Abstract
Aims: Carbohydrate-deficient transferrin (%CDT) is a well-established and highly specific biomarker for sustained heavy consumption of alcohol. However, in pregnant women, the specificity of this biomarker might be affected by advanced gestational age, even after accounting for increased transferrin concentrations in pregnancy. The goal of this prospective study was to assess the variability in %CDT during pregnancy among alcohol-abstaining patients. Methods: Patients were recruited during one of the first prenatal care visits and followed-up to term. Abstinence was confirmed by maternal self-report and by alcohol biomarkers. Biomarkers assessed in the mother included serum gamma-glutamyltranspeptidase, urine ethyl glucuronide and ethyl sulfate, and whole blood phosphatidylethanol (PEth). In addition, PEth was measured in a dry blood spot card obtained from a newborn. For %CDT analysis, serum samples were collected at baseline and at term and analyzed by an internationally validated high-performance liquid chromatography and spectrophotometric detection method. Results: At recruitment (mean gestational age 22.6 ± 7.3 weeks), the mean %CDT concentration was 1.49 ± 0.30%, while at term, it increased to 1.67 ± 0.28% (P = 0.001). Using a conventional cutoff concentration %CDT >1.7%, 22.9 and 45.7% of the sample would be classified as ‘positive’ for this biomarker at recruitment and at term, respectively (P = 0.011 ). Conclusion: These results suggest that a conventional cutoff of 1.7% might be too low for pregnant women and would generate false-positive results. We propose that %CDT >2.0% be used as a cutoff concentration indicative of alcohol exposure in pregnant women. The sensitivity of %CDT at this cutoff for heavy drinking during pregnancy needs to be assessed further.
INTRODUCTION
Fetal alcohol spectrum disorder (FASD) is an umbrella term that encompasses a continuum of clinical presentations caused by prenatal alcohol exposure from the most severe case of fetal alcohol syndrome (FAS), to partial FAS, alcohol-related birth defects and alcohol-related neurodevelopmental disorder. Increasing evidence indicates that the majority of children adversely affected by fetal alcohol exposure do not present with birth defects (reviewed by Jacobson et al., 1998). In the absence of characteristic facial dysmorphia, a confirmation of maternal drinking is required as a diagnostic criterion for FASD (American Academy of Pediatrics, 2000). However, confirmation of maternal drinking during pregnancy is often problematic because of the stigma associated with alcohol use during pregnancy and the unreliability of maternal self-report. While screening questionnaires, such as TWEAK or Alcohol Use Disorders Identification Test (AUDIT), can be quite sensitive to the identification of risky drinking behavior (≥14 drinks/week; Sokol et al., 1989; Russell et al., 1996; Bradley et al., 1998; Chang et al., 1999), their validity for identification of more moderate alcohol use is largely unknown. Biomarkers of alcohol consumption, defined as direct ethanol metabolites/conjugation products or ethanol-induced protein alterations, could augment or supplant maternal report, since they present an objective measure of recent alcohol exposure over and above that which might be reported by patients. Prior research indicates that a positive test outcome for two or more alcohol biomarkers in pregnant women is more predictive of having a child with FASD than self-report measures (Stoler et al., 1998).
Carbohydrate-deficient transferrin (CDT) is a well-established and highly specific biomarker for sustained heavy consumption of alcohol. CDT in this paper refers to the amount of disialo-transferrin as a percentage of the total transferrin isoforms and labeled as %CDT. Currently, CDT is the only laboratory test approved by the US Food and Drug Administration for the detection of heavy drinking (reviewed by Anton, 2001). CDT is usually elevated after an intake of 50–80 g (4–6 standard drinks) of ethanol per day during the previous 1–3 weeks (reviewed by Bakhireva and Savage, 2011). Owing to a relatively short half-life, CDT levels usually normalize between 1 and 4 weeks of abstinence with a corresponding half-life of 9.5 days (Jeppsson et al., 1993; Helander et al., 2001). Some prior studies, which used older techniques to measure CDT, indicated that serum CDT concentrations are higher in non-pregnant women than in men (reviewed by Arndt, 2001); however, studies based on high-performance liquid chromatography (HPLC) analysis found no gender differences (Helander et al., 2003; Bergstrom and Helander, 2008b). Earlier studies also reported that pre-menopausal status and serum estradiol levels correlated positively with CDT concentrations (reviewed by Arndt, 2001); however, the observed effect in these studies could be due to elevations in total transferrin rather than CDT per se. Furthermore, two recent reports suggested that the disialo isoform (expressed as %CDT in this report) increases with advanced gestational age during pregnancy (Bianchi et al., 2011; Kenan et al., 2011), which, based on the current cutoffs of 1.7%, could lead to false-positive results. The purpose of this prospective observational study was to examine changes in %CDT concentration in serum samples collected from a well-characterized cohort of alcohol-abstaining pregnant women and to determine whether a revision in the cutoff level of %CDT might be warranted.
