Abstract
Objective
To determine the effects of length of cochlear implant use and other demographic factors on the development of sustained visual attention in prelingually deaf children and to examine the relations between performance on a test of sustained visual attention and audiological outcome measures in this population.
Design
A retrospective analysis of data collected before cochlear implantation and over several years after implantation. Two groups of prelingually deaf children, one >6 years old (N = 41) and one <6 years old (N = 47) at testing, were given an age-appropriate Continuous Performance Task (CPT). In both groups, children monitored visually presented numbers for several minutes and responded whenever a designated number appeared. Hit rate, false alarm rate, and signal detection parameters were dependent measures of sustained visual attention. We tested for effects of a number of patient variables on CPT performance. Multiple regression analyses were conducted to determine if CPT scores were related to performance on several audiological outcome measures.
Results
In both groups of children, mean CPT performance was low compared with published norms for normal-hearing children, and performance improved as a function of length of cochlear implant use and chronological age. The improvement in performance was manifested as an increase in hit rate and perceptual sensitivity over time. In the younger age group, a greater number of active electrodes predicted better CPT performance. Results from regression analyses indicated a relationship between CPT response criterion and receptive language in the younger age group. However, we failed to uncover any other relations between CPT performance and speech and language outcome measures.
Conclusions
Our findings suggest that cochlear implantation in prelingually deaf children leads to improved performance on a test of sustained visual processing of numbers over 2 or more years of cochlear implant use. In preschool-age children who use cochlear implants, individuals who are more conservative responders on the CPT show higher receptive language scores than do individuals with more impulsive response patterns. Theoretical accounts of these findings are discussed, including cross-modal reorganization of visual attention and enhanced phonological encoding of visually presented numbers.
Prelingually deaf children with cochlear implants are a unique clinical population to study because they have experienced a period of auditory deprivation before the development of significant speech and language skills. The success of many deaf children in acquiring spoken language from the electrical stimulation provided by a cochlear implant is impressive. Although clinical outcomes have been found to be quite variable in this population (Blamey et al., 2001; Pisoni, Cleary, Geers, & Tobey, 2000; Sarant, Blamey, Dowell, Clark, & Gibson, 2001), the scores of most children on a range of speech perception, language, and speech intelligibility tests improve with cochlear implant use (Miyamoto, et al., 1997; Svirsky, Robbins, Kirk, Pisoni, & Miyamoto, 2000; Tyler et al., 1997).
Physical device limits such as reduced spectral resolution and compressed dynamic range clearly play a role in limiting the speech perception abilities developed by prelingually deaf children who use cochlear implants. Nevertheless, many prelingually deaf children are able to use these degraded signals to develop age-appropriate speech perception and language skills with a cochlear implant. In contrast, other prelingually deaf children obtain only awareness of sound with a cochlear implant and their speech and language skills remain significantly delayed. The sources of this outcome variability have yet to be fully revealed. Studies examining predictors of cochlear implant performance have reported effects of early implantation, communication mode, device type, and dynamic range (Geers, Brenner, & Davidson, 2003; Geers, Nicholas, & Sedey, 2003; Kirk, 2000; Tobey, Geers, Brenner, Altuna, & Gabbert, 2003). However, a large portion of outcome variance still remains unexplained by these traditional demographic and medical factors (Pisoni et al., 2000; Sarant et al., 2001).
A major paradigm shift in the study of pediatric cochlear implant outcomes is underway, based on recent investigations of the cognitive abilities of prelingually deaf children (Pisoni et al., 2000). The premise of this approach is that individual differences in speech and language outcomes in prelingually deaf children may reflect underlying differences in central auditory processing and related cognitive functions such as working memory, attention, and executive functions. These processes are not specific to audition nor to language, yet they may play important roles in perceiving speech, acquiring language, and developing the sensory-motor abilities crucial to producing highly intelligible speech (Pisoni & Cleary, 2004).
Two major research objectives can be identified in the study of cognitive processes of prelingually deaf children with cochlear implants. The first is to describe how individual cognitive processes may be altered in children who have experienced a period of auditory deprivation. The second is to assess relationships between individual cognitive functions and speech and language outcomes. Both objectives may reveal important predictors of benefit and new sources of individual differences in clinical outcomes with a cochlear implant. Furthermore, research on central auditory and cognitive factors may provide the theoretical basis for new therapeutic cognitive and behavioral interventions for deaf children who, despite having access to sound with a cochlear implant, do not demonstrate significant improvement in spoken language skills.
A number of visual information processing studies conducted with prelingually deaf adults have revealed compelling evidence that auditory deprivation leads to reorganization of visual attention processes (Bavelier et al., 2001; Bavelier et al., 2000; Neville & Lawson, 1987a, 1987b, 1987c; Proksch & Bavelier, 2002; Rothpletz, Ashmead, & Tharpe, 2003). In general, the findings from electrophysiological, fMRI, and behavioral studies have demonstrated that deaf adults, compared with normal-hearing adults, show evidence for increased processing of peripheral visual stimuli and wider spatial distribution of selective attention. The deaf adults appeared to have developed a visual system geared toward processing a wider spatial area than typically observed in normal-hearing participants (Proksch & Bavelier, 2002).
The deaf adults tested in these experiments typically used American Sign Language (ASL, a manual language that is linguistically distinct from any spoken language). To test the hypothesis that reorganization of visual attention is due to auditory deprivation per se rather than a result of acquiring expertise in a manual language, several studies have tested normal-hearing adults who learned ASL at an early age. These adults typically acquired ASL from their deaf parents. The effect of ASL fluency itself did not turn out to be significant, suggesting that the reorganization of peripheral visual processing and selective attention results from auditory deprivation and not from acquisition of a manual language (Bavelier et al., 2001; Neville & Lawson, 1987c; Proksch & Bavelier, 2002).
Although no studies to date have examined development of selective visual attention in prelingually deaf children of different ages, there have been several developmental studies of sustained visual attention in this population of children. Sustained attention is a form of attention that is responsible for the continuous allocation of processing resources for the detection of rare events (Parasuraman, 1998). This ability is crucial for efficient exploration of the environment and supports other perceptual and cognitive processes. Experimentally, sustained visual attention capacity can be measured by a continuous performance task (CPT), in which participants are asked to respond to low-frequency target stimuli. Experimental manipulations of these tasks in normal-hearing participants have demonstrated the fragile nature of sustained visual attention. Performance has been shown to decline with increased task length, lower signal salience, faster stimulus rate, and higher memory load (Parasuraman, 1998).
The CPT was originally developed by Rosvold, Mirskey, Sarason, Bransome, & Beck (1956) for assessing patients with brain damage. Several newer CPTs have since been designed and normed for clinical use (Conners, 2000; Gordon, McClure, & Aylward, 1996; Greenberg, 1991). All of these tests have moderate clinical validity for detecting attentional Disorders such as Attention Deficit Hyperactivity disorder in children at risk for these conditions (Barkley, 1991; Forbes, 1998). In a CPT, children are asked to monitor a stream of visually presented stimuli and respond by pressing a key each time they detect a target stimulus. Raw scores are generally expressed as the number or rate of correct responses (or hits) and incorrect responses. Because CPTs are visual information processing tasks that have no auditory demands, they have been used to assess the sustained visual attention skills of both deaf children and adults.
Although CPTs have been widely used to assess sustained visual attention skills, these tasks are quite complex and sensitive to a number of different cognitive factors. CPTs require not only sustained attention but also the ability to hold the knowledge of the target sequence in working memory. Furthermore, CPT performance is negatively affected by impulsivity (an inability to inhibit responses to the nontarget stimuli). This complexity is described by Parasnis, Samar, & Berent (2003) in their discussion of the raw scores obtained from a CPT. Parasnis et al. argue that more attentive participants will demonstrate increased hit rates and lower false alarm rates than less attentive participants. In contrast, less impulsive participants will show both decreased false alarm rates and decreased hit rates than more impulsive participants. Thus, as Parasnis et al. point out, the raw scores on a CPT cannot be said to be independent measures of any one cognitive factor. As a result, Signal Detection Theory (SDT; Green & Swets, 1966) can be applied to compute two variables from the hit rate and false alarm rate: d′, or perceptual sensitivity (ability to distinguish the targets from nontargets), and β (response criterion). These two factors, d′ and β, have been widely used as dependent measures in CPTs (Parasuraman, 1998). Perceptual sensitivity is an index of the degree to which participants can discriminate between the target signals and the nontargets. In contrast, response criterion is an index of the participant’s likelihood to respond to both target and nontarget stimuli. A low β is consistent with impulsivity because the response rate is much higher for a given target presentation rate, whereas a high β reflects a more conservative, less impulsive response criterion.
In the first study of sustained visual attention skills of prelingually deaf children with cochlear implants, Quittner, Smith, Osberger, Mitchell, & Katz (1994) administered a CPT to three groups of children: prelingually deaf children with cochlear implants, prelingually deaf children who used hearing aids, and normal-hearing children. Two experiments are reported by Quittner et al. In the first experiment, children were assigned to one of two age groups: younger (6 to 8 years) and older (9 to 13 years). Overall, Quittner et al. found that children in the older group performed better than children in the younger group, demonstrating an effect of chronological age on CPT performance. In addition, Quittner et al. reported a main effect of group. Across age groups, normal-hearing children performed better than both children with cochlear implants and children with hearing aids. However, the authors also found an interaction between age and group. In the early age range, both groups of prelingually deaf children had lower mean d′ scores than the normal-hearing children. In the older age range, however, the children with cochlear implants performed as well as normal-hearing children, whereas the mean d′ of children with hearing aids was below that of both the children with cochlear implants and the normal-hearing children.
In the second experiment, Quittner et al. (1994) reported findings from a longitudinal study of CPT performance in prelingually deaf children with cochlear implants and a group of deaf children who used hearing aids. Each child was tested twice, with approximately 8 months between testing sessions. The children with cochlear implants were tested only after implantation (about 10 and 18 months after implant). The results from this experiment mirrored the first experiment: children with cochlear implants showed greater increases in d’ than children with hearing aids over the 8-month interval. A similar interaction was found for β scores: Children with cochlear implants showed a decrease in β, whereas children with hearing aids showed an increase in β over the 8-month interval. On the basis of the results from these two experiments, Quittner et al. concluded that children who experienced a period of profound prelingual deafness before cochlear implantation are delayed in their development of visual attention skills. Moreover, they interpreted their findings as evidence that access to sound with a cochlear implant produced more typical development of visual attention skills than did a hearing aid in prelingually deaf children. In the first experiment, a significant interaction between age group and access to hearing was observed on perceptual sensitivity alone, whereas in the second experiment, the interaction was found on both d′ and β.
Although no attempt was made to control for length of cochlear implant use, Quittner et al. (1994) suggested that the effect of a cochlear implant on CPT performance was relatively rapid, as indicated by their findings in Experiment 2. They also suggested that CPT performance was unlikely to be related to speech and language outcomes in the children with cochlear implants because gains in linguistic skills typically emerge more slowly over time, after several years of cochlear implant use (Fryauf-Bertschy, Tyler, Kelsay, & Gantz, 1992; Miyamoto, Osberger, Robbins, Myres, & Kessler, 1993). However, Quittner et al. did not report any speech and language outcome data, nor did they assess the relations between CPT performance and speech/language outcomes in either of their two experiments.
