Abstract
Background
Human twin studies have shown that certain responses to alcohol, including subjective perceptions, are genetically influenced. Previous studies have provided evidence that a low level of response to alcohol predicts future alcohol use disorders in humans. Recent genetic studies suggest an association between alcohol dependence and genetic variation in the γ-aminobutyric acid A (GABAA) receptor α2 subunit gene (GABRA2). Based on a haplotypic association of alcohol dependence with GABRA2, we investigated whether GABRA2 alleles are associated with the subjective responses to clamped alcohol concentration.
Methods
One hundred and ten healthy social drinkers (53 men) underwent the alcohol clamp. Fifteen minutes after the start of an intravenous infusion of alcohol, the breath alcohol concentration was clamped at a target of 50 ± 5 mg/dl for 165 minutes. Subjective physiologic responses to alcohol and stimulant and sedative effects of alcohol were measured repeatedly during the alcohol clamp. Because aldehyde dehydrogenase 2 (ALDH2) has been shown to have a great impact on the subjective responses to alcohol, we divided subjects by ALDH2 genotype for further analyses. To examine the role of genetic variation in GABRA2, 7 single nucleotide polymorphisms (SNPs) that were informative in association studies were included as factors in the analysis.
Results
Among these 7 SNPs, 3 SNPs (rs279869, rs279858, and rs279837) located in the middle of the GABRA2 gene showed significant associations with subjective effects of alcohol. Subjects with 1 or 2 copies of the more common allele showed greater subjective responses to alcohol than did individuals homozygous for the alcohol dependence–associated allele regardless of ALDH2 genotype.
Conclusions
These findings confirm and extend the observation that the GABRA2 alleles affect the subjective responses to alcohol, and suggest that the genetic variations in GABRA2 might play a role in the risk of alcohol use disorders by moderating the subjective effects of alcohol.
Keywords: GABA, GABRA2, Alcohol, Subjective Response, Alcohol Clamping, ALDH2
Alcohol use disorder is a common and complex disorder with a well-documented highly hereditary nature (Higuchi et al., 2006; Roh et al., 2008). Subjective response to alcohol is also known to be a genetically influenced characteristic (Schuckit et al., 2007; Viken et al., 2003). This suggests that genetic influences on individual variation in subjective response to alcohol may underlie the effects of genes on alcohol-related disorders. A low level of response to the acute effects of alcohol has been associated with an increased risk of both excessive alcohol intake (Hinckers et al., 2006; Schuckit et al., 2007) and alcohol dependence (Schuckit, 1994; Schuckit and Smith, 1996; Schuckit et al., 2004), which are well known to be highly heritable (Kendler, 2001).
There is consistent evidence that the GABAA receptor regulates the alcohol self-administration in animal models, probably by stimulating reward circuitry in the mesolimbic system (Chester and Cunningham, 2002; Eiler and June, 2007; Harvey et al., 2002; June et al., 2003). Several GABAA receptor subunits have been implicated in alcohol effects, so that the specific subunit composition of the receptor may be an important determinant of alcohol’s CNS effects. The α2 subunit of the GABAA receptor mediates the anxiolytic effects of benzodiazepines (Low et al., 2000; Rudolph et al., 1999) and enhances the hypnotic, but not the sedative, effects of combined exposure to alcohol and benzodiazepines (Tauber et al., 2003). Two genome-wide scans in humans have provided evidence of linkage of alcohol dependence to a region of chromosome 4p that includes a cluster of 4 genes encoding γ-aminobutyric acid A (GABAA) receptor subunits (Long et al., 1998; Reich et al., 1998). A previous study (Edenberg et al., 2004) found that 31 single nucleotide polymorphisms (SNPs) in GABAA receptor α2 subunit gene (GABRA2), but only 1 of the 20 SNPs in the flanking genes, showed significant association with alcohol dependence. Additional studies have provided the replication of this association in a region of GABRA2 among various ethnic groups (Covault et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005; Soyka et al., 2008); though, there is a negative association study between GABRA2 and alcohol dependence (Matthews et al., 2007). Previous studies have also provided evidence that GABAA receptors mediate several behavioral effects of alcohol such as ethanol self-administration and motor impairment (Davies, 2003; Grobin et al., 1998; Hanchar et al., 2005). GABRA2 gene was associated with the differences in the subjective effects of alcohol including blushing sensations, stimulant and sedative effects (Pierucci-Lagha et al., 2005), and the variance in drinking behavior (Bauer et al., 2007). In the former study (Pierucci-Lagha et al., 2005), the more common A allele of the rs279858 SNP within the GABRA2 gene showed greater subjective effects of alcohol than did individuals with 1 or 2 copies of the alcohol dependence–associated G allele. These findings underscore the potential contributions of variation at GABRA2 to the differences in the subjective responses to alcohol, the variance of drinking behavior, and the risk for alcohol dependence.