METHODS
Study design and measures
The study was approved by the University of New Mexico (UNM) Human Research Review Committee (HRRC) and utilized a prospective cohort design (HRRC 09-004). Women attending a special UNM substance abuse clinic during pregnancy were recruited during one of the first prenatal care visits. To be eligible, patients had to give consent in English or Spanish, be at least 18-years old, have a singleton pregnancy confirmed by ultrasound and be <35 weeks gestation at enrollment. An on-site study coordinator administered a brief screening questionnaire to determine patients' eligibility for placement in either (a) an alcohol-exposed or (b) a no drinking ‘control’ group. This report focused on results obtained from the ‘control’ group to examine the effect of advanced gestational age on %CDT concentrations and to eliminate the confounding effect of variable alcohol consumption patterns. To be classified into the control group, a patient could be a recipient of an opioid-maintenance therapy (OMT) and/or be a recreational drug user, but had to meet the following three criteria with respect to alcohol consumption: (a) no binge drinking (consumption of ≥4 drinks on occasion) episodes in the month before the last menstrual period (LMP); (b) consumption of an average of no more than four drinks per week in the month before the LMP and (c) abstinence from alcohol since the LMP. Patients who met these eligibility criteria were administered an informed consent and participated in the baseline interview that ascertained their socio-demographic characteristics, medical and reproductive history (e.g. presence of chronic conditions, including hepatobililary disorders, gravidity, parity, pregnancy dates, any complications during pregnancy and prior adverse perinatal outcomes), use of multi-vitamins and iron supplements and past and current substance use. In addition, the AUDIT questionnaire and the Timeline Follow-back Procedure (TLFB) were administered, and the reported quantity and frequency of alcohol use on TLFB were converted into absolute ounces of alcohol per day and per drinking day (Bowman et al., 1975; Jacobson et al., 2002). At the same visit, maternal serum, whole blood and urine were collected for biomarker analyses.
Repeat specimens from patients were obtained at delivery at term gestation. Upon admission to the hospital for delivery, specimen electronic collection orders for the mother (serum, urine) and newborn child [dry blood spot (DBS) card] were activated. An additional DBS card with capillary blood, obtained by a heel puncture, was collected during a routine neonatal screening. A follow-up interview with the mother, including a repeated TLFB questionnaire to capture any changes in alcohol consumption since the first interview, was conducted by a study coordinator before discharge. Data on maternal and newborn perinatal outcomes (e.g. gestational age at delivery, complications during pregnancy and the newborn period) were abstracted from the electronic medical records and reviewed for completeness by a study neonatologist and an obstetrician.
Laboratory measures
A battery of maternal alcohol biomarkers ascertained in this study included serum gamma-glutamyltranspeptidase (GGT) and %CDT, urine ethyl glucuronide (EtG) and ethyl sulfate (EtS) and whole blood phosphatidylethanol (PEth). Serum and urine specimens for analysis of GGT, %CDT and EtG/EtS were collected at both the baseline and follow-up visits, while whole blood for analysis of PEth was collected at the baseline visit only. Maternal serum specimens were sent to the TriCore Reference Laboratory (Albuquerque, NM, USA) and to the Medical University of South Carolina (Charleston, SC, USA) for GGT and %CDT analyses, respectively. GGT was analyzed as part of a liver panel by the enzymatic rate method for GGT activity on a Beckman Synchron LX analyzer. CDT was analyzed by an internationally validated HPLC and spectrophotometric detection method (Helander et al., 2010) and expressed as a %CDT. For the HPLC analysis, coefficient of variability was <6% and translated into a variation of 0.1% in %CDT. Maternal urine and whole blood specimens were sent to the US Drug Testing Laboratory (Des Plaines, IL, USA) for analysis of EtG, EtS and PEth, respectively, by liquid chromatography-tandem mass spectrometry (LC/MS/MS) methods as previously described (Aradottir and Olsson, 2005; Bean, 2006; Jones et al., 2011). Newborn PEth was also analyzed in a DBS card, collected by routine heel prick and analyzed by LC/MS/MS as previously described (Jones et al., 2011). The Clinical and Laboratory Standards Institute protocol for blood collection on filter paper was followed (Clinical and Laboratory Standards Institute, 2007). The samples were identified only by a unique study identification number, and contract laboratories were blinded to the patients' exposure status.
