Abstract
Kaposi sarcoma (KS) is a complex cancer that arises from the initial infection of an appropriate endothelial or progenitor cell by Kaposi Sarcoma Herpesvirus/Human Herpesvirus-8 (KSHV/HHV8). However, the majority of KS cases occur when infected patients also suffer from some coincident form of immune deregulation, providing a favorable microenvironment for tumor development. Cellular hallmarks of KS progression include both the hyper-proliferation of KSHV-infected cells and the infiltration of immune modulatory cells into KS lesions, which together result in chronic inflammation, the induction of angiogenesis and tumor growth. This review describes the current understanding of the interactions between KSHV and host responses that result in this unusual cancer, along with existing treatments and prospects for future therapeutic approaches.
Introduction
Kaposi sarcoma (KS) is characterized by angioproliferative multifocal tumors of the skin, mucosa and less frequently the viscera. A close inspection of KS lesions reveals that they are largely comprised of cells of endothelial origin with a unique spindled morphology, and these are accompanied by a variable chronic inflammatory infiltrate. From an epidemiological standpoint, four different forms of KS are recognized: Classic (sporadic), African (endemic), acquired immune deficiency syndrome (AIDS)-associated (epidemic), and Transplant or immunosuppression-associated (iatrogenic) KS (Table 1). Although these various forms of KS have different environmental and immunological components, the development of each depends upon prior infection with KSHV. However, KSHV infection alone is insufficient for the development of KS. For most cases of AIDS and Transplant KS it is well established that in addition to infection with KSHV, some form of immunodeficiency is also necessary for disease progression. However, several natural history studies indicate that additional factors may contribute to the development of KS in asymptomatic KSHV-infected persons. For example, not all AIDS patients develop KS even in the face of profound immunosuppression, only a minority of KSHV-infected transplant recipients develop iatrogenic KS, and people with classic or endemic KS are not typically immunosuppressed [1, 2]. The co-factors involved in classic and endemic KS have yet to be definitively elucidated. Various environmental and genetic factors have been implicated, as well as age, sex and malnutrition. The association of KS with the Koebner phenomenon, a condition where lesions initiate or recur at inflammatory sites of injury or trauma [3] and the recrudescent KS (KS flare) seen with the immune constitution inflammatory syndrome (IRIS) [4] suggest that inflammation plays a contributing role in oncogenesis. Progression of KS disease likely depends on a complex and as yet incompletely understood interplay between KSHV and the host immune system that allows for the establishment of a tumor-promoting environment. Like all herpesviruses, KSHV establishes a life-long infection in the host that depends upon virus-encoded immune evasion genes and genes that influence cellular proliferation, survival, migration, angiogenesis and cytokine/chemokine production. The host responds to persistent viral infection with its own chronic inflammatory response, thereby facilitating events that, particularly in the context of immunosuppression, could drive viral oncogenesis. Understanding the dynamic relationship between host and viral factors that drives KS oncogenesis is central to mounting effective strategies to prevent or ameliorate tumor development. In addition, deciphering KS pathogenesis will enhance our understanding of novel virus-induced mechanisms of tumorigenesis. There is a growing appreciation of the role that chronic inflammation plays in cancer development, and KS offers a unique opportunity to explore this topic.
Table 1.
KS clinical presentation
| Classic (sporadic) KS |
|
| African (endemic) KS |
|
| AIDS-related (epidemic) KS |
|
| Transplant (iatrogenic) KS |
|
Historical Perspective
In the late 19th century, Moritz Kaposi first described KS as a rare and relatively indolent disease of the lower extremities (classic KS). Throughout the 20th century, endemic KS was recognized in parts of Africa and the Middle East as a common cancer of children and adults. For example, in the pre-AIDS era, endemic KS in central Africa comprised up to 9% of malignant tumors in males [5]. Today, KS is the most prevalent malignancy among AIDS patients worldwide, and it has become the most common cancer in several sub-Saharan African countries where KSHV is endemic and HIV is widespread. Compared to the other forms of KS, AIDS-associated KS is an aggressive disease typically manifesting with disseminated and even visceral involvement, and has an untreated median survival of less than 2 years. KS occurs in AIDS patients who have not had highly active antiretroviral therapy (HAART) around 20,000× more often than in the general population [6]. In areas where highly active antiretroviral therapy (HAART) is widely available, there has been a dramatic decline in KS incidence. However, access to HAART is not universal, and this has led to a KS epidemic in many parts of Africa, along with the resultant potential for disease re-emergence elsewhere. KSHV-seropositive individuals with other forms of immunodeficiency (genetic, iatrogenic, idiopathic) are also at increased risk (~500× greater than the general population) for KS development [6]. In addition to KS, KSHV is also involved in two AIDS-associated B-cell cancers, primary effusion lymphoma (PEL), and the plasma cell variant of multicentric Castleman’s disease (MCD). KSHV seroprevalence varies geographically and demographically, and both sexual and non-sexual routes of transmission have been proposed. In the USA, prevalence ranges from approximately 5% among random blood donors to as high as 80% among groups of homosexual men [7]. In sub-Saharan Africa, where KS was endemic prior to the AIDS epidemic, seroprevalence ranges from 30–100% [8].