This study examined the moderating effects of GABRA2 alleles on subjective and physiologic effects of alcohol in healthy social drinkers. We used the alcohol clamp method for alcohol administration, which uses an intravenous infusion of alcohol at rates adjusted online to close the gap between measurements of breath alcohol concentration (BrAC) and the target concentration. The clamp method reduces experimental variance in BrAC (O’Connor et al., 1998) which can be caused by the substantial pharmacokinetic variability following oral alcohol administration. Therefore, the alcohol clamp used in this study is a more exhaustive objective measure than the oral loading of alcohol that was used in the previous study of the effects of GABRA2 on the subjective effects of alcohol assessed only during the ascending limb of the BrAC (Pierucci-Lagha et al., 2005). Our method allowed the evaluation of the initial response to alcohol following the ascending limb of the BrAC as well as the adaptive response to alcohol during the clamped BrAC interval. Based on the previous studies, we hypothesized that GABRA2 alleles would moderate the subjective responses to alcohol measured during not only the ascending limb of BrAC curve but also the clamped BrAC interval. As we expected, subjects with ALDH2*1/*2 showed higher initial response than those with ALDH2*1/*1 (Matsushita et al., manuscript in preparation). Therefore, we divided subjects by ALDH2 genotype and performed further analyses separately in each ALDH2 genotypic group.
SUBJECTS AND METHODS
Subjects
The study population consisted of 110 Japanese subjects (53 men, 48.2%) and was recruited from Yokosuka, Kanagawa, Japan. Subjects were aged 20 to 59 (mean age ± SD, 36.7 ± 10.5 years). All were healthy without apparent history of physical and psychiatric illness, determined by questionnaires about health status and past medical history. A questionnaire was used to quantify the subjects’ alcohol use and to evaluate the family history of alcohol-related problems. Most of them were social drinkers but included small percentage of heavy drinkers who drink more than 4 drinks on any day or 14 per week in men (3 drinks on any day or 7 per week in women) as shown in Table 1. We compared initial response, which will be defined in the following, to alcohol between social and heavy drinkers. Because we did not find any significant differences in initial response to alcohol between them, we combined social and heavy drinkers and performed subsequent analyses. Exclusion criteria included apparent medical history of renal, hepatic, cardiovascular, pulmonary, or gastrointestinal disease; a personal history of any DSM-IV (American Psychiatric Association, 1994) axis I disorder, including alcohol-related disorders; homozygous for inactive aldehyde dehydrogenase 2 (ALDH2) allele (ALDH2*2/*2) because of the possibility of acute intoxication caused by ALDH deficiency. The Ethics Committee of the National Hospital Organization Kurihama Alcoholism Center approved the protocol, and all subjects gave written informed consent before participation.
Table 1.
Demographic and Drinking Characteristics of Subjects
| Variables | Prevalence (%)
|
|
|---|---|---|
| Male (n = 53) | Female (n = 57) | |
| Gender | 48.2 | 51.8 |
| Age [Mean (SD), years]a | 36.8 (10.5) | 36.7 (10.6) |
| Family history of alcohol problemsa | ||
| Alcohol abuse | 5.6 | 8.3 |
| Alcoholic liver disease | 1.9 | 3.3 |
| Alcohol dependence | 3.7 | 1.7 |
| Drinking frequencya | ||
| 1–3/month | 30.6 | 48.0 |
| 1–2/week | 16.7 | 28.0 |
| 3–4/week | 25.0 | 16.0 |
| 5–6/week | 11.1 | 4.0 |
| Everyday | 16.7 | 4.0 |
| Amounts of alcohol consumption per occasionb | ||
| <10 g | 0.0 | 13.0 |
| <30 g | 55.6 | 58.7 |
| <40 g | 2.8 | 6.5 |
| <50 g | 22.2 | 17.4 |
| <60 g | 0.0 | 4.4 |
| ≤100 g | 8.3 | 0.0 |
| >100 g | 11.1 | 0.0 |
No significant differences between gender groups.
There were significant differences between gender groups (p = 0.013).
Preparation for Testing
Subjects arrived in the Kurihama Alcoholism Center laboratory at 9:00 AM, having been instructed to abstain from alcohol for at least 36 hours and from food for at least 12 hours. Abstinence was verified by BrAC measurement. After measuring height, weight, body temperature, and blood pressure, subjects ate a 350-kilocalorie breakfast that was composed of cornflakes and milk. An indwelling catheter was inserted into a vein in the antecubital fossa of each arm, the dominant arm for the infusion and the nondominant arm for blood sampling.