Data analysis
To examine the effect of gestational age on %CDT and avoid variability due to different alcohol consumption patterns, a sample size was initially restricted to 39 controls who completed their follow-up visits by February 2012 and had %CDT measures available at both visits. The sample size was further restricted to 35 abstinent (by self-report on the TLFB procedure) pregnant women who were also negative on four maternal biomarkers (i.e. GGT, PEth, EtG, EtS) at both visits and PEth in DBS of a newborn to ensure that these patients were true abstainers from alcohol use in the period immediately prior to %CDT measurements and presumable fully during pregnancy. Mean %CDT concentrations between the two visits were compared by the paired t-test. In addition, a proportion of patients who scored above the conventional cutoff of 1.7% CDT at each visit were compared by McNemar's test for paired proportions. To examine the effect of covariates on %CDT, a multiple linear regression was conducted with %CDT at the follow-up visit as the dependent variable and %CDT at the baseline and other potential risk factors as independent variables. A list of potential predictors included maternal age, active viral hepatitis infection, use of multi-vitamins and iron supplements, weight gain during pregnancy, parity, smoking and use of OMT and illicit drugs during pregnancy. Predictors were examined individually and included in the final model if they were associated with %CDT at the follow-up visit at P ≤ 0.1 after accounting for the effect of the baseline %CDT concentration. Maternal age, cocaine/crack-cocaine and methamphetamines use, viral hepatitis status and smoking were included in the final model; %CDT values were log-transformed for regression analyses. Analyses were conducted in SAS® 9.2 (SAS Institute, Cary, NC, USA) and R (R Development Core Team, Vienna, Austria).
RESULTS
The sample included a large proportion of ethnic minorities (77.1% Hispanic/Latina, 5.7% American Indian) and socially disadvantaged (48.6% less than high school education, 100% public health insurance) pregnant women (Table 1). The mean maternal age at recruitment was 25.2 ± 4.4 years. Given that the population was recruited from the UNM substance abuse clinic designated for pregnant women, use of illicit drugs, OMT and tobacco products was prevalent, and 17.1% of patients had hepatitis C.
Table 1.
Description of the study population
| Maternal characteristics (n = 35) | Mean ± SD or % |
|---|---|
| Maternal age (years) | 25.2 ± 4.4 |
| Gestational age at recruitment (weeks): range: 11–34 | 22.6 ± 7.3 |
| Gestational age at delivery (weeks): range: 24–41 | 38.0 ± 3.0 |
| Weight gain in pregnancy (kg) | 15.1 ± 7.6 |
| Marital status | |
| Single | 48.6% |
| Married/co-habitating | 45.7% |
| Separated/divorced | 5.7% |
| Education | |
| Less than high school | 48.6% |
| High school or equivalent | 25.7% |
| Some college/vocational school or higher | 25.7% |
| Race | |
| White | 91.4% |
| Black or African American | 2.9% |
| American Indian | 5.7% |
| Ethnicity: Hispanic | 77.1% |
| Gravidity: Primigravida | 77.1% |
| Parity: Nulliparous | 65.7% |
| Health insurance: Medicaid | 91.4% |
| Other public insurance | 8.6% |
| Multi-vitamin use in pregnancy | 85.7% |
| Use of iron supplements during pregnancy | 14.3% |
| Hepatitis C | 17.1% |
| Substance use (anytime in pregnancy) | |
| Opioid-maintenance therapy and/or opiates | 88.6% |
| Marijuana | 40.0% |
| Cocaine/crack-cocaine | 31.4% |
| Methamphetamines | 17.1% |
| Benzodiazepines | 22.9% |
| Smoking (anytime during pregnancy) | 62.9% |
SD, standard deviation.
