Abstract
The adult human skin harbors a variety of leukocytes providing immune surveillance and host defense, but knowledge about their ontogeny is scarce. In this study we investigated the number and phenotype of leukocytes in prenatal human skin (dermal dendritic cells (DDCs), macrophages, T cells (including FoxP3+ regulatory T cells), and mast cells) to unravel their derivation and to get a clue as to their putative function in utero. By flow cytometry and immunofluorescence, we found a distinction between CD206+CD1c+CD11c+ DDCs and CD206+CD209+CD1c− skin macrophages by 9 weeks estimated gestational age (EGA). T cells appear at the end of the first trimester, expressing CD3 intracytoplasmatically. During midgestation, CD3+FoxP3− and CD3+FoxP3+ cells can exclusively be found in the dermis. Similarly, other leukocytes such as CD117+ (c-kit) mast cells were not identified before 12–14 weeks EGA and only slowly acquire a mature phenotype during gestation. Our data show at which time point during gestation antigen-presenting cells, T cells, and mast cells populate the human dermis and provide a step forward to a better understanding of the development of the human skin immune system.
Introduction
The dermis harbors a variety of leukocytes, which interact to provide immune surveillance and host defense (Bangert et al., 2011; Teunissen et al., 2012). Recently, we reported that CD45+HLA-DR+ leukocytes are already present by 9 weeks estimated gestational age (EGA) in human embryonic skin and that their development and attraction depends on various growth and differentiation factors present in developing skin, leading to the sequential acquisition of the phenotypic repertoire, exemplified by the development of epidermal Langerhans cells (Schuster et al., 2009). Despite these recent advances in the understanding of the development of the skin immune system, the leukocytes of the embryonic and fetal human dermis have remained poorly characterized. In particular, our understanding of the diversity, origin as well as function of antigen-presenting cells (APCs) and mast cells along with the development of cells of the adaptive immunity in skin is still scarce and needs further elucidation.
Dermal dendritic cells (DDCs) and skin macrophages are APCs that share a similar surface marker profile. They express HLA-DR and various monocyte/macrophage markers (e.g., CD11c, CD14, CD36, CD68, FXIIIa, or CD206 (mannose receptor)) to a varying extent (Meunier et al., 1993; Nestle et al., 1993; Ebner et al., 2004; Angel et al., 2007; Zaba et al., 2009; Geissmann et al., 2010). Yet, these markers have failed to unambiguously distinguish between DDCs and macrophages. Recently, Zaba et al. (2007) proposed CD1c (BDCA-1) as a reliable marker for resident skin DCs. Controversy exists about the expression of CD209 (DC-SIGN), which has been described by some groups as a specific marker for skin macrophages (Ochoa et al., 2008; Canard et al., 2011), while other groups find co-expression with DDC subsets (Geijtenbeek et al., 2000; Zaba et al., 2007). With regard to the distinction of macrophages and dermal DCs in prenatal skin, we and others have shown that CD36+, CD1c+, FXIIIa+, and HLA-DR+ cells can be identified during the first trimester, but detection of co-expression and inclusion of other markers is necessary to gain better insight into their development (Foster et al., 1986; Fujita et al., 1991; Gibran et al., 1996; Schuster et al., 2009).
As part of the adaptive immune system, T cells fulfill important roles in immunosurveillance (Foster and Elbe, 1997; Clark et al., 2006; Clark, 2010). In adult skin, a large pool of trafficking memory T cells allows for a rapid response in case of antigenic challenge. The entry of T cells into skin is a multistep process, initialized by the binding of cutaneous lymphocyte-associated antigen (CLA)-positive T cells to E-selectin on post capillary venules (Chong et al., 2004). CCL17/thymus and activation-regulated chemokine (Hijnen et al., 2004; Saeki and Tamaki, 2006) and CCL27/cutaneous T-cell-attracting chemokine (Morales et al., 1999) have a central role for the attraction of T cells. Of note, CCL27, a chemokine constitutively produced in skin by epidermal keratinocytes, selectively attracts CLA+CCR10+ memory T cells into skin (Morales et al., 1999). In contrast to the well-studied ontogeny of T cells in fetal liver, spleen, and thymus, the phenotype of T cells in human prenatal skin has been poorly investigated. While ultrastructural studies failed to detect T cells in developing skin (Breathnach, 1977), a recent report identified naive T cells (CD4+>CD8+) at 18 weeks EGA using immunohistochemistry (Di Nuzzo et al., 2009). In the course of the development of the immune system, T cells are selected or regulated to become tolerant to self-antigens and reactive against foreign antigens. In addition to the deletion of self-reactive cells, it has been demonstrated that regulatory T cells increase in numbers during the very early stages of pregnancy and help to suppress fetal immune responses in humans (Sasaki et al., 2004; Somerset et al., 2004; Jasper et al., 2006; Mold et al., 2008; Tilburgs et al., 2008; Yang et al., 2008).
Information about mast cell ontogeny in human prenatal skin is scarce (Fujita et al., 1969; Breathnach, 1978; Omi et al., 1991). Mast cells are bone marrow–derived cells expressing the pan-leukocyte marker CD45 as well as CD117 (c-kit; Metcalfe et al., 1997). Their function in skin is essentially unknown, but probably involves both homeostatic regulation of nerves and blood vessels, as well as host defense (Galli et al., 2008). The most characteristic feature of mature mast cells is cytoplasmic granules, which contain numerous preformed mediators that are released at the time of stimulation. Proteoglycans within the mast cell granule permit the binding of a variety of cationic dyes, such as toluidine blue or Giemsa, which stain the mast cell metachromatically. Additionally, antibodies specific for two granule-associated serine proteases, tryptase and chymase, can be used to identify skin mast cells (Irani et al., 1989). Ultrastructural studies performed in the seventies and eighties of the last century identified unanimous mast cells at around 20–26 weeks EGA, containing specific granules as well as immature pre-stages (Omi et al., 1991). During the first trimester, only cells containing small vesicles with no clearly recognizable granules were rarely observed ultrastructurally representing either melanoblasts or immature mast cells (Breathnach, 1978).
Given that our understanding of multiple aspects of the development of the skin immune system is incomplete in humans and that the situation in mice differs in significant aspects (Fadel and Sarzotti, 2000; Adkins et al., 2004; Levy, 2007; Elbe-Bürger and Schuster, 2010), we believe that the study of the ontogeny of skin leukocytes has the potential to yield valuable information about the origin and relationship of skin leukocytes and the general status of the skin immune system in utero.
