Abstract
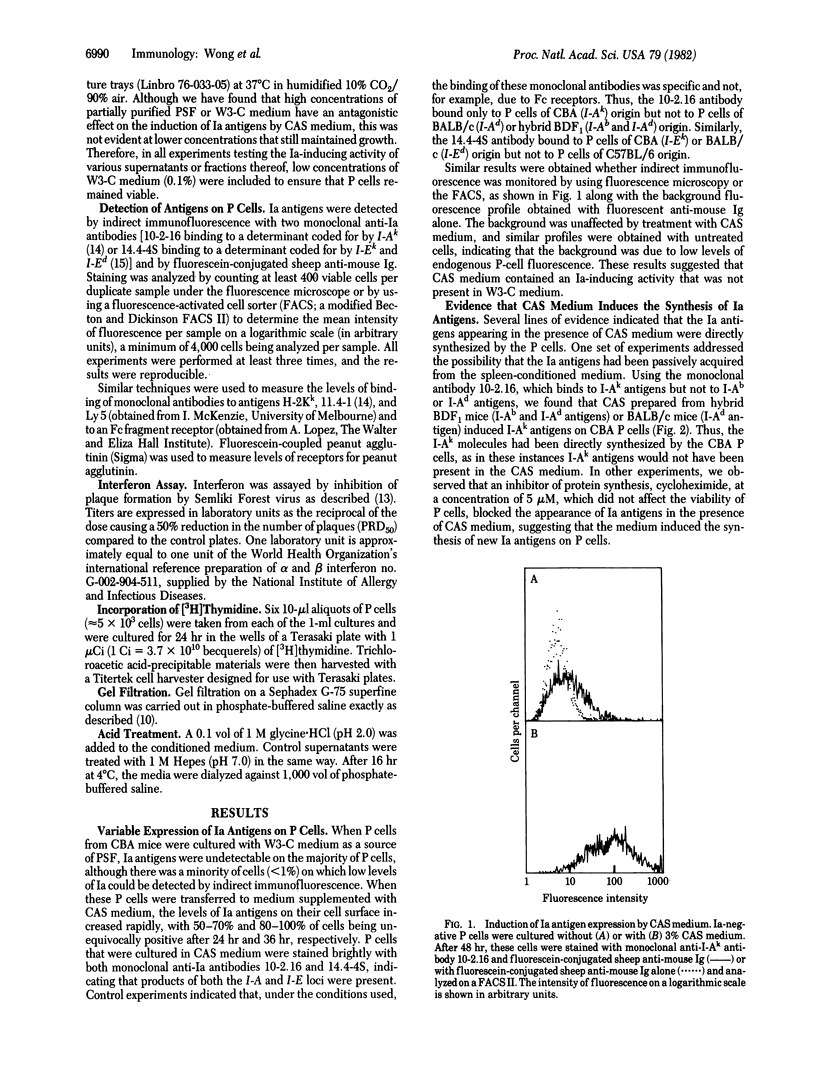
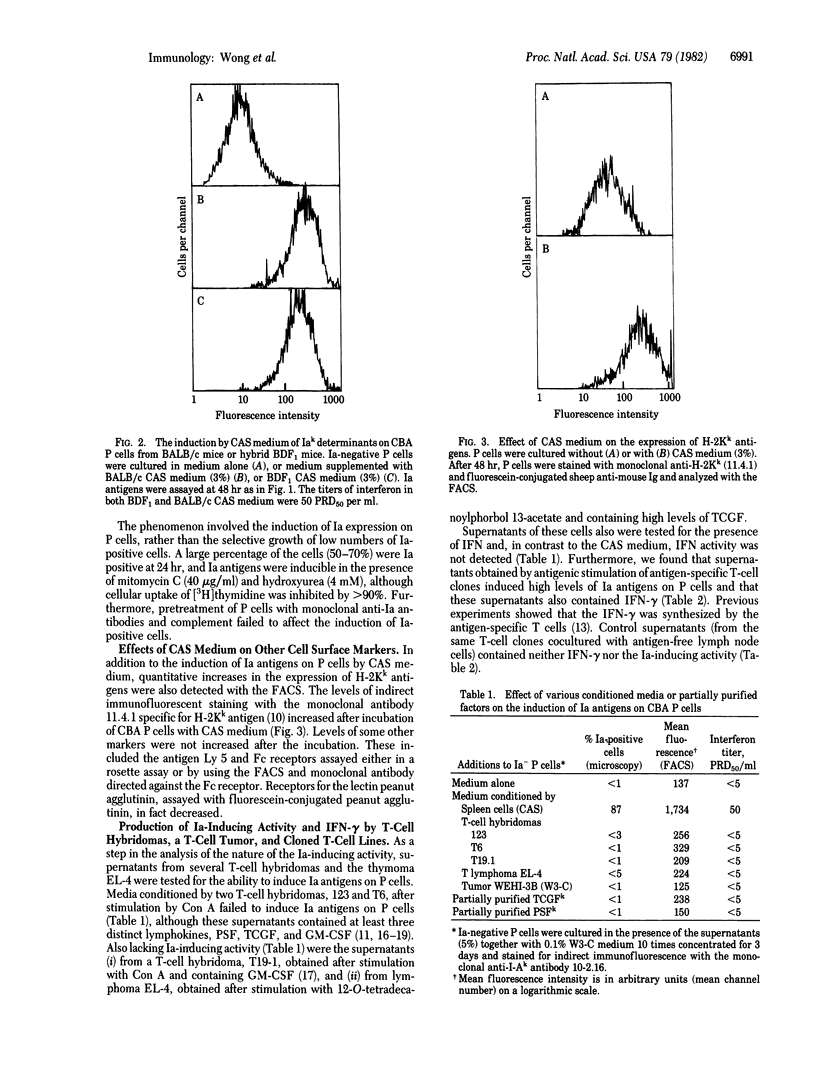
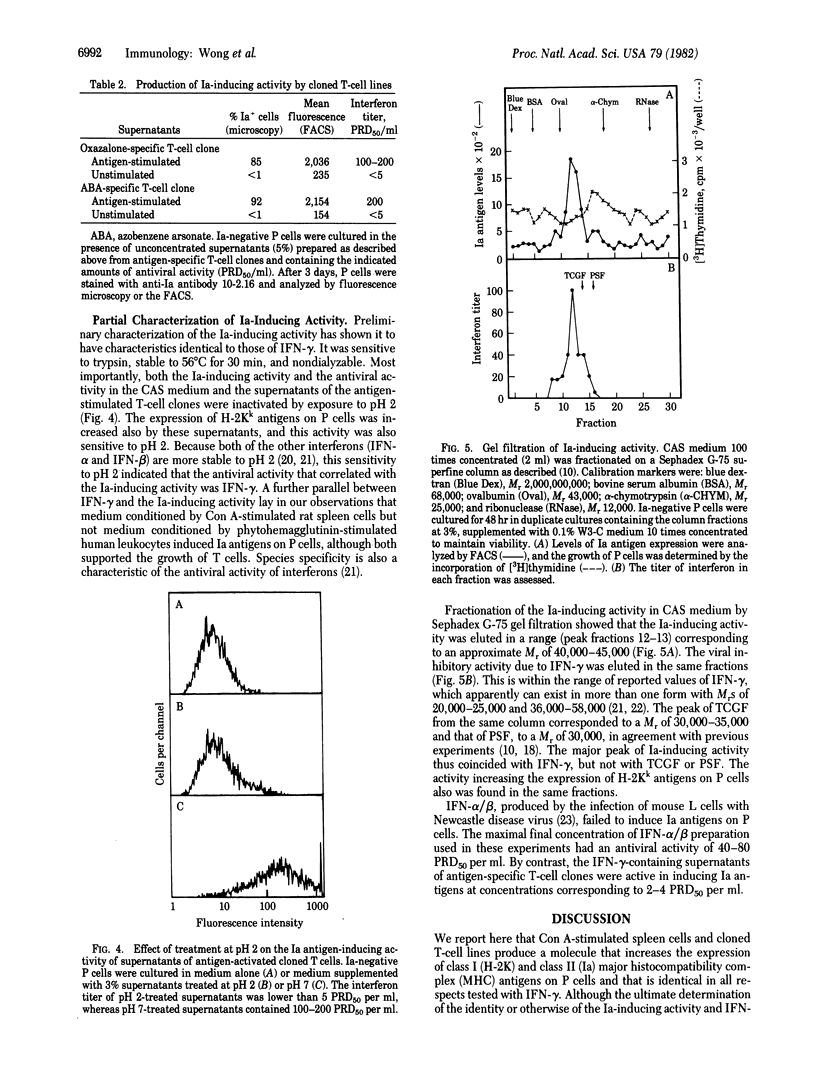
Persisting (P) cells (murine cells that resemble mast cells and grow continuously in vitro for prolonged periods in the presence of a specific growth factor) did not express detectable levels of Ia antigens (murine class II major histocompatibility antigens) when their growth was supported by partially purified P cell-stimulating factor. However, when these Ia-negative P cells were transferred to medium conditioned by concanavalin A-stimulated spleen cells, Ia antigens appeared within 24 hr. The increase in Ia antigens was due to induction of synthesis of Ia antigens by P cells and not to absorption of Ia antigens from the conditioned medium or selective growth of Ia-positive cells from a low number of Ia-positive cells in the original population. The Ia-inducing activity was also found in supernatants from antigen-stimulated cloned T-cell lines, but not from certain T-cell hybridomas or the T lymphoma EL-4. The presence of Ia-inducing activity correlated with the presence of interferon-gamma (IFN-gamma). The gel filtration profiles of IFN-gamma activity and Ia-inducing activity were coincident and corresponded to an apparent molecular weight of 40,000-45,000. Both the IFN-gamma and Ia-inducing activity were destroyed by treatment at pH 2. These results indicate that IFN-gamma or a closely related molecule induces Ia antigens on P cells and suggest that regulation of Ia antigen expression may be an important aspect of the effects of IFN-gamma on the immune and hemopoietic systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basham T. Y., Bourgeade M. F., Creasey A. A., Merigan T. C. Interferon increases HLA synthesis in melanoma cells: interferon-resistant and -sensitive cell lines. Proc Natl Acad Sci U S A. 1982 May;79(10):3265–3269. doi: 10.1073/pnas.79.10.