As evident from Table 2, the mean AUDIT score for alcohol use before pregnancy was 2.6 ± 5.1 and only one patient scored above the cutoff. In the TLFB interview the vast majority of patients (94.3%) reported no or less than once-a-month alcohol use during the periconceptional period, and none reported alcohol use during pregnancy. Except for %CDT, all other maternal and newborn biomarkers for these patients measured at both visits were below the established cutoff values, confirming abstinence.
Table 2.
Alcohol consumption pattern and alcohol biomarkers
| Alcohol consumption pattern (n = 35) | Mean ± SD or % |
|---|---|
| Self-reported alcohol use before pregnancy | |
| AUDIT score (12 months before baseline interview) | 2.6 ± 5.1 |
| AUDIT ≥8 (12 months before baseline interview) | 2.9% |
| Alcohol use during the periconceptional period | |
| Frequency of alcohol consumption (1 month around LMP) | |
| Never | 71.4% |
| Less than monthly | 22.9% |
| Once a week | 5.7% |
| Grams of pure alcohol per drinking day | 7.0 ± 14.0 |
| Binge drinking (≥4 drinks/occasion) a month around LMP | 0% |
| Self-reported alcohol use during pregnancy | |
| Per TLFB procedure | 0 |
| Maternal alcohol biomarkers | |
| GGT1 (U/l) | 16.0 ± 8.3 |
| GGT1 >55 U/l | 0% |
| GGT2 (U/l) | 16.8 ± 11.6 |
| GGT2 >55 U/l | 0% |
| PEth1 (ng/ml) | 0 |
| UEtG1 (ng/ml) | 0 |
| UEtG2 (ng/ml) | 0 |
| UEtS1 (ng/ml) | 0 |
| UEtS2 (ng/ml) | 0 |
| %CDT1 | 1.49 ± 0.30 |
| %CDT1>1.7% (mean+2 SD for non-pregnant controls) | 22.9% |
| %CDT2 | 1.67 ± 0.28 |
| %CDT2 >1.7% (mean+2 SD for non-pregnant controls) | 45.7% |
| Newborn alcohol biomarkers | |
| PEth (in dried blood spots) | 0% |
Subscript 1 refers to biomarkers assessed at the baseline visit (22.6 gestational weeks on average).
Subscript 2 refers to biomarkers assessed at term.
GGT, gamma-glutamyltranspeptidase; PEth, phosphatidylethanol; UEtS, urine ethyl glucuronide; UEtS, urine ethyl sulfate; %CDT, carbohydrate-deficient transferrin; SD, standard deviation; TLFB, Timeline Follow Back procedure.
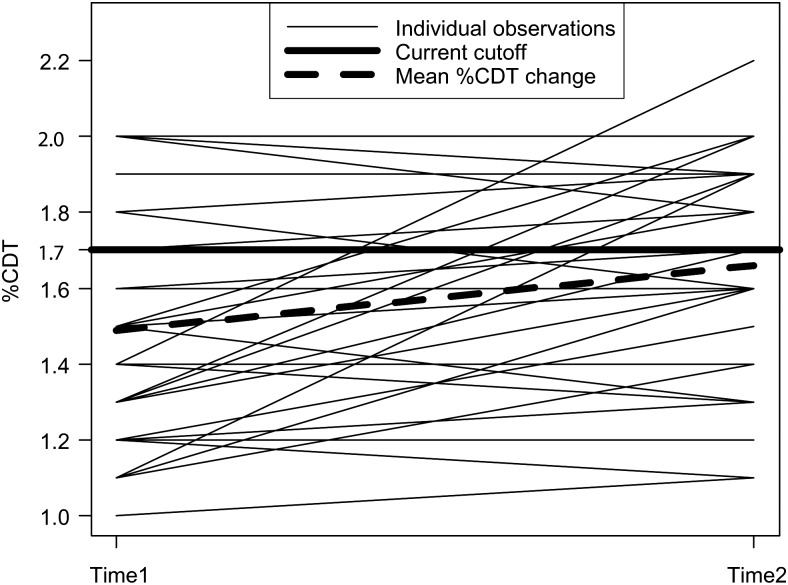
The mean %CDT concentrations were 1.49 ± 0.30 at the first blood draw (mean 22.6 gestational weeks) and 1.67 ± 0.28 at term (mean 38.0 gestational weeks) (P = 0.001). Individual changes for each patient between the two visits are shown in Fig. 1. Using a conventional cutoff concentration of %CDT >1.7%, corresponding to 2 SD above the mean or a concentration above the 97.5th percentile for non-pregnant controls (Helander et al., 2003; Bergstrom and Helander, 2008a), 22.9 and 45.7% of the samples would be classified as being ‘positive’ for this biomarker at recruitment and at term, respectively (P = 0.011). However, only one patient had a %CDT concentration above 2.0 (%CDT = 2.2), corresponding to more than 3 SD above the mean for non-pregnant controls (Helander et al., 2003), which was at term. As shown in Table 3, the results of the multivariate analysis indicated that the baseline %CDT concentration (regression coefficient = 0.48; P < 0.001) and marijuana use in pregnancy (regression coefficient = −0.05; P = 0.022) were the only significant predictors of the %CDT at the follow-up visit after controlling for maternal age, cocaine/crack-cocaine and methamphetamine use, viral hepatitis and smoking.