In a follow-up study with a larger sample of children, Smith, Quittner, Osberger, & Miyamoto (1998) attempted to replicate the earlier findings reported by Quittner et al. (1994). Two experiments were reported in this study. In the first experiment, CPT scores were obtained from prelingually deaf children with cochlear implants, prelingually deaf children with hearing aids, and normal-hearing children, each of which were subdivided into 6 age groups: 6, 7, 8, 9, 11, and 13 years old. Like Quittner et al., Smith et al. found an interaction between chronological age and group. At 6, 7, and 8 years of age, both groups of deaf children had lower mean d′ scores than normal-hearing children. In contrast, at 9, 11, and 13 years of age, deaf children with cochlear implants performed equally to normal-hearing children based on mean d′, whereas both groups had higher mean d′ scores than the children with hearing aids.
To explain the interaction between cochlear implantation and chronological age, Smith et al. (1998) hypothesized that access to sound, either through normal acoustic or electrical hearing, before or during a critical developmental period, was important for children to acquire typical sustained visual attention skills as assessed by the CPT. They argued that cochlear implantation of deaf children before or during the critical period leads to faster maturation of visual attention skills, compared with children with hearing aids, until they reached age appropriate levels compared with children with normal hearing. The authors argued that before the onset of the critical period for visual attention development, deaf children with cochlear implants would show no developmental advantage over deaf children with hearing aids in acquiring these skills. Smith et al. attributed the lack of an effect of a cochlear implant in the 6- to 8-year-old children as evidence that this critical period began at around age 7 to 8 years.
In the original CPT, children were asked to detect a two-number sequence. In the easier CPT, the younger children only were asked to detect single digits in the number stream. This new version had a lower working memory load than the original CPT and is recommended and normed for children ages 3 to 5 years (Gordon et al., 1996). Smith et al. found that children with cochlear implants displayed higher mean d′ scores than children with hearing aids, indicating that cochlear implant use lead to improved perceptual sensitivity on the CPT in younger children than were tested previously. Therefore, the lack of an effect of a cochlear implant in 6- to 8-year-old children on the original CPT may have been due to the difficulty of the task. The second experiment by Smith et al. found an effect of auditory experience on CPT skills in 4- to 7-year-old children, using an easier version of the original CPT. Although a critical period during which access to sound leads to typical performance on a CPT might exist, the age of onset and upper age limit remained undefined by the results reported by Smith et al.
Any interpretation of the interaction between chronological age and cochlear implantation reported by Quittner et al. (1994) and Smith et al. (1998) is limited by the fact that neither study controlled for length of cochlear implant use, which varied between 6 months and 6 years. This confounding variable may have been responsible for the observed interactions because children in the younger cochlear implant group may have had fewer years of experience with their devices than children in the older group. Although Smith et al. reported that length of cochlear implant use and CPT performance were not correlated in their study, this finding did not rule out an effect of length of cochlear implant use over the first few years of cochlear implant use. Indeed, Quittner et al. concluded that the effect of a cochlear implant on visual attention skills was relatively rapid. However, because chronological age and length of cochlear implant use were not independently controlled, the source of the interaction between chronological age and cochlear implantation cannot be determined from the findings reported by Quittner et al. or Smith et al.
Although Smith et al. (1998) and Quittner et al. (1994) both concluded that a cochlear implant leads to more typical development of sustained attention skills in deaf children, they did not systematically examine the role of demographic, medical, or audiological factors that may have influenced CPT performance. They did assess the effects of communication mode and found no differences in CPT performance between children in oral communication programs (deaf children who learn to communicate primarily through listening, lipreading, and oral skills) and total communication programs (deaf children who incorporate a manual correlate of their spoken language along with oral skills). However, Smith et al. and Quittner et al. did not assess other demographic variables such as cause of deafness, cochlear implant type, gender, or other medical factors, which may have contributed to their findings.
Why should access to sound provided by a cochlear implant, and not a hearing aid, affect visual information processing and performance on a visual CPT? The aided auditory thresholds of the two groups of deaf children studied by both Smith et al. (1998) and Quittner et al. (1994) were quite similar, indicating that both devices were equal in terms of their effect on detection of near threshold sounds. Perhaps something about the nature of the auditory information provided by a cochlear implant lead to the improved sustained visual attention skills in this population? Hearing aids function by amplifying sound to stimulate the functional hair cells remaining in a cochlea damaged by sensorineural hearing loss. Processing of this amplified signal by the profoundly deaf cochlea often leads to a significant amount of spectral distortion, limiting the effectiveness of hearing aids for improving speech perception (Niparko et al., 2000). In contrast, cochlear implants extract specific cues encoded in the time-varying spectrum of speech signals and use this information to directly stimulate the auditory nerve (Niparko et al., 2000). Despite carrying impoverished acoustic-phonetic speech information, a cochlear implant is better suited than a hearing aid for speech perception in profoundly deaf individuals who have great difficulty understanding speech (Niparko et al., 2000).
It is also possible that the observed benefits of a cochlear implant for speech and language development in profoundly deaf individuals are related to the neural and cognitive mechanisms responsible for the improvements in sustained visual attention skills with a cochlear implant. For instance, gains in perceptual skills and other cognitive processes may be related to spoken language development, which enables deaf children to perform better on the CPT. One way to test these predictions would be to conduct a set of multivariate analyses to determine whether CPT scores obtained from children with cochlear implants explain a significant portion of the variance in their speech/language test scores. However, relations between CPT scores and speech/language scores were not examined by either Smith et al. (1998) or Quittner et al. (1994) or by other researchers who have used the CPT to assess sustained visual attention in deaf children.
In a recent report, Tharpe, Ashmead, & Rothpletz (2002) raised several criticisms about the conclusions of Smith et al. (1998) and Quittner et al. (1994) and suggested that their CPT results may have reflected differences in IQ between the test populations. To deal with this concern, Tharpe et al. conducted a study of CPT performance in prelingually deaf children with cochlear implants, prelingually deaf children with hearing aids, and normal-hearing children in which nonverbal IQ of each participant was measured and used as a covariate in all analyses. Tharpe et al. failed to find any differences in mean d′; between children with cochlear implants and deaf control children with hearing aids and were unable to replicate the earlier differences in CPT scores between normal-hearing children and either group of deaf children reported by Quittner et al. and Smith et al. Tharpe et al. interpreted their new results as evidence that the benefit of a cochlear implant on sustained visual attention reported by Quittner et al. and Smith et al. was due to differences in IQ rather than differences in visual attention.
Although the conclusions of Tharpe et al. (2002) might be correct, their findings must be interpreted cautiously for several reasons. First, the sample size used in their study was extremely small (only 8 participants in each participant group) compared with the much larger samples used by Smith et al. (1998) and Quittner et al. (1994). Second, the experimental design used by Tharpe et al. was fundamentally different from the designs used by Quittner et al. and Smith et al., in which chronological age was an independent variable. Although Tharpe et al. raised important issues about the earlier work on sustained visual attention in children with cochlear implants and suggested further areas of research, their study cannot be considered a valid replication of either of the earlier studies.
Finally, the CPT scores reported by Tharpe et al. (2002) were close to ceiling performance. The participants tested by Tharpe et al. had a mean d′ of 3.5 and above, which strongly suggests that ceiling effects may have influenced their results. The ranges of d′ obtained by Smith et al. (1998) and Quittner et al. (1994), using a different CPT, were much wider than reported by Tharpe et al. (1.75 to 4.25 in Quittner et al., and 2.0 to 4.75 in Smith et al.). Therefore, it is possible that Tharpe et al. failed to detect differences in CPT performance between deaf children with cochlear implants and deaf children with hearing aids because the CPT they used was too easy for these children.
To summarize, three studies on the development of sustained visual attention in prelingually deaf children with cochlear implants have been reported in the literature to date. In the recent study by Tharpe et al. (2002), no differences between deaf children with cochlear implants, deaf children with hearing aids, or normal-hearing children were observed when they controlled for nonverbal IQ. However, several methodological factors make their findings difficult to compare with the earlier results from the studies by Quittner et al. (1994) and Smith et al. (1998), which both found a beneficial effect of a cochlear implant on the development of perceptual sensitivity on a visual CPT. Clearly, additional research is needed to understand the effect of length of cochlear implant use and chronological age because these variables were not analyzed independently in any of the previous studies. Furthermore, we do not know what other demographic and medical variables might influence sustained visual attention skills of deaf children. Finally, the relations between auditory/oral speech and language skills and sustained visual attention skills have not yet been systematically assessed. The present study was designed to investigate these unanswered questions.
Our first aim was to assess the effect of length of cochlear implant use on sustained visual attention skills. Although we could not explicitly control the chronological age of our participants, we used a statistical model capable of testing for the relative independent effects of length of cochlear implant use and chronological age on CPT performance. We hypothesized that CPT scores would be affected by both length of cochlear implant use and chronological age. We were also interested in determining the time-course of the effects of cochlear implant use: Do visual attention skills increase rapidly during the first year of use, as suggested by Smith et al. (1998) and Quittner et al. (1994), or do they change more gradually over time after implantation? Finally, we were interested in determining if there was an interaction between length of cochlear implant use and chronological age, as predicted by the critical period hypothesis proposed by Smith et al. We hypothesized that the effect of cochlear implant use would be seen in children during a critical age range but not before or after this age range.
Our second aim was to determine if any medical, audiological, or demographic characteristics of prelingually deaf children would affect the development of sustained visual attention skills after cochlear implantation. Although several of these factors have been shown to play a role in the development of auditory/oral speech and language skills with a cochlear implant, their effects on other cognitive skills in prelingually deaf children, such as sustained visual attention, are largely unknown.
Finally, our third aim was to assess the relations between CPT scores and several traditional audiological outcome measures of speech perception, language development, and speech intelligibility. As mentioned previously, no attempt was made in any of the previous studies of CPT performance in deaf children to examine these relations. If postimplant CPT scores of deaf children are related to scores on standard clinical measures of speech and language skills, this would have important implications for explaining individual variability in audiological outcomes of children with a cochlear implant in terms of underlying differences in cognitive abilities such as perceptual sensitivity, impulsivity, and working memory.
We report two experiments with similar longitudinal designs, measures, and independent variables. The major difference between the two experiments was the age range of the children tested. The first experiment included children older than 6 years of age; the second experiment included a younger group of children. Two different age-appropriate CPTs were used to assess sustained visual attention skills in each group of children.
Experiment 1
Methods
Participants
We conducted a retrospective analysis of longitudinal clinical data gathered at the Indiana University School of Medicine Cochlear Implant Program. The participants were part of a larger comprehensive, prospective clinical study of speech and language outcomes in deaf children with cochlear implants. The sample used here consisted of a group of 41 prelingually deaf children (profoundly deaf by 3 years old) who received cochlear implants by 9 years of age. All children were implanted with Nucleus 22 devices. Mean age at implantation was 6.2 years old and ranged from 2.5 to 8.9 years. Fifteen children used oral communication and 26 used total communication at the time of testing. More than 68% had a congenital hearing loss, and the most common acquired cause was meningitis. A subset of children (N = 19) had pre-implant nonverbal IQ scores (WISC or WIPSI) in their charts, and the mean standardized score was 101.3. Table 1 provides a summary of the medical, audiological, and demographic characteristics of our sample.
TABLE 1.