Alcohol Administration by Alcohol Clamp
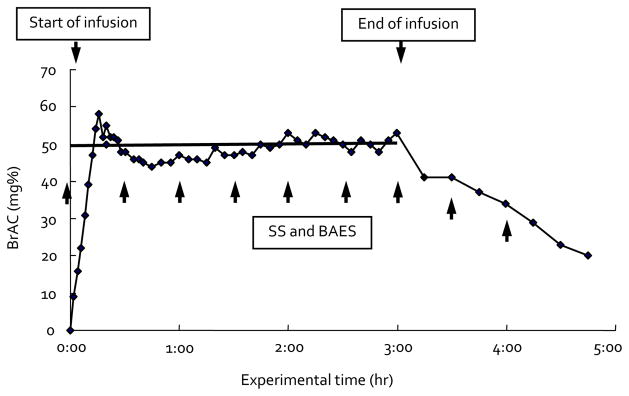
At 10:00 AM, the intravenous infusion of 6% (v/v) ethanol in Ringer’s lactate was begun, using a precomputed rate profile. The profile was derived by forcing a physiologically based pharmacokinetic model of the individual’s alcohol distribution and elimination to follow the desired time course of BrAC as a function of time: a linear ascending limb reaching 50 ± 5 mg/dl at 15 minutes, and then constant for 165 minutes by the method of Ramchandani et al. (1999a). We chose the target level, 50 mg%, as a low to moderate level that would be well tolerated by all subjects without major adverse effects and that would give us measurable effects with sufficient interindividual variance to examine the genetic determinants of interest. Based on serial BrAC measurement, small and intermittent adjustments of the infusion rate were made to maintain the clamped BrAC within 5 mg/dl of the target concentration. After 165 minutes of clamping, the infusion was stopped, the catheter was removed, and the subject was provided with lunch. The BrAC was tracked at 15-minute intervals until it fell below 20 mg/dl when the subject was dismissed from the laboratory. The duration of a typical study session was 5 hours. The whole BrAC clamping paradigm is shown in Fig. 1.
Fig. 1.
Representative sample of breath alcohol concentration clamping paradigm. SS, Sensation Scale; BAES, Biphasic Alcohol Effects Scale.
Measures of Subjective Responses
A battery of dependent measures of subjective responses to alcohol was administered in the baseline condition before the infusion and every 30 minutes after the infusion. Subjective responses were measured using the following self-report questionnaires.
Sensation scale (SS) (Maisto et al., 1980) consists of 26 items that are divided into 6 subscales measuring the subject’s current perceptions about a variety of sensations that often are associated with alcohol but are not specifically attributed to alcohol during testing: Gastro-Intestinal subscale measures sensations felt in the stomach (4 items), Anesthetic subscale measures sensations associated with loss of feeling or decreased sensitivity to feelings (9 items), Central Stimulant subscale measures sensations involving effects on the brain, or what is commonly called “getting high” (4 items), Impaired Function subscale measures perceived changes in certain abilities or skills (3 items), Warmth/Glow subscale measures effects associated with blushing sensations (3 items), and Dynamic Peripheral subscale measures sensations associated with excitation, including changed breathing and heart rate (3 items). We used 5 subscales in our analysis and excluded the Gastro-Intestinal subscale that does not appear to discriminate effectively between subjects who consumed alcoholic and nonalcoholic beverages (Maisto et al., 1980). We added General subscale incorporating all items except the items “warm” and “relaxed” instead (Ramchandani et al., 1999b).
Biphasic alcohol effects scale (BAES) (Martin et al., 1993) is a 14-item unipolar adjective rating scale designed to measure both stimulant and sedative effects of alcohol. The BAES is composed of a 7-item Stimulant subscale and a 7-item Sedative subscale. Subjects rated the adjectives on a scale of 0 (not at all) to 10 (extremely). Item scores were summed into each subscale.
Assessment of Initial Response and Acute Adaptation
Initial and adaptive responses to alcohol were assessed using scalar indices of change (Morzorati et al., 2002; Ramchandani et al., 1999b). One index assessed “initial response” (improvements or impairments) in brain function after alcohol. The other index assessed “acute adaptation” (tolerance or sensitization) to alcohol while the brain’s exposure to alcohol was held constant. We defined initial response as the difference in the SS or the BAES between the baseline and the first 30 minutes after the beginning of the infusion. The index of acute adaptation was computed as the changes in the measurement of subjective responses from 30 minutes to 4 hours after the infusion by measuring every 30 minutes 8 times total as shown in Fig. 1.
Genotyping
DNA was purified from venous blood samples using the DNA Extractor WB Kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan). ALDH2 genotyping was performed by a previously described polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method (Harada and Zhang, 1993). A total of 7 SNPs from GABRA2 gene were selected according to the prior research (Lappalainen et al., 2005): rs567926, rs534459, rs529826, rs279869, rs279858, rs279837, and rs9291283. These SNPs were genotyped using the TaqMan method on an ABI GeneAmp PCR System 9700 apparatus (Applied Biosystems, Foster City, CA). The location of the 7 GABRA2 SNPs is presented in the previous study (Covault et al., 2004).
Statistical Analyses
The differences in initial response by ALDH2 and GABRA2 genotypes were analyzed by analysis of variance (ANOVA), whereas the data for acute adaptation were examined using repeated measures ANOVA. We compared the differences in initial response and acute adaptation among subjects with different genotypes of 7 GABRA2 SNPs divided by ALDH2 genotype because ALDH2 genotype has been proved to have strong effects on the level of response to alcohol (Cook et al., 2005; Luczak et al., 2002; Wall et al., 1999).