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Schrader J. W. Biochemical characterization of regulatory factors derived from T cell hybridomas and spleen cells. I. Separation of T cell growth factor and T cell replacing factor from granulocyte-macrophage colony-stimulating factor. J Immunol. 1982 Jan;128(1):168–174. [PubMed] [Google Scholar]
- Clark-Lewis I., Schrader J. W. Biochemical characterization of regulatory factors derived from T cell hybridomas and spleen cells. II. Evidence for glycosylation of T cell growth factor, T cell replacing factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1982 Jan;128(1):175–180. [PubMed] [Google Scholar]
- Clark-Lewis I., Schrader J. W., McKimm-Breschkin J. L. Preparation of T cell growth factor free from interferon and factors stimulating hemopoietic cells and mast cells. J Immunol Methods. 1982;51(3):311–322. doi: 10.1016/0022-1759(82)90398-2. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Schrader J. W. P cell-stimulating factor: biochemical characterization of a new T cell-derived factor. J Immunol. 1981 Nov;127(5):1941–1947. [PubMed] [Google Scholar]
- Cline M. J., Billing R. Antigens expressed by human B lymphocytes and myeloid stem cells. J Exp Med. 1977 Oct 1;146(4):1143–1145. doi: 10.1084/jem.146.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J. L., Jr, Glasgow L. A., Kern E. R., Youngner J. S. Inhibition of murine osteogenic sarcomas by treatment with type I or type II interferon. J Natl Cancer Inst. 1978 Sep;61(3):871–874. [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Fleischmann W. R., Jr, Klimpel K. D. Gamma interferon (IFN gamma) and IFN alpha/beta suppress murine myeloid colony formation (CFU-C)N: magnitude of suppression is dependent upon level of colony-stimulating factor (CSF). J Immunol. 1982 Jul;129(1):76–80. [PubMed] [Google Scholar]