Results
Embryonic skin contains significant populations of DDCs and macrophages
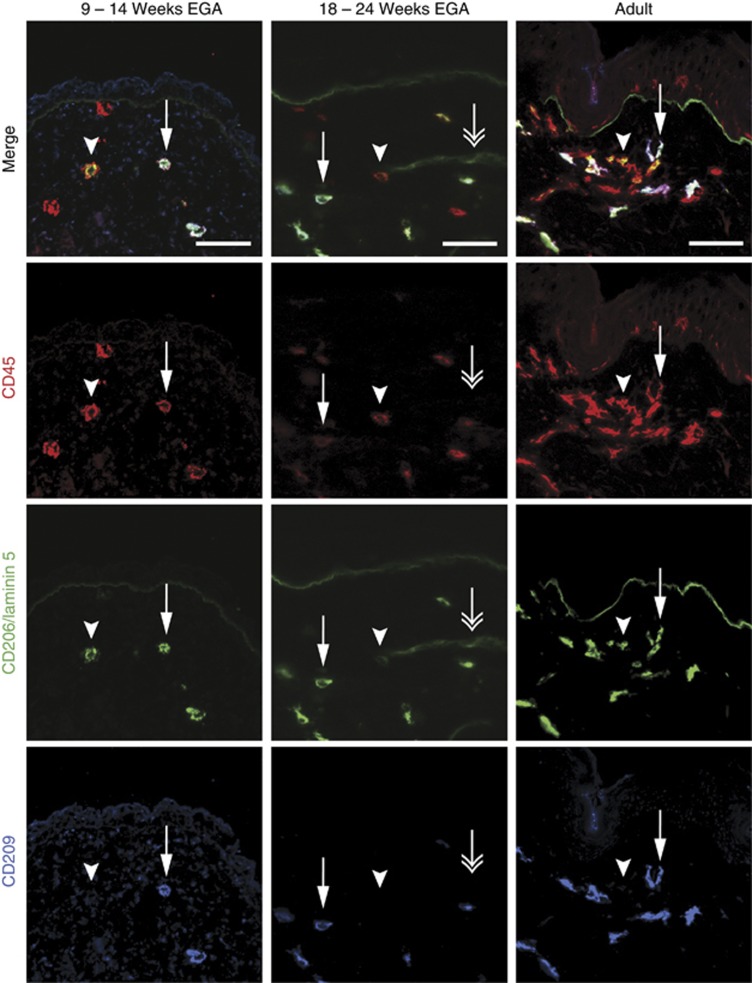
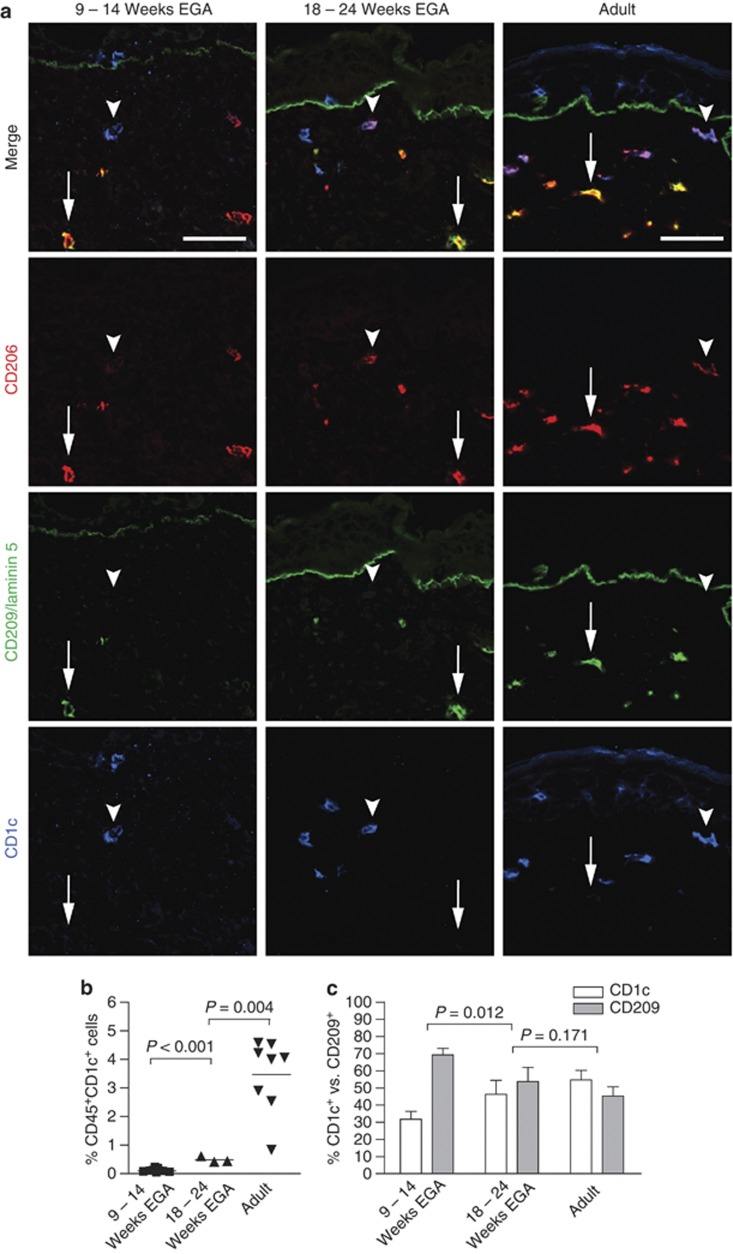
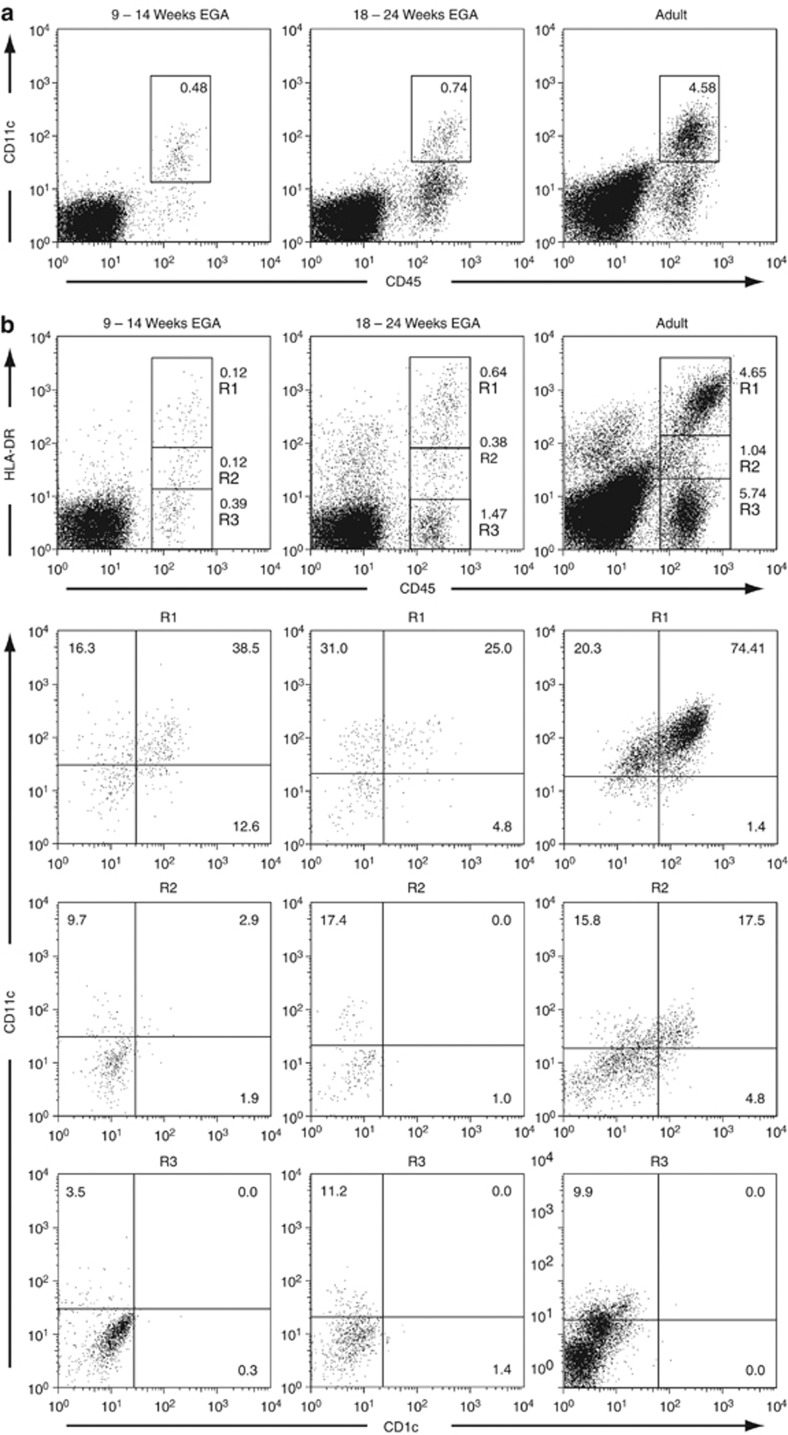
To assess whether macrophages in prenatal human dermis express important lineage molecules, skin of defined gestational age groups and, in parallel, of adults was immunostained for CD45, CD206, and CD209. Similar to adult skin, CD45+CD206+CD209+ cells (Figure 1, arrows), most probably macrophages, as well as CD45+CD206+CD209− cells (Figure 1, arrrowheads) are identified already in embryonic dermis. The staining intensity of CD206 and CD209 in embryonic skin is mostly comparable to adult skin, yet some cells show only weak expression (compare Figure 2, left column, arrowhead), implying that the upregulation of these markers occurs in the skin. Of note, CD206 staining of linear structures, most likely blood vessels, is observed occasionally in prenatal but not in adult skin (Figure 1, middle column, double arrow). Epidermal CD45+ cells at all age groups investigated lack CD206 and CD209. To evaluate whether CD1c and CD209 are mutually exclusive, triple immunofluorescence staining was performed. We found virtually nonoverlapping populations of CD206+CD1c+ DDCs (Figure 2, arrowhead) and CD206+CD209+ macrophages (arrow) already in prenatal dermis, similar to what has been reported in adult dermis, with the exception that CD1c+ cells more often lack or weakly express CD206. Statistical analysis revealed that the frequency of CD1c+ leukocytes significantly increases with gestational age (Figure 2b) and that CD209+ cells outnumber CD1c+ cells between 9 and 14 weeks EGA, reaching adult-like ratios already by midgestation (Figure 2c). By flow cytometry, we found that in all age groups investigated CD11c+ cells are detectable and that CD11c expression is restricted to CD45+ leukocytes (Figure 3a). In depth analysis of HLA-DRhighCD45+ cells reveals that CD1c+CD11c+ cells are already present