The Duncan test was used for the post hoc analysis. The dependent measures examined in the analyses were the initial response and acute adaptation by calculating 6 SS subscales scores and the 2 BAES subscale scores. The statistical analysis of the comparison data was performed using SAS program (version 9.1.3, SAS Institute Inc., Cary, NC). The statistical significance level was 0.05. We used the software Haploview to visualize linkage disequilibrium (LD) relationships between GABRA2 SNPs (Barrett et al., 2005).
RESULTS
Drinking Features
Of participants, 2.6% had a positive family history of alcohol dependence. The demographic and drinking data of participants are given in Table 1. As shown in Table 1, the gender groups did not differ on demographic or drinking characteristics except drinking amount per occasion. The genotype frequencies of ALDH2 and GABRA2 SNPs are shown in Table 2.
Table 2.
Genotype Frequencies of ALDH2 and GABRA2 SNPs
| Gene | SNP name (this study) | ABa assay ID | NCBIb SNPc reference ID | Genotype | Frequency (%) |
|---|---|---|---|---|---|
| ALDH2d | ALDH2*1 /*1:ALDH2*1 /*2 | 76.4:23.6 | |||
| GABRA2e | 1 | 7537087 | rs567926 | TT:TC:CC | 57.8:31.4:10.8 |
| 2 | 8262855 | rs534459 | TT:TC:CC | 9.2:32.1:58.7 | |
| 3 | 1836784 | rs529826 | GG:GA:AA | 9.4:30.2:60.4 | |
| 4 | 8262927 | rs279869 | CC:CA:AA | 41.4:43.3:15.4 | |
| 5 | 2073557 | rs279858 | GG:GA:AA | 15.4:43.3:41.4 | |
| 6 | 8263070 | rs279837 | TT:TC:CC | 39.5:44.0:16.5 | |
| 7 | 8262290 | rs9291283 | TT:TC:CC | 0.9:7.3:91.7 |
Applied Biosystems.
National Center for Biotechnology Information.
Single nucleotide polymorphism.
Aldehyde dehydrogenase 2 gene.
γ-Aminobutyric acid A receptor α2 subunit gene.
Linkage Disequilibrium Within GABRA2
A high degree of LD was observed across the GABRA2 gene in the subjects of this study. The estimated pairwise LD (D′) (Lewontin, 1988) values between SNPs 1 to 7 were 1 (SNPs 1 to 2), 1 (2 to 3), 1 (3 to 4), 1 (4 to 5), 0.92 (5 to 6), and 1 (6 to 7). Almost complete LD was observed among these SNPs. A detailed illustration of LD within GABRA2 is presented in Fig. 2. Best estimates generated by Haploview for the 5-marker haplotypes defined by SNPs 1 to 5 identified 3 common haplotypes, TCACA, CTGAG, and TCAAG, which together represented 99.2% of chromosomes. The frequencies of each haplotype were 62.0, 25.9, and 11.3%, respectively.
Fig. 2.
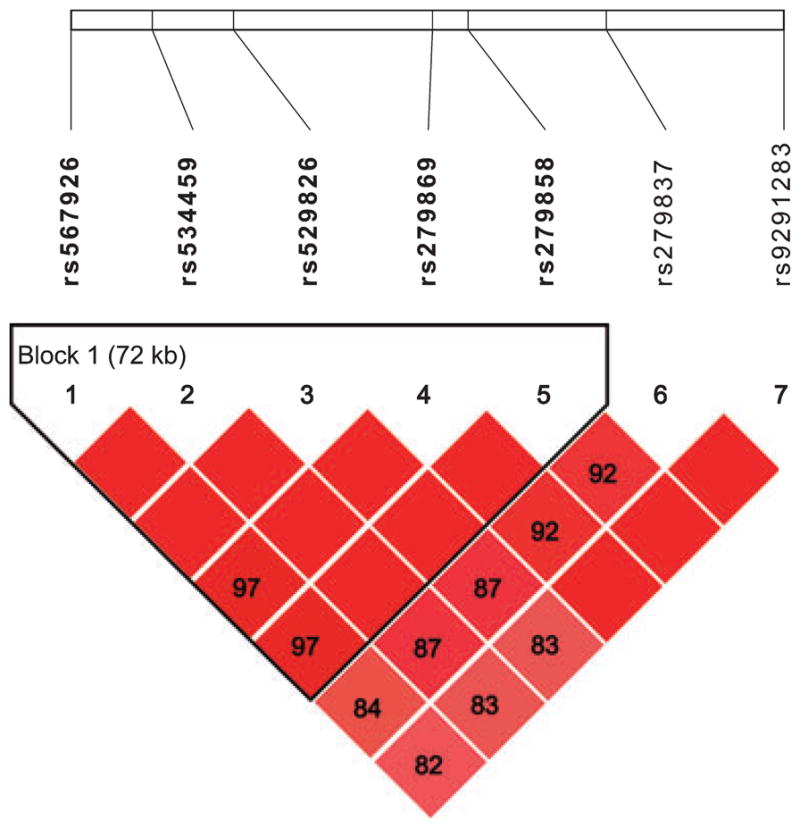
Pattern of linkage disequilibrium (LD) within GABRA2 identified by SNPs 1 to 7. The numbers on the top correspond to each SNP as named in Table 2. Each number in the diamonds represents LD (D′) values for the respective SNP pairs. ◆, Absolute LD (D′ = 1); 97, D′ = 0.97 between SNPs 1 and 4.
