Abstract
Around a fifth of melanomas exhibit an activating mutation in the oncogene NRas that confers constitutive signaling to proliferation and promotes tumor initiation. NRas signals downstream of the major melanocyte tyrosine kinase receptor c-kit and activated NRas results in increased signaling via the extracellular signal–regulated kinase (ERK)/MAPK/ERK kinase/mitogen-activated protein kinase (MAPK) pathways to enhance proliferation. The Ras oncogene also activates signaling via the related Rho GTPase Rac1, which can mediate growth, survival, and motility signaling. We tested the effects of activated NRasQ61K on the proliferation, motility, and invasiveness of melanoblasts and melanocytes in the developing mouse and ex vivo explant culture as well as in a melanoma transplant model. We find an important role for Rac1 downstream of NRasQ61K in mediating dermal melanocyte survival in vivo in mouse, but surprisingly NRasQ61K does not appear to affect melanoblast motility or proliferation during mouse embryogenesis. We also show that genetic deletion or pharmacological inhibition of Rac1 in NRasQ61K induced melanoma suppresses tumor growth, lymph node spread, and tumor cell invasiveness, suggesting a potential value for Rac1 as a therapeutic target for activated NRas-driven tumor growth and invasiveness.
Introduction
Mutations in the Ras proto-oncogene family (HRas, NRas, and KRas) are among the most frequently mutated genes in human cancers, occurring in 20–30% of human tumors (Downward, 2003). Residue G12 in the phosphate-binding loop and the catalytic residue Q61 are most commonly mutated (Malumbres and Barbacid, 2003). Constitutively active NRasQ61K is frequently found in nevi and early-stage melanomas (Platz et al., 2008). Expression of human NRasQ61K in the mouse melanocyte lineage results in hyperpigmented skin (Ackermann et al., 2005), which can progress to cutaneous melanoma on an INK4a-deficient background.
The mitogen-activated protein kinase pathway, modulated by Raf directly interacting with Ras, is a key downstream effector in Ras signaling (Cook and McCormick, 1994; Marshall, 1995; Gray-Schopfer et al., 2007). However, the recruitment of other Ras targets is indispensable to elicit a full Ras biological response. Among these Ras-dependent, Raf-independent pathways are those connecting Ras to the Rho subfamily of small GTPases. There are no published reports of activating Rho-family mutations in human tumors, but Rho-family proteins often exhibit upregulation of their activity in tumors (Sahai and Marshall, 2002).
The small GTPase Rac1 is required for cell cycle regulation and Ras-induced transformation in vitro (Qiu et al., 1995), but relatively little is known about its role in cancer in vivo. Rac1 is required for KRasG12D-driven formation of lung tumors (Kissil et al., 2007) and oral papillomas (Samuel et al., 2011). However, activated Ras isoforms can exert profoundly different effects in different forms of cancer (Hancock, 2003; Whitwam et al., 2007; Karreth and Tuveson, 2009). Here, we show that Rac1 activity is required for NRasQ61K-induced dermal melanocyte survival in vivo and for increased invasiveness conferred by NRasQ61K on primary melanocytes in vitro and in vivo. In addition, genetic deletion or pharmacological inhibition of Rac1 in NRasQ61K-expressing melanoma tumors suppressed tumor growth and lymph node spread. Thus, many of the crucial downstream effects of NRasQ61K in melanoma are likely to be mediated by Rac1 and implicate Rac1 and its downstream partners as potential key targets for melanoma therapy.
Results
Rac1 is required for anchorage-independent growth (AIG) of NRasQ61K-expressing primary murine melanocytes in culture
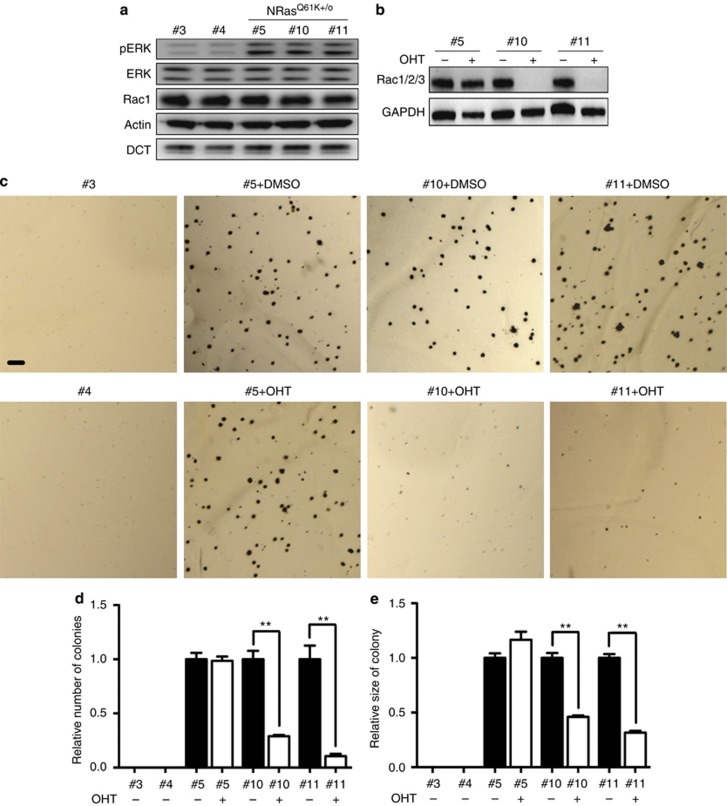
In our recent study (Li et al., 2011), we generated two independent immortalized Rac1 f/f Ink4a−/− Tyr∷CreERt2+/o melanocyte cell lines #3 and #4, which were unable to grow in soft agar (Figure 1c–e). To test the importance of Rac1 downstream of NRasQ61K in oncogenic transformation for melanocytes, we generated melanocyte cell lines from 1-day-old Tyr∷NRasQ61K+/o Rac1 f/f Ink4−/− Tyr∷CreERt2+/o (#10 and #11) and Tyr∷NRasQ61K+/o Rac1 f/f Ink4a−/− Tyr∷CreERt2o/o (#5) littermate mice (also littermate of #3 and #4). As expected, NRasQ61K+/o-expressing melanocytes (#5, #10 and #11) have elevated activation of mitogen-activated protein kinase signaling compared with Rac1 f/f Ink4a−/− Tyr∷CreERt2+/o melanocyte cell lines #3 and #4 (Figure 1a; Ackermann et al., 2005; Whitwam et al., 2007; Li et al., 2011), but Rac1 expression level was not affected by expression of NRasQ61K (Figure 1a). Rac1 deletion was induced with tamoxifen analog 4-hydroxytamoxifen (OHT) in cell lines that express CreERt2 (#10 and #11), but not in cell line #5, which is CreERt2 negative (Figure 1b). Neither Rac2 nor Rac3 was detectable, regardless of Rac1 deletion (Figure 1b). All the NRasQ61K-expressing melanocyte cell lines (#5, #10 and #11) were able to induce rapid AIG in soft agar and form large colonies (Figure 1c–e). Rac1 deletion reduced colony number by about 80% and the average colony size by about 60% (Figure 1c–e). Rac1-deleted NRasQ61K-expressing melanocytes additionally showed reduced proliferation (Supplementary Figure S1A online). 12-O-Tetradecanoylphorbol 13-acetate acts as a growth factor for cultured melanocytes, but is not required for growth when melanocytes are transformed by constitutively active c-kit receptor (Larue et al., 1992). Similarly, 12-O-tetradecanoylphorbol 13-acetate is not required for NRasQ61K-expressing melanocytes growth on either 2D or in soft agar (Supplementary Figure S1 online). Thus, NRasQ61K confers Rac1-dependent AIG on primary melanocytes. This suggests an important, but previously undescribed, role for Rac1 in mediating oncogenic transformation induced by NRasQ61K in melanocytes.