KSHV Biology
KSHV is a γ-herpesvirus in the Rhadinovirus genus encoding approximately 87 open reading frames (ORFs), 15 of which have no homologues in the other human herpesviruses. Like most herpesviruses, KSHV has two major modes of replication. In the lytic phase, entry, uncoating, and nuclear import are followed by a coordinated sequence of viral transcription, DNA replication, and assembly, followed by the final release of nascent virions. KSHV can also undergo a “latent” life cycle where only a small subset of viral genes is expressed. Here, after entry and translocation to the nucleus, the viral DNA circularizes, and multiple copies are maintained as episomes attached to the host chromosome via the viral latency associated nuclear antigen (Lana-1). Viral genomes are then replicated at roughly the same rate as the host chromosome, such that each daughter cell receives several copies of the viral genome at cell division. Most cells in both KS lesions and KSHV-infected cultures are latently infected, with lytic replication occurring in only a subset of infected cells. However, as discussed elsewhere and summarized in Table 2, it appears that both phases of the virus life cycle play significant roles in the pathogenesis of KS.
Table 2.
KSHV-encoded and KSHV-induced proteins involved in KS pathogenesis
| KSHV encoded protein or miRNA | Expression Type [119] | Oncogenic | Anti-Apoptotic | Immune Modulatory | Pro-inflammatory | Angiogenic |
|---|---|---|---|---|---|---|
| ORF73 (Lana-1) | Latent | X | X | |||
| ORF72 (vCyc) | Latent | X | X | X | ||
| ORF71 (vFlip) | Latent | X | ||||
| K12 (Kaposin A) | Latent/TPA Inducible | X | ||||
| ORF74 (vGPCR) | Lytic early | X | X | X | ||
| K1 | Lytic early and latent* | X | X | X | X | X |
| K9 (vIRF1) | Lytic early | X | X | X | ||
| K11/11.1 (vIRF2) | Lytic | X | ||||
| K10.5/10.6 (vIRF3 Lana-2) | Latent | X | X | |||
| ORF45 (tegument) | Immediate Early | X | ||||
| ORF50 (RTA) | Immediate Early | X | ||||
| K2 (vIL-6) | Lytic early and latent* | X | X | X | ||
| ORF4 (KCP) | Lytic late | X | ||||
| K3 (MIR1) | Immediate Early | X | ||||
| K5 (MIR2) | Immediate Early | X | ||||
| K8 (KbZIP) | Immediate Early | X | ||||
| K7 (vIAP) | Lytic late | X | ||||
| ORF16 (vBcl-2) | Lytic early | X | ||||
| K6, K4, K4.1 (vCCL-1,-2,-3) | Immediate Early | X | X | |||
| Kaposin B | Latent/TPA Inducible | ? | ||||
| miRK1-12 | Latent | X | X | X | X | X |