Characteristics of participants in experiments 1 and 2
| Experiment 1 (School-age CPT) |
Experiment 2 (Preschool CPT) |
|
|---|---|---|
| Cause of hearing loss | 1 genetic, 1 CMV | 3 genetic |
| 11 meningitis | 13 meningitis | |
| 28 unknown | 31 unknown | |
| Ear of implantation | 18 right, 23 left | 27 right, 20 left |
| Pure-tone average (dB HL) | 112.6 (5.66) | 111.1 (5.78) |
| Number of active electrodes | 19.8 (4.14) | 19.8 (3.42) |
| Age at implantation (yr) | 6.2 (1.60) | 4.8 (1.34) |
| Communication mode | 15 OC, 26 TC | 22 OC, 25 TC |
| Nonverbal IQ | 101.3 (16.53) | 99.4 (14.43) |
For continuous variables, the mean value for each experiment is given with standard deviation in parentheses. Nonverbal IQ scores were available for 19 of 41 children in Experiment 1 and 37 of 47 children in Experiment 2.
Children were tested once before implantation and every 6 to 12 months after implantation for up to 3 years of device use. Interval data were collapsed into one of four intervals: pre-implant, 1 year after implant, 2 years after implant, and 3 years after implant. Not all children were tested at each interval, as is common in clinical populations, creating missing data cells. Missing data occurred for several reasons. Some children moved away from the Indianapolis area after implantation and were unable to continue the longitudinal study. Also, because our clinical participants are given a large number of tests during each visit, they are often too tired or not cooperative enough to complete all of the tests at a given session.
Procedures
The CPT used in the present study was the “school-age CPT,” (Gordon et al., 1996) the same CPT used in the studies reported by Smith et al. (1998) and Quittner et al. (1994). This test format is recommended for use in normal-hearing children from 6 to 16 years old. The experimental apparatus consists of a free-standing button box with one blue key below an LCD display. The school-age CPT is a 9-minute CPT in which target and nontarget numbers appear at random in the center of a viewing screen at 1-second intervals. Children were instructed to press a key every time a “9” appeared after a “1.” They were instructed to refrain from pressing the key when nontarget numbers appeared or when “9” appeared after any number other than “1.” Over 9 minutes, a total of 540 numbers are presented with a relative target frequency of 0.083 (45 targets). Instructions were given in the child’s preferred mode of communication. Each child was allowed a few practice trials. Testing began only when they had demonstrated that they understood the task. Children were tested with an experimenter but not their caregiver present in the room. No feedback was given during the task other than general encouragement.
Each time a correct response (key press) was made when the target sequence appeared, the computer internally scored a “hit.” Each time a key press was made when the target sequence had not appeared, the computer internally scored a “false alarm.” The Gordon CPT apparatus automatically computes the total number of hits, misses, and false alarms from which the hit rates and false alarm rates were computed manually (Gordon et al., 1996).
Perceptual sensitivity, or d′, was computed as the difference between the z scores for hit rate and false alarm rates. This measure reflects the participant’s ability to discriminate the visual target sequence from nontargets. Response criterion, or β, was computed as the ordinate for hit rate divided by the ordinate for false alarm rate. β reflects the response bias of the participant. More conservative (i.e., less impulsive) responders will have higher β scores than less conservative, or more impulsive responders. Although there is some controversy over the use of parametric measures in cases where the assumptions of SDT are not necessarily true (Pastore, Crawley, Berens, & Skelly, 2003), we included these measures in our analyses along with hit rate and false alarm rate because earlier studies of CPT performance in deaf children and adults have used d′ and β as measures of sustained visual attention. Thus, we report results in terms of d′ and β to make comparisons to previous studies; however, we also report results in terms of hit rate and false alarm rate where appropriate.
Because we did not collect data from a control group of age-matched normal-hearing children, we used the published percentile norms as a benchmark for comparative purposes. The Gordon CPT manual publishes norms for typically developing children from 6 to 16 years old (Gordon et al., 1996). We did not perform any statistical analyses using these standardized data but used them only for descriptive purposes.
At our implant center, a large battery of tests are routinely used to assess oral speech and language skills of children with cochlear implants. From this battery, we selected four tests as representative outcome measures for our sample of children. Open-set word recognition was measured using the Phonetically Balanced-Kindergarten (PBK) test (Haskins, 1949). This test is administered using live-voice presentation and is scored by word (PBK-w) and phoneme (PBK-p). Children hear a spoken word and are asked to repeat the word aloud to the examiner. The words used in this test are phonetically balanced, English monosyllables.
Vocabulary knowledge was assessed with the Peabody Picture Vocabulary Test-III (Dunn & Dunn, 1997). At our center, this test (PPVT) is administered in the child’s preferred mode of communication. Each vocabulary item is presented either orally or orally and manually. The child then chooses from four pictures, one of which correctly corresponds to the meaning of the word.
The Reynell Developmental Language Scales 3rd edition (Reynell & Huntley, 1985) was administered to assess both receptive and expressive language skills. The receptive scales (RDLS-rec) measure 10 different skills including word recognition, sentence comprehension, and verbal comprehension of ideational content. The expressive language scales (RDLS-exp) assess skills such as spontaneous expression of speech and picture description. Like the PPVT, the RDLS was administered in the child’s preferred mode of communication.
Speech intelligibility was assessed by using the Beginner’s Intelligibility Test (Osberger, Robbins, Todd, & Riley, 1994). Audio recordings were made of children repeating a list of 10 sentences presented to them by a clinician. These recordings were then presented to three naïve adult listeners who were asked to transcribe what they thought the children said. Intelligibility scores (BIT) are based on the number of words correctly transcribed by the adult listeners.
Each of these tests, including the CPT, was administered in an otolaryngology clinical setting by clinical audiologists or speech-language pathologists. These clinicians received special training in working with children with cochlear implants and in communicating using Signed Exact English.
Results
As noted earlier, because our participants were drawn from a larger clinical population enrolled in a long-term longitudinal study of cochlear implant outcomes, we could not always obtain CPT scores for each child at each test interval. A traditional repeated-measures ANOVA would, therefore, eliminate data from any child who was not tested at each interval. However, such an analysis can often lead to skewed results as well as underestimates of variability (Schafer & Graham, 2002). We therefore used a statistical technique called the SAS Mixed Procedure* to analyze our data. The SAS Mixed Procedure is a mixed-effects model that uses a maximum-likelihood estimation method to create a model without eliminating any participants (Schafer & Graham). This model makes the assumption that the probability of missing data is not related to the actual missing score (Schafer & Graham). Scores from participants tested at two or more time intervals are used by the model to compute an estimation of the mean score at each time interval. Scores from all participants (including those tested at only one interval) are used to compute the variance of the mean score at each time interval. With this procedure, systematic selection biases can be minimized by using data from all children, even those who were not tested at each interval, in the test design (Schafer & Graham). Table 2 lists the number of participants who were tested on the CPT at each of the test intervals.
TABLE 2.
Number, age, and age ranges of participants with CPT scores at each test interval
| Before implant |
Year 1 | Year 2 | Year 3 | |
|---|---|---|---|---|
| Experiment 1 | ||||
| No. of cases | 6 | 17 | 30 | 15 |
| Mean age (yr) | 8.03 | 8.13 | 8.42 | 8.59 |
| Age range (yr) | 6.5–8.9 | 6.3–9.9 | 4.5–10.9 | 6.1–11.7 |
| Experiment 2 | ||||
| No. of cases | 19 | 30 | 23 | NA |
| Mean age (yr) | 5.25 | 5.67 | 6.43 | NA |
| Age range (yr) | 3.4–7.2 | 3.2–7.6 | 4.2–9.5 | NA |
CPT Performance of Prelingually Deaf Children With Cochlear Implants Compared With a Normative Sample
As described in the previous section, we calculated normative percentile scores from the CPT raw scores. Table 3 shows the percentile means for hit and false alarm rates at each interval along with the number of participants tested at each interval. Examination of these scores reveals that the prelingually deaf children performed poorly on the school-age CPT compared with the normative sample on both hit and false alarm rates. The highest mean normative scores were found in children who had used their implants for 2 years; however, these means were only at the 36th and 16th percentiles for hit and false alarm rates, respectively. The Gordon Diagnostic Manual does not publish norms for d′ or β (Gordon et al., 1996).
TABLE 3.
Mean percentile CPT scores at each test interval
| Before implant |
Year 1 | Year 2 | Year 3 | |
|---|---|---|---|---|
| Experiment 1 | ||||
| No. of cases | 3 | 16 | 26 | 15 |
| Hit rate | 23 (27) | 27.5 (34.8) | 36.6 (32.9) | 17.9 (25.8) |
| False alarm rate | 3.0 (0.0) | 12.5 (11.2) | 16.3 (18.9) | 14.9 (22.0) |
| Experiment 2 | ||||
| No. of cases | 19 | 30 | 23 | NA |
| Hit rate | 20.6 (25.6) | 20.2 (23.3) | 38.5 (36.1) | NA |
| False alarm rate | 13.8 (14.8) | 22.7 (23.0) | 24.4 (20.6) | NA |
Percentile scores were calculated for those children who were within the normative age range at time of testing. Mean percentile scores are shown with standard deviations in parentheses.
Effects of Chronological Age
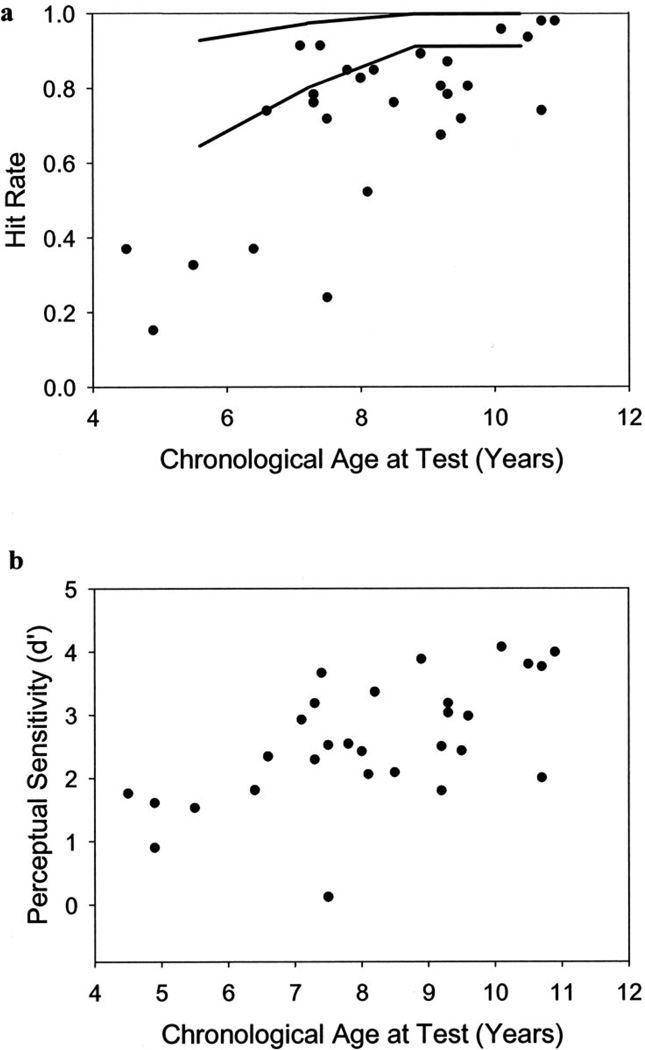
This effect is illustrated in Figure 1 (a and b). The results obtained from the SAS mixed model revealed significant effects of chronological age on hit rate, F(1, 34.7) = 15.93, p < 0.01, and d′, F(1, 39.5) = 13.15, p < 0.01. No significant effects were found for β or false alarm rate. Thus, our sample of prelingually deaf children with cochlear implants demonstrated increases in hit rate and perceptual sensitivity as a function of age.
