for perturbation directions of 60 , 90 , 120 , 240 , 270 and 300 and for frontal plane torques, perturbation directions of 0 , 30 , 150 , 180 , 210 and 330 were used (see Figure 1c for a definition of perturbation directions).
EMG integrals were computed for each of 12 muscles at each of 12 perturbation directions across four epochs: baseline (−250 to −50 ms relative to perturbation onset) and three 75 ms epochs spanning from 25-250ms following perturbation onset. Each epoch was divided by its duration (baseline 200 ms, other epochs 75 ms) to obtain an average value for each epoch. These averages were normalized by dividing by the maximum value for each muscle (determined across all 12 perturbation directions and each of the 4 epochs), for each subject, individually; thus, the direction and epoch with the largest value, for a given subject, was set equal to 100% and the averages for all other directions and epochs were scaled accordingly. Although raw magnitude differences could not be compared in this manner between groups, the spatial patterns of each muscle could be discerned as well as the relative contribution of the muscle for a given direction and epoch.
Data analysis
Descriptive statistics were computed for all measures. Demographic measures (age, height, weight, BMI, stance width, foot angle) were compared between groups using independent samples T-tests. Pain measures were compared using Wilcoxon Ranked Sum Tests. The joint torque responses to the surface translation perturbations were analyzed using a mixed model repeated measures analysis of variance for each joint torque, with subject as the random effect, perturbation direction as the repeated factor (across 6 directions) and ALBP vs. NLBP as the grouping factor (SPSS Inc., Chicago, Il, USA; SAS Institute, Inc., Cary, NC, USA). EMG integrals were analyzed using a repeated measures analysis of variance for each muscle, with subject as the random effect, epoch (across 4 epochs) and perturbation direction (across 12 directions) as the repeated factors and ALBP vs. NLBP as the grouping factor (SAS Institute, Inc., Cary, NC, USA). For each significant interaction found, post hoc tests were performed, including tests of simple effects and pairwise comparisons. An alpha level of P<0.05 was considered statistically significant for main effect comparisons.
Results
Overall, individuals with ALBP demonstrated active joint torque responses at proximal and distal joints that were similar in magnitude and rate of development (slope) but peaked earlier than individuals without LBP. Individuals with ALBP generally demonstrated altered muscle activity distally at the lower limbs and proximally at the trunk. The subjects’ trunk muscle activity was dependent upon direction of perturbation, with increased activation in directions where the muscles act as prime movers and reduced activation in directions where muscles may act as stabilizers.
Subjects
The 16 ALBP (8 female) and 16 NLBP (8 female) groups did not differ on measures of age, height, weight, body mass index (BMI), stance width or toe-out angle (0.193<P<0.892, Table 1). Individuals with ALBP demonstrated significantly higher pain ratings (NPRS, P<0.001; McGill Number of Words Score, P<0.001, Table 1) than the NLBP cohort. Most individuals with ALBP reported bilateral pain (13 of 16), with two individuals reporting unilateral pain (1 left, 1 right) and one who reported no pain at the time of testing. All individuals in the ALBP group did not have specific diagnoses and had experienced pain for 8.4 years on average (range: 1.5-25 yrs). In addition, this ALBP cohort demonstrated significantly higher pain ratings (NPRS, mean ± S.D., 3.5±1.9; P=0.008) than our previously studied cohort (NPRS, 1.9±1.4) who were in a quiescent period of their pain (Jacobs et al. 2011; Jones et al. 2012).
Table 1.
Subject demographic information
| Parameter [Mean ( SD)] | aLBP (n = 16) | NLBP (n = 16) | P-value |
|---|---|---|---|
| Age (years) | 33.9 (6.2) | 33.5 (9.0) | 0.892 |
| Height (m) | 1.75 (0.09) | 1.71 (0.07) | 0.278 |
| Weight (kg) | 74.0 (8.9) | 68.9 (12.3) | 0.193 |
| BMI (kg/m2) | 24.3 (2.6) | 23.4 (3.2) | 0.382 |
| Gender (# Male/ # Female) | 8 /8 | 8 / 8 | N/A |
| Stance Width (cm) | 21.9 (3.0) | 22.2 (5.3) | 0.849 |
| Toe-out Angle (°) | 105.0 (11.1) | 102.8 (5.2) | 0.473 |
| McGill Pain Questionnaire* …(# of Words Score) |
5 (2-11) | 0 (0-3) | <0.001 |
| Numeric Pain Rating Scale*(/10) | 3 (0-7) | 0(0-1) | <0.001 |
| Oswestry Disability Index (/100) | 19.8 (8.8) | N/A | N/A |
| Duration of Symptoms (yrs) | 8.4 (7.5) | N/A | N/A |
Median (Range) values are provided.