Association Between GABRA2 and Initial Response
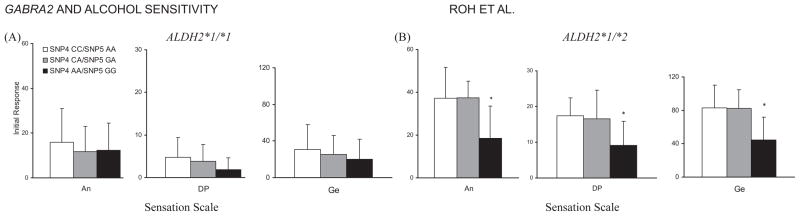
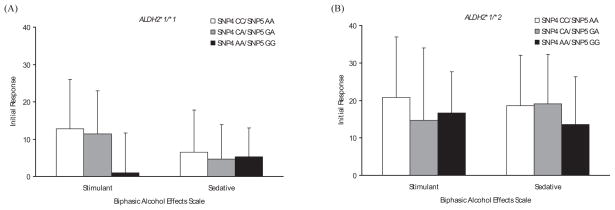
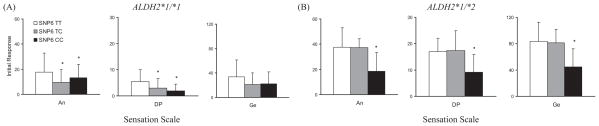
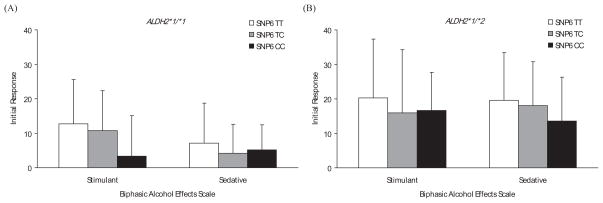
SNPs 1, 2, 3, and 7 showed no significant association with the initial response to alcohol (data not shown), whereas SNPs 4 to 6 were significantly associated with the initial response on several subscales in SS. As the distribution of genotypes between SNPs 4 and 5 is identical in all subjects, we combined the results from these 2 SNPs (SNP4 A-allele = SNP5 G-allele; SNP4 C-allele = SNP5 A-allele). Among individuals with ALDH2*1/*2, subjects with 1 or 2 copies of SNP4 C-allele (SNP5 A-allele) had greater increased scores in Anesthetic [F (2, 20) = 5.23, p = 0.015], Dynamic Peripheral [F (2, 22) = 4.14, p = 0.030], and General [F (2, 18) = 4.82, p = 0.021] subscales of SS than those homozygous for SNP4 A-allele (SNP5 G-allele) (Fig. 3B). In individuals with ALDH2*1 /*1, there was a trend for higher scores among SNP4 C-allele (SNP5 A-allele) carriers than SNP4 A-allele (SNP5 G-allele) homozygotes in Stimulant [F (2, 74) = 2.74, p = 0.071] subscale of the BAES (Fig. 4A). In SNP6, the above-mentioned 3 SS subscale scores were also statistically significant: In subjects with ALDH2*1/*1, there were significant differences in Anesthetic [F (2, 74) = 3.58, p = 0.033] and Dynamic Peripheral [F (2, 77) = 4.39, p = 0.016] subscale scores among TT, TC, and CC genotypes (Fig. 5A). Among subjects with ALDH2*1 /*2, the increase in Anesthetic [F (2, 20) = 5.24, p = 0.015], Dynamic Peripheral [F (2, 22) = 4.09, p = 0.031], and General [F (2, 18) = 4.84, p = 0.021] subscale scores following alcohol administration was greater in the T-allele carriers than in the C-allele homozygotes (Fig. 5B). Without regard to ALDH2 genotype, all participants showed no significant differences in BAES by the genotype of GABRA2 SNP6 (Fig. 6).
Fig. 3.
Estimated mean (SD) for the initial response measured by Sensation Scale by genotypes of ALDH2 and GABRA2 SNPs 4 and 5. (A) Among subjects with ALDH2*1 /*1, initial responses did not differ by the genotype of GABRA2 SNPs 4 and 5. (B) Among subjects with ALDH2*1 /*2, those with 1 or 2 copies of the SNP4 C-allele (SNP5 A-allele) of GABRA2 showed significantly more increased initial responses in Anesthetic (p = 0.015), Dynamic Peripheral (p = 0.030), and General (p = 0.021) subscales than those homozygous for the SNP4 A-allele (SNP5 G-allele). Asterisks indicate significant differences (p < 0.05). An, Anesthetic subscale; DP, Dynamic Peripheral subscale; Ge, General subscale.