Fig. 1.
School-age vigilance CPT performance improves as a function of chronological age. Hit rate (a) and perceptual sensitivity (b) both increase with chronological age. Each data point represents the CPT score (mean hit rate or mean d′) for one participant tested at the 2-year interval of cochlear implant use. Several data points overlap because some participants had identical mean CPT scores. Two lines representing the 85th and 15th percentiles for hit rate based on normative data are included in (a) for comparison purposes.
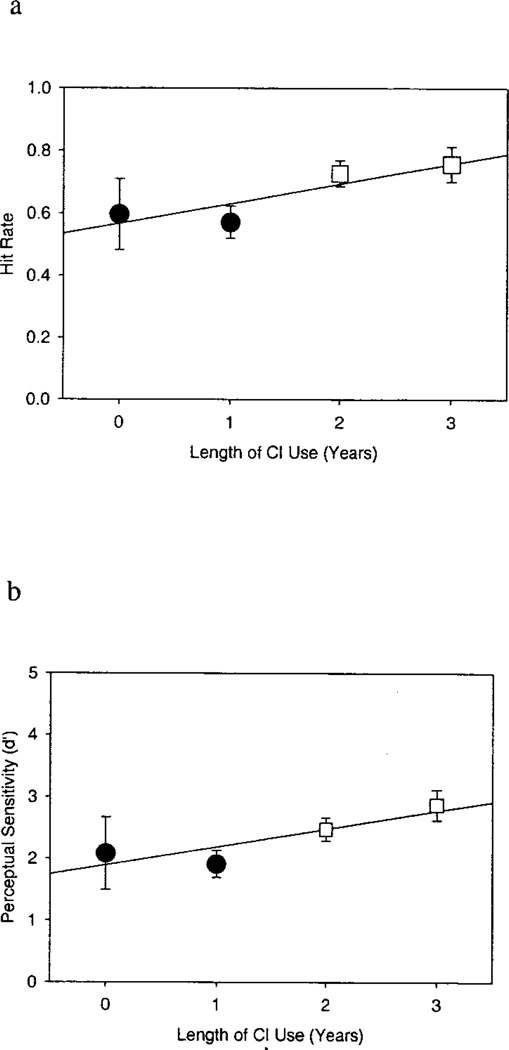
Effects of Length of Cochlear Implant Use
Figures 2 (a and b) illustrates the significant effects of length of cochlear implant use on CPT hit rate and d′. The SAS mixed model produces estimated means for hit rate, false alarm rate, d′, and β as a function of length of cochlear implant use while partialing out the effect of variables in the model. Thus, the estimated mean CPT scores in Figure 2 (a and b) demonstrate the effect of length of cochlear implant use as if chronological age were held constant. The estimated mean hit rate increased from 0.60 (SE = 0.11) before cochlear implantation to 0.76 (SE = 0.06) after 3 years of cochlear implant use. This increase in hit rate was statistically significant, F(3,27.9) = 3.08, p < 0.05. The estimated mean for d′ also increased significantly with length of cochlear implant use from 2.08 (SE = 0.57) before implantation to 2.86 (SE = 0.25) after 3 years of use, F(3,23.8) = 4.46, p < 0.05. In contrast, length of cochlear implant use did not significantly affect β or false alarm rate. The estimated mean false alarm rate was 0.054 (SE = 0.03) before implantation and 0.048 (SE = 0.01) after 3 years of cochlear implant use, and the estimated mean β was 2.63 (SE = 5.0) before implantation and 8.18 (SE = 2.0) after 3 years of cochlear implant use (neither effect was significant, p > 0.05). No significant interaction between chronological age and length of cochlear implant use was found for any of the measures of CPT performance.
Fig. 2.
School-age vigilance CPT performance improves as a function of length of cochlear implant use. Mean hit rate (a) and d′ (b) both increase with length of cochlear implant use. Each data point represents the estimated mean CPT performance from the SAS mixed model at a given year of cochlear implant use. Error bars indicate standard error. Different shaped/shaded data points represent significant differences in mean CPT performance as determined by post hoc analyses. The exception is that mean performance at year 0 did not differ significantly from performance at any subsequent interval. This probably is due to the large degree of variability in CPT performance before implantation.
Tukey’s post hoc comparisons were carried out to assess differences between each postimplant interval. In Figure 2 (a and b), means that differed significantly from each other are represented by data points of different shapes. Figure 2a shows that hit rate increased significantly, as a function of length of cochlear implant use, between postimplant years 1 and 2 (Tukey’s p < 0.05) and years 1 and 3 (Tukey’s p < 0.05), but no significant increase was found between years 2 and 3 (Tukey’s p = 0.97). Figure 2b shows that perceptual sensitivity (d′) followed a similar pattern, increasing between years 1 and 2 (Tukey’s p < 0.01) and years 1 and 3 (Tukey’s p < 0.01) but not between years 2 and 3 (Tukey’s p = 0.39).
We identified 16 participants who were tested on the school-age CPT at least twice (at two or more intervals of cochlear implant use). The raw hit rate and d′ scores for these 16 participants at each interval of testing are given in Table 4. Four of these participants showed near ceiling hit rates (above 90%) on the initial test and demonstrated relatively flat hit scores over time. Ten participants showed some improvement in hit rate over subsequent testing intervals consistent with the mixed model. Thirteen of the 16 participants showed an increase in d′ over subsequent testing intervals.
TABLE 4.
School-age CPT scores of participants tested at two or more intervals
| Before Implant | Year 1 | Year 2 | Year 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Participant No. | HR | d′ | HR | d′ | HR | d′ | HR | d′ |
| 99 | 0.76 | 2.09 | 0.71 | 2.36 | ||||
| 135 | 0.41 | 1.32 | 0.76 | 2.29 | 0.87 | 2.84 | ||
| 140 | 0.98 | 3.76 | 0.89 | 3.88 | ||||
| 169 | 0.91 | 3.03 | 0.85 | 2.59 | ||||
| 196 | 0.80 | 2.31 | 0.74 | 2.00 | ||||
| 201 | 0.59 | 2.42 | 0.78 | 3.18 | ||||
| 210 | 0.60 | 2.51 | 0.59 | 2.24 | 0.83 | 2.42 | ||
| 221 | 0.80 | 2.93 | 0.93 | 3.80 | 0.84 | 3.06 | ||
| 222 | 0.67 | 1.80 | 0.78 | 2.12 | ||||
| 231 | 0.37 | 1.81 | 0.87 | 3.76 | ||||
| 279 | 0.57 | 2.27 | 0.98 | 3.76 | 0.91 | 3.76 | ||
| 363 | 0.57 | 2.08 | 0.72 | 2.43 | ||||
| 375 | 0.91 | 3.66 | 0.98 | 4.11 | ||||
| 376 | 0.54 | 1.27 | 0.72 | 2.52 | ||||
| 383 | 0.96 | 3.72 | 0.96 | 4.07 | ||||
| 397 | 0.65 | 2.43 | 0.80 | 2.50 | ||||
Effects of Medical, Audiological, and Demographic Variables
The effects of medical, audiological, and demographic variables on CPT scores were also examined in separate analyses using SAS mixed models that controlled for chronological age and length of cochlear implant use. We found no significant effects on school-age CPT scores for any of the variables listed in Table 1.
Relations Between CPT Performance and Speech and Language Outcome Measures
We conducted a series of stepwise multiple regression analyses to investigate the relations between CPT performance and individual outcome measures (Cohen & Cohen, 1983). In each model, we used CPT scores from postimplant interval year 2 as predictor variables because this interval had the largest number of participants with CPT scores (N = 30). Length of cochlear implant use and age at implantation were also included as predictor variables. We used the most recent speech and language outcome score for each participant as the dependent variable in our analyses. Correlations of the predictor variables indicated that CPT hit rate and d′ were highly correlated (r = 0.864, p < 0.0001). As a result, we only included d′ and β as predictor variables to prevent effects of colinearity from confounding our results. All other screening correlations were low to moderate.
We conducted six stepwise multiple regression analyses, one for each outcome measure. Inclusion and exclusion criteria were p < 0.05 and p > 0.10, respectively. Table 5 describes the final regression models for each outcome measure. Length of use was a significant predictor of PPVT and BIT scores. Age at implantation was a negative predictor of PBK scores both for words and phonemes. Response criterion was a significant predictor of receptive language age equivalent scores on the RDLS. Neither β nor d′ were significant predictors of any other outcome measures.
TABLE 5.
Final stepwise regression models for speech and language outcome measures: Experiment 1
| Dependent variable |
Predictor(s) | df | p | β | R2 (adjusted) |
|---|---|---|---|---|---|
| PPVT | Cochlear implant use | 1,25 | 0.008 | 0.498 | 0.218 |
| RDLS-rec | β | 1,16 | 0.028 | 0.516 | 0.433 |
| RDLS-exp | None | ||||
| PBK-p | Age of implant | 1,25 | 0.005 | −0.521 | 0.242 |
| PBK-w | Age of implant | 1,25 | 0.004 | −0.532 | 0.255 |
| BIT | Cochlear implant use | 1,24 | 0.005 | 0.530 | 0.251 |
PPVT, RDLS scores are in age equivalents. PBK, BIT scores are in percent correct. For each predictor, standardized coefficient (β), t value, and p value are listed. For each model, adjusted R2 is listed.
Discussion
The low mean percentile CPT scores demonstrated by the children in our study replicate the earlier findings reported by Quittner et al. (1994) and Smith et al. (1998) that across ages, prelingually deaf children show atypical sustained visual attention skills compared with normal-hearing children. Depending on the length of cochlear implant use, approximately one third to one half of children would have fallen into the “abnormal” clinical range as defined in the Gordon CPT manual (Gordon et al., 1996). However, similar proportions of children did perform in the “normal” range, suggesting that not all deaf children displayed atypical sustained visual attention skills. Based on mean percentile scores, false alarm rates were more atypical than hit rates in this sample of children.
The SAS mixed model used here allowed us to measure the effects of chronological age and length of cochlear implant use separately as continuous independent variables. Similarly to Smith et al. (1998) and Quittner et al. (1994), we found a significant effect of chronological age on both d′ and hit rate. We also found that these CPT measures increased as a function of length of cochlear implant use. Over 3 years of cochlear implant use, prelingually deaf children showed an increase in hit rate and perceptual sensitivity when the effect of chronological age was partialed out. However, unlike Quittner et al., we found no effect of chronological age or length of cochlear implant use on false alarm rate or β. Although there was a trend for older children to have slightly higher β and for β to increase with greater cochlear implant use, neither effect reached significance.
These findings suggest that performance of prelingually deaf children on the school-age CPT begins to improve after 1 year of cochlear implant use. In Experiment 1, this improvement appears to be due to increasing perceptual sensitivity for distinguishing the target sequence from the nontargets and not due to a change in levels of impulsivity. We cannot say whether this improvement levels off or continues to increase with longer periods of cochlear implant use. Given the previous findings of Smith et al. (1998) and Quittner et al. (1994), we would expect continued improvement in CPT performance as a function of cochlear implant use until the deaf children are, on average, performing equally to normal-hearing children. We did not find a significant interaction between chronological age and length of cochlear implant use to suggest a critical period for development of sustained visual attention skills. Also, we did not find any significant effects for the demographic, medical, or audiological variables on CPT performance.