An active episode of LBP shortens the latencies of joint torque responses
Peak torques
Individuals with and without ALBP modulated their peak joint torque responses in the sagittal and frontal planes in accordance with perturbation direction (Direction: Trunk Extension-Flexion P=0.043, all other joints, P<0.001). Compared to the NLBP group, the ALBP group demonstrated no differences in peak torque magnitude in either the frontal or sagittal planes (0.250≤P≤0.957 for group main effects). Only one group by direction effect was significant, namely Trunk Left-Right Side Bending torque (P=0.041), although post hoc analysis detected only a trend to reduced torque by the ALBP group following 330 perturbations (post hoc group effect, P=0.078).
Rate of Torque Development
The rate of torque development varied with perturbation direction in both groups (Direction: all joints, P<0.0001; Late Trunk Side Bending torque, P=0.044; with the exception of the Late Trunk Flexion-Extension torque, P=0.46). The groups did not differ in their rates of torque development in either the frontal or sagittal planes (P values ranged from 0.22 to 0.98).Only one group by direction effect was significant, namely right Knee Flexion-Extension (P=0.022); post hoc analysis detected that the ALBP had a significantly greater rate of torque development for the 120° perturbation direction.
Peak torque latencies
Both groups demonstrated latencies to peak torque that varied with perturbation direction in both groups (Direction: 0.001<P=0.023, with the exception of right Hip Extension-Flexion, P=0.593). Despite the lack of torque magnitude differences between the two groups, temporal differences were significant between the ALBP and NLBP groups. The ALBP group demonstrated earlier sagittal peak joint torques at the ankles (Group: left, P=0.042; right, P=0.060), knees (Group: left, P=0.012; right, P=0.003), hips (Group: left, P=0.033; right, P=0.037) and for the initial trunk response (Group: P=0.003) but not the later trunk response (Group: P=0.385) (Figure 2).
Fig. 2.
Peak joint torque latencies in the sagittal plane for trunk (early and late responses), and left and right hips, knees and ankles. Polar plots depict group means comparing individuals with LBP (ALBP; black circles) to those without LBP (NLBP; grey circles), with the radial axis reporting latency in ms following perturbation onset. Significant group effects (P < 0.050) are denoted by #.
The two groups also differed on the timing of peak torque in the frontal plane; subjects with ALBP demonstrated earlier torque peaks at the ankle (Group: left, P=0.058; right, P=0.020) but not at the hips (Figure 3). Additionally, the ALBP group demonstrated earlier initial and later trunk side-bending peak torques following left and rightward perturbations that had either a forward or backward component. Specifically, the ALBP group, compared to the NLBP group, demonstrated earlier latencies for the Early Trunk Left-Right Side Bending torques for 30 and 330 perturbation directions (Group X Direction: P=0.032) and for Late Trunk Left-Right Side Bending torques at 30 , 150 , 210 and 330 perturbation directions (Group X Direction: P<0.001).
Fig. 3.
Peak joint torque latencies in the frontal plane for trunk (early and late responses), and left and right hips and ankles. Polar plots depict group means comparing individuals with LBP (ALBP; black circles) to those without LBP (NLBP; grey circles), with the radial axis reporting latency in ms following perturbation onset. Significant group effects (P < 0.020) are denoted by #, while significant group by direction interactions (P < 0.040) are denoted by &. Post hoc group differences are denoted by * at the directions that achieved significance.