in the developing skin. The HLA-DRhighCD45+ population contains a similar percentage of CD1c−CD11c+ cells but, in contrast to adult skin, a higher percentage of CD1c−CD11c− cells (Figure 3b, R1). In addition, HLA-DRlow/negCD45+ cells express CD11c to a similar extent at all time points investigated (Figure 3b, R2 and R3).
Figure 1.
CD45+CD206+CD209+ and CD45+CD206+CD209− cells are present in embryonic dermis. Immunofluorescence triple staining was performed on cryostat sections of embryonic, fetal, and adult skin. Arrows indicate triple positive cells, arrowheads CD45+CD206+ double-positive cells, and double arrows indicate CD206+ vascular structures. One of at least three experiments per group is shown. Bars=50 μm. EGA, estimated gestational age.
Figure 2.
CD206+CD1c+ DDCs can be distinguished from CD206+CD209+ skin macrophages in embryonic skin. (a) Arrows indicate CD206+CD209+ cells, and arrowheads indicate CD1c+ DCs in all indicated age groups. One of at least four experiments per group is shown. Bars=50 μm. (b) Dot graph shows the increase of CD45+CD1c+ cells in developing skin using flow cytometry. (c) Shown are relative numbers of CD1c+ and CD209+ dermal cells at selected developmental time points analyzed by immunoflourescence (n=4–5 per group). DC, dendritic cell; DDC, dermal dendritic cell; EGA, estimated gestational age.
Figure 3.
CD11c+ leukocytes are present throughout skin development. (a, b) Multiparameter flow cytometry of freshly isolated single cell suspensions of embryonic, fetal, and adult skin was performed by incubation with mAbs against the cell surface markers indicated. Gates in dot plots were set according to isotype-matched control staining. Dead cells were excluded by 7-amino-actinomycin-D uptake. Dot plots are representative of 3–6 experiments. (b) CD45+HLA-DRhigh (R1), CD45+HLA-DRweak (R2), and CD45+HLA-DR− (R3) cells from the indicated age groups are compared with regard to expression of CD11c and CD1c (lower panels). EGA, estimated gestational age.