Figure 1.
Rac1 is required for NRasQ61K-induced anchorage-independent growth. (a) Western blots of primary melanocyte cell lines with antibodies as indicated. (b) Western blot of #5, #10, and #11 primary melanocyte cell lines treated with DMSO or 4-hydroxytamoxifen (OHT) for 5 days were probed with antibodies as indicated. (c) Representative images of primary melanocyte cell lines treated with DMSO or OHT were plated in soft agar in the presence of 200 nM 12-O-tetradecanoylphorbol 13-acetate (TPA) for 2 weeks. (d) Relative number of colonies and (e) relative size of each colony in the presence of 200 nM TPA. All error bars show mean±SEM from three independent experiments. Bar=500 μm; **P<0.01 by t-test. DCT, dopachrome tautomerase; ERK, extracellular signal-–regulated kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
NRasQ61K does not confer excess melanocyte accumulation in embryos, but loss of Rac1 leads to melanocyte proliferation defects
We next examined the number and position of melanoblasts in Tyr∷NRasQ61K-expressing embryos. Strikingly, Tyr∷NRasQ61K-expressing E15.5 embryos or P0.5 newborn pup skin did not show a statistically significant increase in the number of melanoblasts/melanocytes (Supplementary Figure S2 online). There was a significant reduction in melanoblast number in Rac1 f/f Tyr∷Cre embryos regardless of the presence of Tyr∷NRasQ61K (Supplementary Figure S2 online), indicating that NRasQ61K does not affect melanoblast numbers or position.
Expression of NRasQ61K causes Rac1-dependent hyperpigmentation in adult mice
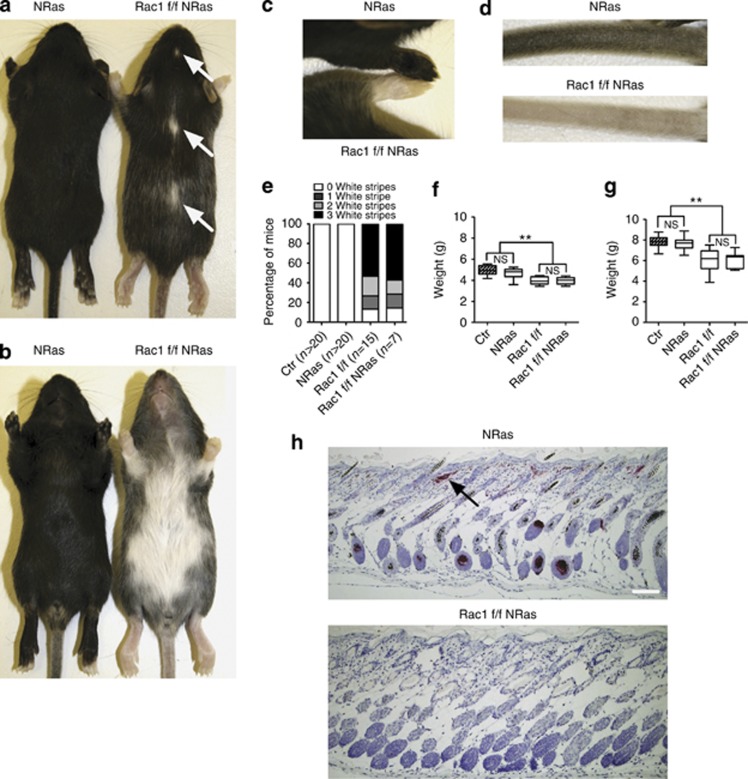
Mice of the C57BL/6 strain normally have light skin and black fur, but when Tyr∷NRasQ61K is expressed, they have black skin and darker fur (Ackermann et al., 2005). Mice lacking Rac1 in melanocytes have white patches on the underside and along the dorsal midline and also a lightening of the paws and tails (Supplementary Figure S2C and D online; Li et al., 2011). At P14, Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre mice had similar pigmentation patterns to Rac1 f/f Tyr∷Cre mice (Figure 2a–d and Supplementary Figure S3A and B online;Li et al., 2011). Furthermore, the number and size of white patches on the dorsal skin between Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre and Rac1 f/f Tyr∷Cre mice was similar (Figure 2a and e and Supplementary Figure S3A online). Mice were born healthy at the expected Mendelian ratio. However, Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre mice were smaller than control littermates and had a similar weight to Rac1 f/f Tyr∷Cre as measured on P7 and P14 (Figure 2f and g and Methods). Histological analysis of P14 Tyr∷NRasQ61K ventral skin revealed melanocytes in hair follicles, dermis and fatty tissue (Figure 2h). Thus, deletion of Rac1 in the melanocyte lineage from mice expressing NRasQ61K resulted in similar pigmentation defects as Rac1 deletion alone.
Figure 2.
Mice expressing melanocyte lineage NRasQ61K retain pigmentation defects caused by Rac1 deletion. Coat color of dorsal (a) and ventral side (b) of P14 Tyr∷NRasQ61K+/o Rac1 f/f Tyr∷Cre+/o (Rac1 f/f NRas) mouse with Tyr∷NRasQ61K+/o (NRas) control (Ctr) littermate. Typical dorsal white patches Rac1 f/f NRas mouse (arrows). Coat color of forelimb (c) and tail (d) of Rac1 f/f NRas mouse with NRas Ctr littermate. (e) Quantification of white patches on dorsal side of mice. Body weight of mice at P7 (f) and P14 (g); n=6 mice from three different litters. Lower, median, and upper quartile are shown. (h) Ventral skin from P14 NRas Ctr and Rac1 f/f NRas mice with anti-dopachrome tautomerase (melanocytes). Typical cluster of dermal melanocytes in NRas Ctr mouse is shown with an arrow. **P<0.01 compared with Ctr by t-test. Bar=100 μm. NS, not significant.