The results from the multiple regression analyses suggest that children who are more conservative responders (higher β) on the CPT have higher receptive language scores on the RDLS than do children who are less conservative responders (lower β). We failed to find any other significant relations between CPT performance and open-set word recognition, expressive language, or speech intelligibility scores. Children with longer periods of cochlear implant use had greater vocabulary and speech intelligibility scores. Children who received cochlear implants at earlier ages showed greater open-set word recognition scores. These findings fail to support the hypothesis that the visual processing skills recruited by the school-age CPT are related to abilities that influence speech and language development in prelingually deaf children with cochlear implants.
Experiment 2
Methods
Participants
Experiment 2 was also a retrospective analysis of longitudinal clinical data gathered at the Indiana University School of Medicine Cochlear Implant Program. Participants included a younger group of 47 prelingually deaf children who received Nucleus 22 cochlear implants by 9 years of age. In this sample of children, mean age at implantation was 4.8 years old, with a range of 2.2 to 7.5 years. The other medical, audiological, and demographic characteristics of the sample are summarized in Table 1.
Testing protocols in this experiment were identical to the previous study. Children were tested once every 6 to 12 months until 2 years after implantation. Interval data were collapsed into one of three intervals: before implant, 1 year after implant, and 2 years after implant. We did not include data from postimplant year 3 in the analyses for Experiment 2 because only 4 children had preschool CPT scores at this interval. As in the previous experiment, not all children were tested on all measures at each interval. The number of children who were tested at each interval are shown in Table 2.
Procedures
The preschool CPT is recommended for use with normal-hearing children from 3 to 5 years of age (Gordon et al., 1996). This 6-minute CPT task uses the same testing apparatus and visual stimuli as the school-age CPT described previously. In this new task, children were also required to monitor a stream of numbers presented visually at 1-second intervals. In contrast to the school-age CPT, however, the preschool CPT required children to only respond whenever a “1” appeared on the screen. This CPT version is easier than the school-age task because children are not required to remember the previously presented number when the target appears. Therefore, the preschool CPT has a lower working memory load than the school-age CPT. The results were scored in the same manner and the same dependent variables for sustained visual attention were computed as described previously for Experiment 1.
The same four traditional speech and language outcome measures used in Experiment 1 were also used as outcome measures in this experiment. However, in this younger population of children, we did not have a sufficient sample size with 2 years of cochlear implant use to carry out correlations with scores obtained at this interval. Therefore, in Experiment 2, we only examined relations between measures obtained after 1 year of cochlear implant use.
Results
Preschool CPT Performance of Prelingually Deaf Children With Cochlear Implants Compared With a Normative Sample
Although the preschool CPT is recommended and normed for children from 3 to 5 years of age, a number of children in our sample were older than 5 years at the time of testing. The most stringent criteria for calculating percentile scores would be to exclude those children who were older than 5 years of age. However, this would leave us with a very small group of children to compare with the normative samples. Therefore, we report normative scores for children who were 6 years old or younger at the time of testing. These data should be interpreted with caution and are included only for descriptive purposes. Table 3 shows the percentile means for hit rate and false alarm rate at each testing interval, along with the number of participants who were normed at each interval. The highest mean normative scores, 38th and 20th percentiles for hit rate and false alarms, respectively, were found in children who had used their cochlear implant for 2 years. These data suggest that like the older deaf children in Experiment 1, younger deaf children with cochlear implants performed poorly on the preschool CPT compared with the normative sample.
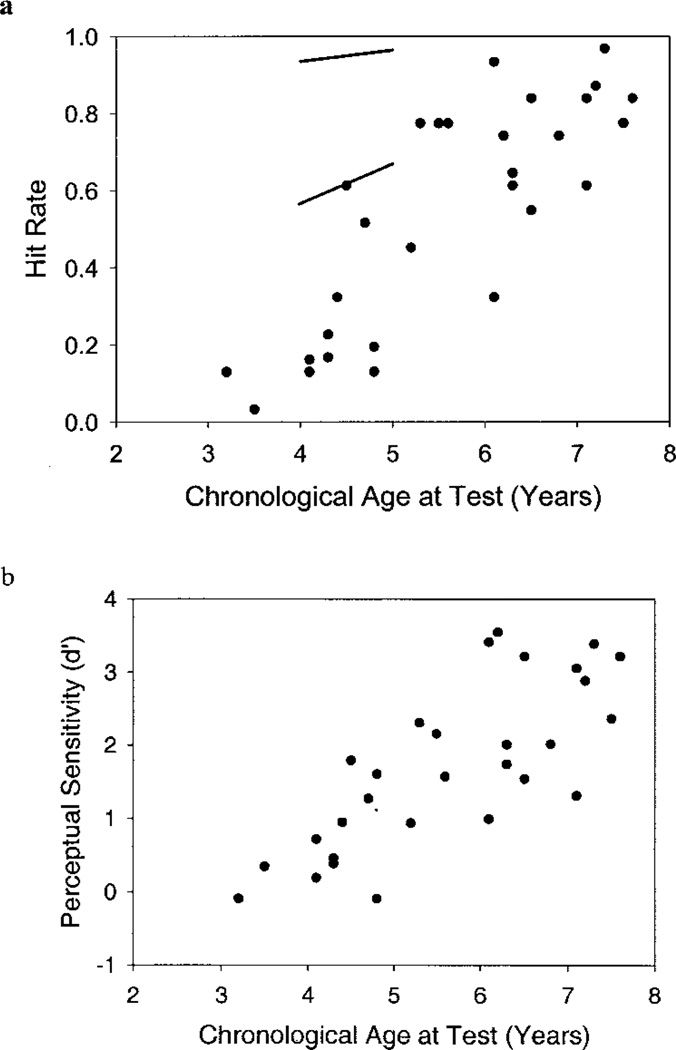
Effect of Chronological Age
To test for independent effects of chronological age and length of implant use, we constructed a model using the SAS Mixed Procedure described earlier. Figure 3 (a and b) illustrates the hit rates and perceptual sensitivity scores of individual participants plotted as a function of chronological age at testing. The results obtained from the SAS mixed model revealed that both hit rate, F(1, 47.7) = 64.69, p < 0.01, and d′, F(1, 50.9) = 55.2, p < 0.01, increased as a function of chronological age. No significant interactions between chronological age and length of cochlear implant use were found for any of the measures of CPT performance. As in Experiment 1, no effect of chronological age was found on β or false alarm rate, although the latter approached significance, F(1,51.6) = 3.18, p = 0.08.
Fig. 3.
Preschool vigilance CPT performance improves as a function of chronological age (a and b). Hit rate (Fig. 1a) and perceptual sensitivity (Fig. 1b) both increase with chronological age. Each data point represents the mean CPT score (mean hit rate or mean d′) for one participant tested at the 1-year interval of cochlear implant use. Several data points overlap because some participants had identical mean CPT scores. Two lines representing the 85th and 15th percentiles for hit rate based on normative data are included in (a) for comparison purposes.
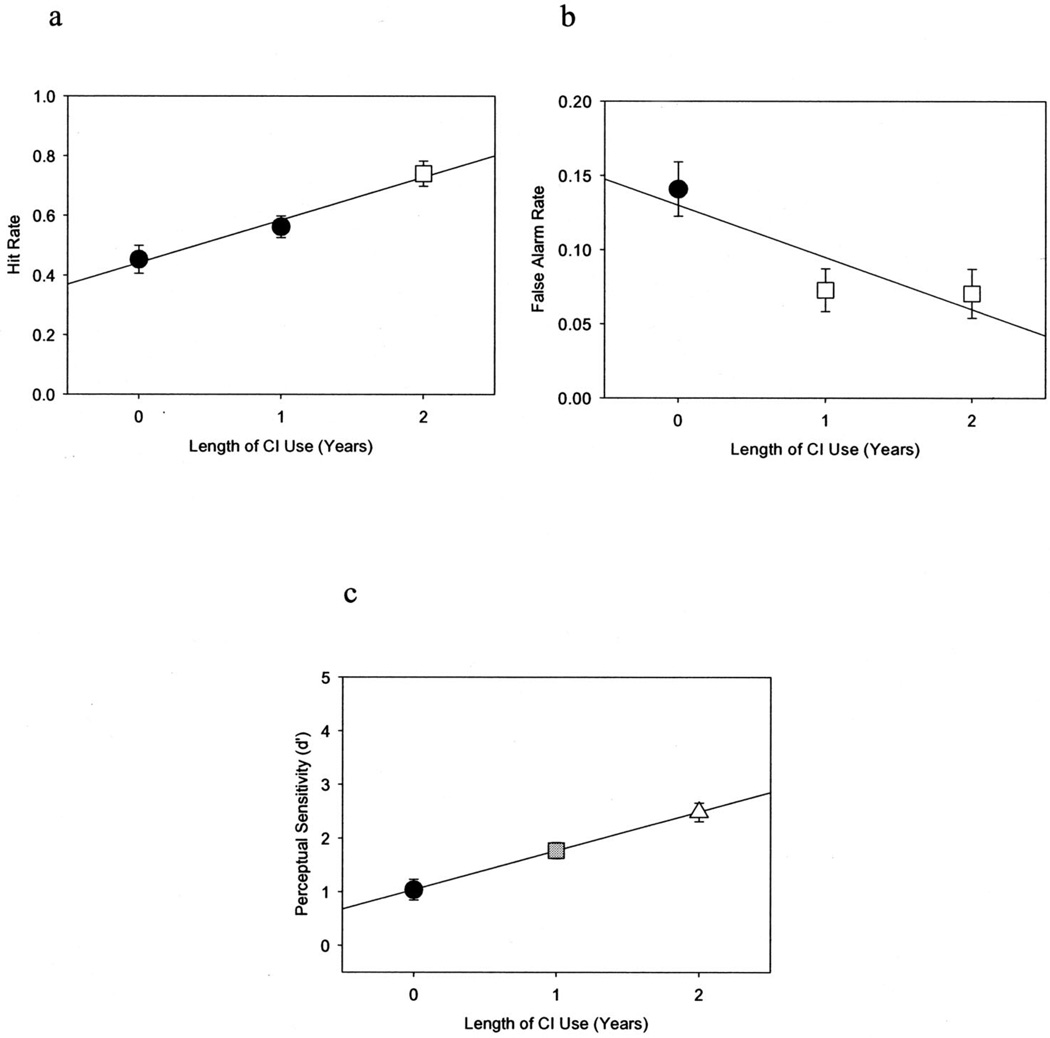
Effect of Length of Cochlear Implant Use
Figure 4 (a through c) illustrates the estimated mean hit and false alarm rates and d′ as a function of length of cochlear implant use. Estimated mean hit rate significantly increased from 0.45 (SE = 0.05) before implantation to 0.74 (SE = 0.04) after 2 years of cochlear implant use, F(2,47.8) = 11.38, p < 0.01. A significant effect of length of cochlear implant use was also found for mean false alarm rate, which decreased from 0.14 (SE = 0.02) before cochlear implantation to 0.07 (SE = 0.02) after 2 years of cochlear implant use, F(251.3) = 5.55, p < 0.01. Finally, d′ significantly increased from 1.04 (SE = 0.19) before implantation to 2.49 (SE = 0.17) after 2 years of cochlear implant use, F(2,50.2) = 16.25, p < 0.01. In contrast, length of cochlear implant use did not significantly affect β (p > 0.05). Estimated mean response criterion was 2.15 (SE = 1.5) before implantation and 2.83 (SE = 1.3) after 2 years of cochlear implant use.
