Abstract
Genetic variants of polyomavirus SV40 are powerful agents with which to define viral effects on cells and carcinogenesis pathways. We hypothesized that differences in biologic variation among viral strains affect the process of viral infection and are reflected in antibody responses to the viral nonstructural large T-antigen (TAg) protein but not in neutralizing antibody responses against the inoculated viral particles. We analyzed the production of TAg antibody and neutralizing antibody in Syrian golden hamsters that were inoculated with SV40 viral strains by intracardiac, intravenous, or intraperitoneal routes and remained tumor free. Compared with the intraperitoneal route, intravascular (that is, intravenous, intracardiac) inoculation resulted in increased frequency of responsiveness to TAg but not in higher TAg antibody titers. The intravascular route was superior both for eliciting neutralizing antibody responses and for higher titers of those responses. Viruses with complex regulatory regions induced TAg antibody more often than did viruses with simple regulatory regions after intraperitoneal but not intravascular injections, with no differences in antibody titers. This viral genetic variation had no effect on neutralizing antibody production after intraperitoneal or intravascular inoculations or on neutralizing antibody titers achieved. These findings confirm that SV40 variants differ in their biologic properties. Route of inoculation combined with viral genetic variation significantly influence the development of serum antibodies to SV40 TAg in tumor-free hamsters. Route of inoculation—but not viral genetic variation—is an important factor in production of neutralizing antibody to SV40.
Abbreviations: TAg, large T antigen
Polyomavirus SV40 was discovered as an unrecognized contaminant of early poliovaccines38 and was shown promptly to be an oncogenic virus.9,15,17,18 Syrian golden hamsters are the small animal model that is susceptible to SV40 tumorigenicity.7,9-13,18,27,35 Since its discovery, SV40 has proven to be an outstanding tool for the discovery of mechanisms underlying carcinogenesis and for viral influences on cellular processes.1,3,5,19,23,30
Genetic variants of SV40 exist.16,20,26,28,33,34,36,37,41 This variation typically occurs in the viral regulatory region, in which some strains have duplications or rearrangements (or both) in the enhancer region, and in the C-terminal region of the large T-antigen (TAg) gene, in which nucleic acid variations may result in amino acid changes in the protein. TAg is an essential viral replication protein and the major viral oncoprotein. An important question is whether SV40 viral variants differ in their biologic properties, including in host responses to infections, as this could affect the spectrum of viral disease pathogenesis. We previously have shown that the viral regulatory region influences SV40 tumor induction in hamsters35,40 and vertical transmission of the virus in hamsters29 and that the route of inoculation influences SV40-mediated carcinogenesis.27 Because TAg is not a component of the virus particle but instead is synthesized in virus-infected cells, we hypothesized that differences in antibody responses to TAg reflect biologic variation among viral strains with respect to the process of viral infection. In contrast, we expected that neutralizing antibody responses arise primarily against the injected viral particles, which represent a single serotype, and therefore are less informative about viral variation. We report here that an analysis revealed that the route of inoculation—in combination with viral genetic variation—significantly influences the development of serum antibodies to SV40 large TAg but not the titer of those antibodies in virus-exposed, tumor-free hamsters. The route of inoculation—but not viral genetic variation—influenced both the frequency of development and the titers of virus-neutralizing antibodies in the same animals.
Materials and Methods
Viruses.
The viruses used in the current study, including the construction of recombinant viruses, have been described previously.16,35 Strain SVCPC and its recombinants contain a simple (1E) regulatory region structure, whereas strain 776 and its recombinants and strain VA45-54 contain complex (2E) regulatory regions that include sequence duplications, rearrangements, or both. Viral stocks of SV40 strains and recombinant viruses were prepared in TC7 cells, and virus titers were quantitated by plaque assay.6
Animal experiments.
Sera collected and analyzed from the intracardiac study performed here were compared with sera collected previously from animals inoculated by intravenous and intraperitoneal routes. Comparable experimental conditions were maintained for those previous experiments, including source of animals, housing conditions, amounts of virus inocula, and use of young adult animals.
Syrian golden hamsters (Mesocricetus auratus) were purchased from Harlan Laboratories (Indianapolis, IN) and were housed in the biohazard facility at the Center for Comparative Medicine (Baylor College of Medicine, Houston, TX). All hamsters were maintained in accordance with the Guide for the Care and Use of Animals,21 the Animal Welfare Act,2 and all other applicable laws and guidelines. The facility and its program are fully AAALAC-accredited. The studies were IACUC-approved.
We previously described the procedures by which 3-wk-old hamsters were inoculated intravenously27 or intraperitoneally35 with 1.0 × 107 pfu virus (0.2 mL IV, 0.5 mL IP) or uninfected cell lysate (0.2 mL IV, 0.5 mL IP). For intracardiac injections, 5- to 7-wk-old hamsters were anesthetized with isoflurane in a biosafety cabinet. The depth of anesthesia was assessed by monitoring respiration and the pedal reflex. The hamsters were injected into the heart under sterile conditions with 1.0 × 107 pfu virus (0.1 mL) by using a 27-gauge needle with a 1-mL syringe. The heart was palpated gently, and the needle was inserted into the skin and positioned at a 15° to 30° angle. The needle was advanced slowly into the thoracic cavity on the animal's left side, between the intercostal spaces and toward the left ventricle of the heart. Needle placement was verified by pulsation of blood into the needle. The inoculum was injected slowly into the heart over 20 s, and then the needle was removed carefully. To decrease trauma and cardiac tamponade, only 2 injection attempts were allowed per animal. Control hamsters were injected with uninfected cell lysate (0.1 mL) under the same conditions. After inoculation, the hamsters were placed in a cage in sternal recumbency, kept warm, and monitored for signs of cardiac or respiratory distress until fully recovered. The hamsters were observed daily for evidence of illness. Survival after this procedure was 99%.
