Abstract
Objectives
To assess the immunohistochemical expression of Ki-67 and Proliferating Cell Nuclear Antigen (PCNA) as proliferative markers to study proliferative activity and CD34 as an endothelial cell marker in order to study vascular proliferation in astrocytomas in correlation with some clinicopathological parameters (age, gender, site of the tumor, and tumor grade).
Methods
A retrospective study wherein a total of 51 formalin-fixed paraffin-embedded brain astrocytoma excisional biopsies covering the period of June 2009 to February 2011 were retrieved from the archival materials of the Specialized Surgical Hospital in Medical City in Baghdad, Iraq. The histopathological diagnosis had been revised and all cases were stained by immunohistochemical technique with Ki-67, PCNA, and CD34 tumor markers. Values were considered statistically significant when p<0.05.
Results
Fibrillary astrocytoma (WHO grade II) was found to be the most common type among astrocytic tumors with the peak age incidence of astrocytomas found in the second and fifth decades of life, and a slight male predominance had been identified. There was a significant correlation between the age of the patients and the grade of the tumor, Ki-67 and PCNA labeling indices, and microvessel density (MVD) detected by CD34 (p<0.05). There was a highly significant correlation between Ki-67 and PCNA labeling indices in astrocytomas (p<0.001).
Conclusion
A significant correlation was found between Ki-67, PCNA labeling indices, and MVD (microvessel density) detected by CD34, and between the clinicopathological variables of astrocytomas (age and grade of tumor). Hence, Ki-67 and PCNA, as markers for proliferation, and MVD as a marker of angiogenesis, could be used as ancillary methods in the differentiation of borderline grades of astrocytomas.
Keywords: Ki-67, PCNA, CD34, Astrocytomas
Introduction
Astrocytic tumors are the most common primary tumors of the central nervous system. These tumors have an inherit tendency for progression and recurrence. The histopathological examination and grading do not always identify the subset of these tumors, especially when the tumor sample is small or inadequate.1 The annual incidence of CNS tumors ranges from 10 to 17 per 100,000 persons for intracranial tumors and 1 to 2 per 100,000 persons for intraspinal tumors; about half to three-quarters are primary tumors and the rest are metastatic. In childhood, the CNS tumors are likely to arise in the posterior fossa, accounting for about 20% of all childhood tumors.2 In Iraq, CNS tumors are the fifth most common tumor in adults and the second most common in children.3 Several grading systems are used to grade astrocytomas. The most widely used system is the World Health Organization (WHO) classification (1979, 1993, 2000, and 2007) that grades astrocytomas (I-IV) based on cytological atypia, mitotic activity, vascular proliferation, and necrosis: pilocytic astrocytoma (grade I), diffuse astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma (grade IV).4
Since proliferative activity is a reliable method to assess tumor biology, there has been continuous research to find such biological markers. These markers may assist significantly in the evaluation of proliferative activity in anaplastic astrocytomas and even have prognostic value. The monoclonal antibody Ki-67/MIB-1 is commonly used and has proven prognostic and diagnostic power in astrocytic tumors.5 Ki-67 is a nuclear antigen expressed in the G1, S, G2, and M phases of the cell cycle. The monoclonal antibody MIB-1 detects this nuclear antigen expressed by proliferating cells during the entire cell cycle. The percentage of immunopositive cells is referred to as the MIB-1 labeling index (L1).6 It has been recorded that the average level of MIB-1 LI varies considerably in the different grades of astrocytomas, but considerable overlap can be observed between them.7 All studies show increasing values of Ki-67/MIB-1 LI with increasing grade of malignancy. Most of them demonstrate that MIB-1 LI differentiates well between diffuse astrocytomas WHO grade II (AII) and anaplastic astrocytomas (AA) and between (AII), as well as glioblastomas (GM), but not between AA and GM. There is, however, considerable overlap of indices between the different malignancy groups. Further, in most studies; positive correlations between MIB-1 LI and survival were found, though the proposed cut-off values vary substantially between the reports. The reviewed studies reported MIB-1 LI as an important prognostic factor in human astrocytomas. Due to the great spread of values between the various tumor grades however, MIB-1 LI cannot be used as a diagnostic factor alone but should be used in combination with established criteria of histological malignancy. It may be especially useful in cases where histology reveals a low-grade astrocytoma, whereas other parameters indicate a more malignant neoplasm.8 Bowers et al. found that children with pilocytic astrocytomas with an MIB-1 LI of more than 2.0 have a shortened progression-free survival.9
Several studies have emphasized the association of PCNA-LI (Proliferating Cell Nuclear Antigen Labeling Index) with tumor malignancy grade and to have prognostic significance in a number of malignancies. It has been found that there is an association between PCNA-LI and histological grade implying that this marker could assist in discriminating between grade II and grade III astrocytoma.10 Studies showed that the highest PCNA and Ki-67 LI values were detected in glioblastoma (GBM) while pilocytic astrocytoma showed the lowest values. In such tumors, the correlation between the increasing grade and PCNA, as well as Ki-67 LI values were statistically significant. A correlation between the clinical outcome and Ki-67, as well as PCNA LI values was also detected.11
Microvessel density (MVD) is quite a useful parameter in the evaluation of angiogenesis. Studies revealed that MVD by CD34 is significantly different between normal brain tissue and astrocytoma. MVD is increased with the progression of the pathological grade of astrocytoma. Significant differences of MVD were found among astrocytomas of different grades. Thus, high MVD may be a contributing factor to the invasive growth of astrocytoma.12 The aim of the present study is to assess the immunohistochemical expression of Ki-67 and PCNA as proliferative markers to detect proliferative activity and the presence of CD34 as an endothelial cell marker to study vascular proliferation in astrocytomas in correlation with some clinicopathological parameters (age, gender, site of the tumor and tumor grade).