Fig. 4.
Preschool vigilance CPT performance improves as a function of length of cochlear implant use. Mean hit rate (a) and d′ (c) both increase, whereas mean false alarm rate (b) decreases with length of cochlear implant use. Each data point represents the estimated mean CPT performance from the SAS mixed model at a given year of cochlear implant use. Error bars indicate standard error. Different shaped/shaded data points represent significant differences in mean CPT performance as determined by post hoc analyses.
Tukey’s post hoc analyses were carried out to assess performance differences on the preschool CPT between each postimplant interval. Figure 4a shows that preschool CPT hit rate increased significantly, as a function of length of cochlear implant use, between postimplant years 1 and 2 (Tukey’s p < 0.01), but not between pre-implant and postimplant year 1, although the latter was a trend (Tukey’s p = 0.06). Figure 4b shows that false alarm rate on this task decreased significantly between the pre-implant interval and postimplant year 1 (Tukey’s p < 0.01), but not between postimplant years 1 and 2 (Tukey’s p = 0.92). Figure 4c shows that perceptual sensitivity (d′) increased significantly from pre-implant to postimplant year 1 (Tukey’s p < 0.01) and then again from postimplant year 1 to postimplant year 2 (Tukey’s p < 0.01).
We identified 21 participants who were tested on the preschool CPT at least twice (at two or more intervals of cochlear implant use). The raw hit rate, false alarm rate, and d′ scores for these 21 participants at each interval of testing are given in Table 6. Only 1 participant showed a near ceiling hit rate (above 90%) on the initial test. Fourteen participants showed an increase in hit rate over subsequent testing intervals consistent with the findings of our mixed model. Similarly, 17 participants showed an increase in d′ over subsequent testing intervals. For false alarm rate, 10 children showed floor performance (<10%) on the initial test and showed relatively flat false alarm rates over time. Ten children showed a decrease in false alarm rate over subsequent testing intervals consistent with the mixed model results.
TABLE 6.
Preschool CPT scores of participants tested at two or more intervals
| Before implant | Year 1 | Year 2 | Year 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant | HR | FA | d′ | HR | FA | d′ | HR | FA | d′ | HR | FA | d′ |
| 141 | 0.65 | 0.05 | 2.01 | 0.87 | 0.03 | 3.04 | ||||||
| 143 | 0.77 | 0.20 | 1.57 | 0.90 | 0.06 | 1.99 | ||||||
| 145 | 0.13 | 0.03 | 0.71 | 0.03 | 0.01 | 0.62 | ||||||
| 146 | 0.77 | 0.08 | 2.15 | 0.73 | 0.03 | 2.46 | ||||||
| 151 | 0.52 | 0.09 | 1.39 | 0.93 | 0.03 | 3.41 | 0.73 | 0.02 | 2.68 | |||
| 153 | 0.32 | 0.27 | 0.15 | 0.19 | 0.01 | 1.6 | ||||||
| 161 | 0.61 | 0.08 | 1.68 | 0.87 | 0.04 | 2.88 | ||||||
| 162 | 0.16 | 0.13 | 0.13 | 0.52 | 0.11 | 1.27 | 0.67 | 0.06 | 1.99 | |||
| 165 | 0.26 | 0.09 | 0.7 | 0.77 | 0.06 | 2.3 | 0.97 | 0.05 | 3.51 | |||
| 166 | 0.45 | 0.14 | 0.93 | 0.97 | 0.10 | 3.12 | ||||||
| 167 | 0.45 | 0.16 | 0.87 | 0.32 | 0.08 | 0.94 | 0.93 | 0.05 | 3.17 | |||
| 197 | 0.13 | 0.15 | −0.1 | 0.03 | 0.01 | 0.62 | ||||||
| 200 | 0.93 | 0.01 | 3.8 | 0.97 | 0.07 | 3.39 | ||||||
| 201 | 0.67 | 0.03 | 2.32 | 0.61 | 0.07 | 1.74 | ||||||
| 204 | 0.20 | 0.08 | 0.56 | 0.74 | 0.00 | 3.55 | ||||||
| 208 | 0.45 | 0.03 | 1.76 | 0.32 | 0.07 | 0.99 | ||||||
| 234 | 0.84 | 0.01 | 3.22 | 1.00 | 0.01 | 5.32 | ||||||
| 236 | 0.68 | 0.18 | 1.38 | 0.55 | 0.08 | 1.54 | 0.70 | 0.15 | 1.57 | |||
| 238 | 0.52 | 0.26 | 0.69 | 0.84 | 0.02 | 3.05 | ||||||
| 390 | 0.70 | 0.31 | 1.02 | 0.61 | 0.15 | 1.31 | ||||||
| 489 | 0.16 | 0.12 | 0.19 | 0.50 | 0.43 | 0.5 | ||||||
Effects of Medical, Audiological, and Demographic Variables on Preschool CPT Performance
No significant effects on preschool CPT scores were found for any of the medical, audiological, or demographic variables with the exception of the number of active electrodes in the implant array. To explore this effect further, we divided the participants into two groups, using a median split, at an electrode number of 21. One group consisted of children with a full array of active electrodes and the second group consisted of children with fewer than 22 active electrodes. Children in the full array group had higher d′ scores on the preschool CPT, F(1,48) = 6.79, p < 0.05, and a higher hit rate, F(1,43.9) = 4.03, p = 0.05, than children who had less than 22 electrodes.
Relations Between Preschool CPT Scores and Speech and Language Outcome Measures
A series of multiple regression analyses was carried out in the same manner as in Experiment 1, with several changes. First, CPT scores from 1 year after implantation were used as predictor variables because the largest number of children (N = 30) had scores at this interval. Second, we included number of electrodes as a predictor variable in the regression analyses because of the previous result that children with full arrays showed greater CPT scores. Screening correlations again showed that hit rate and d′ were highly related (r = 0.909, p = 0.0001), whereas other correlations were low to moderate. Therefore, we used d′ and β only in the regression analyses. Inclusion and exclusion criteria for the stepwise regressions were the same as in Experiment 1.
Table 7 describes the final regression models obtained for each outcome measure. Length of use was a significant predictor of PPVT, RDLS-exp, RDLS-rec, PBK-p, PBK-w, and BIT scores. Number of electrodes was also a significant predictor of RDLS-exp and RDLS-rec scores. Neither β nor d′ were significant predictors of any of the outcome measures.
TABLE 7.
Final stepwise regression models for speech and language outcome measures: Experiment 2
| Dependent variable |
Predictor(s) | df | p | β | R2 (adjusted) |
|---|---|---|---|---|---|
| PPVT | Cochlear implant use | 1,25 | 0.0001 | 0.802 | 0.629 |
| RDLS-rec | Cochlear implant use | 1,24 | 0.008 | 0.443 | 0.433 |
| Elec No. | 1,24 | 0.012 | 0.419 | ||
| RDLS-exp | Cochlear implant use | 1,24 | 0.012 | 0.427 | 0.392 |
| Elec No. | 1,24 | 0.012 | 0.423 | ||
| PBK-p | Cochlear implant use | 1,23 | 0.003 | 0.563 | 0.287 |
| PBK-w | Cochlear implant use | 1,22 | 0.007 | 0.537 | 0.256 |
| BIT | Cochlear implant use | 1,24 | 0.0001 | 0.755 | 0.552 |
PPVT, RDLS scores are in age equivalents. PBK, BIT scores are in percent correct. For each predictor, standardized coefficient (β), t value, and p value are listed. For each model, adjusted R2 is listed. In models with multiple predictor variables, listed R2 reflects the combination of both variables.
Discussion
The children in Experiment 2 who were younger than 6 years of age performed poorly on the preschool CPT compared with the normative sample tested by Gordon et al. (1996). Although no study to date has compared the preschool CPT scores of prelingually deaf children with those of normal-hearing children, the present results suggest that atypical development of sustained visual attention in deaf children can be detected by 3 to 6 years of age. However, future comparisons between deaf and normal-hearing children on the preschool CPT will help to confirm this prediction.
As we showed in Experiment 1 with the school-age children, scores on the preschool CPT improved as a function of length of cochlear implant use. Over 2 years of cochlear implant use, prelingually deaf children showed an increase in hit rate, a decrease in false alarm, and an increase in perceptual sensitivity when the effect of chronological age was partialed out. Although the decrease in false alarm rate on the preschool CPT leveled off after 1 year of cochlear implant use, the hit rate did not increase until after 1 year of use. Perceptual sensitivity, d′, increased significantly at each interval of cochlear implant use. Given the earlier findings of Smith et al. (1998) and Quittner et al. (1994), we would expect continued improvement in CPT performance as a function of cochlear implant use until the deaf children are, on average, performing at levels that are comparable to normal-hearing children. As we found in Experiment 1, improvement in CPT performance with cochlear implant use appears to be due to an increase in perceptual sensitivity and not to changes in response criterion or impulsivity.
We failed to find any effects of the medical, audiological, or demographic variables on preschool CPT scores except for the number of active electrodes. This is the first report of an effect for the number of active electrodes on performance of a visual processing task in children with cochlear implants. Our sample size and skewed distribution of electrode number do not allow us to determine whether this finding was carried by a small number of children who have fewer active electrodes. Other studies have found that a small number (fewer than 12) of electrodes or spectral channels are required to achieve maximal speech perception in quiet or noise with cochlear implants or cochlear implant simulations (Dorman, Loizou, Kemp, & Kirk, 2000; Fishman, Shannon, & Slattery, 1997; Friesen, Shannon, Baskent, & Wang, 2001).
There are at least two reasons why some children with cochlear implants may not have a full array of active electrodes. First, some of these children may have had partial insertions due to anatomic cochlear abnormalities such as a Mondini malformation. Second, during programming it is sometimes necessary to deactivate electrodes that are functioning inappropriately (stimulating the facial nerve, requiring a disproportionately large amount of current, or not working at all). Thus, it is hard to determine precisely whether the effect we found was due to the actual number of stimulation points in the cochlea or to some other confounding variable associated with a lower number of electrodes.
The results from our multiple regression analyses in Experiment 2 failed to find any significant relations between preschool CPT performance and open-set speech perception, receptive language, expressive language, or speech intelligibility scores. Children who used their cochlear implants for longer periods of time showed greater performance on all outcome measures than children who had used their cochlear implant for shorter periods of time. Children who had more active electrodes showed higher expressive and receptive language scores than children with fewer electrodes. These findings fail to support the hypothesis that the processing skills required by the preschool CPT are related to abilities that affect speech and language development in prelingually deaf children with cochlear implants.
General Discussion
Experiments 1 and 2 were designed to investigate the performance of two groups of prelingually deaf children who used cochlear implants on a CPT of sustained visual attention. Each task measured a child’s ability to monitor a sequence of visual digits and detect target stimuli from nontarget stimuli. Although these tasks involved different working memory requirements in the complexity of the target stimulus and were administered to children of two different age ranges, the results obtained from both CPT tasks were consistent with the earlier findings of Quittner et al. (1994) and Smith et al. (1998): Mean performance of prelingually deaf children on the CPT is atypical compared with published norms for normal-hearing children. The effect of cochlear implant use on CPT performance, independent from chronological age, suggests that auditory experience leads to a gradual maturation of sustained visual attention skills over a period of several years. In both Experiments 1 and 2, the improvement in CPT performance with chronological age and cochlear implant use appears to be due to an increase in the ability to discriminate the target sequence from the nontargets. We found no evidence that response criterion, as assessed by the school-age or preschool CPT, changes with chronological age or cochlear implant use.