Both male and female hamsters were used in all experiments, except that only female hamsters were used for intracardiac inoculations. At the termination of experiments, hamsters were euthanized by deep anesthesia with isoflurane followed by exsanguination by cardiocentesis. Either serum or plasma was collected. Necropsies were conducted on all hamsters inoculated by the intracardiac and intravenous routes and on many of those inoculated intraperitoneally.
Antibody assays.
Indirect immunofluorescence was used to detect and titer IgG antibodies against SV40 TAg.8,40 Briefly, SV40-transformed hamster cells expressing nuclear TAg were cultured on round glass coverslips and then were harvested and fixed for 3 min in acetone at room temperature. Sera or plasma were screened at a dilution of 1:10 or 1:5 by using a secondary rabbit antibody against hamster IgG that was labeled with fluorescein isothiocyanate. Plasma Sample Diluent (BioFX Laboratories, Owings Mills, MD) was used to dilute the plasma samples. Ascites fluid from an SV40 tumor-bearing hamster served as a positive control antiserum against TAg. Each sample was tested in duplicate. A plaque reduction test was used to detect SV40 neutralizing antibodies.6,27 Sera were heat-inactivated for 30 min at 56 °C, were diluted 1:10, and then were mixed with an equal volume of an SV40 virus dilution containing 50 to 100 pfu per 0.1 mL. The virus–serum mixture was incubated for 30 min at 37 °C, and then 0.2 mL was inoculated onto TC7 cell monolayers. After adsorption for 2 h at 37 °C, the monolayers were overlaid with an agar–Eagle medium mixture. Plaques were counted on day 15. Positive samples reduced the number of plaques by 50% or more as compared with the virus-only sample. A hyperimmune rabbit serum against SV40 was used as a positive control virus-neutralizing antibody. Each sample was tested in triplicate.
Statistical analysis.
Nonparametric statistics were used, because they matched the level of measurement of the data. The Z test for the comparison of proportions was used to determine statistical differences in the frequency of SV40 antibody responses between groups according to route of inoculation and viral strain. The χ2 test was used to compare distributions of titer cut-offs. A P value of 0.05 or less was considered statistically significant. All statistical analyses were performed by using SAS software (version 9.2, SAS Institute, Cary, NC).
Results
Characterization of antibody responses to SV40 viral proteins in hamsters.
The majority of SV40 tumor-bearing hamsters have antibodies against SV40 TAg (the viral oncoprotein). In addition, many virus-exposed hamsters respond immunologically to TAg yet do not develop tumors.27,35,40 Factors affecting the T-antibody responses in the absence of tumors have not been analyzed. The current study examined T-antibody responses in archival sera from SV40-inoculated hamsters that had remained tumor-free for 9 to 12 mo after inoculation. We compared 3 routes of inoculation, 2 of which involved injection into the bloodstream (intracardiac, intravenous); the other was injection into the intraperitoneal cavity (Table 1). In addition, we assessed multiple SV40 strains, including those with either simple (1E) or complex (2E) regulatory regions, to examine viral genetic influences on serologic responses. A total of 167 virus-exposed, tumor-free hamsters were analyzed, as well as 43 control animals that had been inoculated with uninfected cell lysate. Table 1 shows the percentage of hamsters positive for TAg antibodies and for viral neutralizing antibodies in each experimental group.
Table 1.
SV40 T-antigen IgG and neutralizing antibody in sera from tumor-free hamsters at 9 to 12 mo after virus inoculation
| SV40 T-antigen IgG |
SV40 neutralizing antibody |
|||||
| Route of inoculation | Virus regulatory region | Virus strain | No. tested | No. positive (%) | No. tested | No. positive (%) |
| ICa | complex | 776-2E | 13 | 10 (77) | 13 | 13 (100) |
| simple | SVCPC | 13 | 12 (92) | 13 | 13 (100) | |
| Controls | 11 | 0 (0) | 11 | 0 (0) | ||
| IVb | complex | VA45-54 | 2 | 2 (100) | 2 | 2 (100) |
| complex | 776-VA | 4 | 4 (100) | 4 | 4 (100) | |
| complex | 776-CPC | 11 | 11 (100) | 11 | 11 (100) | |
| simple | SVCPC | 3 | 2 (67) | 3 | 3 (100) | |
| Controls | 12 | 0 (0) | 12 | not done | ||
| IP-Aa | complex | 776-2E | 18 | 13 (72) | 18 | 13 (72) |
| simple | SVCPC | 16 | 10 (62) | 16 | 10 (62) | |
| simple | SVCPC-776 | 5 | 0 (0) | 5 | 3 (60) | |
| Controls | 10 | 0 (0) | 10 | 0 (0) | ||
| IP-Bc | complex | 776-2E | 27 | 22 (81) | 27 | 16 (59) |
| complex | VA45-54 | 10 | 6 (60) | 10 | 2 (20) | |
| complex | 776-VA | 19 | 9 (47) | 19 | 6 (32) | |
| simple | SVCPC | 15 | 7 (47) | 15 | 8 (53) | |
| simple | SVCPC-776 | 11 | 4 (36) | 11 | 4 (36) | |
| Controls | 10 | 0 (0) | 10 | 0 (0) | ||
This report.
From reference 27.
From reference 35.
After intravascular (intracardiac, intravenous) inoculation, many of the virus-exposed hamsters produced TAg antibodies, regardless of virus strain, sometimes reaching 100% of animals per group. In comparison with those inoculated intravascularly, hamsters exposed through the intraperitoneal route revealed a more variable pattern of serologic responsiveness (Table 1). Two independent experiments involving intraperitoneal injections are shown. There was a wider range in frequency of SV40 TAg antibody production among the groups of virus strains, with viruses with complex regulatory regions appearing to elicit antibody more frequently than did those with simple regulatory regions.