FoxP3+ T cells are present in fetal human skin
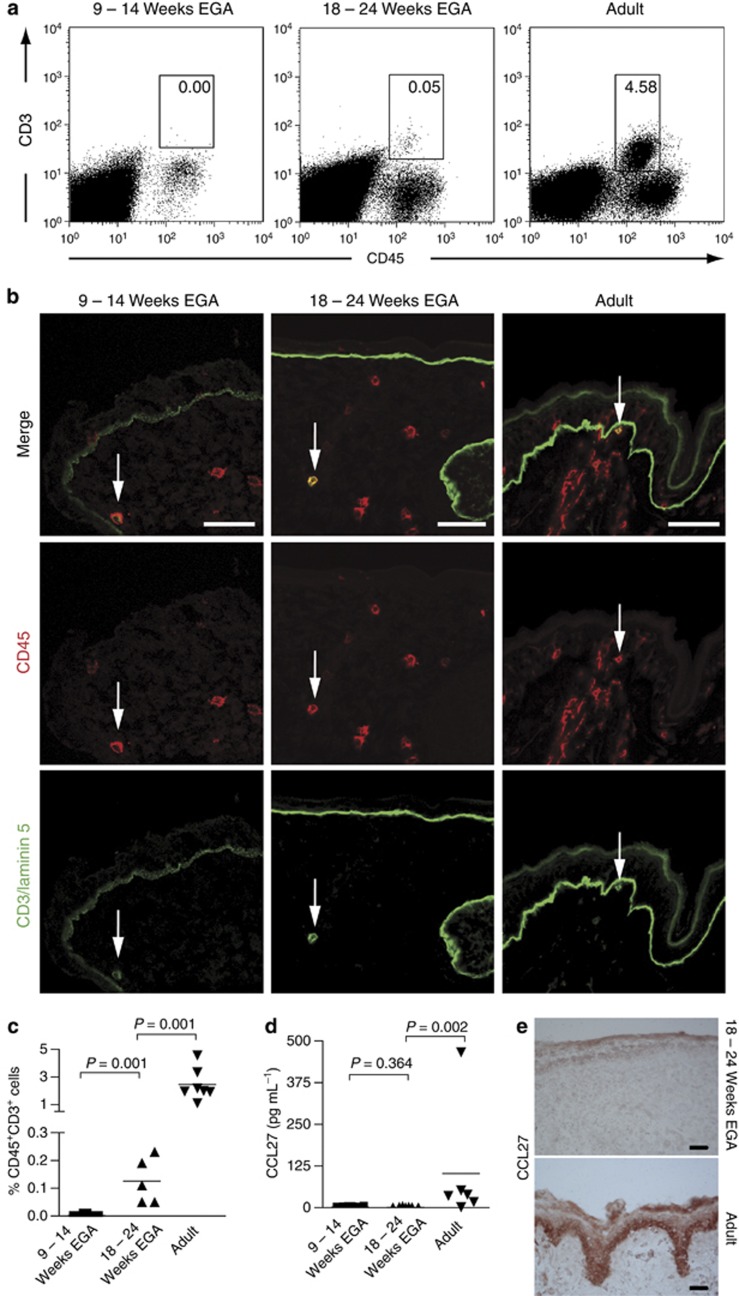
CD45+CD3+ T cells were not detectable in skin during the first trimester in six out of eight samples (Figure 4a-c), whereas two samples (11 and 14 weeks EGA) show a minute population, comprising 0.01% of total skin single cell suspensions. In these embryonic samples, CD3 was rarely found by immunofluorescence and seemed to be expressed only intracellulary (Figure 4b). During midgestation, a moderate increase in T cells takes place, but numbers are approximately 15- to 20-fold lower than in adult skin. CD3+CD45+ cells are exclusively found in the dermis during skin development.
Figure 4.
Skin T cells can be identified at midgestation. (a) Multiparameter flow cytometric analysis was performed after incubation with mAbs against the cell surface markers indicated. Gates in dot plots were set according to isotype-matched control staining. Dead cells were excluded by 7-amino-actinomycin-D uptake. Dot plots are representative of 5–8 experiments. (b) Immunofluorescence double staining was performed on cryostat sections of embryonic, fetal, and adult skin. One of at least three experiments per group is shown. Arrows denote CD45+CD3+ T cells. (c) Graphs show an increase of T-cell numbers in developing prenatal skin analyzed by flow cytometry (n=5–8). (d) Single cell suspensions of embryonic, fetal, and adult skin were cultured for 48 hours, supernatants were harvested, and CCL27 levels were analyzed by ELISA. Bars represent the mean. (e) Immunohistochemical staining was performed on cryostat sections of fetal and adult skin, and CCL27 binding was visualized using amino-ethyl-carbazole. Bars=50 μm. EGA, estimated gestational age.
Given the prominent role of CCL27 in the cutaneous recruitment of memory T cells, we analyzed skin cell culture supernatants from embryonic, fetal, and adult single cells, and found that CCL27 concentrations are significantly higher in adult than in embryonic and fetal skin (Figure 4d). In line with these data, immunohistochemical analysis of CCL27 expression revealed a lower staining intensity in fetal compared with adult epidermis (Figure 4e). Of note, skin appendages in fetal skin showed a similar staining intensity as adult skin (data not shown). Taken together, these findings indicate that fetal keratinocytes produce less CCL27 than adult keratinocytes. As the majority of skin-homing T cells in fetal skin are naive (Di Nuzzo et al., 2009) and, thus, not attracted by CCL27 (Morales et al., 1999), our finding suggests that the influx of predominately naive T cells during skin development might be independent of CCL27.
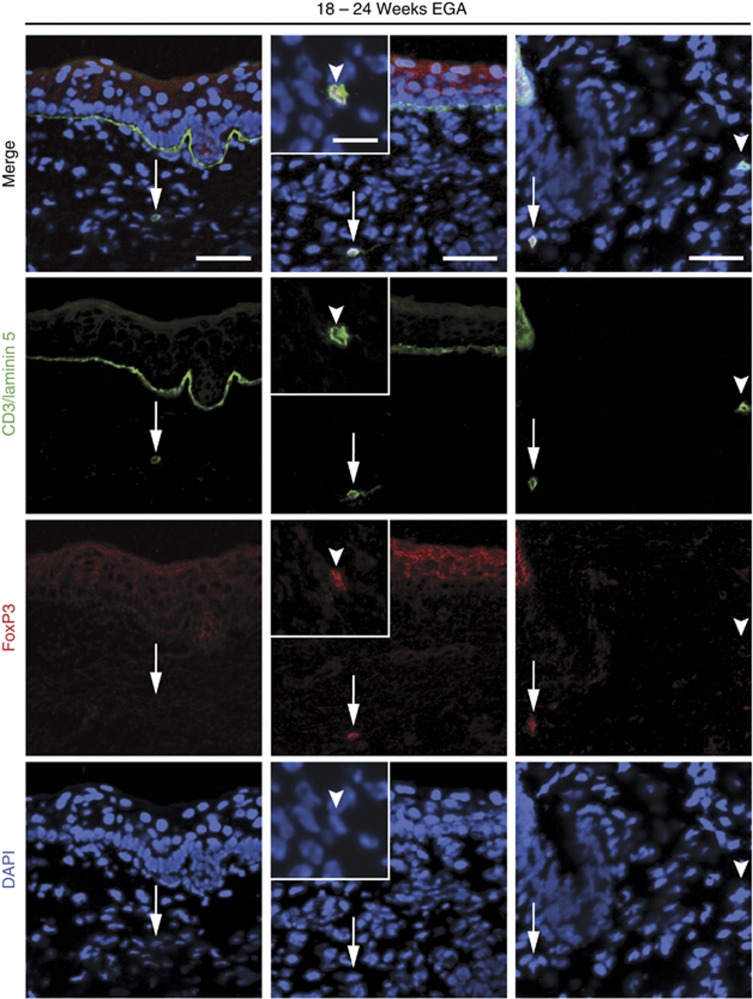
Given the key role of regulatory T cells controlling and maintaining tolerance to self-antigens and the linkage of FoxP3 to this function, we further investigated whether these cells may already be present in prenatal skin. Indeed, FoxP3 expression was found in 20.0% (SD 6.5% n=5) of all T cells (5.9 CD3+ cells cm−1, SD 2.9; n=5), a frequency comparable to what is found in the adult skin (Figure 5; Clark and Kupper, 2007; Agius et al., 2009).