| KSHV induced protein | ||||||
| c-Kit | Cellular | X | ||||
| PDGF-R | Cellular | X | X | |||
| RDC1 (CXCR7) | Cellular | X | X | X | ||
| IL-6 | Cellular | X | X | |||
| MMPs | Cellular | X | X | |||
| Ang-2 | Cellular | X | ||||
| HIF1α, HIF1β | Cellular | X | ||||
| Cox-2 | Cellular | X | X | X |
Recent evidence suggests that K1 and vIL-6 mRNAs can also be detected latently [120]
KS Pathology
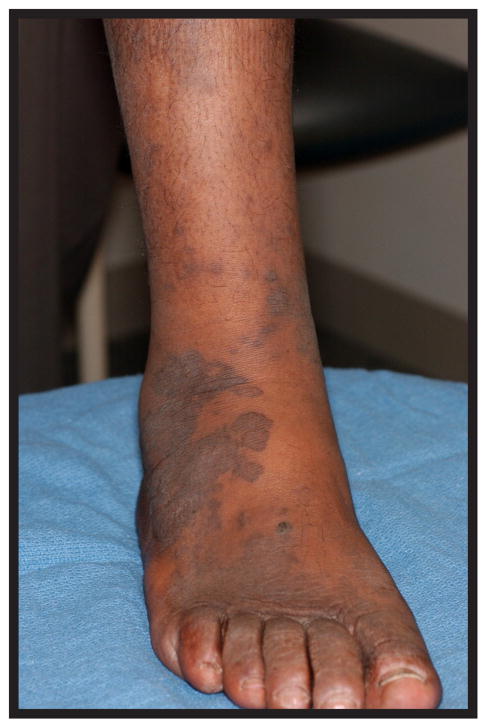
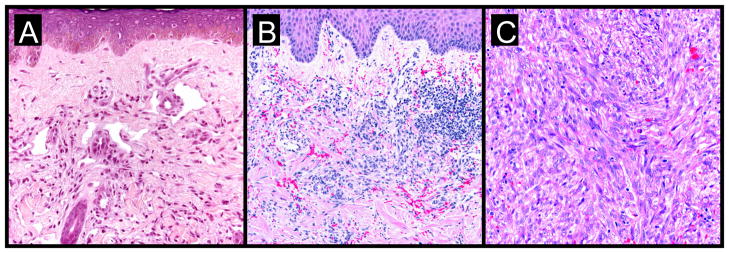
Well-developed KS lesions (Figure 1) are comprised of spindled-shaped tumor cells, abnormal vessels, and a variable chronic inflammatory infiltrate. Various histological variants (e.g. anaplastic KS, pyogenic-granuloma like KS, lymphangioma-like KS, amongst others) exist, that may mimic other vascular lesions [9, 10]. A premalignant stage of KS (so-called lymphedematous KS or KS in-situ) has been described in the setting of chronic lymphedema [11]. Chronic lymphedema results in local immune incompetence that, when coupled with lymphangiogenesis from developing collateral vessels, results in the development of KS lesions [12]. Depending on the inflammatory milieu and patient immune status, KS lesions may progress or regress. Progression evolves through distinct clinico-pathologic stages (Figure 2): they evolve from early (patch stage) flat macules into plaques (plaque stage) and subsequently nodular lesions (tumor stage). KS flare (so-called KS exacerbation) may occur as a result of IRIS following HAART, after corticosteroids, and with rituximab (chimeric monoclonal antibody against the protein CD20) therapy [13]. Regressing lesions are characterized by a partial or complete reduction of spindle cells accompanied by a prominent lymphocytic infiltrate [14]. The histologic appearance of KS is virtually identical in the different epidemiologic KS types. However, the clinical presentation varies among the different forms of KS (Table 1). Apart from molecular detection methods, KSHV can be identified and localized within KS lesional cells using immunohistochemistry. The commercial monoclonal antibody often used for this purpose is directed against the C-terminus of the latency-associated nuclear antigen (LNA-1 or LANA-1) encoded by ORF-73. LNA-1 immunoreactivity in KS cells usually appears as stippled nuclear staining.
Figure 1.
KS plaques of the lower extremity
Figure 2. KS progression: a histological view.