Methods
In this retrospective study, a total of 51 formalin-fixed paraffin-embedded brain excisional biopsies from Iraqi patients covering the period from June 2009 to February 2011. The study included 22 cases of diffuse fibrillary astrocytomas, 16 cases of glioblastomas, 7 cases of pilocytic astrocytomas and 6 cases of anaplastic astrocytomas were retrieved from the archival materials of specialized surgical hospital in medical city. All the clinicopathological parameters (such as age, gender, site of tumor and tumor grade) were obtained from the available histopathological reports. Ethical approval was obtained from the institution in which the study was carried out in order to enable us to record patients’ clinical data from their files.
From each block, 4 sections of 4 µm thickness were taken. One section was stained with hematoxylin and eosin (Fig. 1) for revision of the histopathological diagnosis, and the other 3 sections were stained immunohistochemically using three steps-indirect streptavidin method for Monoclonal Mouse Anti-Human Ki-67 Antigen (MIB-1), clone M 7240, (manufactured by DAKO) Denmark, Monoclonal Mouse Anti-Rat Proliferting Cell Nuclear Antigen (PCNA), Clone PC10, (manufactured by DAKO) and Monoclonal Mouse Anti-Human CD34, clone QBEnd-10, (manufactured by DAKO, Denmark). Technical negative controls were obtained by omitting the primary antibody for the three markers under identical test condition, respectively. Sections from a lymph node with follicular lymphoid hyperplasia known to be immunoreactive for Ki-67, PCNA and CD34 were used as a positive control (as recommended by the manufacturer).
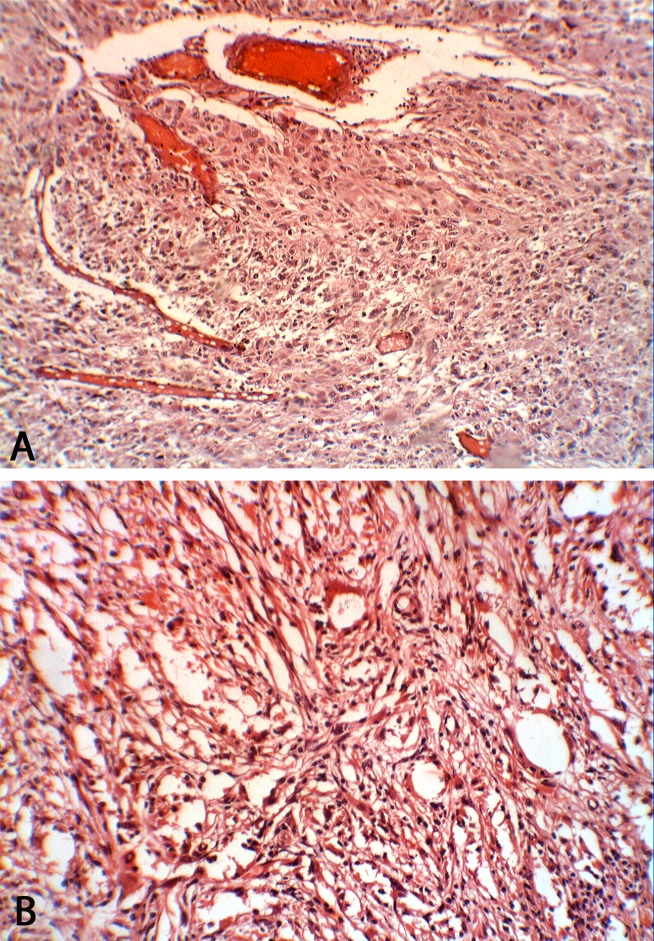
Figure 1.
Glioblastoma multiforme (grade IV) stained with H& E demonstrating marked cellularity with marked hyperchromatism and pleomorphism. Note the prominent vascularity as well as the area of necrosis. (A) At power (×10); (B) At power (×40).
Positive immunohistochemical staining for Ki-67 is brown nuclear with a diffuse pattern, (Fig. 2). Ki-67 expression was evaluated semi-quantitatively. It was scored by counting at least 1000 cells in 10 high-power fields. Every brown stained nucleus was considered positive, irrespective of intensity. The percentage of positive stained cells was recorded as Ki-67 labeling index (Ki-67 LI).13 For PCNA, cells labeled by the antibody displayed a staining almost entirely confined to the nucleus and with a diffuse or granular pattern or a mixture of both, the color of the stain is brown, (Fig. 3). PCNA expression was evaluated semi-quantitatively. It was scored by counting 1000 cells in 10 high-power fields. Every brown stained nucleus was considered positive, irrespective of intensity. The percentage of positive stained cells was recorded as PCNA labeling index (PCNA LI).14
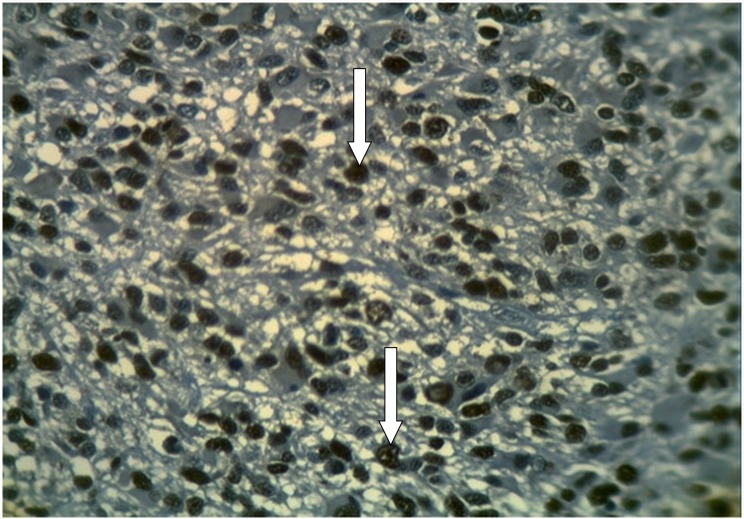
Figure 2.