- Lampert I. A., Suitters A. J., Chisholm P. M. Expression of Ia antigen on epidermal keratinocytes in graft-versus-host disease. Nature. 1981 Sep 10;293(5828):149–150. doi: 10.1038/293149a0. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- McKimm-Breschkin J. L., Mottram P. L., Thomas W. R., Miller J. F. Antigen-specific production of immune interferon by T Cells lines. J Exp Med. 1982 Apr 1;155(4):1204–1209. doi: 10.1084/jem.155.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Osborne L. C., Georgiades J. A., Johnson H. M. Classification of interferons with antibody to immune interferon. Cell Immunol. 1980 Jul 15;53(1):65–70. doi: 10.1016/0008-8749(80)90426-8. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Pelus L. M., Saletan S., Silver R. T., Moore M. A. Expression of Ia-antigens on normal and chronic myeloid leukemic human granulocyte-macrophage colony-forming cells (CFU-GM) is associated with the regulation of cell proliferation by prostaglandin E. Blood. 1982 Feb;59(2):284–292. [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Differential efficacies of human type I and type II interferons as antiviral and antiproliferative agents. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5928–5932. doi: 10.1073/pnas.77.10.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R. K., Adler W. H., Nordin A. A. Modulation of natural cytotoxicity by alloantibodies. IV. A comparative study of the activation of mouse spleen cell cytotoxicity by anti H-2 antisera, interferon, and mitogens. Cell Immunol. 1981 Sep 1;63(1):28–41. doi: 10.1016/0008-8749(81)90026-5. [DOI] [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Arnold B., Clark-Lewis I. A con A-stimulated T-cell hybridoma releases factors affecting haematopoietic colony-forming cells and B-cell antibody responses. Nature. 1980 Jan 10;283(5743):197–199. doi: 10.1038/283197a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Clark-Lewis I. T cell hybridoma-derived regulatory factors. I. Production of T cell growth factor following stimulation by concanavalin A. J Immunol. 1981 Mar;126(3):1101–1105. [PubMed] [Google Scholar]