- subtle proliferation of irregular vascular channels between normal stromal collagen
- the extravasation of erythrocytes and hemosiderin into the stroma
- detection of lymphoplasmacytic infiltrate
- proliferating spindled cells that form interlacing bundles closely approximated with blood-filled vascular spaces
- intracellular hyaline globules within lesional cells (likely representing phagocytosed erythrocytes within lysozomes)
- increased inflammatory infiltrate consisting of lymphocytes, plasma cells, macrophages, and dendritic cells
- formation of intersecting fascicles and sheets of proliferating spindled cells
Origin of KS lesion cells
Unlike most tumors that arise from the expansion of a single transformed cell, the majority of KS tumors are polyclonal [15, 16], although a small percentage have been shown to be monoclonal [17]. The majority of tumor cells that comprise a KS lesion have a distinctive spindle morphology and express endothelial markers such as CD31 (also called PECAM-1 for platelet endothelial cell adhesion molecule) and CD34 [18]. In addition to spindle cells (SC), KS tumors contain other cell types such as T-cell lymphocytes, monocytes, macrophages, erythrocytes and dendritic cells [19, 20]. Over the past 15 years many studies have been published in an attempt to pinpoint the origin of the SC. Initial histologic studies suggested that SC were of lymphatic endothelial cell (LEC) origin [21]. The majority of SC express LEC-specific markers such as LYVE-1, D2-40 (podoplanin), and VEGFR-3, but markers of blood vascular endothelial cells (BEC) such as factor VIII (von Willebrand factor) and PAL-E have also been identified [22–25]. In addition, KS lesions are not found in areas devoid of lymphatics, such as the brain [26]. Two independent groups used microarray analysis in an attempt to resolve this question. Using comparative transcriptomics, Wang et al. found that the gene expression profile of KS tissue more closely matched that of LEC, confirming the majority of the histology data [27]. They further compared the transcriptomes of KSHV infected and uninfected LEC and BEC, and discovered that KSHV infection resulted in a reprogramming, such that their gene profiles converged. Hong et al. performed a transcriptomic analysis of KSHV-infected BEC and likewise concluded that infected BEC were reprogrammed towards a lymphatic expression profile, as exemplified by induction of the LEC specific marker PROX1 [28]. KSHV-induced expression of the lymphangiogenic molecule VEGF-C has been implicated in the reprogramming of BEC to LEC [29]. In addition, the viral miRNAs, miR-K6 and miR-K11 have recently been implicated in reprogramming LEC by downregulating the transcription factor musculoaponeurotic fibrosarcoma oncogene homolog (MAF) [30]. An alternate explanation for an increased LEC phenotype in vivo is that infected LEC may have a proliferative advantage over BEC. Support for this notion comes from in vitro studies demonstrating that the KSHV copy number is higher in infected LEC than BEC [27]. Another possibility is that the originator of the KS spindle cell is an uncommitted endothelial progenitor cell (EPC) defined as being CD34+ and VEGFR2+. A recent study identified the presence of uninfected EPC in biopsies from all stages of KS development, suggesting an active recruitment of potential target cells [31]. In addition, circulating EPC were increased in peripheral blood of classic KS patients [32] and two independent studies report direct infection of CD146+ EPC isolated from the blood of classic KS patients [33, 34]. The mechanism of the SC precursor infection is also unclear. In addition to plasma virus, KSHV has been detected in peripheral blood mononuclear cells (PBMC) such as B cells, T cells and monocytes of persons with KS [35]. Thus, precursor SC may be primary targets or could be infected following contact with PBMC, possibly during extravasation. The fact that KSHV affects cellular markers used to identify LEC and BEC, and the possibility that KSHV may infect progenitors and influence lineage commitment, prevents a definitive conclusion about the cellular origin of KS.
Mechanisms of KS tumorigenesis
Cellular transformation is a multi-step process involving increased proliferation, apoptosis prevention, and autocrine signaling pathways. Evaluating the neoplastic nature of the KS SC has been problematic, primarily because these cells do not behave like typical cancer cells. For example, KS explant cells grown ex vivo still require serum and growth factors for maintenance and do not readily grow in soft agar or form tumors in nude mice [36]. The lack of specific chromosomal abnormalities in the spindle cells also suggests an atypical neoplasm [37]. Interestingly, upon passage in culture, cells derived from KS tissue lose the KSHV genome suggesting either limitations in the in vitro culture systems or the inability of the virus to confer a fully immortalized or transformed phenotype. In agreement, KSHV infection of primary endothelial cells in vitro does not readily lead to immortalization or transformation, with the exception of one study [38]. Taken together, the current data suggest that the KS tumor microenvironment must play a critical role in maintaining the KSHV transformed spindle cell in vivo. Interestingly, a recent study suggests that KSHV induces the Warburg effect (a common tumor phenotype involving increased glycolysis with a concomitant decrease in oxygen utilization), which may be responsible for maintaining the latently infected endothelial cells [39].