Expression of NRasQ61K promotes survival and accumulation of epidermal/dermal melanocytes via Rac1 over the hair cycle
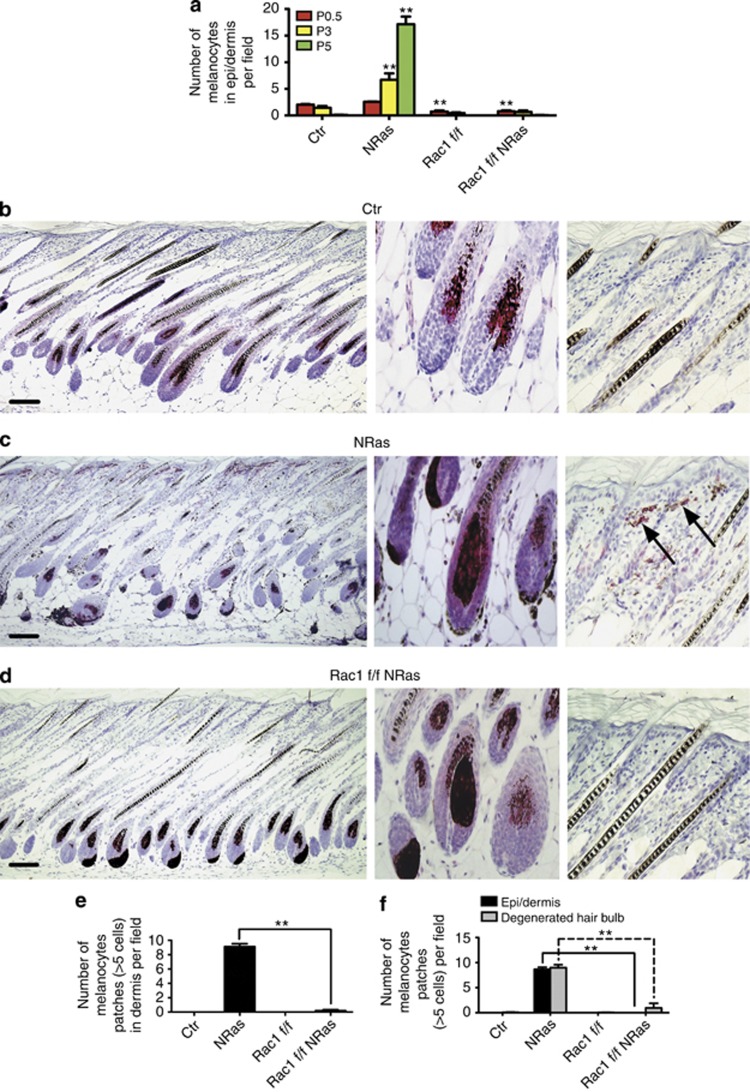
Before birth, murine melanoblasts reside in the dermis and epidermis (Luciani et al., 2011), but shortly after birth they reside only in hair follicles (Kelsh et al., 2009). We investigated the origin of Tyr∷NRasQ61K dermal melanocyte clusters (Figure 2h, arrow) and the effect of Rac1 loss during the first hair cycle, which is synchronous (Fuchs, 2007). Dorsal skin from control, Tyr∷NRasQ61K control, Rac1 f/f Tyr∷Cre and Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre mice at P0.5, 3, 5, 14, and 21 was sectioned and stained with dopachrome tautomerase antibody to localize melanocytes. In control, Rac1 f/f Tyr∷Cre and Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre mice, melanocytes in epidermis and dermis gradually disappeared from P0.5 to P5 (Figure 3a, Supplementary Figure S4A and C online and data not shown), leaving only hair follicle melanocytes (Hirobe, 1984; Kelsh et al., 2009). However, in Tyr∷NRasQ61K control mice, melanocytes in epidermis survived from P0.5 and number of melanocytes in epidermis increased over the hair cycle (Figure 3a, Supplementary Figure S4B online), indicating that expression of NRasQ61K promotes Rac1-dependent postnatal melanocyte survival and/or growth in epidermis.
Figure 3.
Rac1 is required for NRasQ61K-induced survival of murine dermal melanocytes. (a) Number of melanocytes in epi/dermis at P0.5, P3, and P5 per field ( × 10 objective) from (⩾3 pups, 3 litters). Dorsal skin from P14 control (Ctr) (b), NRas Ctr (c), and Rac1 f/f NRas (d) mice with anti-dopachrome tautomerase (melanocytes). High-magnification images of hair follicle and dermis shows typical cluster of excess dermal melanocytes in NRas mouse (arrow). (e) Number of patches (⩾5 cells) of melanocytes in dermis per field at P14 (from ⩾3 pups, 3 litters). (f) Number of patches (⩾5 cells) of melanocytes in dermis or former hair bulb per field at P21 (from ⩾3 pups, 3 litters). **P<0.01 by t-test. Bars=100 μm.
At P14, Tyr∷NRasQ61K control (Figure 3c) and Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre (Figure 3d) skin showed excess melanocytes in hair follicles (Figure 3b). However, in Tyr∷NRasQ61K control mice, melanocytes also accumulated in clusters in the epidermis/dermis and fatty tissue (Figure 3c, arrows) but these were absent from Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre mice (Figure 3d and e).
The transition from anagen to telogen leads to apoptosis of most follicular melanocytes (Sharov et al., 2005). In the telogen phase of the first hair follicle cycle (P21), only the permanent part of the hair follicles remained in dorsal skin taken from control mice (Supplementary Figure S4A online). Most of follicular melanocytes remained in the dermis even after the hair follicles in Tyr∷NRasQ61K mouse degenerated (Figure 3f and Supplementary Figure S4B online, arrows). Which, however, failed to be maintained when Rac1 was deleted (Figure 4f, Supplementary Figure S4C online). Thus, NRasQ61K protects dermal melanocytes from being cleared from the skin in a Rac1-dependent manner.
Figure 4.