- Schrader J. W. In in vitro production and cloning of the P cell, a bone marrow-derived null cell that expresses H-2 and Ia-antigens, has mast cell-like granules, and is regulated by a factor released by activated T cells. J Immunol. 1981 Feb;126(2):452–458. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Nossal G. J. Strategies for the analysis of accessory-cell function: the in vitro cloning and characterization of the P cell. Immunol Rev. 1980;53:61–85. doi: 10.1111/j.1600-065x.1980.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Paul W. E., Shevach E. M. Guinea-pig Ia antigens: functional significance and chemical characterization. Transplant Rev. 1976;30:174–196. doi: 10.1111/j.1600-065x.1976.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Sharrow S. O., Mathieson B. J., Singer A. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J Immunol. 1981 Apr;126(4):1327–1335. [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Oppenheim J. J. Regulation of murine macrophage Ia-antigen expression by products of activated spleen cells. J Exp Med. 1980 Dec 1;152(6):1734–1744. doi: 10.1084/jem.152.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nogueira N., Witmer M. D., Tydings J. D., Mellman I. S. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Moore R. N., Rosenstreich D. L. Correction of defective macrophage differentiation in C3H/HeJ mice by an interferon-like molecule. J Immunol. 1982 Jan;128(1):380–387. [PubMed] [Google Scholar]
- Winchester R. J., Ross G. D., Jarowski C. I., Wang C. Y., Halper J., Broxmeyer H. E. Expression of Ia-like antigen molecules on human granulocytes during early phases of differentiation. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4012–4016. doi: 10.1073/pnas.74.9.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]