Figure 5.
FoxP3+ T cells are present in fetal human skin. Immunofluorescence double staining was performed on cryostat sections of fetal skin (n=5). Arrows denote CD3+FoxP3− T cells (left panel) and CD3+FoxP3+ T cells (middle panel). Arrowheads indicate intracellular FoxP3 staining in the insert. Demonstration of a FoxP3+ and a FoxP3− T cell on the same section (right panel). Bars=50 μm, bar in inset=20 μm. DAPI, 4,6-diamidino-2-phenylindole; EGA, estimated gestational age.
Immature mast cells appear during the first trimester
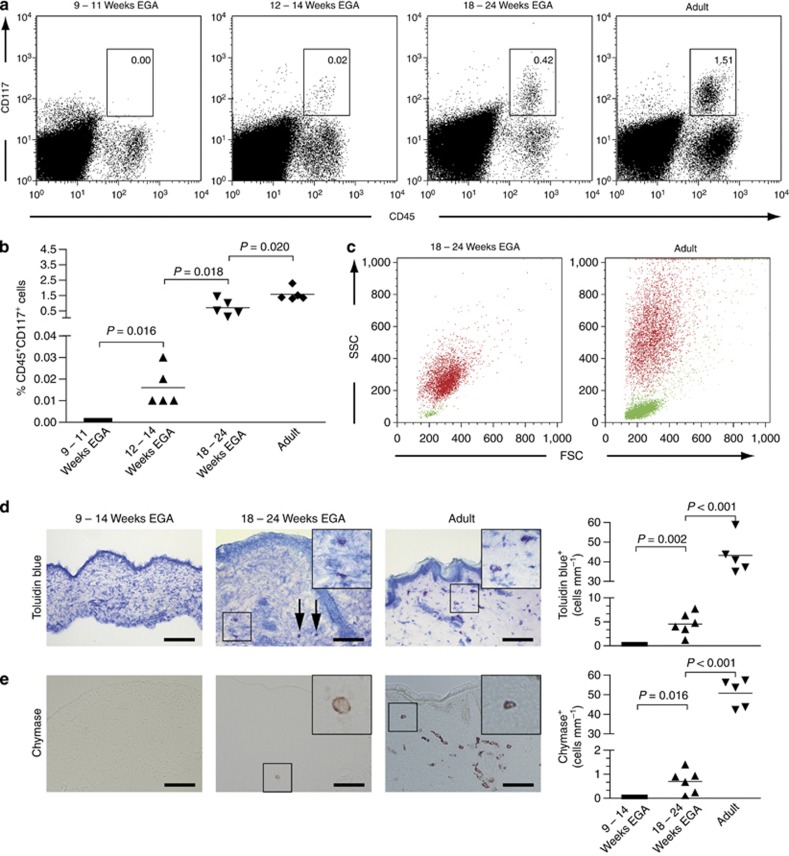
By flow cytometry, CD45+CD117+ mast cells are not detectable in human prenatal skin until 11 weeks EGA (Figure 6a and b). Coinciding with the beginning of bone marrow hematopoiesis a minute, though distinct, population of mast cells can be first identified at 12–14 weeks EGA. The frequency of CD45+CD117+ cells increases considerably during the second trimester, occasionally reaching adult-like levels (Figure 6a and b). A comparison of the scatter profiles of fetal and adult CD45+CD117+ skin mast cells reveals a substantially weaker granularity of fetal than adult mast cells (Figure 6c, red dots), indicating immaturity of fetal mast cells due to the lack of specific granules. By contrast, CD45+CD3+ T cells in fetal and adult skin have a similar forward and side scatter profile, but significantly differ in numbers (Figure 6c, green dots). In line with the suspected mast cell immaturity, we could not detect mast cells by their metachromatic staining with toluidin blue up to 14 weeks EGA (Figure 6d). Toluidin blue–expressing cells were first identified in the second trimester, but their numbers were approximately 8-fold lower than in adult skin. Similar to adult skin, these cells are preferentially found around vessels and in vicinity to skin appendages. Mature, chymase-expressing mast cells are rarely found in fetal skin (Figure 6e), but can be easily identified in adult skin. The ratio of toluidin blue/chymase-positive cells changes from 6.4:1 in fetal skin to 0.9:1 in adult skin.
Figure 6.
Mast cells appear during the first trimester but are not fully mature at midgestation. (a) Flow cytometric analysis of freshly isolated single cell suspensions of embryonic, fetal, and adult skin revealed the presence of immature mast cells in fetal skin. Shown are representative dot plots of five experiments per group. (b) Graphs show the increase in mast cell numbers of developing skin analyzed by flow cytometry. Five specimens were investigated per age group. Bars represent the mean of investigated groups. (c) The size, granularity, and numbers of CD45+CD117+ cells (red dots) and CD45+CD3+ cells (green dots) between fetal and adult skin are compared. (d, e) Toluidin blue (d) and chymase (e) stainings were performed on cryostat sections of embryonic, fetal, and adult skin (left panel). Arrows denote toluidine blue-expressing cells in fetal dermis. Bar=100 μm. The numbers of metachromatic cells (d, right panel) and chymase+ cells (e, right panel) were determined and bars represent the mean of investigated groups (n=5–6 per group). EGA, estimated gestational age; FCS, forward scatter; SSC, side scatter.
Discussion
We have shown that human embryonic skin contains almost mutually exclusive, committed CD209+ skin macrophages and CD1c+ DDCs. In addition, we have found that important cells of the skin immune system colonize the developing skin at different time points, i.e., APC by 9 weeks EGA (the earliest time point investigated), T cells during the second trimester, and mast cells at 12 weeks EGA.