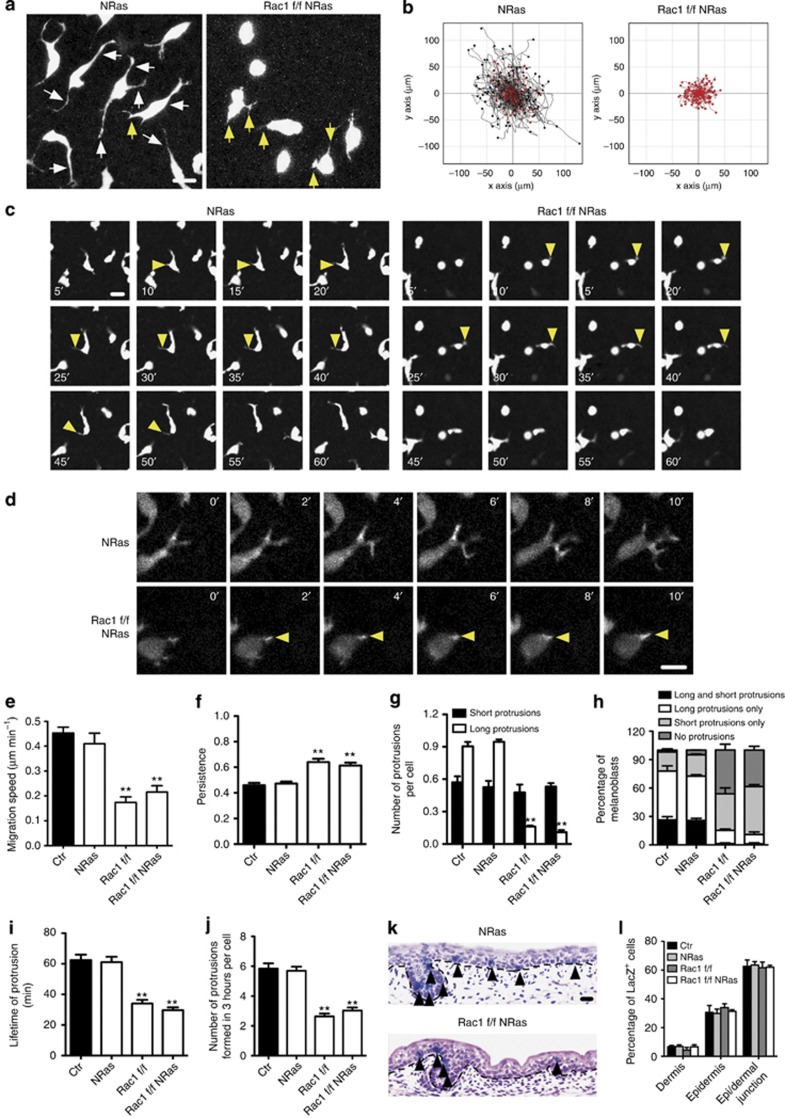
Expression of NRasQ61K does not affect melanoblast migration in epidermis. All experiments show embryo skin explants or embryos. (a) Combined Z-stack confocal images of melanoblasts from Z/EG NRas and Z/EG Rac1 f/f NRas embryo skin. Long (⩾cell body width) and short (⩽cell body width) protrusions, are shown with white and yellow arrows, respectively. (b) Three hour tracks of melanoblasts. Black tracks migrated faster and red slower than average of control (Ctr). (c) Live imaging of cell protrusion (yellow arrow) dynamics. (d) Live imaging of melanoblast in GFP-Lifeact NRas Ctr or GFP-Lifeact Rac1 f/f NRas skin. Yellow arrows indicate protrusions. (e) Migration speed. (f) Persistence. (g) Number of long/short protrusions per melanoblast. (h) Proportion of melanoblasts with long/short protrusions. (i) Lifetime of growing protrusions. (j) Frequency of protrusion formation. (k) Photos and (l) quantification of melanoblasts in E15.5 DCT∷LacZ embryos. Melanoblasts are indicated with black arrows. Black dotted lines represent epi/dermal junction. Error bars indicate mean±SEM. **P<0.01, by t-test. Bars (a, c)=10 μm and (k) 20 μm.
NRasQ61K does not affect the motility of melanoblasts in embryonic skin or their ability to cross the embryonic dermal/epidermal basement membrane
Melanoma metastasis has analogies with embryonic development (Hendrix et al., 2007; Yang and Weinberg, 2008). NRasQ61K is frequently found in human metastatic melanoma (Demunter et al., 2001), thus may contribute to abnormal invasiveness and migration of melanoma cells (Mishra et al., 2010). We studied the motility of melanoblasts in the epidermis of embryo skin explants by live imaging (Mort et al., 2010). We asked whether NRasQ61K could affect melanoblast motility and whether this was Rac1 dependent, as Rac1 is important for speed and protrusion formation of melanoblasts (Li et al., 2011). Melanoblasts expressing NRasQ61K had a similar morphology to control melanoblasts, showing both long and short protrusions (Figure 4a) in the same proportions (Figure 4g and h). NRasQ61K-expressing melanoblasts extended dynamic short protrusions that elongated and often dictated the direction of migration (Figure 4c yellow arrows and Supplementary Movie S1 online). Loss of Rac1 in NRasQ61K-expressing melanoblasts produced a phenotype indistinguishable from Rac1 null melanoblasts, with short stubby protrusions leading migration but rarely elongating (Figure 4c, yellow arrows, Supplementary Movie S1 online). Furthermore, by labeling melanoblasts with lifeact-GFP (Li et al., 2011), we also confirmed that the short stubby protrusions in Tyr∷NRasQ61K Rac1 f/f Tyr∷Cre melanoblasts were actin-rich spiky protrusions and not bleb-based (Figure 4d, yellow arrows, Supplementary Movie S2 online). Expression of activated NRasQ61K did not measurably affect the migration speed (Figure 4b and e), persistence (Figure 4f), lifetime (Figure 4i), or frequency of protrusions (Figure 4j). Therefore, we conclude that NRasQ61K does not affect melanoblast motility in any detectable way. Furthermore, there was no effect of expression of NRasQ61K on the ratio of melanoblasts in the dermis versus epidermis (Figure 4k and l). In summary, NRasQ61K expression has no detectable effect on melanoblast motility or basement membrane crossing during embryogenesis, whereas signaling through Rac1 is limiting for melanoblast proliferation and motility.