Glioblastoma multiforme (grade IV) stained immunohistochemically with anti-Ki-67 showing positive brown nuclear expression of Ki-67 with large number of stained nuclei reflecting active cellular proliferation (arrows) (×40).
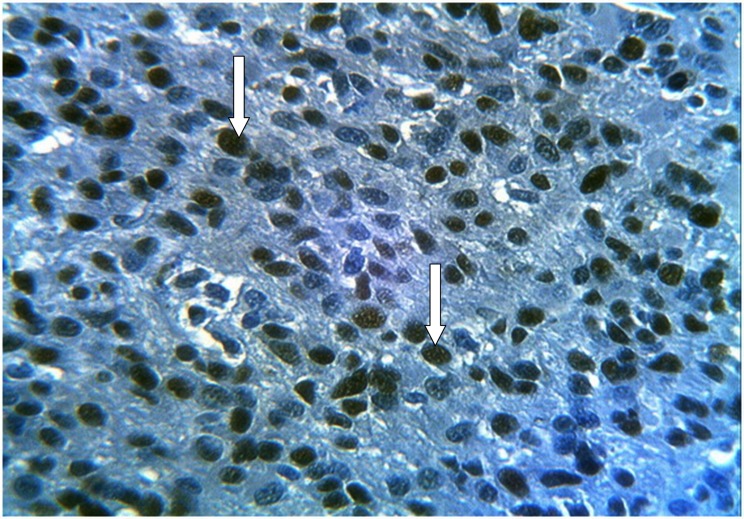
Figure 3.
Glioblastoma multiforme (grade IV) stained immunohistochemically with anti-PCNA showing positive brown nuclear expression of PCNA (diffuse or granular stained nuclei) with large number of stained nuclei reflecting increased cellularity (arrows) (×40).
Brown cytoplasmic staining of endothelial cells of CD34 is considered positive reaction, (Fig. 4). MVD was calculated according to Weidner’s method. Sections were scanned at low power (×10) looking for hot spots (the hot spot area is an area with the most dense vessel growth). Only hot spots close to the tumor cell clusters in viable areas (non-necrotic and non-sclerotic areas) were included in this study. Once the regions of interest were defined, microvessel count (MVC) was performed by counting the individual stained blood microvessels (at power ×20) representing a field size of 0.74 mm2. Three hot spots were chosen, and MVD was calculated by dividing the mean of the MVC of the examined fields on the high-power field area which is 0.74 mm2.15
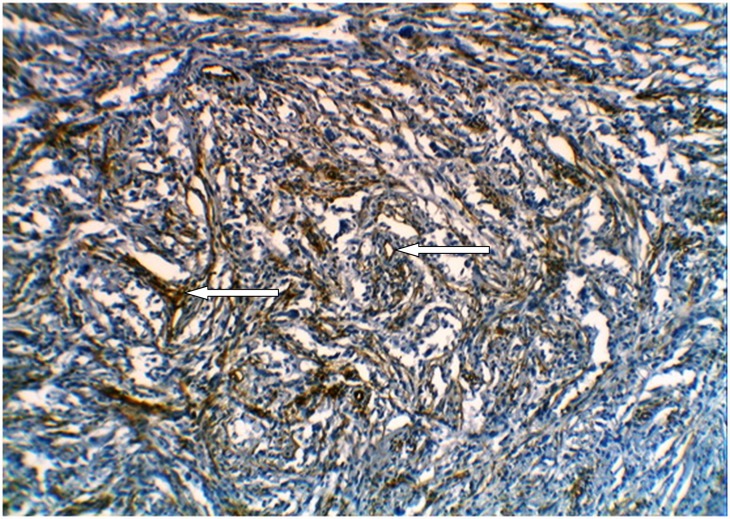
Figure 4.
Glioblastoma multiforme (grade IV) stained immunohistochemically with anti-CD34 showing positive brown cytoplasmic staining of endothelial cells and marked microvessels proliferation(arrows) (×10).
Statistical analysis was performed using SPSS V. 17 (statistical package for social sciences) and Excel 2007 programs. Data analysis was done using t-test and Spearman rank correlation for two continuous variables, ANOVA (analysis of variance) test for more than two continuous variables. Data were expressed as mean±SEM (the standard error of the mean). Values were considered statistically significant when p-value is less than 0.05.
Results
The mean±SEM of age for cases enrolled in the present study was 35.98±2.67 years with a range of 2-68 years. Statistical analysis showed that the two peak incidences of astrocytomas were found in the second and fifth decade of life. Gender distribution of brain astrocytoma cases showed slight male predominance with 27 (53%) cases compared to female with 24 (47%) cases; male to female ratio was 1.125:1. Regarding the site of astrocytoma, 17 (33.3%) cases were frontal, 16 (31.4%) cases were cerebellar, 13 (25.5%) cases were parietal and 5 (9.8%) cases were temporal. When the tumor grade is taken into consideration, 7 (13.2%) cases were pilocytic astrocytomas (WHO grade I), 22 (43.1%) cases were fibrillary astrocytomas (WHO grade II), 6 (11.8%) cases were anaplastic astrocytomas (WHO grade III) and 16 (31.4%) cases were glioblastomas (WHO grade IV).