Although we did not find direct evidence for a critical period for CPT performance as suggested by Smith et al. (1998) and Quittner et al. (1994), the lack of an interaction between length of cochlear implant use and chronological age in our study does not disprove their critical period hypothesis. It is possible that the age ranges of children in the present study fell within a critical period during which cochlear implant experience influences sustained visual attention development. Further research on the sustained visual attention skills of our youngest and oldest children with cochlear implants may reveal evidence of a critical period.
We found little evidence that the cognitive skills measured by either the school-age CPT or the preschool CPT were related to standard audiological outcome measures. In Experiment 1, children who were less impulsive on the school-age CPT showed higher receptive language scores than children who were more impulsive. Impulsivity and other externalizing behavior problems were previously found to be negatively correlated with speech/language skills of prelingually deaf children with several years of cochlear implant experience (Knutson, Ehlers, Wald, & Tyler, 2000). However, school-age β was not related to any other outcome measures, nor did we find a similar relation between preschool β and receptive language skills. Therefore, we are hesitant to conclude that our results support Knutson et al. Further study comparing the long-term audiological outcomes of children who perform more impulsively on a CPT with those of children who perform less impulsively is needed to determine how robust a relationship there is between response criterion and cochlear implant outcomes.
We found little evidence to suggest that demographic, medical, and audiological variables influenced CPT performance other than the effect of number of active electrodes on preschool CPT performance. We also failed to find interactions of these variables with the effect of length of cochlear implant use. Taken together, our results support the conclusion of Quittner et al. (1994) and Smith et al. (1998) that experience with a cochlear implant appears to help shape the development of cognitive processing of visually presented digits as measured by a CPT. However, the underlying neural and cognitive processes responsible for deaf children’s atypical CPT performance and for the effect of cochlear implant use have yet to be sufficiently defined.
The hypothesis proposed by Quittner et al. (1994) and Smith et al. (1998) was that early auditory deprivation leads to remodeling of visual attention processes. Reasoning that deaf children are forced to use vision to monitor their environment, Quittner et al. and Smith et al. argued that visual attention processes in these children would reorganize and adapt to maintain a wide spatial focus rather than a narrow, task-specific focus such as a CPT. In describing their “division of labor” hypothesis, Smith et al. suggested that normal-hearing children learn to sustain focused visual attention with a remarkable degree of acuity in part because of their ability to use auditory signals to detect environmental events. Because many environmental events may occur outside the field of visual attention, they proposed that audition frees the visual system from using capacity-demanding resources to detect these events.
However, the CPT used in the present study and earlier investigations does not explicitly assess selective allocation of visual attention. In selective attention experiments, distracting stimuli are used to test for effects on visual target processing time or accuracy (Parasnis et al., 2003). Although selective attention may be useful in any task requiring concentration (there are almost always small sounds/events occurring in the environment), we do not know to what degree these abilities play a role in performance on sustained attention tasks. To test the predictions of the division of labor hypothesis, therefore, the appropriate type of task is a selective attention task and not a sustained attention task as used by Smith et al. (1998) and Quittner et al. (1994).
The Gordon Diagnostic System includes a task called the distractibility CPT (Gordon et al., 1996). This task is similar to the previous CPTs (which we will call vigilance CPTs for distinction) except for the addition of two additional streams of numbers that appear to the right and left of the original number sequence. Participants are instructed to ignore these two additional number sequences and focus only on the numbers that appear in the center of the display. By comparing performance on the distractibility CPT with the vigilance CPT, the ability of a participant to selectively attend to the center display can be measured (Gordon et al., 1996).
Mitchell & Quittner (1996) administered both the distractibility and vigilance CPTs to a group of normal-hearing children and a group of prelingually deaf children who used hearing aids. They found that the prelingually deaf children scored lower on average than the normal-hearing children on both CPTs. The authors computed a distraction decrement score by subtracting each participant’s score on the distractibility CPT from their score on the vigilance CPT. They reported that prelingually deaf children with hearing aids showed greater mean distraction decrements than the normal-hearing children. Thus, prelingually deaf children were less able to ignore the distracting numbers that flanked the stimulus stream of interest. This finding is consistent with the prediction of Smith et al. (1998) that selective visual attention skills of prelingually deaf children develop atypically compared with normal-hearing children.
None of the previous studies of sustained or selective visual attention in deaf children can tell us at what age this visual reorganization occurs. However, the results reported by Mitchell and Quittner (1996) suggest that some reorganization occurs in deaf children by the school-age years. The findings we reviewed earlier from studies of deaf adults suggest that this reorganization involves enhancement of peripheral visual processing and spatial widening of the visual attention filter (Bavelier et al., 2001; Bavelier et al., 2000; Neville & Lawson, 1987a, 1987b, 1987c; Proksch & Bavelier, 2002; Rothpletz et al., 2003). However, it is not clear how this reorganization would affect performance on the CPTs used in the current study because no distracting stimuli were present in the display. Therefore, a visual selective attention task such as the one used by Bavelier and colleagues should be developed to assess the skills of deaf and normal-hearing preschool and school-age children. Future studies may reveal the age at which reorganization of visual attention begins in deaf children and whether access to auditory input with a cochlear implant leads to more typical development of this domain.
As mentioned previously, the CPTs used in Experiments 1 and 2 are not pure measures of sustained visual attention skills. Visually presented digits are stimuli that must be encoded into phonological storage and maintained in working memory. Use of verbal stimuli by these CPTs complicates the interpretation of our results. Although general properties of visual attention, such as perceptual sensitivity, may play an important role in these results, we cannot exclude factors that are known to affect perceptual and cognitive processing of verbal stimuli such as phonological encoding, fluency, and subvocal rehearsal.
Several recent studies of immediate sequence memory in prelingually deaf children with cochlear implants have reported evidence that verbal encoding and rehearsal skills appear to be atypical in deaf children. These children have been shown to have impaired immediate recall for sequences of stimuli regardless of modality of presentation provided that the stimuli can be verbally encoded (Cleary, Pisoni, & Geers, 2001; Dawson, Busby, McKay, & Clark, 2002; Pisoni & Cleary, 2003). Compared with normal-hearing children, prelingually deaf children with cochlear implants also display reduced immediate memory spans for auditory presentation of numbers, visual presentation of presentation of colored lights, auditory presentation of color names, and for auditory color names and lights presented together (Cleary et al., Pisoni & Cleary). Dawson et al. tested deaf children with cochlear implants on a number of immediate recall tasks using either auditory or visually presented stimuli. They found impairments in deaf children on the auditory tasks as well as several visual tasks. However, performance of deaf children on visual tasks appeared to depend largely on whether the stimuli could be verbally encoded or named. On the visual tasks that used verbal stimuli, deaf children showed shorter memory spans than normal-hearing children. However, they did not show impairments in recall of nonverbal visual stimuli.
Taken together, studies of immediate recall suggest that verbal encoding of stimuli is atypical in young deaf children regardless of whether these stimuli are heard or seen, or simultaneously heard and seen. Therefore, fundamental deficits in verbal encoding of visually presented digits may have played a limiting role in the performance of deaf children on the CPT. In other words, the deaf children might have normal visual attention mechanisms yet have difficulty encoding and processing stimuli into phonological representations. Indeed, phonological storage and subvocal rehearsal of stimulus representations via the phonological loop have been shown to protect stimulus representations in working memory from time-related decay (Baddeley, Gathercole, & Papagno, 1998). Studies that manipulate use of the phonological loop by participants have shown that deaf children do not take advantage of this mechanism to the same degree as normal-hearing children (Chincotta & Chincotta, 1996; Pisoni & Cleary, 2003). Therefore, the CPT used in this and the earlier studies may be biased against prelingually deaf children who have atypical verbal encoding skills.
Differences in verbal encoding skills may explain the effect of number of active electrodes on preschool CPT performance reported in Experiment 2. Although it would be hard to explain this effect in terms of a direct effect on the visual attention system, it is more intuitive to hypothesize that the degree of spectral resolution that a child receives with a cochlear implant has an effect on the verbal encoding skills that they develop over time. The underlying assumption here is that the phonological representations of speech obtained through the auditory or visual modalities are influenced by the quality and nature of auditory input. To be verified, this hypothesis requires more investigation into the development of verbal encoding skills of deaf children with cochlear implants. Nevertheless, it is consistent with a number of studies in deaf children with cochlear implants that have demonstrated strong relations between the child’s ability to encode, temporarily store, and reproduce sequential visual stimuli and performance on a range of speech perception, production, and language tests (Burkholder & Pisoni, 2003; Dawson et al., 2002; Pisoni & Cleary, 2003, 2004; Surowiecki et al., 2002).
To date, only two studies of sustained visual attention in normal-hearing adults and prelingually deaf adult ASL users have used CPTs in which stimuli were unlikely to be verbally encoded. Dittmar, Berch, and Warm (1982) used a CPT that required 45 minutes of visual monitoring of a light-bar that executed a string of paired movements. The task was to respond when the degree of movement differed between the two paired movements. Dittmar et al. reported that deaf adults showed higher levels of performance on this task than normal-hearing adults, a result that appears to contradict the present results. However, the visual processing required by this task is clearly different from the current CPT because visual detection of movement differences does not involve verbal encoding. Thus, differences in CPT performance between deaf and normal-hearing individuals may depend on the type of visual stimulus used and whether or not this stimulus can be verbally encoded.
More recently, Parasnis et al. (2003) reported CPT results obtained from prelingually deaf adult ASL users and normal-hearing adults. Their CPT used visually presented geometrical shapes as stimuli and the task was to detect a certain type of shape based on whether the object had a hole toward its top or bottom. In contrast to the findings of Dittmar et al. (1982), Parasnis et al. reported deficits in performance of deaf adults compared with normal-hearing participants. It is unclear to what degree the geometric stimuli were verbally encoded (for instance, as “top” or “bottom”). If we assume that verbal encoding was used by participants who took the geometric CPT, then the results of Parasnis et al. are consistent with the earlier studies by Quittner et al. (1994) and Smith et al. (1998) and the present findings, which found deficits in the performance of deaf children on a CPT that used numbers as stimuli. Future work on sustained visual attention skills of deaf children with cochlear implants should include tasks that do not require verbal encoding.
We propose two general mechanisms to explain the effects of a cochlear implant on the performance of deaf children on a CPT of sustained attention. One explanation is that afferent auditory input from a cochlear implant leads to cross-modal changes in the ability to maintain visual attention to discriminate target from nontarget stimuli. A second explanation is that atypical phonological encoding and verbal rehearsal skills, which result from a period of early auditory deprivation, begin to improve after cochlear implantation. It is possible that both processes may interact to affect CPT performance in deaf children. Future research on sustained visual attention skills of deaf children with cochlear implants should include tasks that do not contain stimuli that can be easily named or encoded phonologically. Testing of deaf children with cochlear implants on tasks of selective attention may help us understand the nature of reorganization of visual attention resulting from auditory experience. In addition, future work on the development of verbal encoding and rehearsal processes in deaf children before and after receiving a cochlear implant may provide insight into how processing and storage of visual digits may be altered by auditory access to and experience with a spoken language. Finally, the testing of children over a wider range of ages may reveal critical periods during which the development of these cognitive processes might be affected by a period of auditory deprivation and modified after cochlear implantation.