All of the hamsters inoculated by the intravenous and intracardiac routes produced SV40 neutralizing antibody, whereas hamsters inoculated intraperitoneally only sporadically produced detectable neutralizing antibody. The viral regulatory region structure appeared to have less influence on the patterns of neutralizing antibody production. None of the control animals from any of the experiments had serologic evidence of SV40 infection.
Factors affecting SV40 antibody responses in hamsters.
Statistical analyses were performed to determine factors that significantly influenced detectable antibody responses to viral antigens (Table 2). For these analyses, the results from the 2 independent intravascular injections (intravenous, intracardiac) were pooled, and the 2 intraperitoneal experiments were combined. Routes of inoculation were compared, and potential contributions of the viral regulatory region were considered. Both TAg IgG antibody and neutralizing antibody responses were evaluated, considering whether sera were antibody-positive or -negative.
Table 2.
Statistical analysis of the effects of route of inoculation and viral genetic variation on the frequency of SV40 antibody responses in tumor-free hamsters
| Statistical analysis |
|||||||
| Group | Route of inoculation | Virus strain | No. positive/ no. tested (%) | Groups compared | P | ||
| SV40 T-antigen IgG | |||||||
| 1 | IC | 776-2E | 10/13 (77) | All viruses, IV+IC (groups 1, 2, 3, 4, 5, 6; 89% positive) | vs | All viruses, IP (groups 7, 8, 9, 10, 11; 59% positive) | 0.001 |
| 2 | SVCPC | 12/13 (92) | |||||
| 3 | IV | SVCPC | 2/3 (67) | 2E viruses, IV+IC (groups 1, 4, 5, 6; 90% positive) | vs | 2E viruses, IP (groups 7, 10, 11; 68% positive) | 0.02 |
| 4 | VA45-54 | 2/2 (100) | |||||
| 5 | 776-VA | 4/4 (100) | |||||
| 6 | 776-CPC | 11/11 (100) | |||||
| 1E viruses, IV+IC (groups 2, 3; 88% positive) | vs | 1E viruses, IP (groups 8, 9; 45% positive) | 0.003 | ||||
| 7 | IP (total)a | 776-2E | 35/45 (78) | 2E viruses, IP (groups 7, 10, 11; 68% positive) | vs | 1E viruses, IP (groups 8, 9; 45% positive) | 0.01 |
| 8 | SVCPC | 17/31 (55) | |||||
| 9 | SVCPC-776 | 4/16 (25) | |||||
| 10 | VA45-54 | 6/10 (60) | |||||
| 11 | 776-VA | 9/19 (47) | |||||
| 2E viruses, IV+IC (groups 1, 4, 5, 6; 90% positive) | vs | 1E viruses, IV+IC (groups 2, 3; 81% positive) | 0.2 | ||||
| 12 | IP-A | 776-2E | 13/18 (72) | All viruses, IC (groups 1, 2; 81% positive) | vs | All viruses, IV (groups 3, 4, 5, 6; 95% positive) | 0.2 |
| 13 | SVCPC | 10/16 (62) | |||||
| 14 | SVCPC-776 | 0/5 (0) | |||||
| All viruses, IP-A (groups 12, 13, 14; 59% positive) | vs | All viruses, IP-B (groups 15, 16, 17, 18, 19; 61% positive) | 0.4 | ||||
| 15 | IP-B | 776-2E | 22/27 (81) | 1E viruses, IP-A (groups 13, 14; 48% positive) | vs | 1E viruses, IP-B (groups 18, 19; 42% positive) | 0.7 |
| 16 | VA45-54 | 6/10 (60) | |||||
| 17 | 776-VA | 9/19 (47) | |||||
| 18 | SVCPC | 7/15 (47) | |||||
| 19 | SVCPC-776 | 4/11 (36) | |||||
| 2E viruses, IP-A (group 12; 72% positive) | vs | 2E viruses, IP-B (groups 15, 16, 17; 69% positive) | 0.8 | ||||
| SV40 neutralizing antibody | |||||||
| 20 | IC | 776-2E | 13/13 (100) | All viruses, IV+IC (groups 20, 21, 22, 23, 24, 25; 100% positive) | vs | All viruses, IP (groups 26, 27, 28, 29, 30; 51% positive) | <0.0001 |
| 21 | SVCPC | 13/13 (100) | |||||
| 22 | IV | SVCPC | 3/3 (100) | 2E viruses, IV+IC (groups 20, 23, 24, 25; 100% positive) | vs | 2E viruses, IP (groups 26, 29, 30; 50% positive) | <0.0001 |
| 23 | VA45-54 | 2/2 (100) | |||||
| 24 | 776-VA | 4/4 (100) | |||||
| 25 | 776-CPC | 11/11 (100) | |||||
| 1E viruses, IV+IC (groups 21, 22; 100% positive) | vs | 1E viruses, IP (groups 27, 28; 53% positive) | 0.001 | ||||
| 26 | IP (total)a | 776-2E | 29/45 (64) | 2E viruses, IP (groups 26, 29, 30; 50% positive) | vs | 1E viruses, IP (groups 27, 28; 53% positive) | 0.73 |
| 27 | SVCPC | 18/31 (58) | |||||
| 28 | SVCPC-776 | 7/16 (44) | |||||
| 29 | VA45-54 | 2/10 (20) | |||||
| 30 | 776-VA | 6/19 (32) | |||||
| 2E viruses, IV+IC (groups 20, 23, 24, 25; 100% positive) | vs | 1E viruses, IV+IC (groups 21, 22; 100% positive) | 1.0 | ||||
| 31 | IP-A | 776-2E | 13/18 (72) | All viruses, IC (groups 20, 21; 100% positive) | vs | All viruses, IV (groups 22, 23, 24, 25; 100% positive) | 1.0 |
| 32 | SVCPC | 10/16 (62) | |||||
| 33 | SVCPC-776 | 3/5 (60) | |||||
| All viruses, IP-A (groups 31, 32, 33; 69% positive) | vs | All viruses, IP-B (groups 34, 35, 36, 37, 38; 44% positive) | 0.01 | ||||
| 34 | IP-B | 776-2E | 16/27 (59) | 1E viruses, IP-A (groups 32, 33; 67% positive) | vs | 1E viruses, IP-B (groups 37, 38; 46% positive) | 0.2 |
| 35 | VA45-54 | 2/10 (20) | |||||
| 36 | 776-VA | 6/19 (32) | |||||
| 37 | SVCPC | 8/15 (53) | |||||
| 38 | SVCPC-776 | 4/11 (36) | |||||
| 2E viruses, IP-A (group 31; 72% positive) | vs | 2E viruses, IP-B (groups 34, 35, 36; 43% positive) | 0.03 | ||||
IP (total; groups 7–11 and 26–30) are the combined totals of animals from the IP-A and IP-B experiments.