Rac1 is required for NRasQ61K-induced invadopodia formation and invasion
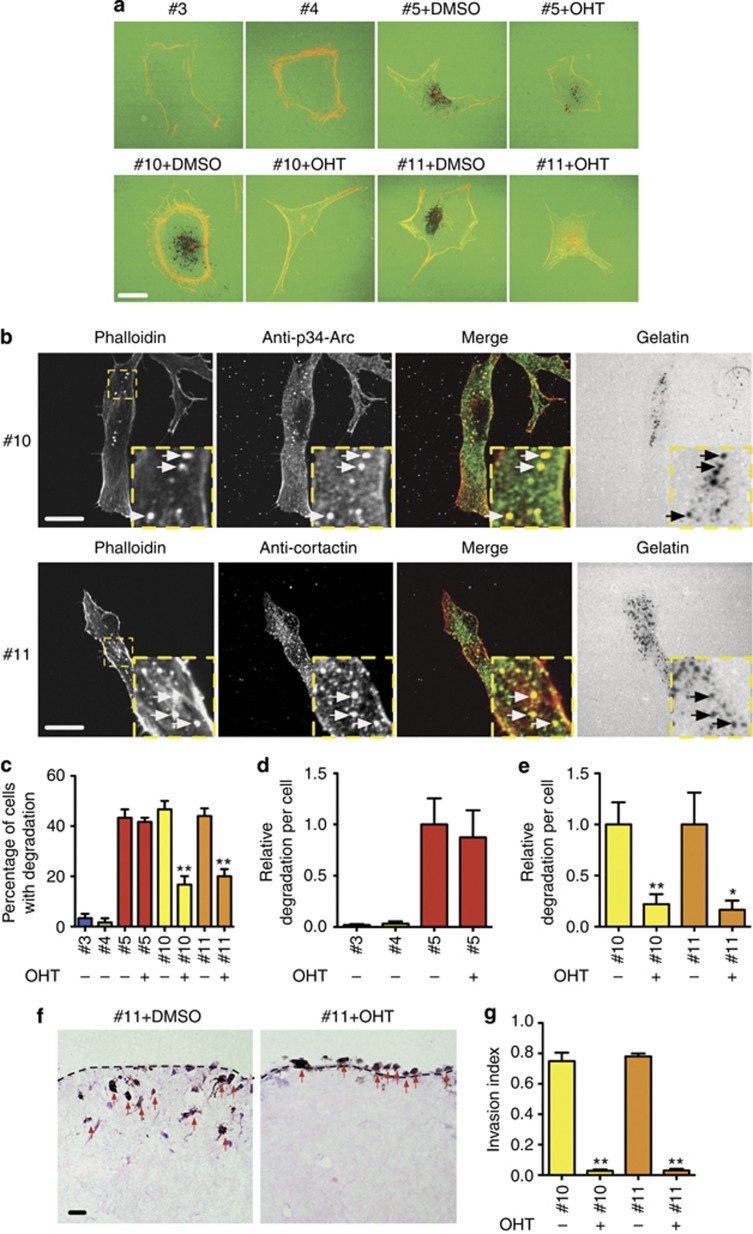
We asked whether expression of NRasQ61K could enhance the ability of melanocytes to degrade extracellular matrix and whether matrix-remodeling capacity was Rac1 dependent. Invadopodia are actin-based membrane protrusions formed in invasive cancer cells that have a matrix degradation activity (Buccione et al., 2004). They are rich in filamentous actin, cortactin, and Arp2/3 complex (Linder, 2007). To examine whether NRasQ61K could induce invadopodia formation, wild-type melanocyte cell lines (#3 and #4) and NRasQ61K melanocyte cell lines (#10 and #11) were cultured on cross-linked gelatin matrix. Wild-type melanocytes (#3 and #4) showed similar morphology compared with NRasQ61K-expressing melanocyte cell lines, but did not display invadopodia (Figure 5a). In contrast, NRasQ61K-expressing melanocyte cell lines (#5, #10 and #11) formed copious invadopodia (Figure 5b). We found ∼45% of NRasQ61K-expressing melanocytes and only 4% of normal melanocytes contained invadopodia (Figure 5c). Furthermore, the matrix degradation for individual cells was negligible in controls (#3 and #4) as compared with NRasQ61K-expressing melanocytes (Figure 5d).
Figure 5.
Rac1 is required for NRasQ61K-induced invadopodia formation and invasion in melanocytes. (a) #3, #4, #5, #10, and #11 primary melanocyte cell lines treated with DMSO or 4-hydroxytamoxifen (OHT) on cross-linked gelatin and stained with rhodamine phalloidin. (b) #10 or #11 melanocyte cells on cross-linked gelatin stained with anti-p34 (Arp2/3 complex), anti-cortactin, and phalloidin. Arrows indicate invadopodia. (c) Percentage of cells degrading matrix. (d) Relative matrix degradation to DMSO control #5 (e) or #10 or #11. All error bars show mean±SEM from 30 cells per experiment, n=3 independent experiments. (f) Organotypic invasion assays and quantification (g) for invasion of #10 or #11 melanocytes treated with DMSO or OHT. Melanocytes are indicated by red arrows. **P<0.01 and *P<0.05 by t-test. Bars=10 μm (a, b) and 20 μm (f). Insets show invadopodia.
#10 and #11 NRasQ61K-expressing melanocytes treated with OHT to induce Rac1 deletion showed about 60% reduction in the percentage of cells degrading matrix (Figure 5c) and 80% reduced degradation (Figure 5a and e). OHT treatment did not affect invadopodia formation by #5 melanocytes, which do not delete Rac1 (Figure 5d). In addition, OHT-treated #10 and #11 melanocytes failed to invade in organotypic assays (Figure 5f and g). We conclude that Rac1 is important for melanocyte invadopodia formation and invasion downstream of NRasQ61K.