In the studied cases, the mean ± SEM of Ki-67 LI was 17.76%± 2.46 with a range of 0% to 70% whereas the mean±SEM of PCNA LI was 20.98% ± 2.71 with a range of 0% to 80%. Regarding the CD34 antibody, the mean ± SEM of MVD was 37.3 ± 3.43 with a range from 4.05-108.1.
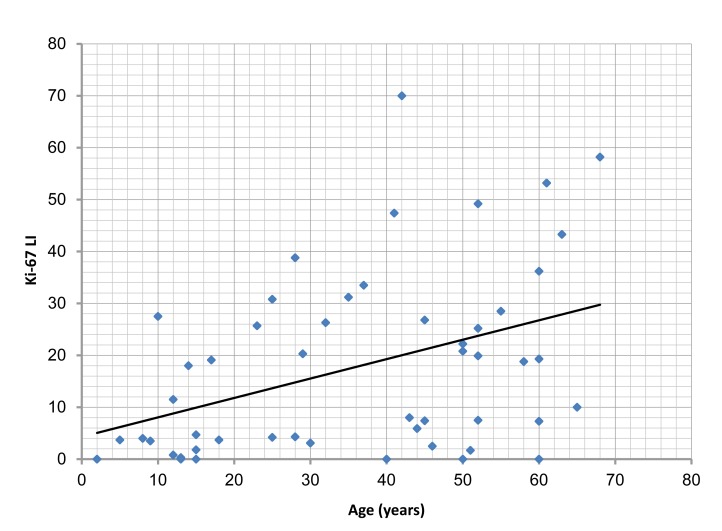
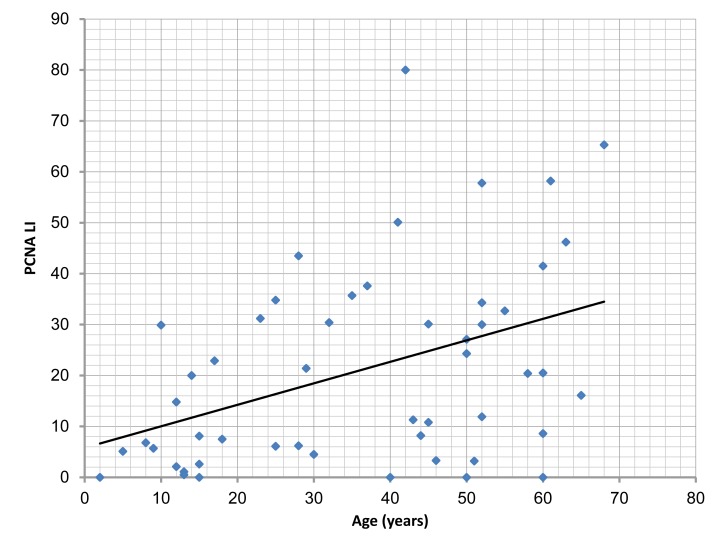
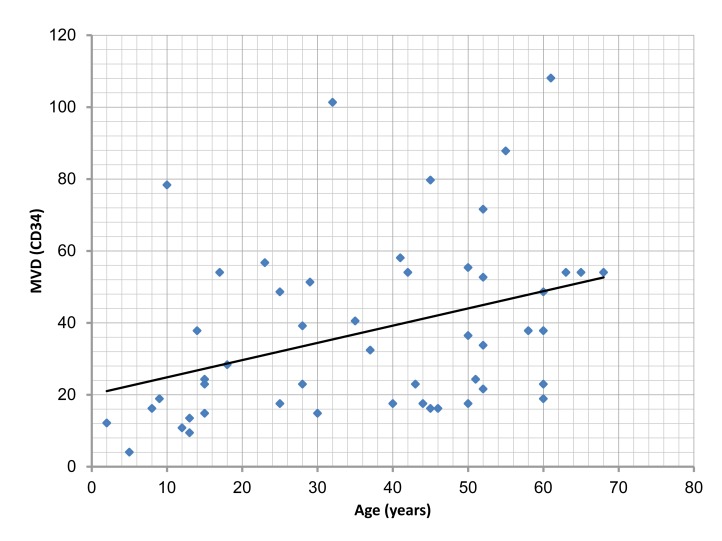
There was a significant correlation between the age of cases studied, and Ki-67 LI, PCNA LI and MVD (p=0.003, 0.002, 0.007, respectively). (Figs. 5, 6, 7,)
Figure 5.
Correlation of Ki-67 labeling index (Ki-67 LI) with the age of the studied cases, (r=0.407; p=0.003).
Figure 6.
Correlation of PCNA labeling index(PCNA LI) with the age of the studied cases, (r=0.416; p= 0.002).
Figure 7.
Correlation of microvessel density (MVD) with the age of the studied cases, (r=0.373; p=0.007).