Analyses of the SV40 TAg IgG antibody responses revealed the strong influence exerted by the route of inoculation (Table 2). This antibody response is dependent on virus infection of cells and the synthesis of TAg (which is not a component of the virus particle). Significantly (P < 0.05) more antibody-positive responses occurred when hamsters were inoculated by the intravascular route as compared with the intraperitoneal route. This observation was consistent whether all the viruses were considered together (intravascular, 89%; intraperitoneal, 59%; P = 0.001) or whether virus variants with complex (2E) or simple (1E) regulatory regions were compared separately with intraperitoneal injections (90% compared with 68%, P = 0.02; 88% compared with 45%, P = 0.003, respectively). Whereas the frequency of generation of antibody to TAg differed between 2E and 1E viruses that were introduced intraperitoneally (68% compared with 45%, P = 0.01), no such difference was apparent after intravascular injection (90% compared with 81%, P = 0.2). There were no differences in T-antibody responses when the intracardiac and intravenous routes were compared (P = 0.2) or when the 2 independent experiments involving intraperitoneal injections were compared for all viruses (P = 0.4), for 1E viruses only (P = 0.7), or for 2E variants only (P = 0.8).
We also compared neutralizing antibody responses, which are directed against the SV40 viral capsid (Table 2). Presumably, this immune response was elicited primarily by the inoculated virus particles and was not dependent on virus replication. The superiority of the intravascular route over the intraperitoneal route of inoculation on SV40 neutralizing antibody production was even more pronounced than that for TAg IgG antibody, with more significant effects for all virus variants compared together (100% compared with 51%, P < 0.0001), for 2E viruses only (100% compared with 50%, P < 0.0001), and for 1E viruses only (100% compared with 53%, P = 0.001). However, in contrast to TAg antibody, frequency of neutralizing antibody responsiveness between the 2E and 1E viruses was not different after intraperitoneal (50% compared with 53%, P = 0.73) or intravascular (100% compared with 100%, P = 1.0) inoculation. This result is not unexpected, given that the viral capsid is the same for all the virus variants. Significant differences in frequency of neutralizing antibody production were not detected in individual experiments involving intracardiac and intravenous injections (P = 1.0) or in the 2 intraperitoneal experiments that compared viruses with simple regulatory regions (P = 0.2). However, a difference in neutralizing antibody responses was detected between the 2 IP experiments that compared 2E viruses (P = 0.03; the IP-A experiment included only a single 2E virus).
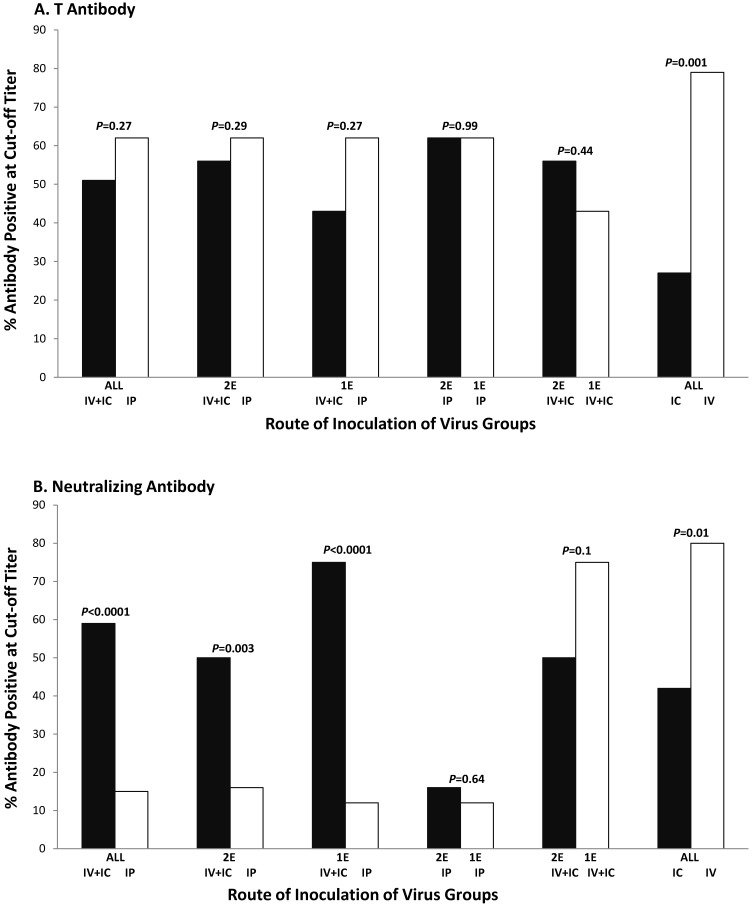
We then assessed whether route of inoculation or viral factors significantly influenced the titers of antibody-positive responses; only antibody-positive sera were included in these analyses. The number of sera considered with each virus group is itemized in Table 3. Several comparisons were made by using different cut-off dilutions; the most informative dilutions are shown in Table 3 (1:100 for TAg antibody, 1:1000 for neutralizing antibody). Bar charts of summary data (Figure 1) illustrate the strikingly different effects of conditions on the titers obtained for TAg antibody and neutralizing antibody responses.