Genetic deletion or pharmacological inhibition of Rac1 impairs NRasQ61K-induced melanoma tumor growth and formation of lymph node metastasis
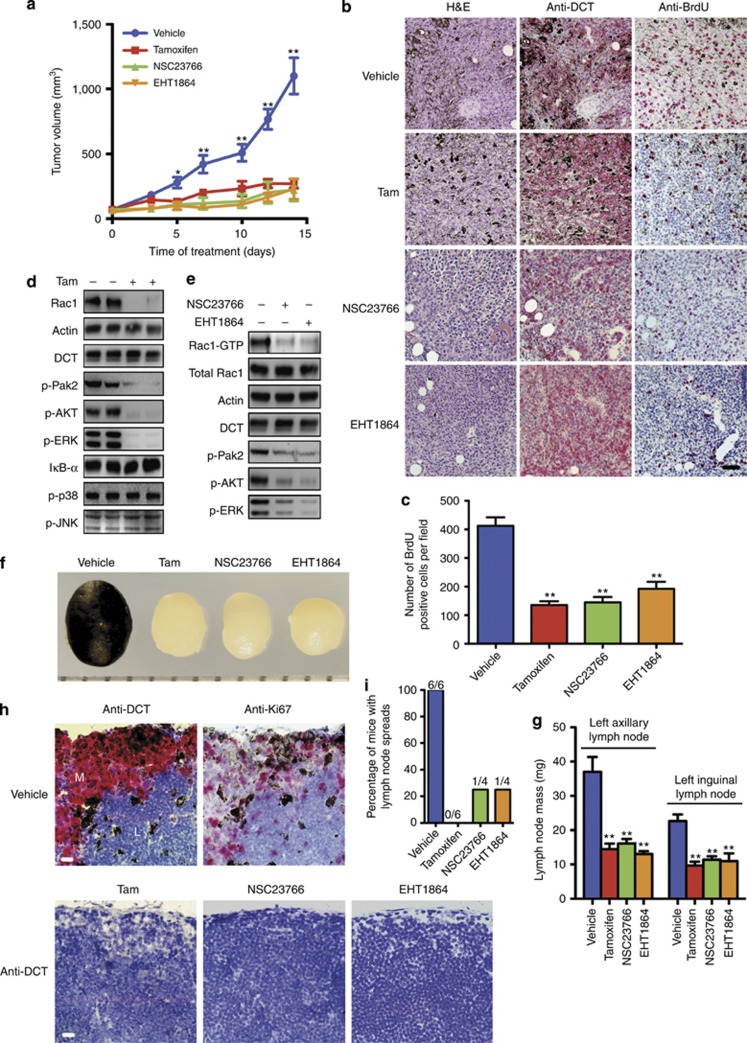
Although Rac function is required for KRasG12D-induced primary squamous cell skin and lung tumor initiation (Kissil et al., 2007; Samuel et al., 2011), the role of Rac1 in NRasQ61K-induced tumor growth and invasiveness in vivo has not been addressed. We transplanted #11 NRasQ61K Rac1 f/f melanocytes subcutaneously into the left flank of nude mice (CD-1). The growth of Rac1-deleted allografts on day 1–3 of tamoxifen treatment was similar to vehicle, likely because it requires 3 days to deplete endogenous Rac1 (Li et al., 2011). However, allograft growth halted at day 5 after tamoxifen treatment (Figure 6a). BrdU incorporation was reduced by 60% in Rac1-deleted allografts (Figure 6b and c). Pak2 (Mouse melanocytes do not express Pak1/3 (Li et al., 2011).), Erk1/2, and AKT activation were reduced in Rac1-deleted allografts. Although the other Rac1-dependent signaling pathways (JNK, p38, and NF-κB; Jaffe and Hall, 2005) remained unchanged (Figure 6d). Rac-specific inhibitors (NSC23766 or EHT1864) showed strong inhibition of Rac1 activity in vivo (Figure 6e) and significant inhibition of allografts growth (Figure 6a) without inducing body-weight loss (Supplementary Figure S5A online). Proliferation was decreased by about 50% (Figure 6b). Consistent with Rac1 deletion results, both NSC23766- and EHT1864-treated allografts showed a strong decrease in Pak2, Erk1/2, and AKT activation (Figure 6e). Interestingly, analysis of vehicle-treated mice systematically revealed pigmentation of left axillary and inguinal skin-draining lymph nodes (Figure 6f and Supplementary Figure S5B online), a characteristic of the metastatic process. Tumor-draining lymph nodes were enlarged (Figure 6f and g and Supplementary Figure S5B online), and contained large numbers of dopachrome tautomerase-positive pigmented proliferating melanocytic cells (Figure 6h) and disrupted architecture (Supplementary Figure S5C online). However, in tamoxifen, NSC23766- or EHT1864-treated mice, lymph nodes retained their normal architecture and weight (Figure 6f and g and Supplementary Figure S5B, C online). All vehicle-treated mice (6/6) had melanocytes in lymph nodes (Figure 6h and i, Supplementary Figure S5C online). Whereas, none of mice (0/6) treated with tamoxifen and only 1 in 4 mice treated with either NSC23766 or EHT1864 showed melanocytes in lymph nodes (Figure 6h and i, Supplementary Figure S5C online). Together, these data indicate that Rac1 activity is required for NRasQ61K-induced tumor growth and lymph node spread in vivo, which suggests that Rac1 may be a valid therapeutic target against NRasQ61K-induced metastatic melanoma.
Figure 6.
Rac1 activity is required for NRasQ61K-induced melanoma growth and lymph node spread. (a) Response of allografts to drug treatment (n⩾4) on day 3 of NSC23766 or EHT1864 treatment (P<0.05). (b) Drug-treated allografts stained with H&E, anti-dopachrome tautomerase (DCT), and anti-BrdU. (c) BrdU-positive cells per field (⩾3 tumors). (d) Western blot of vehicle/tamoxifen (Tam)-treated allografts. (e) Rac1 activity and western blot of vehicle-, NSC23766-, or EHT1864-treated allografts. (f) Left inguinal lymph node and (g) average lymph node mass for drug-treated mice (n⩾4). (h) Lymph nodes with anti-DCT or with anti-Ki67. L=lymph node tissue; M=melanocytes. (i) Percentage of mice with DCT-positive lymph nodes. Error bars show mean±SEM, **P<0.01 and *P<0.05 by t-test. Bars=50 μm. H&E, hematoxylin and eosin.
Discussion
NRasQ61K does not affect either migration or proliferation during embryogenesis nor compensate for Rac1 loss
As NRasQ61K is a major driver of human melanoma (Hendrix et al., 2007), we investigated whether expression of NRasQ61K would alter the growth, survival, or motility of melanoblasts during embryogenesis. However, NRasQ61K-expressing embryos showed a normal distribution and numbers of melanoblasts until birth. Although we cannot formally discount the possibility that NRasQ61K may enhance melanoblast proliferation and simultaneously promote apopotosis, we did not observe any apoptotic melanoblasts using live ex vivo imaging (Supplementary Movie S1 online) or by histology (not shown). This may indicate a high basal level of NRas activity in melanoblasts during embryogenesis, as cells are proliferating and populating the skin and that KitL (kit ligand) is expressed in the embryo epidermis and decreases after birth (Balch et al., 2011). Other factors besides Ras may be limiting for the growth and accumulation of melanoblasts such that expressing NRasQ61K does not further enhance proliferation or survival. Melanoblasts require the membrane-bound form of KitL for survival and migration in the epidermis, suggesting cell–cell interactions might be involved (Abraham et al., 2012). Rac1 has been shown to mediate the formation of dendritic structures in melanoma cells in response to melanocyte-stimulating hormone and UV light in vitro (Scott and Cassidy, 1998; Balch et al., 2011). However, whether dendritic structures mediated by Rac1 in melanocytes or melanoma cells in vitro are analogous to long protrusions mediated by Rac1 during melanoblast migration in vivo is still not clear. We emphasize here that, in addition to our previous study (Li et al., 2011), Rac1 is an important mediator of dynamic long protrusions, which contribute to melanoblast migration in epidermis.