Fig. 4.
Estimated mean (SD) for the initial response measured by Biphasic Alcohol Effects Scale by genotypes of ALDH2 and GABRA2 SNPs 4 and 5. (A) Among subjects with ALDH2*1 /*1, there was a trend for higher initial responses in Stimulant (p = 0.071) subscale among the SNP4 C-allele (SNP5 A-allele) carriers than the SNP4 A-allele (SNP5 G-allele) homozygotes. (B) Among subjects with ALDH2*1 /*2, there were no significant differences in the initial responses by the genotypes of GABRA2 SNPs 4 and 5.
Fig. 5.
Estimated mean (SD) for the initial response measured by Sensation Scale by genotypes of ALDH2 and GABRA2 SNP6. (A) Among subjects with ALDH2*1 /*1, there were significant differences in Anesthetic (p = 0.033) and Dynamic Peripheral (p = 0.016) subscale scores among the SNP6 TT, TC, and CC genotypes. (B) Among subjects with ALDH2*1 /*2, those with 1 or 2 copies of the SNP6 T-allele of GABRA2 showed significantly more increased initial responses in Anesthetic (p = 0.015), Dynamic Peripheral (p = 0.031), and General (p = 0.021) subscales than those homozygous for the SNP6 C-allele. Asterisks indicate significant differences (p < 0.05). An, Anesthetic subscale; DP, Dynamic Peripheral subscale; Ge, General subscale.
Fig. 6.
Estimated mean (SD) for the initial response measured by Biphasic Alcohol Effects Scale by genotypes of ALDH2 and GABRA2 SNP6. (A) Among subjects with ALDH2*1 /*1, there were no significant differences in the initial responses by GABRA2 SNP6. (B) Among subjects with ALDH2*1 /*2, there were also no significant differences in the initial responses by the genotype of GABRA2 SNP6.
Association Between GABRA2 and Acute Adaptation
All but the SNP6 had no significant association with acute adaptation (data not shown). In the GABRA2 SNP6, subjects with ALDH2*1/*1 showed significant association with acute adaptation in Dynamic Peripheral [F (2, 72) = 6.39, p = 0.003] subscale of SS and Stimulant [F (2, 70) = 4.01, p = 0.023] and Sedative [F (2, 69) = 5.49, p = 0.006] subscales of BAES. By contrast, there were no significant effects of the SNP6 on the acute adaptation in those with ALDH2*1/*2. Significant levels of all these results are shown in Table 3.
Table 3.
Significance Levelsa of Association Between the SNP6 in GABRA2 and Acute Adaptation by ALDH2 Genotype
| ALDH2*1 /*1 | ALDH2*1 /*2 | |
|---|---|---|
| Sensation scale | ||
| Anesthetic | 0.067 | 0.579 |
| Central stimulant | 0.093 | 0.483 |
| Impaired function | 0.063 | 0.355 |
| Warmth/glow | 0.566 | 0.055 |
| Dynamic peripheral | 0.003* | 0.284 |
| General | 0.203 | 0.730 |
| Biphasic alcohol effects scale | ||
| Stimulant | 0.023* | 0.092 |
| Sedative | 0.006* | 0.657 |
Significance levels were expressed in p-value by repeated measures ANOVA.
Significant p-values are indicated with an asterisk.
DISCUSSION
This is the first association study of GABRA2 polymorphisms with subjective responses to alcohol using the objective method of intravenous alcohol administration, i.e., the alcohol clamp. The most consistent finding in this study was an effect of GABRA2 SNPs 4 to 6 (rs279869, rs279858, and rs279837) on subjective responses to alcohol in nondependent drinkers.
In the present study, subjects with 1 or more C allele at SNP4 (A allele at SNP5) and with ALDH2*1/*2 reported greater initial responses to alcohol (as measured on the SS) compared with subjects who were homozygous for the A allele at SNP4 (G allele at SNP5). In subjects with ALDH2*1 /*1, however, there was no significant effect of the genotype of SNPs 4 and 5 on the initial response with the exception that SNP4 C-allele (SNP5 A-allele) carriers showed a trend for higher scores in Stimulant subscale of the BAES than SNP4 A-allele (SNP5 G-allele) homozygotes. On the other hand, subjects with the T allele at SNP6 presented greater subjective responses to alcohol than did individuals homozygous for the C allele, without regard to ALDH2 genotype. The results for initial responses might be interpreted in the context that the role of GABRA2 in the subjective responses to alcohol could be independent of ALDH2. As for the acute adaptation, only participants with ALDH2*1/*1 reported significantly different subjective effects of alcohol according to the genotype of GABRA2 SNP6. These results for acute adaptation can be interpreted in the following way: the effects of ALDH2*1/*2 on the subjective responses to alcohol were so overwhelming that the effects of GABRA2 might be masked in subjects with ALDH2*1/*2.