There was no significant correlation between gender and site of the tumor, and Ki-67 LI, PCNA LI, and MVD. (Tables 1, 2)
Table 1. Correlation of Ki-67 labeling index (Ki-67 LI), PCNA labeling index (PCNA LI) and microvessel density (MVD) with the gender of the cases.
| Marker | Gender | Mean ± SEM | p |
|---|---|---|---|
| Ki-67 LI | Female | 17.46%±3.97 | 0.909 |
| Male | 18.03%±3.10 | ||
| PCNA LI | Female | 20.63%±4.38 | 0.906 |
| Male | 21.30%±3.41 | ||
| CD34 (MVD) | Female | 37.04±4.98 | 0.944 |
| Male | 37.53±4.83 |
Table 2. Correlation of Ki-67 labeling index (Ki-67 LI), PCNA labeling index (PCNA LI) and microvessel density (MVD) with the site of astrocytomas.
| Marker | Site | Mean | SEM | p |
|---|---|---|---|---|
| Ki-67 LI | Cerebellum | 17.56% | 5.22 | 0.999 |
| Frontal | 17.61% | 4.09 | ||
| Temporal | 17.14% | 10.74 | ||
| Parietal | 18.44% | 3.63 | ||
| PCNA LI | Cerebellum | 20.45% | 5.78 | 0.998 |
| Frontal | 21.53% | 4.40 | ||
| Temporal | 20.16% | 11.86 | ||
| Parietal | 21.24% | 4.16 | ||
| CD34 (MVD) | Cerebellum | 31.41 | 6.64 | 0.135 |
| Frontal | 33.54 | 4.25 | ||
| Temporal | 33.23 | 8.60 | ||
| Parietal | 51.03 | 7.93 |
Significant increases in Ki-67 LI and PCNC LI, as well as MVD among astrocytoma grades were identified. (Table 3)
Table 3. Correlation of Ki-67 labeling index (Ki-67 LI), PCNA labeling index (PCNA LI) and microvessel density (MVD) with the histopathological grade of the cases.
| Marker | Grade | Mean | SEM | p |
|---|---|---|---|---|
| Ki-67 LI | Pilocytic | 1.51% | 0.64 | <0.001 |
| Fibrillary | 6.42% | 1.26 | ||
| Anaplastic | 23.13% | 2.25 | ||
| glioblastoma | 38.45% | 3.45 | ||
| PCNA LI | Pilocytic | 2.60% | 0.94 | <0.001 |
| Fibrillary | 8.50% | 1.41 | ||
| Anaplastic | 28.88% | 2.59 | ||
| glioblastoma | 43.23% | 3.86 | ||
| CD34 (MVD) | Pilocytic | 11.57 | 1.52 | <0.001 |
| Fibrillary | 23.82 | 2.13 | ||
| anaplastic | 43.46 | 4.17 | ||
| glioblastoma | 64.77 | 5.20 |
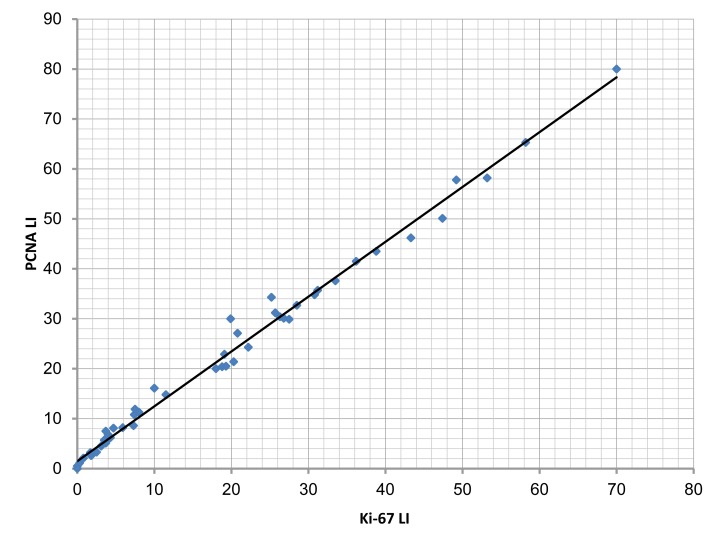
From a statistical point of view there was a positive correlation between Ki-67 LI and PCNA LI in all types of astrocytomas, this correlation was highly significant (p<0.001). (Fig. 8)
Figure 8.
The correlation between Ki-67 labeling index (Ki-67 LI) and PCNA labeling index (PCNA LI) in the studied cases (r=0.995; p<0.001).
Discussion
One of the best and well known immunohistochemical methods for evaluating the proliferation rate is quantitation of Ki-67, which was developed and introduced by Gerdes in 1993. A technique which shows all phases of the cell cycle except cells in G0 phase and because the use of Ki-67 antigen is restricted to frozen material, a new antibody (MIB-1) to an epitope of the Ki-67 antigen is used on paraffin-embedded tissue. Although this marker is a helpful marker for evaluating cellular proliferation, previous studies that used it in grading gliomas have shown conflicting results.1
In the present study, there was a significant correlation between the age and the Ki-67 LI (p<0.05). This result is supported by the Egyptian study done by Abdelaziz et al.16 and by a study done by Rodriquez-Pereira et al.17 While Isolan et al.18 revealed no correlation between age and the expression of Ki-67. This variation may be due to different antibodies used for immunohistochemical detection of Ki-67 antigen (Ki-67 antibody was used in the study of Isolan et al. while MIB-1 was used in this study. MIB-1 antibody can be used on formalin-fixed paraffin-embedded section, and this is its advantage over the original Ki-67 antibody), in addition to differences in population size and ethnic groups.1
The present study found no significant correlation between gender and Ki-67 LI (p>0.05). This result is in agreement with the results of the studies done by Mahzouni et al.1 Abdelaziz et al.16 and Liwei and Xiuzhen19; however, a study done by Yonghua et al.20 demonstrated a significant correlation between gender and immunohistochemical expression of Ki-67. This discordance could be attributed to environmental, racial and geographical differences, in addition to the difference in the sample size and antibodies used for the detection of Ki-67 antigen.