An active episode of LBP selectively alters muscle activation responses
Distal Muscles
Significant group by epoch by direction interactions were found for the TA (P=0.0002) and GA (P<0.0001) muscles. Post hoc testing revealed that individuals with NLBP increased TA activation following perturbation directions in which a given muscle would act as a prime mover, namely perturbation directions with a forward component. Specifically the ALBP group had greater TA activation during the 25-100 ms epoch for 30° and 60° perturbations (P=0.0064 and 0.0060, respectively) and during the 100-175 ms epoch for directions from 30°-120° (P ranged from <.0001 to 0.034) (Figure 4). For the dorsal muscle, GA, a similar pattern of prime mover activation emerged with the ALBP group demonstrating increased activity across backwards perturbation directions. Specifically the ALBP had greater GA activation during the 25-100 ms epoch for perturbation direction 300° (P=0.021) and during the 100-175 ms epoch for the 270°-330° directions (P=0.0022 for 270° and P<0.0001 for 300° and 330°) (Figure 4). However, the ALBP also demonstrated decreased activity primarily across forward or off-axis perturbation directions. The ALBP group had reduced activity of GA during the 25-100 ms epoch for 0° (P=0.010) and 90° (P=0.031) perturbation directions, during the 100-175 ms epoch for directions 90° (P=0.0061) and 150° (P=0.040) (Figure 4) and during the 175 to 250 ms epoch at 30°, 60°, 90°, 120°, 150°, 180° and 330° perturbation directions (P values ranged from <0.0001 to 0.026). Significant group by epoch (P<0.0001 for both muscles) and group by direction (P<0.0001 for both) interactions were found for both the TA and GA muscles (Figure 5), however these as well as main effects will not be interpreted due to the presence of the 3-way interaction.
Fig. 4.
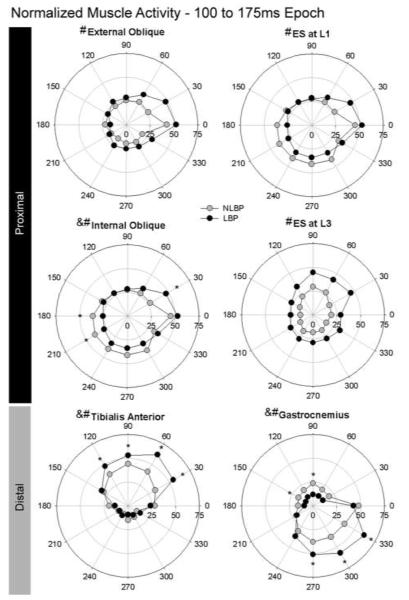
Average normalized muscle activities for the left proximal and distal muscles for the 100-175 ms epoch. Polar plots depict group means of the left External and Internal oblique muscles, left Erector Spinae at L1 and L3, Tibialis Anterior and Gastrocnemius muscles comparing individuals with LBP (ALBP; black circles) and those without LBP (NLBP; grey circles), with the radial axis reporting the % normalized muscle activity. Significant group by epoch by direction interactions (P < 0.050) are denoted by ampersand, significant group by epoch interactions are denoted by hash symbol, with post hoc group differences denoted by asterisks at the directions that reached significance.
Fig. 5.
Average normalized muscle activities for the left proximal and distal muscles averaged across direction depicting group by epoch interactions. Plots depict group means ± S.E. comparing individuals with LBP (ALBP; black circles) to those without LBP (NLBP; grey circles) across four epochs, Epoch 1 (baseline: 250 to 50 ms prior to perturbation), Epoch 2 (25 to 100 ms after perturbation), Epoch 3 (100 to 175 ms prior to perturbation), Epoch 4 (175 to 250 ms after perturbation). Significant group by epoch by direction interactions (P<0.050) are denoted by #. Significant post hoc group differences (P<0.050) are denoted by *.