Previous association studies have shown the SNP4 A-allele, SNP5 G-allele, and SNP6 C-allele to be over-represented among subjects with alcohol dependence (Covault et al., 2004; Lappalainen et al., 2005). The risk of alcohol dependence has been associated with a low level of response to alcohol, as measured by subjective feeling of intoxication following an alcohol challenge (Bauer and Hesselbrock, 1993; Schuckit, 1984, 1994; Schuckit et al., 1996). A family history of alcohol dependence has also been associated with a diminished response to alcohol in nonalcoholics (Moss et al., 1989; Newlin and Thomson, 1990; O’Malley and Maisto, 1985; Pollock, 1992; Savoie et al., 1988; Schuckit, 1984; Schuckit et al., 2000). Monozygotic twins show greater similarity in sensitivity to an alcohol challenge than do dizygotic twins (Heath and Martin, 1992; Martin et al., 1985; Viken et al., 2003), providing evidence that alcohol sensitivity is an inherited trait. In view of this, our findings are consistent with the hypothesis that the risk of alcohol dependence associated with the A allele at SNP4, the G allele at SNP5, and the C allele at SNP6 in 2 population studies (Covault et al., 2004; Lappalainen et al., 2005) may, in part, be mediated by the decreased subjective responses to alcohol in individuals homozygous for these alleles.
Our results indicate that individuals with 1 or 2 copies of the more common SNP4 C-allele, SNP5 A-allele, or SNP6 T-allele experience a greater initial response after their BrAC rises to the target level of 50 mg%. It is possible that, because of these greater initial subjective responses, such individuals may be less likely to continue drinking. On the other hand, individuals homozygous for the alcohol dependence–associated A allele at SNP4, the G allele at SNP5, or the C allele at SNP6 experience a lower initial response after their BrAC rises. Thus, these individuals may need to drink more alcohol to achieve a comparable level of alcohol-induced subjective response and may be more likely to drink if given access to alcohol. However, studies are needed to determine the effects of GABRA2 alleles on drinking behavior as it occurs in natural settings or using self-administration paradigms.
Although genotypic differences at SNPs in the GABRA2 gene are correlated with differences in subjective responses to alcohol, the functional effects of the allelic variation at GABRA2 are not understood (Covault et al., 2004; Dick et al., 2006; Edenberg et al., 2004; Lappalainen et al., 2005; Soyka et al., 2008). To our knowledge, no common functional coding sequence variants have been described in the GABRA2 gene. The SNPs 4 and 6 are located in introns 6 and 3 of GABRA2, respectively; thus, we know that these are not coding SNPs. The SNP5 is located in exon 5 of GABRA2, but this type of SNP is known as a silent mutation. There are no additional data on possible functions of these SNPs. It is unclear whether these particular SNPs are directly involved in the subjective responses to alcohol or whether the SNPs are in linkage disequilibrium with the actual variant in GABRA2 that causes differences in the alcohol effects. Therefore, additional research is required to elucidate the potential mechanism by which the gene influences the subjective responses to alcohol.
One of the major strengths of this study is the use of the alcohol clamp as the method of alcohol administration. The alcohol clamp minimizes the experimental variance in the brain’s exposure to alcohol and makes it possible to maintain long intervals at a target concentration that is the same for all subjects (O’Connor et al., 1998). By avoiding uncertainties associated with gastric emptying and absorption, the method allows the clamp to be established within 20 minutes after beginning ethanol infusion (Ramchandani et al., 1999a). Other strengths of the study include the choice of various SNPs within GABRA2 to predict the responses to alcohol based on findings from recent association studies (Covault et al., 2004; Edenberg et al., 2004; Lappalainen et al., 2005) that yielded highly convergent results of an allelic association with alcohol dependence. In addition, the relatively large number of subjects that participated in this experiment compared with previous study (Pierucci-Lagha et al., 2005) made it possible to examine the separate effects of the alleles by using all 3 genotype groups to examine the responses to alcohol.
The results of our study must nonetheless be viewed in the context of the study’s limitations. Although the instruments that were chosen are used widely to evaluate alcohol-induced subjective effects, the primary outcome measures used in this study were based on participants’ self-report. Subsequent studies of the impact of GABRA2 on behavioral measures of alcohol’s effects in humans should also include physiologic measures that are sensitive to alcohol effects, such as static ataxia, and neuropsychological tests by which changes in cognitive function can also be assessed. Another limitation of the study could be that we included only Japanese subjects; therefore, we should be careful to generalize these findings to other ethnic groups. Despite these limitations, the results of this study suggest an important association between the subjective responses to alcohol and GABRA2 polymorphisms. These findings provide further evidence for the role of GABRA2 in the subjective effects of alcohol and in the development of alcohol use disorders.