Fig. 1.
Individual changes in %CDT levels for 35 pregnant women in mid-pregnancy (22.6 gestational weeks, on average) and at term (38 gestational weeks, on average).*Mean %CDT concentrations increased in 60% of patients.
Table 3.
Predictors of %CDT at term
| Potential predictorsa | Regression coefficient | P-value |
|---|---|---|
| Baseline %CDT | 0.48 | <0.001 |
| Maternal age | −0.01 | 0.053 |
| Marijuana | −0.05 | 0.022 |
| Cocaine | 0.01 | 0.909 |
| Methamphetamines | −0.03 | 0.284 |
| Viral hepatitis | −0.04 | 0.123 |
| Tobacco | −0.03 | 0.196 |
aOther potential predictors (use of multi-vitamins and iron supplements, weight gain during pregnancy, parity and use of other illicit drugs) were not significant in univariate analysis and were not included in the final model. %CDT values were log-transformed.
DISCUSSION
Our results indicate that %CDT concentrations are elevated in pregnant women, especially in late pregnancy. These results are in accord with two other investigations. A study by Bianchi et al. (2011) reported that %CDT was strongly correlated with gestational age in 64 alcohol-abstaining pregnant women, even after adjusting for an increase in total transferrin in pregnancy (partial correlation r = 0.61). However, owing to the cross-sectional nature of that study, the increase in %CDT could not be evaluated in the same subjects with repeated measures. Another recent prospective study (Kenan et al., 2011) examined %CDT changes in 24 pregnant women by analyzing an average of seven repeated samples per person. Their results indicate that %CDT levels increase from 1.07 ± 0.17% during the 9th to the 16th weeks of gestation to 1.61 ± 0.23% in samples obtained within 1 week prior to delivery (∼50% increase) and that the levels began to increase in the second trimester. Moreover, that study demonstrated that all CDT glycoform levels returned to the baseline concentrations within 8–15 weeks postpartum. However, no other biomarkers were evaluated in that study to confirm false-positive results.
Other factors that might affect CDT concentrations have been proposed during the past two decades, including age, smoking, body composition, lipid profile, iron deficiency and anemia, hypertension, viral hepatitis and other liver conditions (reviewed by Arndt, 2001). However, many of these studies captured the confounding effect of these factors on total transferrin rather than %CDT per se (reviewed by Fleming et al., 2004). Among an array of risk factors evaluated in our study, only marijuana use in pregnancy was inversely associated with %CDT at term. The reason for this association is unclear, but warrants further study.
The relatively small sample size and the polysubstance use in this study population might limit the generalizability of our results. However, drug use was carefully captured and adjusted for in multivariate analysis. In addition, we feel that given the similarity of our findings with two previous reports in alcohol-abstaining pregnant women recruited from the general population, OMT and illicit drug use are not major contributors to %CDT changes in pregnancy. Mean %CDT concentrations reported in our study and those reported by Kenan et al. at term are very similar (1.67 and 1.61, respectively). This is a first report to examine accuracy of %CDT among polysubstance-using, pregnant women, predominantly on OMT, who are more likely to be examined for alcohol use in pregnancy than low-risk pregnant women.