Trunk Muscles
A significant group by epoch by direction interaction was found for the LIO (P=0.0082) but was not found for any other trunk muscles. Post hoc testing revealed that individuals with ALBP had reduced activation of the LIO in directions with either a leftward or leftward/backward component across the 25-100 ms (90°, 210°, 240°; P values were 0.032, 0.047 and 0.045, respectively) and 100-175 ms (180°, 210°; P values were 0.024 and 0.017, respectively) epochs, and increased LIO activation during the 100-175 ms epoch in 30° perturbations (P<0.0001), a direction in which the LIO could contribute to a hip/trunk strategy. All trunk muscles demonstrated significant group by epoch effects (P-values ranged from <0.0001 to 0.027; Figure 5), although for RRA this did not reach significance (P=0.079). Post hoc tests revealed that the ALBP group had greater activation of the LEO during the 100-175 ms epoch (P=0.019) and the LES3 during the epochs that spanned 25-175 ms post-perturbation (P=0.030 and P=0.0062) and reduced activation of the REO during the baseline epoch (P=0.044) than did the NLBP cohort. For all muscles, the ALBP group demonstrated a greater rate of increase of muscle activity across epochs, particularly between the 25-100 and 100-175 ms epochs. Significant group by direction interactions were present for the Left and Right RA (P=0.023 and 0.027, respectively), the LEO (P=0.047) and the LIO (P=0.008) muscles but not for the REO (P=0.025) or RIO (P=0.75) or any of the erector spinae muscles (P=0.26 to 0.59) (Figure 6). For the LRA, the ALBP and NLBP groups did not differ at any direction, however, the ALBP group demonstrated increased activation in rightward compared to leftward perturbation directions (P values ranged from <0,0001 to 0.026). For the RRA, the ALBP group demonstrated reduced activity compared to the NLBP group across most directions (P values ranged from 0.004 to 0.048 but not significant at 30° and 60°, with a trend at 210°, P=0.059). The LEO activation levels did not differ between the ALBP and NLBP groups at any direction but the ALBP demonstrated more direction-specific differences than the NLBP. Specifically, the ALBP demonstrated more significant differences between the forward vs. lateral perturbations compared to all other directions (P values ranged from <0.0001 to 0.041), whereas the NLBP group demonstrated greater more significant differences between backwards perturbation directions and all other directions (P values ranged from 0.044). The interaction for the LIO will not be interpreted given the significant 3-way interaction for this muscle.
Fig. 6.
Average normalized muscle activities for the left (left panel) and right (right panel) trunk muscles for the 100-175 ms epoch, depicting side-specific group differences. Polar plots depict group means comparing individuals with LBP (ALBP; black circles) to those without LBP (NLBP; grey circles). Significant group by direction interactions (P < 0.050) are denoted by #. Post hoc group differences (P<0.050) are denoted by * at the directions that reached significance.
Discussion
In response to support surface translations, the ALBP group demonstrated joint torque responses that were of similar magnitude compared to the NLBP group. However, the ALBP group generated their peak torques earlier but at the same rate compared to the NLBP group. In general, individuals with ALBP used a greater proportion of their maximal muscle response activation, proximally and distally, in directions of muscle stretch (also directions in which muscle would act as a prime mover), although there were right/left specific differences in abdominal muscle activity between the groups.
Individuals with ALBP demonstrate temporal not spatial alterations in joint torque strategies
Individuals with ALBP demonstrated earlier peak torque responses across proximal and distal joints, regardless of the perturbation direction (Figures 2 and 3). These earlier peak torque response latencies could be explained either by earlier onsets of the torque responses or similar onsets but with an increased rate of torque development, thus reaching their peak along an earlier time course. Given that the ALBP and NLBP groups did not differ in their rates of torque development, it is likely that the ALBP group may demonstrate earlier onsets of corrective torque.
In general, individuals with LBP demonstrate increased muscle latencies, particularly at the trunk, demonstrating delays in both anticipatory (Hodges and Richardson 1996; Hodges 2001; Jacobs et al. 2009) and automatic postural responses (Magnusson et al. 1996; Radebold et al. 2000; Cholewicki et al. 2002). However, individuals with LBP during a quiescent period demonstrated earlier ES muscle onset latencies in response to predictable loading, specifically on the previously painful side (Moseley et al. 2004; MacDonald et al. 2010). Similarly, healthy individuals who were anticipating induced back pain demonstrated earlier latencies in the superficial abdominal muscles following predictable loading (Moseley et al. 2004). Earlier muscle onset latencies in response to postural perturbation could be due to enhanced muscle spindle sensitivity as a result of increased fusimotor drive. While changes of muscle spindle sensitvity have not been demonstrated with experimentally induced muscle or skin pain (Birznieks et al. 2008), increased fusimotor drive has been demonstrated in humans under conditions of increased arousal (Ribot-Ciscar et al. 2000). Given that earlier onset latencies have been demonstrated prior to predictable loading (MacDonald et al. 2010) and in anticipation of pain (Moseley et al. 2004), it is possible that the early peak torque latencies we have demonstrated could be related to increased arousal in our ALBP cohort, perhaps due to pain-related fear or fear of movement.