Acknowledgments
This study was supported by a block grant to the Division of Clinical Research, National Hospital Organization Kurihama Alcoholism Center funded by the Ministry of Health, Labour and Welfare, Japan. The authors gratefully acknowledge the expert technical assistance of Ms. Imazeki.
References
- American Psychiatric Association. Diagnositic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, Kranzler HR. Variation in GABRA2 predicts drinking behavior in project MATCH subjects. Alcohol Clin Exp Res. 2007;31:1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol. 1993;54:577–589. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Cook TA, Luczak SE, Shea SH, Ehlers CL, Carr LG, Wall TL. Associations of ALDH2 and ADH1B genotypes with response to alcohol in Asian Americans. J Stud Alcohol. 2005;66:196–204. doi: 10.15288/jsa.2005.66.196. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–274. [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr, Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, June HL. Blockade of GABA(A) receptors within the extended amygdala attenuates D(2) regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropharmacology. 2007;52:1570–1579. doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S, Zhang S. New strategy for detection of ALDH2 mutant. Alcohol Alcohol Suppl. 1993;1A:11–13. doi: 10.1093/alcalc/28.supplement_1a.11. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Genetic differences in psychomotor performance decrement after alcohol: a multivariate analysis. J Stud Alcohol. 1992;53:262–271. doi: 10.15288/jsa.1992.53.262. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Kashima H. New findings on the genetic influences on alcohol use and dependence. Curr Opin Psychiatry. 2006;19:253–265. doi: 10.1097/01.yco.0000218595.54054.7a. [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry. 2006;60:282–287. doi: 10.1016/j.biopsych.2005.12.009. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: an update. Arch Gen Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Lewontin RC. On measures of gametic disequilibrium. Genetics. 1988;120:849–852. doi: 10.1093/genetics/120.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–221. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Elvine-Kreis B, Shea SH, Carr LG, Wall TL. Genetic risk for alcoholism relates to level of response to alcohol in Asian-American men and women. J Stud Alcohol. 2002;63:74–82. [PubMed] [Google Scholar]
- Maisto SA, Connors GJ, Tucker JA, McCollam JB, Adesso VJ. Validation of the Sensation Scale, a measure of subjective physiological responses to alcohol. Behav Res Ther. 1980;18:37–43. doi: 10.1016/0005-7967(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin NG, Oakeshott JG, Gibson JB, Starmer GA, Perl J, Wilks AV. A twin study of psychomotor and physiological responses to an acute dose of alcohol. Behav Genet. 1985;15:305–347. doi: 10.1007/BF01070893. [DOI] [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: within-family association and linkage analyses. J Stud Alcohol Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O’Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res. 2002;26:1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Moss HB, Yao JK, Maddock JM. Responses by sons of alcoholic fathers to alcoholic and placebo drinks: perceived mood, intoxication, and plasma prolactin. Alcohol Clin Exp Res. 1989;13:252–257. doi: 10.1111/j.1530-0277.1989.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. [PubMed] [Google Scholar]
- O’Malley SS, Maisto SA. Effects of family drinking history and expectancies on responses to alcohol in men. J Stud Alcohol. 1985;46:289–297. doi: 10.15288/jsa.1985.46.289. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Am J Psychiatry. 1992;149:1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999a;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999b;23:1320–1330. [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genomewide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Roh S, Matsushita S, Higuchi S. Genetic influences on alcohol use and dependence. In: Sher L, editor. Research on the Neurobiology of Alcohol Use Disorders. Nova Science Publishers; New York: 2008. pp. 291–320. [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Savoie TM, Emory EK, Moody-Thomas S. Acute alcohol intoxication in socially drinking female and male offspring of alcoholic fathers. J Stud Alcohol. 1988;49:430–435. doi: 10.15288/jsa.1988.49.430. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk – a 20-year prospective study. Alcohol Clin Exp Res. 2004;28:1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R, Chan G. The ability of the Self-Rating of the Effects of Alcohol (SRE) Scale to predict alcohol-related outcomes five years later. J Stud Alcohol Drugs. 2007;68:371–378. doi: 10.15288/jsad.2007.68.371. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol. 1996;57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42:184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Tauber M, Calame-Droz E, Prut L, Rudolph U, Crestani F. alpha2-gamma-Aminobutyric acid (GABA)A receptors are the molecular substrates mediating precipitation of narcosis but not of sedation by the combined use of diazepam and alcohol in vivo. Eur J Neurosci. 2003;18:2599–2604. doi: 10.1046/j.1460-9568.2003.02988.x. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK. Subjective intoxication in response to alcohol challenge: heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol Clin Exp Res. 2003;27:795–803. doi: 10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
- Wall TL, Johnson ML, Horn SM, Carr LG, Smith TL, Schuckit MA. Evaluation of the self-rating of the effects of alcohol form in Asian Americans with aldehyde dehydrogenase polymorphisms. J Stud Alcohol. 1999;60:784–789. doi: 10.15288/jsa.1999.60.784. [DOI] [PubMed] [Google Scholar]