The current work showed that Ki-67 LI was not significantly affected by the site of astrocytomas (p<0.05). This observation agrees with study done by Liwei and Xiuzhen.19
There was a highly significant correlation between the histopathological grade of astrocytomas and Ki-67 LI (p<0.001). This result agrees with the studies done by Abdelaziz et al.,16 Mahzouni et al.,1 Atik et al.,21 Kayaselcuk et al.,11 Liwei and Xiuzhen.,19 Huang.,22 Rodriquez-Pereira et al.,17 and Scott et al.23
Proliferating cell nuclear antigen (PCNA) is a 36-kd DNA polymerase delta auxiliary protein that complexes with cyclin D and cyclin-dependent kinases, involved in the proliferation of neoplastic as well as non-neoplastic cells, and it is specifically expressed in proliferating cell nuclei. This specific antibody recognizes PCNA protein, which is at the maximum level in the late G1 and S phase of proliferating cells.24 The current study reported a significant correlation between the age and PCNA LI (p<0.05), this result agrees with the study done by Anq et al.25 However, it disagrees with a Chinese one done by Ripple.26 This variation may be due to different antibodies used for immunohistochemical detection of PCNA antigen in addition to genetic, environmental factors, racial and sample size differences. There was no significant correlation between gender and PCNA LI (p>0.05). This result is in accordance with a Chinese study done by Chu et al.27 Regarding the site of astrocytomas, there was no significant correlation between the site of astrocytomas and the PCNA LI (p>0.05). This result is supported by studies done by Chu et al.27 and Lebelt et al.28 There was a highly significant correlation between histopathological grade of astrocytomas PCNA LI (p<0.001). This result agrees with studies done by others.11,25,27,29-34
The present study recorded a significant correlation between the age of patients and MVD (p<0.05). This result is supported by studies conducted by Leon et al.35 and Izycka-Swieszewska et al.36 in which a significant correlation between age and MVD in glioblastomas had been identified, while Lebelt et al.37 revealed no significant difference between age and MVD (detected by CD31 and anti-von Willebrand factor). This difference is attributed to many factors, including differences in sample size, markers used to detect MVD, and population. There was no significant correlation between gender and MVD (p>0.05). This result agrees with other studies done by El-Sayed and Taha,38 and Chu et al.27 Taking the site in consideration, there was no significant correlation between the site of astrocytomas and MVD (p>0.05). This result is in concordance with that obtained by El-Sayed and Taha.38 Chu et al.,27 and Lebelt et al.37 The present study demonstrated a highly significant correlation between MVD and the grade of astrocytoma (p<0.001). This result goes with studies done by others,22,27,38-42 but disagrees with an Iranian study done by Mahzouni et al.43 who used factor VIII-related antigen (von Willebrand factor) to detect the MVD. This discordance may be attributed to different sample size and different markers used to assess angiogenesis. This study showed a significant correlation between Ki-67 LI and PCNA LI as markers of proliferation in cases of astrocytomas (r=0.995; p<0.001), a finding similar to other studies.44-47
Conclusion
A significant correlation has been found between Ki-67, PCNA labeling indices and MVD detected by CD34 immunohistochemical expression and clinicopathological variables of astrocytomas (age and grade of tumor); hence, Ki-67 and PCNA, as markers for proliferation, and MVD as a marker of angiogenesis, could be used as ancillary methods in the differentiation of borderline grades of astrocytomas.
Acknowledgements
The authors would like to thank the College of Medicine/Al-Nahrain University particularly Department of Pathology and Forensic Medicine, and the staff at the teaching laboratories of Al Khadhmiya Teaching in Hospital in Baghdad/Iraq, for their help and support throughout the work. The authors reported no conflict of interest and no funding was received in this work.
References
- 1.Mahzouni P, Mokhtari M, Amirmansour B. Differentiation between reactive gliosis and astrocytomas by MIB-1/ki67 immunostaining. JRMS 2007;12(5):241-245 [Google Scholar]
- 2.Kumer V, Abbas AK, Fausto N, Mitchell RN. In: Robbins and Cotran, Basic Pathology, 8th ed, Elsevier, Philadelphia, USA, 2007:860-882. [Google Scholar]
- 3.Iraqi cancer registry, 2005.