Rac1 is required for NRasQ61K-induced survival of dermal melanocytes
Adult mice expressing NRasQ61K in the melanocyte lineage accumulated more dermal and hair follicle melanocytes than normal mice. Rac1 deletion in melanocytes of NRasQ61K-expressing mice resulted in loss of dermal melanocytes similar to normal mice, suggesting that Rac1 has a very important role downstream of NRas in regulating melanocyte survival in the dermis. However, we failed to detect any apoptotic melanocytes in dermis with cleaved caspase-3 antibody in both P0.5 and P3 skin sections (data not shown). Cells may be rapidly cleared or die via a non-apoptotic mechanism.
c-Kit signaling triggers proliferation and survival via activation of Ras via mitogen-activated protein kinase/extracellular signal-–regulated kinase signaling (Mackenzie et al., 1997) and PI3 kinase. KitL accumulates in the hair bulbs of murine anagen hair follicles with very little or no expression in the adult epidermis (Peters et al., 2003). Lack of KitL survival signals in the epidermis after birth may explain why in normal mice, melanocytes cannot survive in epidermis. However, expression of activated NRasQ61K in melanocytes may overcome the requirement for KitL and thus promote melanocyte survival in epidermis in a Rac1-dependent manner. It will be of interest to see whether the Rac1 function can be compensated by ectopic expression of KitL in epidermis (Kunisada et al., 1998) or by deleting Rac1 in mice lacking the Ras-GAP Nf1 (McCalmont and Bastian, 2012).
Rac1 is required for NRasQ61K-induced AIG and invasive matrix-degrading capacity of melanocytes
Knockdown of Rac1 in melanoma cells resulted in defective proliferation in vitro (Bauer et al., 2007) and Rac1 is important downstream of KRasG12D in a lung cancer model (Kissil et al., 2007). We explored the possibility that Rac1 was required for AIG conferred by NRasQ61K on primary mouse melanocytes in culture. NRasQ61K-expressing melanocytes showed Rac1-dependent AIG (Figure 1). Survival of AIG may be important for melanoblasts during their migration through the dermis and epidermis. This could point to an importance of Rac1 in melanoma development, progression, and metastatic spread, as AIG may be important for these processes.
In addition to its contribution to AIG, Rac1 was surprisingly important for invasive and matrix degradative capacity of cultured melanocytes (Figure 5). Normal immortalized melanocytes did not form invadopodia or degrade gelatin matrix, but NRasQ61K-expressing melanocytes did so in a Rac1-dependent manner (Yamaguchi et al., 2005; Linder, 2007). Rac1 has been previously implicated in invadopodia using dominant negatives (Nakahara et al., 2003; Furmaniak-Kazmierczak et al., 2007) but to our knowledge it has not been previously reported that Rac1 is essential for invadopodia and can work downstream of NRasQ61K in melanoma cells. This has important implications for understanding how Ras confers invasion potential on cells and raises the question of which Rac1 targets are key in invadopodia formation.
Rac1 inhibition as potential therapy for melanoma
Finally, loss of Rac1 or its pharmacological inhibition halted tumor growth in vivo and prevented metastatic spread, suggesting that Rac1 is a key downstream effector of NRasQ61K in melanoma tumor growth and progression. It should be noted that although sentinel node biopsy is now widely recommended for the staging of melanomas and has proven prognostic value (Balch et al., 2011), some interesting controversies remain. For example, two recent studies highlight lymph node spread of an apparently benign melanocytic nevus, raising the possibility that even in nevi that are not genetically unstable, lymph node spread can occur (Abraham et al., 2012; McCalmont and Bastian, 2012). However, larger studies such as Balch et al. (2001) do identify lymph node spread as a significant predictor of survival. In our study, Rac1 loss or downregulation by treatment of mice with Rac inhibitors NSC23766 or EHT1864 decreased key growth signaling pathways via PAK2, AKT, and extracellular signal–regulated kinase, and slowed proliferation in tumors, as well as halting lymph node spread. Thus, we propose that Rac1 is an important candidate target for melanoma tumor growth and metastasis downstream of NRas and targeting Rac1 or its effectors could be effective therapies to pursue.
Materials and Methods
Transgenic mice and genotyping
All experiments were performed according to UK Home Office regulations. See also Supplemental Materials online.
Ex vivo imaging of melanoblast migration
Experimental setup was previously reported (Li et al., 2011).
Whole-mount staining and immunohistochemistry
Whole-mount staining of dopachrome tautomerase-LacZ-positive mouse embryos and dorsal skin was previous reported (Li et al., 2011). Immunohistochemistry was done with standard methods (see Supplementary Material online).
Growth in soft agar
A total of 1 × 104 cells were plated in 1 ml of 0.35% SeaPlaque agarose/DMEM/10% fetal bovine serum (Lonza Rockland, Rockland, ME) on top of 1.5 ml of 0.7% SeaPlaque agarose/DMEM/10% fetal bovine serum in 6-well dishes. Once solidified, the agarose was covered with 2.5 ml F-12/10% fetal bovine serum with or without 200 nM 12-O-tetradecanoylphorbol 13-acetate, which was changed twice weekly. Images were captured 2 weeks later at room temperature using Zeiss Stemi 2000C (Carl Zeiss, Welwyn Garden City, UK) dissection microscope at × 0.65 magnification. The number of colonies (>0.1 mm in diameter) and the relative size of colonies were determined 2 weeks after plating.
Fluorescent gelatin degradation assay
Gelatin degradation assay was done as previously described (Li et al., 2010).
Cell culture
Primary mouse melanocytes were isolated from 1–day-old Rac1 f/f Ink4a−/− Tyr∷CreERT2+/o (#3 and #4), Tyr∷NRasQ61K+/o Rac1 f/f Ink4a−/− Tyr∷CreERT2+/o (#10 and #11), and Tyr∷NRasQ61K+/o Rac1 f/f Ink4a−/− Tyr∷CreERT2o/o littermate mice and cultured as previously reported (Li et al., 2011).
Effector domain pulldown assay
The relative levels of GTP-bound Rac1 in tumors treated with vehicle or Rac inhibitors (NSC23766 or EHT1864) were determined by an effector pulldown assay as described previously (Vidali et al., 2006).
Organotypic invasion assay
Organotypic cultures were set up as described (Edward et al., 2005). See Supplementary Materials online for modifications.
Tumor transplantation and in vivo treatment studies
For tumor grafting experiments, #11 primary immortalized mouse melanocytes cell line were introduced into mice by subcutaneous injection of ∼1 × 106 cells in 0.1 ml. Phosphate-buffered saline into the lower flank of mouse (CD-1 nude females 6-week-old, Charles River Lab, Wilmington, MA). See also Supplementary Materials online.