The distinction of DDCs and skin macrophages by phenotypic terms can be difficult, owing to their high plasticity and/or different lineage affiliation (Meunier et al., 1993; Nestle et al., 1993; Ebner et al., 2004; Angel et al., 2007; Zaba et al., 2009; Geissmann et al., 2010). Recently, various groups proposed new markers for the identification of DDCs and skin macrophages, namely CD1c and CD209, respectively (Ochoa et al., 2008; Zaba et al., 2009; Schäkel and Hänsel, 2011). We show in this study that already by 9 weeks EGA skin macrophages and DCs can be phenotypically separated by the almost distinct expression of CD1c and CD209. Of note, both populations express CD206, making this marker suitable for detecting dermal APCs. On the basis of the expression of these molecules and on earlier results one may reason that both DCs and skin macrophages possess the ability to recognize and ingest foreign antigen in case the epithelial barrier has been breached, e.g., under the condition of microbial invasion of the amniotic fluid. The weak expression of CD206 on some CD1c+ cells as well as the fact that CD206 expression is not found on potential blood precursors (Shepherd et al., 1982) suggest an upregulation of this marker in situ, which can be induced by high levels of M-CSF and IL-6, cytokines already found in embryonic skin (Gersuk et al., 2005; Schuster et al., 2009).
The elucidation of the origin of DCs and skin macrophages is generally complicated by the diversity and plasticity of DDCs. Similar to epidermal Langerhans cells, they co-express CD11c and CD1c, yet, also a subset of CD11c+CD1c− DDCs positive for CD141 has been described (Zaba et al., 2007). In developing skin, HLA-DRhighCD45+ cells are more heterogeneous with regard to the expression of CD11c and CD1c. From an ontogenetic point of view, it is conceivable that CD11c−CD1c− cells act as precursors of CD11c+CD1c+ via CD11c+CD1c− cells, especially given the higher percentage of CD11c−CD1c−HLA-DRhigh leukocytes in prenatal skin. Whether these cells express CD141 remains to be investigated.
T cells are the prototypical cells of the adaptive immune system. In adult, noninflamed skin, the majority of resident T cells are CLA+CD45RO+ memory cells, which are recruited into skin mainly by two chemokines, CCL27 and CCL17 (Morales et al., 1999; Clark et al., 2006; Saeki and Tamaki, 2006). During in utero development, T-cell precursors are first detectable in the fetal liver at 7 weeks of gestation and are found in the developing thymus and circulation by 8–9 weeks EGA (Campana et al., 1989; Holt and Jones, 2000). With regard to function, alloreactivity is found in the fetal liver at 7 weeks EGA and T cells are believed to be immunocompetent by midgestation (Fadel and Sarzotti, 2000; Holt and Jones, 2000; Hermann et al., 2002; Michaelsson et al., 2006; Mold et al., 2008; Vermijlen et al., 2010). By immunofluorescence, we occasionally identified CD45+CD3+ cells in the first trimester, which appear to have intracellular CD3 expression and were, thus, not identified by conventional flow cytometry. CD3+ T cells are detectable in the fetal circulation at about 15–16 weeks of gestation (Haynes et al., 1988). In line with this we found that by midgestation, CD45+CD3+ cells are present in higher numbers, yet, still in numbers much smaller than in adult skin. Two possibilities—or a combination thereof—may explain this observation. First, as almost no exogenous antigen is presented in secondary lymphatic tissues in utero, skin-homing CD45RO+ memory cells simply do not develop. This is corroborated by the recent finding that T cells in fetal skin have a largely naive (CD45RA+) phenotype and do not express CLA, suggesting that T cells may nonspecifically be recruited into prenatal skin (Di Nuzzo et al., 2009). Second, it appears that developing skin contains lower levels of CCL27 than adult skin, thus, preventing the influx of memory T cells from the circulation (Morales et al., 1999). The expression of other cytokines critical for the attraction of lymphocytes in the steady state, such as CCL17, remains to be investigated. With regard to the phenotype of skin lymphocytes, we found that the majority of CD3+ cells expressed CD4, thus, confirming previous results described by Di Nuzzo et al. (2009). We exclusively observed TCR αβ+CD3+-expressing cells in fetal human skin (C Schuster, unpublished data) making the existence of a human analog of murine dendritic epidermal T cells (Steiner et al., 1988) and the recently described murine TCR γδ dermal T cells (Gray et al., 2011; Sumaria et al., 2011) in prenatal skin quite unlikely. Whether skin-homing fetal T cells have a particular function, e.g., in inducing or maintaining tolerance, is currently not known (Mold et al., 2010). Of note, fetal dermatitis after microbial invasion of the amniotic fluid results in inflammatory infiltrates including CD3+ T cells (Kim et al., 2006). This finding suggests that circulating T cells can be attracted under certain conditions.
It has been shown that fetal tissues and blood contain higher numbers of regulatory T cells to control and at least partially maintain tolerance to self-antigens (Somerset et al., 2004; Mold et al., 2008). We were surprised to find similar numbers of FoxP3+ cells in fetal and adult skin, supporting the concept for the existence of various tolerance mechanisms.
The importance of mast cells in skin immunity and homeostasis is increasingly recognized (Metcalfe, 2008). Early electron microscopy studies failed to detect definitive mast cells in human first trimester skin. At the end of the first trimester, Breathnach (1977) identified cells of unclear affiliation containing vesicles compatible with both melanoblasts and mast cells. Using flow cytometry, we showed that mast cells are not present in embryonic skin until 11 weeks but appear after 12 weeks EGA. Yet, we also observed CD45−CD117+ cells, probably representing melanoblasts and/or melanocytes. Of note, the appearance of CD45+CD117+ cells coincides with the beginning of bone marrow hematopoiesis (Tavian and Peault, 2005). Whether these CD45+CD117+ cells are derived from the newly formed bone marrow or are attracted from other hematopoietic stem cell sources due to changing microenvironmental factors is not known. Also hematopoietic progenitor cells can express CD117 in certain stages of development (Tavian and Peault, 2005). It appears quite unlikely that CD45+CD117+ cells represent hematopoietic progenitor cells—either as blood contamination or as resident cells in skin—given that these cells were not observed between 9 and 11 weeks of EGA. CD45+CD117+ mast cells during the first trimester stain neither for toluidin blue nor for chymase, suggesting that no specific granules are present and thus confirming earlier assumptions (Fujita et al., 1969). During midgestation, toluidin blue+ and chymase+ cells are first detectable, although flow cytometric analysis using the side scatter profile as well as the density of toluidin blue- and chymase-staining indicates that the mast cells are immature with regard to both granule numbers as well as granule content. Of note, just a minority of mast cells express chymase, whereas all cells in adult skin stain for this marker (Irani et al., 1989). To date, nothing is known about the functional potential of mast cells in human fetal skin. Interestingly, we occasionally observed degranulation, probably resulting from tissue preparation and cutting. This might indicate that mast cells already in fetal skin possess the capacity to exert their ancestral function. In addition, it is not known whether distinct developmental pathways or environmental factors contribute to the maturation of mast cells. The most important cytokine for mast cell development, stem cell factor, is found at highest concentration in embryonic skin, perhaps providing an important chemoattractant for blood-borne precursors (Schuster et al., 2009).