Table 3.
Statistical analysis of the effects of viral genetic variation and route of inoculation on titers of SV40 antibody responses in tumor-free hamsters
| Statistical analysis of antibody-positive sera |
|||||||
| Group | Route of inoculation | Virus strain | No. of positive sera analyzed | Groups compared | P | ||
| SV40 T-antigen IgG | |||||||
| 1 | IC | 776-2E | 10 | All viruses, IV+IC (groups 1, 2, 3, 4, 5, 6; 51% positive a) | vs | All viruses, IP (groups 7, 8, 9, 10, 11; 62% positive a) | 0.27 |
| 2 | SVCPC | 12 | |||||
| 3 | IV | SVCPC | 2 | 2E viruses, IV+IC (groups 1, 4, 5, 6; 56% positive) | vs | 2E viruses, IP (groups 7, 10, 11; 62% positive) | 0.29 |
| 4 | VA45-54 | 2 | |||||
| 5 | 776-VA | 4 | |||||
| 6 | 776-CPC | 11 | |||||
| 1E viruses, IV+IC (groups 2, 3; 43% positive) | vs | 1E viruses, IP (groups 8,9; 62% positive) | 0.27 | ||||
| 7 | IP (total)b | 776-2E | 35 | 2E viruses, IP (groups 7, 10, 11; 62% positive) | vs | 1E viruses, IP (groups 8, 9; 62% positive) | 0.99 |
| 8 | SVCPC | 17 | |||||
| 9 | SVCPC-776 | 4 | |||||
| 10 | VA45-54 | 6 | |||||
| 11 | 776-VA | 9 | |||||
| 2E viruses, IV+IC (groups 1, 4, 5, 6; 56% positive) | vs | 1E viruses, IV+IC (groups 2, 3; 43% positive) | 0.44 | ||||
| 12 | IP-A | 776-2E | 13 | All viruses, IC (groups 1, 2; 27% positive) | vs | All viruses, IV (groups 3, 4, 5, 6; 79% positive) | 0.001 |
| 13 | SVCPC | 10 | |||||
| 14 | SVCPC-776 | 0 | |||||
| All viruses, IP-A (groups 12, 13, 14; 65% positive) | vs | All viruses, IP-B (groups 15, 16, 17, 18, 19; 60% positive) | 0.7 | ||||
| 15 | IP-B | 776-2E | 22 | 1E viruses, IP-A (groups 13, 14; 60% positive) | vs | 1E viruses, IP-B (groups 18, 19; 64% positive) | 0.86 |
| 16 | VA45-54 | 6 | |||||
| 17 | 776-VA | 9 | |||||
| 18 | SVCPC | 7 | |||||
| 19 | SVCPC-776 | 4 | |||||
| 2E viruses, IP-A (group 12; 69% positive) | vs | 2E viruses, IP-B (groups 15, 16, 17; 59% positive) | 0.53 | ||||
| SV40 neutralizing antibody | |||||||
| 20 | IC | 776-2E | 13 | All viruses, IV+IC (groups 20, 21, 22, 23, 24, 25; 59% positive) | vs | All viruses, IP (groups 26, 27, 28, 29, 30; 15% positive) | <0.0001 |
| 21 | SVCPC | 13 | |||||
| 22 | IV | SVCPC | 3 | 2E viruses, IV+IC (groups 20, 23, 24, 25; 50% positive) | vs | 2E viruses, IP (groups 26, 29, 30; 16% positive) | 0.003 |
| 23 | VA45-54 | 2 | |||||
| 24 | 776-VA | 4 | |||||
| 25 | 776-CPC | 11 | |||||
| 1E viruses, IV+IC (groups 21, 22; 75% positive) | vs | 1E viruses, IP (groups 27, 28; 12% positive) | <0.0001 | ||||
| 26 | IP (total)b | 776-2E | 29 | ||||
| 27 | SVCPC | 18 | |||||
| 28 | SVCPC-776 | 7 | |||||
| 29 | VA45-54 | 2 | |||||
| 30 | 776-VA | 6 | |||||
| 2E viruses, IV+IC (groups 20, 23, 24, 25; 50% positive) | vs | 1E viruses, IV+IC (groups 21, 22; 75% positive) | 0.1 | ||||
| 31 | IP-A | 776-2E | 13 | All viruses, IC (groups 20, 21; 42% positive) | vs | All viruses, IV (groups 22, 23, 24, 25; 80% positive) | 0.01 |
| 32 | SVCPC | 10 | |||||
| 33 | SVCPC-776 | 3 | |||||
| All viruses, IP-A (groups 31, 32, 33; 19% positive) | vs | All viruses, IP-B (groups 34, 35, 36, 37, 38; 11% positive) | 0.37 | ||||
| 34 | IP-B | 776-2E | 16 | 1E viruses, IP-A (groups 32, 33; 15% positive) | vs | 1E viruses, IP-B (groups 37, 38; 8% positive) | 0.59 |
| 35 | VA45-54 | 2 | |||||
| 36 | 776-VA | 6 | |||||
| 37 | SVCPC | 8 | |||||
| 38 | SVCPC-776 | 4 | |||||
| 2E viruses, IP-A (group 31; 23% positive) | vs | 2E viruses, IP-B (groups 34, 35, 36; 12% positive) | 0.4 | ||||
Percentage positive at 1:100 dilution.