Assaying proliferation in vivo
Proliferation levels were assessed by measuring bromodeoxyuridine (BrdU; Amersham Biosciences, Piscataway, NJ) incorporation. Mice were injected with 250 μl of BrdU 2 hours before being killed. Immunohistochemical staining for BrdU was then performed using an anti-BrdU antibody (BD Bioscience, Oxford, UK). At least three mice were used from each treatment for analysis.
Acknowledgments
We thank Colin Nixon and his team for excellent help with histology, Michael O'Neill and Gaurang Daruwala for allograft assistance, Ian Jackson (MRC Human Genetics, Edinburgh) for dopachrome tautomerase-LacZ reporter mice, Victor Tybulewicz (NIMR, London) for Rac1 floxed mice, and Peter D Adams (Beatson institute, Glasgow) for comments on the manuscript. We thank CRUK for core funding to LMM, OJS, and KB.
Glossary
- AIG
anchorage-independent growth
- OHT
4-hydroxytamoxifen
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Abraham RM, Ming ME, Elder DE, et al. An atypical melanocytic lesion without genomic abnormalities shows locoregional metastasis. J Cutan Pathol. 2012;39:21–24. doi: 10.1111/j.1600-0560.2011.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann J, Frutschi M, Kaloulis K, et al. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65:4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- Balch CM, Gershenwald JE, Soong SJ, et al. Update on the melanoma staging system: the importance of sentinel node staging and primary tumor mitotic rate. J Surg Oncol. 2011;104:379–385. doi: 10.1002/jso.21876. [DOI] [PubMed] [Google Scholar]
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- Bauer NN, Chen Y-W, Samant RS, et al. Rac1 activity regulates proliferation of aggressive metastatic melanoma. Exp Cell Res. 2007;313:3832–3839. doi: 10.1016/j.yexcr.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- Cook S, McCormick F. Ras blooms on sterile ground. Nature. 1994;369:361–362. doi: 10.1038/369361a0. [DOI] [PubMed] [Google Scholar]
- Demunter A, Stas M, Degreef H, et al. Analysis of N- and K-Ras mutations in the distinctive tumor progression phases of melanoma. J Investig Dermatol. 2001;117:1483–1489. doi: 10.1046/j.0022-202x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Edward M, Gillan C, Micha D, et al. Tumour regulation of fibroblast hyaluronan expression: a mechanism to facilitate tumour growth and invasion. Carcinogenesis. 2005;26:1215–1223. doi: 10.1093/carcin/bgi064. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmaniak-Kazmierczak E, Crawley SW, Carter RL, et al. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circulation Res. 2007;100:1328–1336. doi: 10.1161/CIRCRESAHA.106.147744. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- Hancock JF. Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol. 2003;4:373–385. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Seftor REB, et al. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- Hirobe T. Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat Rec. 1984;208:589–594. doi: 10.1002/ar.1092080414. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. RHO GTPASES: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Karreth FA, Tuveson DA. Modelling oncogenic Ras/Raf signalling in the mouse. Cur Opin Genet Dev. 2009;19:4–11. doi: 10.1016/j.gde.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh RN, Harris ML, Colanesi S, et al. Stripes and belly-spots—a review of pigment cell morphogenesis in vertebrates. Semin Cell Dev Biol. 2009;20:90–104. doi: 10.1016/j.semcdb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissil JL, Walmsley MJ, Hanlon L, et al. Requirement for Rac1 in a K-ras, induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Yoshida H, Yamazaki H, et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- Larue L, Dougherty N, Porter S, et al. Spontaneous malignant transformation of melanocytes explanted from Wf/Wf mice with a Kit kinase-domain mutation. Proc Natl Acad Sci USA. 1992;89:7816–7820. doi: 10.1073/pnas.89.16.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Dawson JC, Forero-Vargas M, et al. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Ma Y, Yu X, et al. Rac1 drives melanoblast organization during mouse development by orchestrating pseudopod-driven motility and cell-cycle progression. Dev Cell. 2011;21:722–734. doi: 10.1016/j.devcel.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Luciani F, Champeval D, Herbette AL, et al. Biological and mathematical modeling of melanocyte development. Development. 2011;138:3943–3954. doi: 10.1242/dev.067447. [DOI] [PubMed] [Google Scholar]
- Mackenzie MAF, Jordan SNA, Budd PS, et al. Activation of the receptor tyrosine kinase kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- McCalmont TH, Bastian BC. An unconventional deep penetrating melanocytic nevus with microscopic involvement of regional lymph nodes. J Cutan Pathol. 2012;39:25–28. doi: 10.1111/j.1600-0560.2011.01850.x. [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Ha L, Rieker J, et al. Dissection of RAS downstream pathways in melanomagenesis: a role for Ral in transformation. Oncogene. 2010;29:2449–2456. doi: 10.1038/onc.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Hay L, Jackson IJ. Ex vivo live imaging of melanoblast migration in embryonic mouse skin. Pigment Cell Melanoma Res. 2010;23:299–301. doi: 10.1111/j.1755-148X.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Otani T, Sasaki T, et al. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes to Cells. 2003;8:1019–1027. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Peters EMJ, Maurer M, Botchkarev VA, et al. Kit is expressed by epithelial cells in vivo. J Investig Dermatol. 2003;121:976–984. doi: 10.1046/j.1523-1747.2003.12478.x. [DOI] [PubMed] [Google Scholar]
- Platz A, Egyhazi S, Ringborg U, et al. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008;1:395–405. doi: 10.1016/j.molonc.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, et al. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Samuel MS, Lourenço FC, Olson MF. K-Ras mediated murine epidermal tumorigenesis is dependent upon and associated with elevated Rac1 activity. PLoS One. 2011;6:e17143. doi: 10.1371/journal.pone.0017143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GA, Cassidy L. Rac1 mediates dendrite formation in response to melanocyte stimulating hormone and ultraviolet light in a murine melanoma model. J Investig Dermatol. 1998;111:243–250. doi: 10.1046/j.1523-1747.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- Sharov A, Tobin DJ, Sharova TY, et al. Changes in different melanocyte populations during hair follicle involution (catagen) J Investig Dermatol. 2005;125:1259–1267. doi: 10.1111/j.0022-202X.2005.23959.x. [DOI] [PubMed] [Google Scholar]
- Vidali L, Chen F, Cicchetti G, et al. Rac1-null mouse embryonic fibroblasts are motile and respond to platelet-derived growth factor. Mol Biol Cell. 2006;17:2377–2390. doi: 10.1091/mbc.E05-10-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwam T, VanBrocklin MW, Russo ME, et al. Differential oncogenic potential of activated RAS isoforms in melanocytes. Oncogene. 2007;26:4563–4570. doi: 10.1038/sj.onc.1210239. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Lorenz M, Kempiak S, et al. Molecular mechanisms of invadopodium formation. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.