- 4.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007. Aug;114(2):97-109 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habberstad AH, Gulati S, Torp SH. Evaluation of the proliferation markers Ki-67/MIB-1, mitosin, survivin, pHH3, and DNA topoisomerase IIα in human anaplastic astrocytomas–an immunohistochemical study. Diagn Pathol 2011;6:43 10.1186/1746-1596-6-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gözü H, Bilgiç B, Hazneci J, Sargın H, Erkal F, Sargın M, et al. Is Ki-67 index a useful labeling marker for invasion of pituitary adenomas? Turkish Journal of Endocrinology and Metabolism 2005;4:107-113 [Google Scholar]
- 7.Ambroise MM, Khosla C, Ghosh M, Mallikarjuna VS, Annapurneswari S. Practical value of MIB-1 index in predicting behavior of astrocytomas. Indian J Pathol Microbiol 2011. Jul-Sep;54(3):520-525 10.4103/0377-4929.85085 [DOI] [PubMed] [Google Scholar]
- 8.Johannessen AL, Torp SH. The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res 2006;12(3):143-147 10.1007/BF02893360 [DOI] [PubMed] [Google Scholar]
- 9.Bowers DC, Gargan L, Kapur P, Reisch JS, Mulne AF, Shapiro KN, et al. Study of the MIB-1 labeling index as a predictor of tumor progression in pilocytic astrocytomas in children and adolescents. J Clin Oncol 2003. Aug;21(15):2968-2973 10.1200/JCO.2003.01.017 [DOI] [PubMed] [Google Scholar]
- 10.Malhan P, Husain N, Bhalla S, Gupta RK, Husain M. Proliferating cell nuclear antigen, p53 and micro vessel density: Grade II vs. Grade III astrocytoma. Indian J Pathol Microbiol 2010. Jan-Mar;53(1):20-23 10.4103/0377-4929.59177 [DOI] [PubMed] [Google Scholar]
- 11.Kayaselçuk F, Zorludemir S, Gümürdühü D, Zeren H, Erman T. PCNA and Ki-67 in central nervous system tumors: correlation with the histological type and grade. J Neurooncol 2002. Apr;57(2):115-121 10.1023/A:1015739130208 [DOI] [PubMed] [Google Scholar]
- 12.Yu-hong Z, Jie-ying Z, Ai-rong C, Yu-mei M, Heng-shu W, Yue-hong L, et al. Expression and significance of TIP30, VEGF and CD34 in brain astrocytoma. Cancer Research and Clinic 2009;21(7):444-446 [Google Scholar]
- 13.Edilson S, Airlane A, Benedito S. Ki-67 and Bcl-2 antigen expression in adenomatous colorectal polyps from women with breast cancer. Society of Surgical Oncology 2010;17(9):2378-2383 [DOI] [PubMed] [Google Scholar]
- 14.Lumachi F, Ermani M, Marino F, Iacobone M, Baldessin M, Cappuzzo G, et al. PCNA-LII, Ki-67 immunostaining, p53 activity and histopathological variables in predicting the clinical outcome in patients with parathyroid carcinoma. Anticancer Res 2006. Mar-Apr;26(2A):1305-1308 [PubMed] [Google Scholar]
- 15.Zhang YH, Yang Q, Cui W, Liu YX, Liu SX, Yao X. Correlation of microvascular density and clinicopathological factors in different subtypes of renal cell carcinoma. Zhonghua Zhong Liu Za Zhi 2010. Feb;32(2):117-122 [PubMed] [Google Scholar]
- 16.Abdelaziz OS, El-Sabaa BM, Abdelaziz A. Characterization of cancer stem cells in patients with brain astrocytomas: a clinicopathological and immunohistochemical study. Bull Alex Fac Med 2010;46(4):357-363 [Google Scholar]
- 17.Rodríguez-Pereira C, Suárez-Peñaranda JM, Vázquez-Salvado M, Sobrido MJ, Abraldes M, Barros F, et al. Value of MIB-1 labelling index (LI) in gliomas and its correlation with other prognostic factors. A clinicopathologic study. J Neurosurg Sci 2000. Dec;44(4):203-209, discussion 209-210 [PubMed] [Google Scholar]
- 18.Isolan GR, Ribas Filho JM, Isolan PM, Giovanini A, Malafaia O, Dini LI, et al. Astrocytic neoplasms and correlation with mutate p53 and Ki-67 proteins. Arq Neuropsiquiatr 2005. Dec;63(4):997-1004 10.1590/S0004-282X2005000600017 [DOI] [PubMed] [Google Scholar]
- 19.Liwei Y, Xiuzhen W. Expression and clinical significance of PTEN and Ki-67 in hmnan astrocytoma. Jiangsu Medical Journal 2010;36(5):73-75 [Google Scholar]
- 20.Yong-hua R, Guo-ying W, Yi W, Xian Z. Study on the expression of VEGF and Ki-67 related to malignant transformation of astrocytoma. Medicine and Pharmacy of Yunnan 2003;1:18-22 [Google Scholar]
- 21.Atik E, Serarslan Y, Bakaris S, Savas N. The importance of proliferative activity and angiogenesis in meningioma and glioma. Turk Neurosurg 2005;15(4):176-181 [Google Scholar]
- 22.Huang Y. Expression and significance of hypoxia inducible factor-1α, Ki-67 and microvessel density in diffuse infiltrating astrocytoma. Clin Exp Med 2010;23:51-56 [Google Scholar]
- 23.Scott IS, Morris LS, Rushbrook SM, Bird K, Vowler SL, Burnet NG, et al. Immunohistochemical estimation of cell cycle entry and phase distribution in astrocytomas: applications in diagnostic neuropathology. Neuropathol Appl Neurobiol 2005. Oct;31(5):455-466 10.1111/j.1365-2990.2005.00618.x [DOI] [PubMed] [Google Scholar]
- 24.Georgescu CV, Săftoiu A, Georgescu CC, Ciurea R, Ciurea T. Correlations of proliferation markers, p53 expression and histological findings in colorectal carcinoma. J Gastrointestin Liver Dis 2007. Jun;16(2):133-139 [PubMed] [Google Scholar]
- 25.Ang LC, Plewes M, Tan L, Begley H, Agranovich A, Shul D. Proliferating cell nuclear antigen expression in the survival of astrocytoma patients. Can J Neurol Sci 1994. Nov;21(4):306-310 [DOI] [PubMed] [Google Scholar]
- 26.Ripple H. P53 oncoprotein, PCNA expression and their signification in human astrocytoma. Master’s thesis posted on August 19, 2010.In: China Papers (online). Available at: http://mt.china-papers.com Accessed February 28, 2012.