IP (total), groups 7–11 and 26–30, are the combined totals of animals from the IP-A and IP-B experiments.
Figure 1.
Effect of route of inoculation and variation in the viral regulatory region on titers of SV40 antibody responses in tumor-free hamsters. (A) Positive responses for SV40 T antibody by group at a cut-off titer of 1:100. (B) Positive responses for SV40 neutralizing antibody by group at a cut-off titer of 1:1000.
For TAg antibody (Table 3), most of the factors that influenced whether a detectable response occurred (Table 2) did not result in different titers of those responses. The only significant difference in TAg antibody titers was observed when all viruses were compared for the intravenous and intracardiac routes (79% compared with 27%, P = 0.001; Figure 1 A).
We then compared the titers of neutralizing antibody responses (Table 3). The superiority of the intravascular route over the intraperitoneal route was again apparent, often affecting the titer of antibodies produced. Higher titers of neutralizing antibodies were induced whether all viruses were compared (intravascular, 59%; intraperitoneal, 15%; P < 0.0001) or 2E (intravascular, 50%; intraperitoneal, 16%; P = 0.003) and 1E (intravascular, 75%; intraperitoneal, 12%; P < 0.0001) viruses separately. Higher titers of neutralizing antibodies were also induced by the intravenous route compared with the intracardiac route when all viruses were included (80% compared with 42%, P = 0.01; Figure 1 B). Antibody titers in positive sera did not differ after intraperitoneal inoculation of 1E and 2E viruses.
Discussion
We used archived serum to evaluate the immune responses to SV40 TAg and the production of viral neutralizing antibodies by weanling Syrian golden hamsters that were exposed to infectious strains of SV40 by different routes of inoculation. The capsid proteins on the surface of a viral particle, against which the neutralizing antibody response is directed, are similar to an inert antigen. However, in the case of a viral antigen that is not part of the mature virion but is synthesized in infected cells (that is, SV40 TAg), important considerations are the exposure of susceptible cells to the virus and the extent to which virus expression occurs after infection. By analyzing immune responses to both the viral capsid and TAg, we were able to evaluate the immune responses of hamsters to 2 different aspects of SV40 infection.
Antibody responses to inert antigens are influenced by multiple factors, including the efficiency of delivery of antigen to lymphoid tissues, the degree to which the antigen is distributed, and whether an adjuvant is used.25 The intravenous and intracardiac routes deliver antigen primarily to the spleen and secondary lymph nodes, whereas intraperitoneal inoculations allow antigen to be taken up by the lymphatic system and transferred to draining lymph nodes and the thoracic duct.25 Different routes of inoculation elicit different types of immune responses. For polyclonal antibody production, intravenous inoculation has been recommended when using particulate antigens, whereas intraperitoneal inoculation is preferred with soluble antigens.25
The current study revealed that both the route of inoculation and the genetics of the inoculated virus significantly influenced the frequency of antibody responses to SV40 antigens. Intravascular inoculation of virus elicited more frequent antibody responses than did intraperitoneal inoculation. This superiority applied to the production of both TAg antibodies and neutralizing antibodies and was observed regardless of the genetics of the viral strain inoculated. This finding suggests that introduction of SV40 virus directly into the bloodstream delivers the inoculum very efficiently to cells involved in the immune response, whereas uptake of virus from the peritoneal cavity is less efficient in some animals. In a study of visceral leishmaniasis,24 different inoculation routes coupled with different doses of parasites evoked particular immune responses. Specifically, a greater Th2 response was detected after intracardiac inoculation of a high dose of leishmania, whereas a better Th1 response was seen following subcutaneous inoculation of a low dose of parasite.24
We also noted an effect of the structure of the viral regulatory region on the host response to viral nonstructural proteins after intraperitoneal injections. TAg IgG antibody seroprevalence was higher after infection with viruses having complex (2E) regulatory regions as compared with simple (1E) viruses. Given that TAg is a nonstructural protein synthesized in infected cells, TAg IgG antibody production is a marker of successful infection and gene expression in susceptible cells by SV40. We speculate that the 2E viruses induced detectable levels of TAg expression more often than did the 1E viruses. Although the number of positive responses was greater, there was no significant difference in the titers of the positive sera. This difference in induction of TAg antibodies did not occur after intracardiac inoculation of 1E and 2E viruses, perhaps reflecting a more efficient exposure of susceptible cells to the infecting virus by this route that negated any regulatory region effects. These observations suggest that a threshold level of expression of the viral TAg protein may be needed to elicit a specific immune response, the immunofluorescence titers of which are relatively low. These data from tumor-free hamsters reemphasize previous observations that virus-inoculated rabbits and monkeys can produce TAg antibody responses32,39,42 and confirm that the development of antibodies to TAg is a sign of SV40 infection and not necessarily a sign of neoplasia.
Observations from systems involving other persistent viral infections similarly have shown that the characteristics of different viral strains may elicit variable immune responses. The lymphocytic choriomeningitis virus model revealed that slowly replicating strains not only induced weaker cytotoxic T cell responses than did more rapidly replicating strains but also were more successful at establishing persistent infections.4 In the mouse polyoma virus system, infection of newborn mice leads to robust viral replication and easily detected persistent infections, often with subsequent tumor formation, whereas healthy adult mice show reduced replication, persistence at low viral loads, and lack of tumor development.31 This difference is thought to reflect the relative maturity of the host immune system. The tissue pattern of murine polyomavirus persistence depends on the route of inoculation of the newborn mice, with intranasal exposure leading to persistence in both lungs and kidneys, whereas intraperitoneal inoculation produces mainly kidney infections.14 In an encephalitis virus model, benign strains of Venezuelan encephalitis virus were cleared more rapidly than were virulent strains after intracardiac inoculation of hamsters.22
We noted several differences in the patterns of SV40 neutralizing antibody responses compared with TAg IgG antibody responses in our weanling hamsters. Compared with intraperitoneal injection, intravascular inoculation of SV40 strains resulted in more neutralizing antibody responses and higher titers of antibody. However, no differences were observed when 2E and 1E viruses were compared directly for frequency of production of neutralizing antibody or in the titers of those responses after inoculation by either the intravascular or intraperitoneal route. These are predicted results, because the exteriors of virus particles containing the different viral genomes were identical. Given that the neutralizing antibody response presumably was generated against viral particles introduced in the original inoculum, viral genetic variation likely had no effect.