Individuals with high pain-related fear have demonstrated reduced performance on lifting tasks (Vlaeyen et al. 1995; Swinkels-Meewisse et al. 2006) and a leg strength test (Verbunt et al. 2005), restricted spinal motion (Thomas and France 2007), and reduced preferred and fast walking velocities compared to individuals with low fear (Al-Obaidi et al. 2003). In addition, individuals with LBP who had high pain-related fear also demonstrated an increased influence of anticipated pain on their movement patterns (Pfingsten et al. 2001; Al-Obaidi et al. 2003). These altered movement patterns may reflect changes in central set, defined as “a central preparatory state within the nervous system related to higher-level task-related intentions and expectations” that can impact both expected and unexpected postural responses (Cacciatore et al. 2005), acting to restrict either the magnitude or the speed of the movement. Indeed, healthy individuals who experienced a postural threat (quiet standing at the edge of a high surface) reported greater conscious control of posture that was related to an altered postural strategy (Huffman et al. 2009). Individuals with chronic LBP demonstrated decreased variability of trunk movements during walking while performing an attention-demanding task (Lamoth et al. 2008) and altered cerebrocortical activity during an arm raise task (Jacobs et al. 2010). In addition, altered motor unit recruitment, characterized by recruitment of different populations of motor units during an isometric strength task, has been demonstrated by healthy individuals in the anticipation of pain without nociceptive stiumulus (Tucker et al. 2012). These findings suggest a greater involvement of the higher brain centers on postural control in both volitional and anticipatory postural coordination by individuals who are experiencing either pain or the threat of pain. It is not known whether a similar reliance on the higher brain centers is present in individuals with LBP for automatic postural coordination. However, postural anxiety has been shown to increase the amplitude of corrective responses to unexpected surface rotation in healthy individuals, supporting that centrally controlled fear-related constructs can impact automatic postural coordination (Carpenter et al. 2004).
Enhanced APRs in the ALBP group may represent a short-term adaptation to an exacerbation of pain
The postural response strategies of the ALBP cohort elicited by unexpected support surface translations differed from those of individuals with chronic LBP who were in a quiescent period of their pain (Jacobs et al. 2011; Jones et al. 2012). Individuals in a quiescent period demonstrated reduced trunk torques, mediated through increased co-activation of the dorsal and ventral musculature about the trunk (Jones et al. 2012) whereas the current cohort, who had recently experienced an exacerbation of their pain, demonstrated no changes in peak torque magnitude or rate of torque development but shorter latencies to peak torque, with direction-specific enhanced muscle activation. The postural alterations in the current ALBP cohort are noteworthy because they are present at both proximal joints and distal joints remote from the site of pain, suggesting that these alterations may be centrally-mediated. These postural alterations may be related to the individuals’ recent pain exacerbation and could represent an adaptation either to increased pain or secondary to pain-related fear.
Interestingly, it appears that the altered distal muscle recruitment patterns (at the TA and GA) in the ALBP cohort, characterized by enhanced direction-specific muscle activation are virtually identical to those demonstrated by individuals with LBP who are in a quiescent period (Jones et al. 2012), despite differences in the trunk activation and torque patterns. It is plausible that there is a relationship between the motor alterations demonstrated by the ALBP cohort and the reduced trunk torques (indicative of trunk stiffening) that persist between pain episodes. Indeed, Hodges and Tucker (2011) suggests that adaptations in motor patterns linked to pain may persist and result in detrimental long-term changes that may be related to recurrence of LBP. For instance, it may be that the enhanced direction-specific muscle activity demonstrated by individuals in the current ALBP cohort, potentially related to anticipation or fear of pain, could give way to the alternate motor recruitment patterns, such as the enhanced baseline activation (Jacobs et al. 2011) and direction-independent trunk muscle activation, demonstrated by individuals with LBP in a quiescent period of pain (Jones et al. 2012).
Altered motor recruitment patterns that persist following bouts of anticipated pain, have been identified (Tucker et al. 2012) and suggest that even though the threat of pain has been removed, the motor strategies that were adopted in the face of expected pain can persist. Persistent patterns may have detrimental effects on the system, as suggested by Hodges (2011), who proposes a model in which changes in movement strategies, such as increasing trunk stiffness, while beneficial in the short term, may actually become harmful, in this instance due to a reduction in movement variability or increased load on the spine. However, rather than representing a short-term change, our results suggest that the pattern of APRs that persists between pain bouts may be that of increased trunk stiffness (Jones et al. 2012) with heightened proximal and distal responses temporally close to a pain episode.
In the future, tracking changes in the APRs before, during and after a pain exacerbation in the same cohort might provide insight into the long term consequences of postural responses that have been altered by a short term pain exacerbation. This information could be used to target appropriate interventions to mitigate LBP symptoms and recurrence over the long-term.