- 27.Chu SH, Yuan XH, Jiang PC, Li ZQ, Zhang J, Wen ZH, et al. The expression of hepatocyte growth factor and its receptor in brain astrocytomas. Zhonghua Yi Xue Za Zhi 2005. Mar;85(12):835-838 [PubMed] [Google Scholar]
- 28.Lebelt A, Szkudlarek M, Guzińska-Ustymowicz K, Lemancewicz D, Zimnoch L, Dziecioł J. Proliferative activity of chosen central nervous system (CNS) neoplasms. Rocz Akad Med Bialymst 2004;49(Suppl 1):242-243 [PubMed] [Google Scholar]
- 29.Yan D, Ling Y, Yu Y, Fulin S, Ming Y, Di JY, et al. Expressions of VEGF, bFGF, PCNA and P53 in human brain astrocytoma. Progress of Anatomical Sciences 2006;12(3):221-223 [Google Scholar]
- 30.Li-jun W, Ke X, Guo-guang F. Magnetic resonance spectroscopy of human astrocytoma and the correlation with the expression of Survivin and PCNA. Chinese Journal of Medical Imaging Technology 2008;24:11-15 [Google Scholar]
- 31.Bin X, Jun S, Luohe X, Zhimin X. Transforming growth factor α and tumor proliferation cell nuclear antigen in astrocytic tumors. Chinese Journal of Clinical and Experimental Pathology 2006;4:93-97 [Google Scholar]
- 32.Maiti AK, Ghosh K, Chatterjee U, Chakrobarti S, Chatterjee S, Basu S. Epidermal growth factor receptor and proliferating cell nuclear antigen in astrocytomas. Neurol India 2008. Oct-Dec;56(4):456-462 10.4103/0028-3886.44827 [DOI] [PubMed] [Google Scholar]
- 33.Sadanaga H. Immunohistochemical analysis of cell proliferation in gliomas using proliferating cell nuclear antigen. Bull Yamaguchi Med Sch 1994;41(3-4):99-104 [Google Scholar]
- 34.Zimnoch L, Kozielec Z, Lewko J, Cylwik B, Mariak Z. Proliferative activity of glial neoplasms of the brain. Neurol Neurochir Pol 1999. Jan-Feb;33(1):87-96 [PubMed] [Google Scholar]
- 35.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 1996. Jan;77(2):362-372 [DOI] [PubMed] [Google Scholar]
- 36.Izycka-Swieszewska E, Rzepko R, Borowska-Lehman J, Stempniewicz M, Sidorowicz M. Angiogenesis in glioblastoma–analysis of intensity and relations to chosen clinical data. Folia Neuropathol 2003;41(1):15-21 [PubMed] [Google Scholar]
- 37.Lebelt A, Dziecioł J, Guzińska-Ustymowicz K, Lemancewicz D, Zimnoch L, Czykier E. Angiogenesis in gliomas. Folia Histochem Cytobiol 2008;46(1):69-72 10.2478/v10042-008-0009-4 [DOI] [PubMed] [Google Scholar]
- 38.El-Sayed M, Taha MM. Immunohistochemical expression of cycloxygenase-2 in astrocytoma: correlation with angiogenesis, tumor progression and survival. Turk Neurosurg 2011. Jan;21(1):27-35 [PubMed] [Google Scholar]
- 39.Yuhong Z, Jie-Ying Z, Ai-Rong C, Yu-Mei M, Heng-Shu M. YueHong L, Wen-Xin W, Xiang-Hong Z. Expression and significance of TIP30, VEGF and CD34 in brain astrocytoma. Cancer Research Clin 2009;21(7):444-449 [Google Scholar]
- 40.Zhi-qin C, Xia G, Yan-xia S. Correlation of MMP-9 and FLT-1 expression with microvessel density in astrocytoma. Chinese Journal of Clinical and Experimental Pathology 2008;24:6-9 [Google Scholar]
- 41.Fulin S. The correlation study of VEGF, PIGF and children astrocytoma grading and prognosis. Available at http://www.lw23.com/lunwen_1016637247/ Accessed on 23/11/2011.
- 42.Jang FF, Wei W, De WM. Vascular endothelial growth factor and basic fibroblast growth factor expression positively correlates with angiogenesis and peritumoural brain oedema in astrocytoma. J Ayub Med Coll Abbottabad 2008. Apr-Jun;20(2):105-109 [PubMed] [Google Scholar]
- 43.Mahzouni P, Mohammadizadeh F, Mougouei K, Moghaddam NA, Chehrei A, Mesbah A. Determining the relationship between "microvessel density" and different grades of astrocytoma based on immunohistochemistry for "factor VIII-related antigen" (von Willebrand factor) expression in tumor microvessels. Indian J Pathol Microbiol 2010. Oct-Dec;53(4):605-610 10.4103/0377-4929.71996 [DOI] [PubMed] [Google Scholar]
- 44.Korkolopoulou P, Christodoulou P, Kouzelis K, Hadjiyannakis M, Priftis A, Stamoulis G, et al. MDM2 and p53 expression in gliomas: a multivariate survival analysis including proliferation markers and epidermal growth factor receptor. Br J Cancer 1997;75(9):1269-1278 10.1038/bjc.1997.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kordek R, Biernat W, Debiec-Rychter M, Alwasiak J, Liberski PP. Comparative evaluation of p53-protein expression and the PCNA and Ki-67 proliferating cell indices in human astrocytomas. Pathol Res Pract 1996. Mar;192(3):205-209 10.1016/S0344-0338(96)80222-6 [DOI] [PubMed] [Google Scholar]
- 46.Choi YM, Kim TY, Yoon KJ, Moon HB, Kim JM. A comparative study on MIB-1, AgNORs and PCNA expressions in astrocytic tumors. J Korean Neurosurgery Soc 1997;26(4):476-485 [Google Scholar]
- 47.Sallinen PK, Haapasalo HK, Visakorpi T, Helén PT, Rantala IS, Isola JJ, et al. Prognostication of astrocytoma patient survival by Ki-67 (MIB-1), PCNA, and S-phase fraction using archival paraffin-embedded samples. J Pathol 1994. Dec;174(4):275-282 10.1002/path.1711740407 [DOI] [PubMed] [Google Scholar]