Our conclusions from the current study are based on analysis of combined data from several independent animal experiments. We believe that pooled data sets are the most appropriate for comparisons of serologic responses, because this condition accommodates the inclusion of all of the various virus strains. Because the viral strain varied among the individual experiments, combining the 2 intravascular experiments (intracardiac, intravenous) and the 2 intraperitoneal experiments (IP-A, IP-B) leads to representation of a common pool of viral strains.
The current study has some limitations. The sera analyzed were considered to be from tumor-free hamsters. Even though many of the animals had been necropsied and appeared to be tumor-free, undetected microtumors might have been present in some animals. Whether microtumors induce detectable TAg antibody is unknown. We interpret the TAg antibody responses as being generated against newly synthesized TAg in the hamsters. Although we consider it unlikely, TAg might have been present in a viral inoculum (as carry-over from the cell cultures used to grow the virus stock) in sufficient amounts to elicit a TAg antibody response.
In conclusion, we found that the intravascular route elicited more frequent serologic responses of both SV40 TAg IgG antibody and neutralizing antibody against capsid protein than did intraperitoneal inoculation. We noted an influence of the regulatory region structure of the virus on the TAg antibody response only after intraperitoneal injection. Route of inoculation and viral genetic factors had minimal effects on the titers of the TAg antibody responses. In contrast, intravascular inoculation of SV40 resulted in higher titers of neutralizing antibody.
Acknowledgments
We acknowledge support to JLS from the Center for Comparative Medicine at Baylor College of Medicine, which is a participant in the Gulf Coast Consortium Postdoctoral Veterinary Training Program in Laboratory Animal Medicine. This study was supported in part by research grant R01 CA134524 from the NIH to JSB.
References
- 1.Ahuja D, Sáenz-Robles MT, Pipas JM. 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24:7729–7745 [DOI] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2007. 7 USC §2131–2159.
- 3.Atkin SJL, Griffin BE, Dilworth SM. 2009. Polyoma virus and simian virus 40 as cancer models: history and perspectives. Semin Cancer Biol 19:211–217 [DOI] [PubMed] [Google Scholar]
- 4.Bocharov G, Ludewig B, Bertoletti A, Klenerman P, Junt T, Krebs P, Luzyanina T, Fraser C, Anderson RM. 2004. Underwhelming the immune response: effect of slow virus growth on CD8+-T–lymphocyte responses. J Virol 78:2247–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butel JS. 2000. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis 21:405–426 [DOI] [PubMed] [Google Scholar]
- 6.Butel JS, Jafar S, Wong C, Arrington AS, Opekun AR, Finegold MJ, Adam E. 1999. Evidence of SV40 infections in hospitalized children. Hum Pathol 30:1496–1502 [DOI] [PubMed] [Google Scholar]
- 7.Butel JS, Lednicky JA. 1999. Cell and molecular biology of simian virus 40: implications for human infections and disease. J Natl Cancer Inst 91:119–134 [DOI] [PubMed] [Google Scholar]
- 8.Butel JS, Ozbun MA.1994. Viral oncoprotein interactions with cellular proteins: simian virus 40 large T antigen and p53 as models, p 282–309. In: Adolph KW, editor. Methods in molecular genetics, vol 4: molecular virology techniques, part A. San Diego (CA): Academic Press.
- 9.Butel JS, Tevethia SS, Melnick JL. 1972. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv Cancer Res 15:1–55 [DOI] [PubMed] [Google Scholar]
- 10.Carbone M, Lewis AM, Jr, Matthews BJ, Levine AS, Dixon K. 1989. Characterization of hamster tumors induced by simian virus 40 small T deletion mutants as true histiocytic lymphomas. Cancer Res 49:1565–1571 [PubMed] [Google Scholar]
- 11.Cicala C, Pompetti F, Carbone M. 1993. SV40 induces mesotheliomas in hamsters. Am J Pathol 142:1524–1533 [PMC free article] [PubMed] [Google Scholar]
- 12.Diamandopoulos GT. 1972. Leukemia, lymphoma, and osteosarcoma induced in the Syrian golden hamster by simian virus 40. Science 176:173–175 [DOI] [PubMed] [Google Scholar]
- 13.Diamandopoulos GT. 1973. Induction of lymphocytic leukemia, lymphosarcoma, reticulum cell sarcoma, and osteogenic sarcoma in the Syrian golden hamster by oncogenic DNA simian virus 40. J Natl Cancer Inst 50:1347–1365 [DOI] [PubMed] [Google Scholar]
- 14.Dubensky TW, Villarreal LP. 1984. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol 50:541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eddy BE, Borman GS, Grubbs GE, Young RD. 1962. Identification of the oncogenic substance in rhesus monkey cell cultures as simian virus 40. Virology 17:65–75 [DOI] [PubMed] [Google Scholar]
- 16.Forsman ZH, Lednicky JA, Fox GE, Willson RC, White ZS, Halvorson SJ, Wong C, Lewis AM, Jr, Butel JS. 2004. Phylogenetic analysis of polyomavirus simian virus 40 from monkeys and humans reveals genetic variation. J Virol 78:9306–9316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraumeni JF, Jr, Ederer F, Miller RW. 1963. An evaluation of the carcinogenicity of simian virus 40 in man. JAMA 185:713–718 [DOI] [PubMed] [Google Scholar]
- 18.Girardi AJ, Sweet BH, Slotnick VB, Hilleman MR. 1962. Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV40. Proc Soc Exp Biol Med 109:649–660 [DOI] [PubMed] [Google Scholar]
- 19.Gjoerup O, Chang Y. 2010. Update on human polyomaviruses and cancer. Adv Cancer Res 106:1–51 [DOI] [PubMed] [Google Scholar]
- 20.Ilyinskii PO, Daniel MD, Horvath CJ, Desrosiers RC. 1992. Genetic analysis of simian virus 40 from brains and kidneys of macaque monkeys. J Virol 66:6353–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press.