As discussed above, some aspects of the immortalization/transformation pathway must be induced by KSHV, and others must be provided by additional environmental, genetic or immunological factors. An extensive and ongoing body of work has addressed the contribution made by KSHV-encoded proteins towards cellular transformation leading to the development of KS. Because of KSHV’s tendency towards latent gene expression and its ability to induce tumors in infected individuals, a great deal of focus has been placed upon the latent gene products as likely mediators of tumorigenesis (Table 3).
Table 3.
Latent viral immortalizing/transforming proteins (reviewed in [121]) and miRNAs
| Lana-1 (ORF73) |
|
| vCyc (ORF72) |
|
| vFLIP(ORF71, K13) |
|
| Kaposin A (K12) |
|
| miRNAs (miR-K1 through 12) |
|
The role of the KSHV lytic cycle in KS tumorigenesis is also a matter of debate. Initial theories postulated that since only a small percentage of the tumor cells express lytic proteins and that the viral lytic cycle results in cell death, it was more likely that latent viral proteins would mediate cellular transformation. While anti-herpesviral drugs that block viral replication such as ganciclovir and foscarnet have been shown to effectively prevent KS development if given prophylactically, there are no data to support the efficacy of these drugs for the treatment of pre-existing tumors [40–42]. Therefore, while KSHV latent genes are essential for genome maintenance, lytic genes probably play an important role in driving tumorigenesis via direct as well as paracrine mechanisms. Despite the limited number of lytic gene products expressed in KS lesions, several lytic proteins have been found to have intrinsic immortalizing or transforming ability (Table 4). One major drawback of these studies is that the transforming function of individual KSHV genes is usually analyzed in over-expression systems, in the absence of other viral genes. Similarly, the majority of the animal assays were conducted in rodent models, which are not natural hosts for KSHV. Another important caveat is that when expressed alone, lytic genes can activate many cellular genes, but during a normal viral infection, a host shutoff function mediated by the lytic SOX (shut off and exonuclease) gene (ORF37) prevents the expression of most cellular genes [43]. Taken as a whole, these data indicate that KSHV encodes many proteins that individually have the ability to immortalize/transform cells in vitro and in vivo, but how they all work together to perform this function in the context of a normal viral infection remains obscure.
Table 4.
Lytic viral immortalizing/transforming proteins
| vGPCR(ORF74) (reviewed in [68]) |
|
| K1 |
|
| vIRF1 (K9) (reviewed in [150]) |
|
Virus-induced cellular oncogenes
In addition to expressing its own potential oncogenes, KSHV induces the expression of a variety of cellular genes with transforming abilities. Analysis of gene expression profiles in KS tissue or EC infected with KSHV in vitro has identified several genes whose functions could conceivably contribute to KS tumorigenesis [44–50]. In order to verify their oncogenic potential, some of these genes have been further studied using over-expression and gene silencing techniques. Using these methods, the receptor tyrosine kinase c-Kit and the chemokine receptor CXCR7 (also RDC-1) have both been shown to play a role in the in vitro transformation of EC by KSHV [44, 45] [51]. c-Kit is an oncogene with an established role in other cancers [52], and CXCR7 has been shown to have oncogenic function [53]. SCF and SDF-1, the ligands for c-Kit and CXCR7, respectively, are both abundantly expressed in KS lesions, thus raising the possibility that proliferation-inducing autocrine or paracrine growth loops are established in KSHV-infected cells. A recent study implicates the KSHV-induced homeobox gene Pax2 as an additional factor in the transformation of human dermal endothelial cells [54].
Immune evasion
Viruses have evolved a variety of mechanisms to evade the host immune system, thus allowing for efficient viral replication, dissemination and persistence. In the case of a tumor virus such as KSHV, the ability to escape immune detection might also enhance the oncogenic potential of the virus. The two main immune evasion strategies used by KSHV are the establishment of latency and the expression of immunomodulatory genes. During latency, the small number of viral genes expressed limits the number of epitopes that the immune system can target. By contrast, during the lytic cycle, more than 80 viral genes are expressed, potentially exposing the virus to immunodetection and subsequent clearance. However, KSHV encodes a number of proteins that actively hinder the innate and adaptive antiviral responses (Table 5).