Side-specific muscular responses to unexpected perturbations
Group comparisons between the ALBP and NLBP cohorts demonstrated side-specific differences in trunk muscle activation patterns, such that group by direction differences were statistically significant on the left side with fewer differences detected on the right (Figure 6). These were not simply statistical similarities between groups but often asymmetric patterns of activation between the left and right muscles for both groups. Individuals with ALBP demonstrated reduced activity of the RRA, although the left and right patterns were similar. The LIO muscle followed the pattern of the distal muscles, with heightened activity by the ALBP cohort in directions of muscle stretch, with a more or less direction-independent response from the RIO in both groups.
Asymmetries in trunk muscle activation, either by magnitude or frequency of firing, have been reported previously, elicited by unexpected forward support surface translations (Newcomer et al. 2002), during volitional trunk bending movements (Larivière C. 2000) and during isometric trunk extension (Alexiev 1994). Lariviere et al. (2000) reported that these asymmetries were not related to anatomical or kinematic differences in trunk movement patterns between groups but could result from hand-dominance or muscle wasting in individuals that may be related to pain. It is not surprising that activation asymmetries exist, particularly given the heterogeneous nature of the chronic LBP population who may have pain locations and duration, severity and frequency of pain episodes that may vary widely. In the current ALBP cohort, all individuals, with the exception of two, reported bilateral or central pain locations, did not have specific diagnoses and had experienced pain for 8.4 years on average (range: 1.5-25 yrs).
The ALBP group could demonstrate different muscle activation patterns compared to the NLBP group because of the effects of pain on neuromuscular control, including reduced strength (Verbunt et al. 2005), peripheral feedback alterations (Brumagne et al. 2000; Van Dieen et al. 2003), tissue modifications (Gombatto et al. 2008) or may be the result of habitual movement patterns that have reinforced asymmetric muscle activation patterns. Although muscle activation patterns were not reported, Van Dillen et al. (2006) reported significantly more asymmetric movement impairments in individuals with LBP who routinely participated in asymmetric leisure activities (e.g. tennis, golf). Individuals with LBP who participated in rotation-related sports demonstrated more asymmetries of passive hip range of motion than individuals who participated in the same sports but did not have LBP (Gombatto et al. 2008). Gombatto (2008) reported asymmetries in passive tissue resistance in both individuals with and without a history of LBP, although the LBP cohort demonstrated greater asymmetries, despite no group differences in passive tissue extensibility. Thus, both asymmetric movement patterns and passive tissue characteristics been linked to repetitive movement patterns. Whether the muscle activation differences reported in the current study relate to underlying differences in repetitive movement patterns is unknown. Future attempts at classifying individuals with chronic LBP to provide more homogeneous subgroups (Van Dillen et al. 2003) may be beneficial in understanding whether asymmetrical muscle activation patterns elicited by unexpected postural perturbations reflect altered, habitual movement patterns that are related to pain.
Conclusions
Individuals with LBP who experienced an exacerbation of their pain demonstrated altered APRs to unexpected support surface translations. The altered APRs were characterized by shorter peak torque latencies observed at proximal and distal joints, and muscle activation levels that were increased in directions that would result in sway-induced muscle stretch. Our previous work showed that during a quiescent period, individuals with a history of LBP demonstrate alterations only at the trunk. Thus, the altered APRs demonstrated by individuals with LBP in an exacerbation may represent a short-term, pain-related adaptation, which, if left unresolved may contribute to LBP symptoms and future recurrent pain episodes.
Acknowledgments
The authors wish to thank Janice Y. Bunn for her assistance with statistical analysis and Kerry A. McCarthy, Tori Deschanneaux and Natalie Moore for their assistance in data reduction. This work was funded by grants awarded to SMH by the University of Vermont College of Nursing and Health Sciences Dean’s Research Incentive Award, and the National Institutes of Health/National Center for Medical Rehabilitation Research/R01 HD04099.