Compared with previous reports, our prospective study confirmed alcohol abstinence by the most comprehensive battery of ethanol biomarkers ever used in the same cohort of pregnant women and their newborn children. Notably, our panel included a highly sensitive and specific biomarker—PEth measured in both maternal and newborn samples to confirm abstinence. Among non-pregnant patients with alcohol dependence, PEth demonstrated a 94.5 (Hartmann et al., 2007) to 100% (Aradottir et al., 2006) sensitivity. A single drink is not enough to trigger an elevation in PEth; however, a close to 100% sensitivity was detected among alcohol-dependent patients who consumed moderate amounts of alcohol (Aradottir et al., 2006). Among women of reproductive age, PEth was detectable in 93% of women averaging >2 drinks/day and in 53% of subjects drinking ≤1 drink/day (Steward et al., 2010). This biomarker was not assessed in the two prior studies of pregnant women and CDT levels. In a non-pregnant population, PEth is elevated by a much lower level of alcohol consumption than CDT (Bakhireva and Savage, 2011), and thus the use of this biomarker in our study further strengthens prior observations that the steady increase in %CDT during pregnancy is not a result of undetected alcohol use. Finally, this was the first study to examine the influence of other maternal risk factors on %CDT changes in pregnancy.
In summary, our results indicate that using cutoffs specified for men and non-pregnant women, the specificity of %CDT to confirm alcohol abstinence might be affected by advanced gestational age, even after accounting for increased transferrin concentrations in pregnancy. The biologic basis for this phenomenon is still unclear and requires further investigation. These results suggest that a conventional cutoff of 1.7% CDT might be too low for pregnant women and would generate false-positive results, especially if evaluated near or at term. Thus, we propose that a %CDT of ≥2.0% be considered a threshold concentration indicative of alcohol exposure in pregnant women. Only one of our patients and none of patients in the Kenan et al. study had %CDT concentrations above 2.0% near term. However, the sensitivity of %CDT at this cutoff for heavy and moderate drinking during pregnancy needs to be evaluated further.
Funding
This work was supported by the Alcohol Beverage Medical Research Foundation (ABMRF) and the National Institutes of Health (P20 AA017608, UL1 RR031977).
Acknowledgments
We are indebted to the nurses at the UNM Milagro clinic (Sylvia Price and Eve Wohlert) and the Clinical & Translational Science Center research nurses (Sandra Brown, Conra Lacy-Backstrom, Theresa Wussow and Carol Hartenberger) for their assistance with recruitment and follow-up of patients. Emily Leonard coordinated the measurement of %CDT at MUSC. We also thank Luis Robles, Mahek Garg and Dr Ronald Schrader for their assistance with the data management and analysis.
References
- American Academy of Pediatrics. Committee on substance abuse and committee on children with disabilities. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106:358–61. doi:10.1542/peds.106.2.358. [PubMed] [Google Scholar]
- Anton RF. Carbohydrate-deficient transferrin for detection and monitoring of sustained heavy drinking. What have we learned? Where do we go from here? Alcohol. 2001;25:185–8. doi: 10.1016/s0741-8329(01)00165-3. doi:10.1016/S0741-8329(01)00165-3. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Olsson BL. Methodological modifications on quantification of phosphatidylethanol in blood from humans abusing alcohol, using high-performance liquid chromatography and evaporative light scattering detection. BMC Biochem. 2005;6:18. doi: 10.1186/1471-2091-6-18. doi:10.1186/1471-2091-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aradottir S, Asanovska G, Gjerss S., et al. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41:431–7. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13–27. [PubMed] [Google Scholar]
- Bakhireva L, Savage D. Biomarkers of fetal alcohol exposure and fetal alcohol effects. Alcohol Res Health. 2011;34:56–63. [PMC free article] [PubMed] [Google Scholar]
- Bean P. Powerful combination of direct biomarkers of alcohol use and abuse promises to improve current detection rates. On the Risk. 2006;22:38–43. [Google Scholar]
- Bergstrom JP, Helander A. Clinical characteristics of carbohydrate-deficient transferrin (%disialotransferrin) measured by HPLC: sensitivity, specificity, gender effects, and relationship with other alcohol biomarkers. Alcohol Alcohol. 2008a;43:436–41. doi: 10.1093/alcalc/agn017. [DOI] [PubMed] [Google Scholar]