Acknowledgments
This work was supported by NIH-NIDCD Training Grant T32DC00012 to Indiana University and NIH-NIDCD Research Grants R01DC00064 and R01DC00423 to the Indiana University School of Medicine. We thank all of the speech/language pathologists and other clinicians who helped in testing the children and Sujuan Gao and Amy Rong Qi for statistical assistance. We also thank Derek Houston, Joshua Bradley, Bill Kronenberger, Rachel Frush-Holt, and Karen Iler Kirk for insightful comments on an earlier version of the manuscript.
Footnotes
Wolfinger, R., & Chang, M. (1995). Comparing the SAS GLM and MIXED procedures for repeated measures. Paper presented at the Proceedings of the Twentieth Annual SAS Users Group International Conference, Orlando, Florida.
References
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Barkley R. The ecological validity of laboratory and analogue assessment methods of ADHD symptoms. Journal of Abnormal Child Psychology. 1991;19:149–178. doi: 10.1007/BF00909976. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Brozinsky C, Tomann A, Mitchell T, Neville H, Liu G. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. Journal of Neuroscience. 2001;21:8931–8942. doi: 10.1523/JNEUROSCI.21-22-08931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Tomann A, Hutton C, Mitchell T, Corina D, Liu G, et al. Visual attention to the periphery is enhanced in congenitally deaf individuals. Journal of Neuroscience. 2000;20:RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey P, Sarant J, Paatsch L, Barry J, Bow C, Wales R, et al. Relationships among speech perception, production, language, hearing loss, and age in children with impaired hearing. Journal of Speech, Language, Hearing, Research. 2001;44:264–285. doi: 10.1044/1092-4388(2001/022). [DOI] [PubMed] [Google Scholar]
- Burkholder R, Pisoni D. Speech timing and working memory in profoundly deaf children after cochlear implantation. Journal of Experimental Child Psychology. 2003;85:63–88. doi: 10.1016/s0022-0965(03)00033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chincotta M, Chincotta D. Digit span, articulatory suppression, and the deaf: a study of the Hong Kong Chinese. American Annals of the Deaf. 1996;141:252–257. doi: 10.1353/aad.2012.0289. [DOI] [PubMed] [Google Scholar]
- Cleary M, Pisoni D, Geers A. Some measures of verbal and spatial working memory in eight- and nine-year-old hearing-impaired children with cochlear implants. Ear and Hearing. 2001;22:395–411. doi: 10.1097/00003446-200110000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied Multiple Regression/correlation Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1983. [Google Scholar]
- Conners C. Conners’ CPT II for Windows. North Tonawanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- Dawson P, Busby P, McKay C, Clark G. Short-term auditory memory in children using cochlear implants and its relevance to receptive language. Journal of Speech, Language, Hearing, Research. 2002;45:789–801. doi: 10.1044/1092-4388(2002/064). [DOI] [PubMed] [Google Scholar]
- Dittmar M, Berch D, Warm J. Sustained visual attention in deaf and hearing adults. Bulletin of the Psychonomic Society. 1982;19:339–342. [Google Scholar]
- Dorman M, Loizou P, Kemp L, Kirk K. Word recognition by children listening to speech processed into a small number of channels: data from normal-hearing children and children with cochlear implants. Ear and Hearing. 2000;21:590–596. doi: 10.1097/00003446-200012000-00006. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test, 3rd Edition. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Fishman K, Shannon R, Slattery W. Speech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. Journal of Speech, Language, Hearing, Research. 1997;40:1201–1215. doi: 10.1044/jslhr.4005.1201. [DOI] [PubMed] [Google Scholar]
- Forbes G. Clinical utility of the test of variables of attention (TOVA) in the diagnosis of attention-deficit/hyperactivity disorder. Journal of Clinical Psychology. 1998;54:461–476. doi: 10.1002/(sici)1097-4679(199806)54:4<461::aid-jclp8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Friesen L, Shannon R, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. Journal of the Acoustical Society of America. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy H, Tyler R, Kelsay D, Gantz B. Performance over time of congenitally deaf and postlingually deafened children using a multichannel cochlear implant. Journal of Speech, Hearing Research. 1992;35:913–920. doi: 10.1044/jshr.3504.913. [DOI] [PubMed] [Google Scholar]
- Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing. 2003;24(suppl.):24–35. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Geers A, Nicholas J, Sedey A. Language skills of children with early cochlear implantation. Ear and Hearing. 2003;24(suppl.):46–58. doi: 10.1097/01.AUD.0000051689.57380.1B. [DOI] [PubMed] [Google Scholar]
- Gordon M, McClure F, Aylward G. The Gordon Diagnostic System (GDS) Instruction Manual & Interpretive Guide. 3rd Edition. Dewitt, NY: Michael Gordon, PhD; 1996. [Google Scholar]
- Green D, Swets J. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- Greenberg L. T.O.V.A. Interpretation Manual. Minneapolis, MN: 1991. [Google Scholar]
- Haskins H. A phonetically balanced test of speech discrimination for children. Unpublished master’s thesis. Evanston, IL: Northwestern University; 1949. [Google Scholar]
- Kirk K. Cochlear implants: New developments and results. Current Opinion in Otolaryngology Head and Neck Surgery. 2000;8:415–420. [Google Scholar]
- Knutson J, Ehlers S, Wald R, Tyler R. Psychological consequences of pediatric cochlear implant use. Annals of Otology Rhinology Laryngology. 2000;185(Suppl.):109–111. doi: 10.1177/0003489400109s1247. [DOI] [PubMed] [Google Scholar]
- Mitchell T, Quittner A. Multimethod study of attention and behavior problems in hearing-impaired children. Journal of Clinical Child Psychology. 1996;25:83–96. [Google Scholar]
- Miyamoto R, Osberger M, Robbins A, Myres W, Kessler K. Prelingually deafened children’s performance with the Nucleus multichannel cochlear implant. American Journal of Otology. 1993;14:437–445. doi: 10.1097/00129492-199309000-00004. [DOI] [PubMed] [Google Scholar]
- Miyamoto R, Svirsky M, Kirk K, Robbins A, Todd S, Riley A. Speech intelligibility of children with multichannel cochlear implants. Annals of Otology Rhinology Laryngology. 1997;168(suppl.):35–36. [PubMed] [Google Scholar]
- Neville H, Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study, I: normal hearing adults. Brain Research. 1987a;405:253–267. doi: 10.1016/0006-8993(87)90295-2. [DOI] [PubMed] [Google Scholar]
- Neville H, Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study, II: congenitally deaf adults. Brain Research. 1987b;405:268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- Neville H, Lawson D. Attention to central and peripheral visual space in a movement detection task, III: separate effects of auditory deprivation and acquisition of a visual language. Brain Research. 1987c;405:284–294. doi: 10.1016/0006-8993(87)90297-6. [DOI] [PubMed] [Google Scholar]
- Niparko J, Kirk K, Mellon N, McConkey-Robbins A, Tucci D, Wilson B. Cochlear Implants Principles & Practices. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- Osberger M, Robbins A, Todd S, Riley A. Speech intelligibility of children with cochlear implants. Volta Review. 1994;96:169–180. [Google Scholar]
- Parasnis I, Samar V, Berent G. Deaf adults without attention deficit hyperactivity disorder display reduced perceptual sensitivity and elevated impulsivity on the Test of Variables of Attention (T.O.V.A.) Journal of Speech, Language, Hearing, Research. 2003;46:1166–1183. doi: 10.1044/1092-4388(2003/091). [DOI] [PubMed] [Google Scholar]
- Parasuraman R. The Attentive Brain. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Pastore R, Crawley E, Berens M, Skelly M. ‘Non-parametric A’ and other modern misconceptions about signal detection theory. Psychonomic Bulletin and Review. 2003;10:556–569. doi: 10.3758/bf03196517. [DOI] [PubMed] [Google Scholar]
- Pisoni D, Cleary M. Measures of working memory span and verbal rehearsal speed in deaf children after cochlear implantation. Ear and Hearing. 2003;24(suppl.):106–120. doi: 10.1097/01.AUD.0000051692.05140.8E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D, Cleary M. Some new findings on learning, memory and cognitive processes in deaf children following cochlear implantation. In: Zeng FG, editor. Springer Handbook of Auditory Research: Auditory Prosthesis. Volume X. New York, NY: 2004. [Google Scholar]
- Pisoni D, Cleary M, Geers A, Tobey E. Individual differences in effectiveness of cochlear implants in children who are prelingually deaf: new process measures of performance. Volta Review. 2000;101:111–164. [PMC free article] [PubMed] [Google Scholar]
- Proksch J, Bavelier D. Changes in the spatial distribution of visual attention after early deafness. Journal of Cognitive Neuroscience. 2002;14:687–701. doi: 10.1162/08989290260138591. [DOI] [PubMed] [Google Scholar]
- Quittner A, Smith L, Osberger M, Mitchell T, Katz D. The impact of audition on the development of visual attention. Psychological Science. 1994;5:347–353. [Google Scholar]
- Reynell JK, Huntley M. Reynell Developmental Language Scales. 2nd edition. Windsor, UK: NFER-Nelson; 1985. [Google Scholar]
- Rosvold H, Mirskey A, Sarason I, Bransome E, Beck L. A continuous performance test of brain damage. Journal of Consulting and Clinical Psychology. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rothpletz A, Ashmead D, Tharpe A. Responses to targets in the visual periphery in deaf and normal hearing adults. Journal of Speech, Language, Hearing Research. 2003;46:1378–1386. doi: 10.1044/1092-4388(2003/107). [DOI] [PubMed] [Google Scholar]
- Sarant J, Blamey P, Dowell R, Clark G, Gibson W. Variation in speech perception scores among children with cochlear implants. Ear and Hearing. 2001;22:18–28. doi: 10.1097/00003446-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Schafer J, Graham J. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Smith L, Quittner A, Osberger M, Miyamoto R. Audition and visual attention: the developmental trajectory in deaf and hearing populations. Developmental Psychology. 1998;34:840–850. doi: 10.1037//0012-1649.34.5.840. [DOI] [PubMed] [Google Scholar]
- Surowiecki V, Sarant J, Maruff P, Blamey P, Busby P, Clark G. Cognitive processing in children using cochlear implants: the relationship between visual memory, attention, and executive functions and developing language skills. Annals of Otology Rhinology Laryngology. 2002;189(suppl.):119–126. doi: 10.1177/00034894021110s524. [DOI] [PubMed] [Google Scholar]
- Svirsky M, Robbins A, Kirk K, Pisoni D, Miyamoto R. Language development in profoundly deaf children with cochlear implants. Psychological Science. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharpe A, Ashmead D, Rothpletz A. Visual attention in children with normal hearing, children with hearing aids, and children with cochlear implants. Journal of Speech, Language, Hearing, Research. 2002;45:403–413. doi: 10.1044/1092-4388(2002/032). [DOI] [PubMed] [Google Scholar]
- Tobey E, Geers A, Brenner C, Altuna D, Gabbert G. Factors associated with development of speech production skills in children implanted by age five. Ear and Hearing. 2003;24(suppl.):36–45. doi: 10.1097/01.AUD.0000051688.48224.A6. [DOI] [PubMed] [Google Scholar]
- Tyler R, Fryauf-Bertschy H, Kelsay D, Gantz B, Woodworth G, Parkinson A. Speech perception by prelingually deaf children using cochlear implants. Otolaryngology Head and Neck Surgery. 1997;117:180–187. doi: 10.1016/s0194-5998(97)70172-4. [DOI] [PubMed] [Google Scholar]