References
- Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, Al-Zaabie N, Nelson RM. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res. 2003;26:101–108. doi: 10.1097/00004356-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Alexiev AR. Some differences of the electromyographic erector spinae activity between normal subjects and low back pain patients during the generation of isometric axial trunk torque. Electromyogr Clinical Neurophysiology. 1994;34:1–5. [PubMed] [Google Scholar]
- Birznieks I, Burton AR, Macefield VG. The effects of experimental muscle and skin pain on the static stretch sensitivity of human muscle spindles in relaxed leg muscles. J Physiol. 2008;586:2713–2723. doi: 10.1113/jphysiol.2008.151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- Cacciatore TW, Horak FB, Henry SM. Improvement in automatic postural coordination following alexander technique lessons in a person with low back pain. Phys Ther. 2005;85:565–578. [PMC free article] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JH. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol. 2004;92:3255–3265. doi: 10.1152/jn.01139.2003. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Greene HS, Polzhofer GK, Galloway MT, Shah RA, Radebold A. Neuromuscular function in athletes following recovery from a recent acute low back injury. J Orthop Sports Phys Ther. 2002;32:568–575. doi: 10.2519/jospt.2002.32.11.568. [DOI] [PubMed] [Google Scholar]
- Corriveau H, Hebert R, Prince F, Raiche M. Postural control in the elderly: an analysis of test-retest and interrater reliability of the COP-COM variable. Arch Phys Med Rehabil. 2001;82:80–85. doi: 10.1053/apmr.2001.18678. [DOI] [PubMed] [Google Scholar]
- Fritz JM, Irrgang JJ. A comparison of a modified oswestry low back pain disability questionnaire and the quebec back pain disability scale. Phys Ther. 2001;81:776–788. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- Gombatto SP, Norton BJ, Scholtes SA, Van Dillen LR. Differences in symmetry of lumbar region passive tissue characteristics between people with and people without low back pain. Clin Biomech. 2008;23:986–995. doi: 10.1016/j.clinbiomech.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SM, Hitt JR, Jones SL, Bunn JY. Decreased limits of stability in response to postural perturbations in subjects with low back pain. Clin Biomech. 2006;21:881–892. doi: 10.1016/j.clinbiomech.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Hodges P. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain. Exp Brain Res. 2001;141:261–266. doi: 10.1007/s002210100873. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient Muscular Stabilization of the Lumbar Spine Associated With Low Back Pain: A Motor Control Evaluation of Transversus Abdominis. Spine. 1996;21(22):2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152:S90–98. doi: 10.1016/j.pain.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Huffman JL, Horslen BC, Carpenter MG, Adkin AL. Does increased postural threat lead to more conscious control of posture? Gait Posture. 2009;30:528–532. doi: 10.1016/j.gaitpost.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Jones SL, Hitt JR, Bunn JY. A history of low back pain associates with altered electromyographic activation patterns in response to perturbations of standing balance. J Neurophysiol. 2011;106:2506–2514. doi: 10.1152/jn.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Nagle KJ. People with chronic low back pain exhibit decreased variability in the timing of their anticipatory postural adjustments. Behav Neurosci. 2009;123:455–458. doi: 10.1037/a0014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Nagle KJ. Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clin Neurophysiol. 2010;121:431–440. doi: 10.1016/j.clinph.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Henry SM, Raasch CC, Hitt JR, Bunn JY. Responses to multi-directional surface translations involve redistribution of proximal versus distal strategies to maintain upright posture. Exp Brain Res. 2008;187:407–417. doi: 10.1007/s00221-008-1312-1. [DOI] [PubMed] [Google Scholar]
- Jones SL, Henry SM, Raasch CC, Hitt JR, Bunn JY. Individuals with non-specific low back pain use a trunk stiffening strategy to maintain upright posture. J Electromyogr Kinesiol. 2012;22:13–20. doi: 10.1016/j.jelekin.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth CJ, Meijer OG, Daffertshofer A, Wuisman PI, Beek PJ. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: changes in motor control. Eur Spine J. 2006;15:23–40. doi: 10.1007/s00586-004-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth CJ, Stins JF, Pont M, Kerckhoff F, Beek PJ. Effects of attention on the control of locomotion in individuals with chronic low back pain. J Neuroeng Rehabil. 2008;5:13. doi: 10.1186/1743-0003-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larivière CGD, Loisel P. The comparison of trunk muscles EMG activation between between subjects with and without chronic low back pain during flexion-extension and lateral bending. J Electromyogr Kinesiol. 2000;10:79–91. doi: 10.1016/s1050-6411(99)00027-9. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Moseley GL, Hodges PW. People with recurrent low back pain respond differently to trunk loading despite remission from symptoms. Spine. 2010;35:818–824. doi: 10.1097/BRS.0b013e3181bc98f1. [DOI] [PubMed] [Google Scholar]