- Bergstrom JP, Helander A. Influence of alcohol use, ethnicity, age, gender, BMI and smoking on the serum transferrin glycoform pattern: implications for use of carbohydrate-deficient transferrin (CDT) as alcohol biomarker. Clin Chim Acta. 2008b;388:59–67. doi: 10.1016/j.cca.2007.10.011. doi:10.1016/j.cca.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Bianchi V, Vivaldi A, Rasping A., et al. Pregnancy and variations of carbohydrate-deficient transferrin levels measured by the candidate reference HPLC method. Alcohol Alcohol. 2011;46:123–7. doi: 10.1093/alcalc/agq092. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. Measurement and interpretation of drinking behavior. I. On measuring patterns of alcohol consumption. II. Relationships between drinking behavior and social adjustment in a sample of problem drinkers. J Stud Alcohol. 1975;36:1154–72. doi: 10.15288/jsa.1975.36.1154. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Boyd-Wickizer J, Powell SH., et al. Alcohol screening questionnaires in women: a critical review. J Am Med Assoc. 1998;280:166–71. doi: 10.1001/jama.280.2.166. doi:10.1001/jama.280.2.166. [DOI] [PubMed] [Google Scholar]
- Chang G, Goetz MA, Wilkins-Haug L. Identifying prenatal alcohol use: screening instruments versus clinical predictors. Am J Addict. 1999;8:87–93. doi: 10.1080/105504999305884. doi:10.1080/105504999305884. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. Blood Collection on Filter Paper for Newborn Screening Programs; Approved Standard. 5th edn. Vol. 27. Wayne, PA: CLSI Publication LA4-A5; 2007. [Google Scholar]
- Fleming MF, Anton RF, Spies CD. A review of genetic, biological, pharmacological, and clinical factors that affect carbohydrate-deficient transferrin levels. Alcohol Clin Exp Res. 2004;28:1347–55. doi: 10.1097/01.alc.0000139815.89794.be. doi:10.1097/01.ALC.0000139815.89794.BE. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Aradottir S, Graf M. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81–4. doi: 10.1111/j.1369-1600.2006.00040.x. doi:10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Helander A, Eriksson G, Stibler H. Interference of transferrin isoform types with carbohydrate-deficient transferrin quantification in the identification of alcohol abuse. Clin Chem. 2001;47:1225–33. [PubMed] [Google Scholar]
- Helander A, Husa A, Jeppsson JO. Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin Chem. 2003;49:1881–90. doi: 10.1373/clinchem.2003.023341. doi:10.1373/clinchem.2003.023341. [DOI] [PubMed] [Google Scholar]
- Helander A, Wielders JP, Jeppsson JO, et al. Toward standardization of carbohydrate-deficient transferrin (CDT) measurements: II. Performance of a laboratory network running the HPLC candidate reference measurement procedure and evaluation of a candidate reference material. Clin Chem Lab Med. 2010;48:1585–92. doi: 10.1515/CCLM.2010.322. doi:10.1515/cclm.2010.322. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, et al. Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res. 1998;22:345–51. doi: 10.1111/j.1530-0277.1998.tb03659.x. doi:10.1111/j.1530-0277.1998.tb03659.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, et al. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–25. doi: 10.1542/peds.109.5.815. doi:10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jeppsson JO, Kristensson H, Fimiani C. Carbohydrate-deficient transferrin quantified by HPLC to determine heavy consumption of alcohol. Clin Chem. 1993;39:2115–20. [PubMed] [Google Scholar]
- Jones J, Jones M, Plate C, et al. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Anal Methods. 2011;3:1101–6. doi:10.1039/c0ay00636j. [Google Scholar]
- Kenan N, Larsson A, Axelsson O, et al. Changes in transferrin glycosylation during pregnancy may lead to false-positive carbohydrate-deficient transferrin (CDT) results in testing for riskful alcohol consumption. Clin Chim Acta. 2011;412:129–33. doi: 10.1016/j.cca.2010.09.022. doi:10.1016/j.cca.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Russell M, Martier SS, Sokol RJ, et al. Detecting risk drinking during pregnancy: a comparison of four screening questionnaires. Am J Public Health. 1996;86:1435–9. doi: 10.2105/ajph.86.10.1435. doi:10.2105/AJPH.86.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863–8. doi: 10.1016/0002-9378(89)90302-5. discussion 868–870. [DOI] [PubMed] [Google Scholar]
- Steward S, Law T, Randall P, et al. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcohol Clin Exp Res. 2010;34:488–92. doi: 10.1111/j.1530-0277.2009.01113.x. doi:10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler JM, Huntington KS, Peterson CM. The prenatal detection of significant alcohol exposure with maternal blood markers. J Pediatr. 1998;133:346–52. doi: 10.1016/s0022-3476(98)70267-7. doi:10.1016/S0022-3476(98)70267-7. [DOI] [PubMed] [Google Scholar]