- 22.Jahrling PB, Scherer WF. 1973. Growth curves and clearance rates of virulent and benign Venezuelan encephalitis viruses in hamsters. Infect Immun 8:456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javier RT, Butel JS. 2008. The history of tumor virology. Cancer Res 68:7693–7706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur S, Kaur T, Garg N, Mukherjee S, Raina P, Athokpam V. 2008. Effect of dose and route of inoculation on the generation of CD4+ Th1–Th2-type of immune response in murine visceral leishmaniasis. Parasitol Res 103:1413–1419 [DOI] [PubMed] [Google Scholar]
- 25.Kendall LV.2007. Production of polyclonal antibodies, p 41–72. In: Howard GC, Kaser MR, editors. Making and using antibodies. A practical handbook. Boca Raton (FL): CRC Press.
- 26.Lednicky JA, Arrington AS, Stewart AR, Dai XM, Wong C, Jafar S, Murphey-Corb M, Butel JS. 1998. Natural isolates of simian virus 40 from immunocompromised monkeys display extensive genetic heterogeneity: new implications for polyomavirus disease. J Virol 72:3980–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNees AL, Vilchez RA, Heard TC, Sroller V, Wong C, Herron AJ, Hamilton MJ, Davis WC, Butel JS. 2009. SV40 lymphomagenesis in Syrian golden hamsters. Virology 384:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman JS, Baskin GB, Frisque RJ. 1998. Identification of SV40 in brain, kidney and urine of healthy and SIV-infected rhesus monkeys. J Neurovirol 4:394–406 [DOI] [PubMed] [Google Scholar]
- 29.Patel NC, Halvorson SJ, Sroller V, Arrington AS, Wong C, Smith EO, Vilchez RA, Butel JS. 2009. Viral regulatory region effects on vertical transmission of polyomavirus SV40 in hamsters. Virology 386:94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pipas JM. 2009. SV40: cell transformation and tumorigenesis. Virology 384:294–303 [DOI] [PubMed] [Google Scholar]
- 31.Ramqvist T, Dalianis T. 2009. Murine polyomavirus tumour specific transplantation antigens and viral persistence in relation to the immune response, and tumour development. Semin Cancer Biol 19:236–243 [DOI] [PubMed] [Google Scholar]
- 32.Rapp F, Tevethia SS, Rawls WE, Melnick JL. 1967. Production of antibodies to papovavirus SV40 tumor antigen in African green monkeys. Proc Soc Exp Biol Med 125:794–798 [DOI] [PubMed] [Google Scholar]
- 33.Rizzo P, Di Resta I, Powers A, Ratner H, Carbone M. 1999. Unique strains of SV40 in commercial poliovaccines from 1955 not readily identifiable with current testing for SV40 infection. Cancer Res 59:6103–6108 [PubMed] [Google Scholar]
- 34.Simon MA, Ilyinskii PO, Baskin GB, Knight HY, Pauley DR, Lackner AA. 1999. Association of simian virus 40 with a central nervous system lesion distinct from progressive multifocal leukoencephalopathy in macaques with AIDS. Am J Pathol 154:437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sroller V, Vilchez RA, Stewart AR, Wong C, Butel JS. 2008. Influence of the viral regulatory region on tumor induction by simian virus 40 in hamsters. J Virol 82:871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart AR, Lednicky JA, Benzick US, Tevethia MJ, Butel JS. 1996. Identification of a variable region at the carboxy terminus of SV40 large T-antigen. Virology 221:355–361 [DOI] [PubMed] [Google Scholar]
- 37.Stewart AR, Lednicky JA, Butel JS. 1998. Sequence analyses of human tumor-associated SV40 DNAs and SV40 viral isolates from monkeys and humans. J Neurovirol 4:182–193 [DOI] [PubMed] [Google Scholar]
- 38.Sweet BH, Hilleman MR. 1960. The vacuolating virus, SV40. Proc Soc Exp Biol Med 105:420–427 [DOI] [PubMed] [Google Scholar]
- 39.Tevethia SS. 1970. Immune response of rabbits to purified papovavirus SV40. J Immunol 104:72–78 [PubMed] [Google Scholar]
- 40.Vilchez RA, Brayton CF, Wong C, Zanwar P, Killen DE, Jorgensen JL, Butel JS. 2004. Differential ability of 2 simian virus 40 strains to induce malignancies in weanling hamsters. Virology 330:168–177 [DOI] [PubMed] [Google Scholar]
- 41.Vilchez RA, Madden CR, Kozinetz CA, Halvorson SJ, White ZS, Jorgensen JL, Finch CJ, Butel JS. 2002. Association between simian virus 40 and non-Hodgkin lymphoma. Lancet 359:817–823 [DOI] [PubMed] [Google Scholar]
- 42.Vonka V, Zavadova H, Kutinova L, Rezacova D. 1967. Development of antibodies against viral and tumor antigens of papovavirus SV40 in monkeys. Proc Soc Exp Biol Med 125:790–794 [DOI] [PubMed] [Google Scholar]