- Magnusson ML, Aleksiev A, Wilder DG, et al. Unexpected load and asymmetric posture as etiologic factors in low back pain. Eur Spine J. 1996;5(1):23–35. doi: 10.1007/BF00307824. [DOI] [PubMed] [Google Scholar]
- McGill SM. Human Kinetics. Champaign, IL: 2002. Low Back Disorders: evidenced-based prevention and rehabilitation. [Google Scholar]
- Melzack R. The short form of the McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Nicholas MK, Hodges PW. Pain differs from non-painful attention demanding or stressful tasks in its effect on postural control patterns of trunk muscles. Exp Brain Res. 2003;156:64–71. doi: 10.1007/s00221-003-1766-0. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Nicholas MK, Hodges PW. Does anticipation of back pain predispose to back trouble? Brain. 2004;127:2339–2347. doi: 10.1093/brain/awh248. [DOI] [PubMed] [Google Scholar]
- Newcomer KL, Jacobson TD, Gabiel DD, Larson DR, Brey RH, An K. Muscles activation patterns in subject with and without low back pain. Arch Phys Med Rehabil. 2002;83:816–821. doi: 10.1053/apmr.2002.32826. [DOI] [PubMed] [Google Scholar]
- Pfingsten M, Leibing E, Harter W, Kroner-Herwig B, Hempel D, Kronshage U, Hildebrandt J. Fear-avoidance behavior and anticipation of pain in patients with chronic low back pain: a randomized controlled study. Pain Med. 2001;2:259–266. doi: 10.1046/j.1526-4637.2001.01044.x. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25(8):947–954. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Rossi-Durand C, Roll JP. Increased muscle spindle sensitivity to movement during reinforcement manoeuvres in relaxed human subjects. J Physiol. 2000;523(Pt 1):271–282. doi: 10.1111/j.1469-7793.2000.t01-1-00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes IA, Fox JR, Henry SM. Trunk muscular activation patterns and responses to transient force perturbation in persons with self-reported low back pain. Eur Spine J. 2006;15:658–667. doi: 10.1007/s00586-005-0893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford PW, Spadoni G. The reliability, consistency and clinical application of a numerical pain rating scale. Physiotherapy Canada. 2001;53:88–91. [Google Scholar]
- Swinkels-Meewisse IE, Roelofs J, Oostendorp RA, Verbeek AL, Vlaeyen JW. Acute low back pain: pain-related fear and pain catastrophizing influence physical performance and perceived disability. Pain. 2006;120:36–43. doi: 10.1016/j.pain.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Thomas JS, France CR. Pain-related fear is associated with avoidance of spinal motion during recovery from low back pain. Spine. 2007;32:E460–466. doi: 10.1097/BRS.0b013e3180bc1f7b. [DOI] [PubMed] [Google Scholar]
- Tucker K, Larsson AK, Oknelid S, Hodges P. Similar alteration of motor unit recruitment strategies during the anticipation and experience of pain. Pain. 2012;153:636–643. doi: 10.1016/j.pain.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Van Dieen JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13:333–351. doi: 10.1016/s1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Caldwell CA, McDonnell MK, Bloom N, Norton BJ. Trunk rotation-related impairments in people with low back pain who participated in 2 different types of leisure activities: a secondary analysis. J Orthop Sports Phys Ther. 2006;36:58–71. doi: 10.2519/jospt.2006.36.2.58. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. J Orthop Sports Phys Ther. 2003;33:126–142. doi: 10.2519/jospt.2003.33.3.126. [DOI] [PubMed] [Google Scholar]
- Verbunt JA, Seelen HA, Vlaeyen JW, Bousema EJ, van der Heijden GJ, Heuts PH, Knottnerus JA. Pain-related factors contributing to muscle inhibition in patients with chronic low back pain: an experimental investigation based on superimposed electrical stimulation. Clin J Pain. 2005;21:232–240. doi: 10.1097/00002508-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- Von Korff M. Studying the natural history of back pain. Spine. 1994;19:2041S–2046S. doi: 10.1097/00007632-199409151-00005. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Seluyanov VN. The mass and inertia characteristics of the main segments of the human body. In: Matsui H, Kabayashi K, editors. Biomechanics VIII-B. Human Kinetics. Champaign, IL: 1983. pp. 1152–1159. [Google Scholar]