Taken together, we found that the myeloid part of the skin-resident immune system (i.e., DDCs, macrophages, mast cells and, as described previously, epidermal Langerhans cells (Schuster et al., 2009; Elbe-Bürger and Schuster, 2010)) shows a high degree of maturity already at a prenatal stage. Indeed, both in vitro experiments as well as in vivo data indicate that the human fetal skin is already capable of recognizing and responding to microbial products (Kim et al., 2006).
Materials and Methods
Skin samples
After legal termination of pregnancy, 42 specimens of human embryonic and fetal trunk skin ranging from 8 to 24 weeks EGA were studied. The age was estimated by crown-rump length and maternal history. Healthy adult (18–59 years) skin was collected after abdominal, back, and breast surgery. The study was approved by the local ethics committee and conducted in accordance with the declaration of Helsinki Principles. Parents/participants gave their written informed consent.
Preparation of skin cell suspensions
After removal of subcutaneous tissue, embryonic, fetal, and adult skin specimens were incubated on 1.2 U ml−1 Dispase II (Roche Diagnostics, Indianapolis, IN) in phosphate-buffered saline overnight at 4 °C. It was not possible to efficiently separate dermis and epidermis in embryonic skin, thus unseparated skin regardless of age was vigorously agitated in a shaking water bath in 0.53 U ml−1 Liberase 3 (Roche Diagnostics) in phosphate-buffered saline at 37 °C for 60 to 90 min. The resulting single cell suspensions were analyzed by flow cytometry or cultured for 48 hour (106 cells ml−1) in RPMI 1640 medium (Invitrogen, Eugene, OR) supplemented with 10% heat-inactivated fetal calf serum (PromoCell, Heidelberg, Germany), 25 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 10 μg ml−1 gentamicin, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 50 μM 2-mercapto-ethanol, and 0.002% antibiotic-antimycotic solution (all Invitrogen).
Flow cytometry
Single cell suspensions were stained with the following mAbs: PE-anti-CD117 (YB5.B8), PE-anti-CD11c (Leu-M5), PE-anti-HLA-DR (L243; all Becton Dickinson, Mountain View, CA), PE-Cy7-anti-CD45 (J.33; both Beckman Coulter, Fullerton, CA), FITC-anti-CD3 (UCHT1, An der Grub, Kaumberg, Austria), and FITC-anti-CD1c (M241; Ancell, Bayport, MN). Appropriate isotype controls were included. Dead cells were excluded with 7-amino-actinomycin-D (Calbiochem, Darmstadt, Germany). Five-color flow cytometry analyses were performed on a LSR-II (Becton Dickinson) and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Immunohistochemistry
Embryonic, fetal and adult skin specimens were embedded in optimum cutting tissue compound (Tissue-Tek, Sakura Finetek, Zoeterwoude, The Netherlands), snap-frozen in liquid nitrogen, and stored at −80 °C until further processing. Six-micrometer sections were cut, air-dried, fixed in ice-cold acetone for 10 minutes and washed in phosphate-buffered saline. Sections were then incubated with an unconjugated anti-CCL27 mAb (124302, R&D Systems, Minneapolis, MN) or anti-chymase mAb (B7, Millipore, Billerica, MA) overnight at 4 °C, followed by blocking of endogenous peroxidase activity by incubating sections for 10 minutes in methanol containing 0.03% hydrogen peroxide. Subsequently, sections were incubated for 2 hours at room temperature with biotin-conjugated goat anti-mouse IgG using the Elite mouse IgG Vectastain Kit (Vector Laboratories, Burlingame, CA). Biotinylated antibodies were detected with horseradish peroxidase–streptavidin and staining was visualized with amino-ethyl-carbazole (Dako, Glostrup, Denmark). Finally, sections were mounted with Aquatex (Merck, Darmstadt, Germany) and examined using a Nikon Eclipse 80 microscope (Nikon, Tokyo, Japan). Appropriate isotype controls (Becton Dickinson) were included.
Immunofluorescence
Fixed sections were stained with uncongujated primary antibodies anti-CD1c (L161; Serotec, Kidlington, UK), anti-CD209 (DCN46, Becton Dickinson), or FoxP3 (236A/E7; eBioscience, San Diego, CA) overnight at 4 °C and then detected with donkey-anti-mouse NorthernLight637 (R&D Systems). Subsequently, sections were blocked with 10% mouse serum and 2% mouse IgG (Becton Dickinson) for 1 hour at room temperature and stained with the following conjugated antibodies overnight at 4 °C: Alexa Flour 555-anti-CD45 (MEM28, Exbio, Vestec, Czech Republic), Alexa Flour 555-anti-CD206 (19.2, Becton Dickinson, both labeled using the Alexa Flour 555 Monoclonal Antibody Labeling Kit, Invitrogen), FITC-anti-CD206 (19.2; Becton Dickinson), and FITC-anti-CD3 (UCHT1, An der Grub). Alexa Flour 488-anti-Laminin 5 (D4B5; Millipore) was used to visualize the dermo–epidermal junction. Slides were mounted using Vectashield (Vector Laboratories) and images were recorded using a confocal laser scanning microscope (LSM 410; Carl Zeiss, Jena, Germany) equipped with three lasers emitting lights at 488, 543, and 633 nm. For FoxP3 staining, nuclear counterstain was performed with Vectashield containing 4,6-diamidino-2-phenylindole (Vector Laboratories).
Cytokine determination in skin cell supernatants
Skin single cell suspensions (1x106 ml−1) were cultured in 24-well plates (Costar, Lowell, MA) in RPMI 1640 supplemented as described above. After 48 hours, supernatants were harvested, snap-frozen and stored at −80 °C until use. CCL27 was determined by ELISA (R&D Systems) according to the manufactures instructions. Experiments were performed in duplicates.
Toluidin blue staining
Six micrometer frozen sections were cut, air-dried, stained in toluidin blue O/methanol (Sigma-Aldrich, St Louis, MI) for 15 seconds. After washing in phosphate-buffered saline, the sections were mounted with Aquatex (Merck) and examined using a Nikon Eclipse 80 microscope (Nikon).
Quantification of cells in skin sections
FoxP3+CD3+, chymase+, and toluidin blue-reactive cells were enumerated by two independent investigators in multiple skin sections with a total length of at least 6 cm ( × 40 object lens, Carl Zeiss; × 20 object lens; Nikon).
Statistical analysis
Differences between groups were assessed with the Mann–Whitney U test or Student's t-test (GraphPad Software, San Diego, CA). The reported P-value is a result of a two-sided test. A P-value <5% is considered statistically significant.
Acknowledgments
This project was funded by an Austrian Science Foundation grant (P19474-B13 to AE-B). We thank Herbert Strobl for his helpful comments and Anton Stift (Department of Surgery, Medical University of Vienna, Austria) for the organization of adult human samples.
Glossary
- APC
antigen-presenting cell
- CLA
cutaneous lymphocyte-associated antigen
- DDC
dermal dendritic cell
- EGA
estimated gestational age
The authors state no conflict of interest.
Footnotes
This study was performed in Vienna, Austria.
References
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- Agius E, Lacy KE, Vukmanovic-Stejic M, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel CE, Lala A, Chen CJ, et al. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int Immunol. 2007;19:1271–1279. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- Bangert C, Brunner PM, Stingl G. Immune functions of the skin. Clin Dermatol. 2011;29:360–376. doi: 10.1016/j.clindermatol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Breathnach AS. Prosser White Oration (1976). Electron micrographs from a collection. Clin Exp Dermatol. 1977;2:2–16. doi: 10.1111/j.1365-2230.1977.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Breathnach AS. Development and differentiation of dermal cells in man. J Invest Dermatol. 1978;71:2–8. doi: 10.1111/1523-1747.ep12543601. [DOI] [PubMed] [Google Scholar]
- Campana D, Janossy G, Coustan-Smith E, et al. The expression of T cell receptor-associated proteins during T cell ontogeny in man. J Immunol. 1989;142:57–66. [PubMed] [Google Scholar]
- Canard B, Vachon H, Fontaine T, et al. Generation of anti-DC-SIGN monoclonal antibodies capable of blocking HIV-1 gp120 binding and reactive on formalin-fixed tissue. Immunol Lett. 2011;135:165–172. doi: 10.1016/j.imlet.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Chong BF, Murphy JE, Kupper TS, et al. E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: a foundation for a cutaneous immunosurveillance system. J Immunol. 2004;172:1575–1581. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nuzzo S, Pavanello P, Masotti A, et al. Densities, distribution and phenotypic expression of T cells in human fetal skin. Arch Dermatol Res. 2009;301:753–755. doi: 10.1007/s00403-009-0943-9. [DOI] [PubMed] [Google Scholar]
- Ebner S, Ehammer Z, Holzmann S, et al. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int Immunol. 2004;16:877–887. doi: 10.1093/intimm/dxh088. [DOI] [PubMed] [Google Scholar]
- Elbe-Bürger A, Schuster C. Development of the prenatal cutaneous antigen-presenting cell network. Immunol Cell Biol. 2010;88:393–399. doi: 10.1038/icb.2010.13. [DOI] [PubMed] [Google Scholar]
- Fadel S, Sarzotti M. Cellular immune responses in neonates. Int Rev Immunol. 2000;19:173–193. doi: 10.3109/08830180009088504. [DOI] [PubMed] [Google Scholar]
- Foster CA, Elbe A.1997Lymphocyte subpopulations of the skinIn: Bos JD (ed).Skin Immune System (SIS),2nd edn. CRC Press: Boca Raton, New York; 85–103. [Google Scholar]
- Foster CA, Holbrook KA, Farr AG. Ontogeny of Langerhans cells in human embryonic and fetal skin: expression of HLA-DR and OKT-6 determinants. J Invest Dermatol. 1986;86:240–243. doi: 10.1111/1523-1747.ep12285201. [DOI] [PubMed] [Google Scholar]
- Fujita H, Asagami C, Murozumi S, et al. Electron microscopic studies of mast cells of human fetal skins. J Ultrastruct Res. 1969;28:353–370. doi: 10.1016/s0022-5320(69)80027-4. [DOI] [PubMed] [Google Scholar]
- Fujita M, Furukawa F, Horiguchi Y, et al. Regional development of Langerhans cells and formation of birbeck granules in human embryonic and fetal skin. J Invest Dermatol. 1991;97:65–72. doi: 10.1111/1523-1747.ep12478115. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, et al. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersuk G, Hiraoka A, Marr KA. Human monocytes differentiate into macrophages under the influence of human KPB-M15 conditioned medium. J Immunol Methods. 2005;299:99–106. doi: 10.1016/j.jim.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Gibran NS, Nickoloff BJ, Holbrook KA. Ontogeny and characterization of factor XIIIa+ cells in developing human skin. Anat Embryol (Berl) 1996;193:35–41. doi: 10.1007/BF00186831. [DOI] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Singer KH, Denning SM, et al. Analysis of expression of CD2, CD3, and T cell antigen receptor molecules during early human fetal thymic development. J Immunol. 1988;141:3776–3784. [PubMed] [Google Scholar]
- Hermann E, Truyens C, Alonso-Vega C, et al. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood. 2002;100:2153–2158. [PubMed] [Google Scholar]
- Hijnen D, De Bruin-Weller M, Oosting B, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell-attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–340. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- Irani AM, Bradford TR, Kepley CL, et al. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12:301–308. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- Kim YM, Romero R, Chaiworapongsa T, et al. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathol. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Meunier L, Gonzalez-Ramos A, Cooper KD. Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions. Identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells. J Immunol. 1993;151:4067–4080. [PubMed] [Google Scholar]
- Michaelsson J, Mold JE, McCune JM, et al. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Zheng XG, Thompson CB, et al. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–6545. [PubMed] [Google Scholar]
- Ochoa MT, Loncaric A, Krutzik SR, et al. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omi T, Kawanami O, Honda M, et al. Human fetal mast cells under development of the skin and airways. Arerugi. 1991;40:1407–1414. [PubMed] [Google Scholar]
- Saeki H, Tamaki K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J Dermatol Sci. 2006;43:75–84. doi: 10.1016/j.jdermsci.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Schäkel K, Hänsel A. News from dendritic cells in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2011;11:445–450. doi: 10.1097/ACI.0b013e32834a977a. [DOI] [PubMed] [Google Scholar]
- Schuster C, Vaculik C, Fiala C, et al. HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J Exp Med. 2009;206:169–181. doi: 10.1084/jem.20081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd VL, Campbell EJ, Senior RM, et al. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982;32:423–431. [PubMed] [Google Scholar]
- Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G, Koning F, Elbe A, et al. Characterization of T cell receptors on resident murine dendritic epidermal T cells. Eur J Immunol. 1988;18:1323–1328. doi: 10.1002/eji.1830180904. [DOI] [PubMed] [Google Scholar]
- Sumaria N, Roediger B, Ng LG, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- Teunissen MB, Haniffa M, Collin MP. Insight into the immunobiology of human skin and functional specialization of skin dendritic cell subsets to innovate intradermal vaccination design. Curr Top Microbiol Immunol. 2012;351:25–76. doi: 10.1007/82_2011_169. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Roelen DL, van der Mast BJ, et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- Vermijlen D, Brouwer M, Donner C, et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med. 2010;207:807–821. doi: 10.1084/jem.20090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Qiu L, Chen G, et al. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, et al. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol. 2009;129:302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]