Table 5.
Host immune functions affected by KSHV (reviewed in [152])
| Interfron response |
| vIRF1 (K9) |
|
| vIRF-2 (K11 and 11.1) |
|
| vIRF-3 (LANA-2, K10.5 and K10.6) |
|
| vIRF-4 (K10/10.1) |
| ORF45 |
|
| RTA (ORF 50) |
|
| vIL-6 (K2) |
|
| LANA-1 (ORF73) |
|
| KSHV binding |
|
| Complement response |
| ORF 4 (KCP) |
| MHC-1 downregulation |
| MIR1 (modulators of immune recognition) (K3) |
|
| MIR2 (K5) |
|
| vIRF-1 (K9) |
|
Apoptosis of infected cells is yet another mechanism employed by the host to combat viral infection, and KSHV has evolved mechanisms to counteract this as well. Because the prevention of premature cell death leads to enhanced cellular proliferation, the expression of anti-apoptotic genes might also contribute to viral immortalizing and transforming properties. KSHV expresses several lytic and latent proteins that block both extrinsic (FAS and TNFR-1 activated) and intrinsic (p53 activated) apoptosis pathways. vFLIP (latent) and K1 (lytic) can inhibit FAS-mediated apoptosis by blocking the activation of caspase 8 [55, 56]. KSHV also encodes several proteins that directly interact with p53 and inhibit p53-mediated apoptosis; Lana-1, vIRF-1, vIRF-3, RTA [57] and K8 (K-bZIP) [58]. K7 or inhibitor of apoptosis (vIAP) is a homologue of the cellular protein survivin [59] and appears to inhibit apoptosis by inducing p53 degradation [60].
Similarly, vIRF-4 has recently been shown to induce p53 degradation through its interaction with murine double mutant 2 (MDM2) [61]. KSHV also expresses a Bcl-2 homologue, vBcl-2 (ORF16) that inhibits p53-mediated apoptosis by blocking BAX [62]. Because the host cell dies from viral lysis, the lytic anti-apoptotic genes may only exert their effect long enough to maximize production of viral progeny. The latent anti-apoptotic genes would, however, ensure the survival of the cell containing the viral episome, thus resulting in long-term persistence of the virus.
The majority of immune evasion mechanisms utilized by KSHV are undertaken by lytic proteins, suggesting that their main purpose is to prevent eradication of cells producing viral progeny. Transient expression of genes such as K5 may also be crucial during both primary infection and initial establishment of latency. Alternatively, some of the lytic immune evasion genes might be expressed during latency, but at levels that are below the limit of experimental detection [63]. In this instance, lytic immune evasion molecules could play an active role in protecting the KS tumor from immune detection. The data reviewed thus far clearly illustrate that the virus has evolved a variety of mechanisms to combat the host antiviral response. However, like the latent gene products discussed above, most of these studies are performed in vitro, and frequently not in the context of the whole virus, thus making it unclear how effective these mechanisms are in vivo, or what role immune evasion plays in KS pathogenesis.
Role of Chronic Inflammation
Inflammation results primarily from the immune system’s response to infection or trauma. Normally, inflammation subsides once the infectious agent is cleared or the wound is healed, but in the case of persistent infections or uncontrolled cellular proliferation, inflammation can continue as long as the stimulus is present. In these instances the inflammatory process is no longer helpful, and instead becomes part of the problem. Many aspects of KS suggest that chronic inflammation associated with the lesion and/or viral infection plays a role in tumor pathogenesis. A role for inflammation in KS is exemplified by the association of KS with the Koebner (or isomorphic) phenomenon (KP). KP is broadly defined as induction by physical or other noxious insult of lesions of the same form (hence isomorphic) characteristic of the initial disease, or the disease to which the individual is predisposed [64]. A variety of cutaneous diseases demonstrate koebnerization. Several infectious agents, including human papilloma virus (HPV) and KSHV have also been associated with this phenomenon. While the types of trauma are varied (e.g., burn, bite, incision), the inflammatory response generated is thought to attract infected cells to the site as well as exacerbate the oncogenic properties of the viruses (reviewed in [3]). The recurrence of KS that sometimes accompanies IRIS (KS flare) is another example of an inflammatory response initiating or exacerbating KS (reviewed in [4]).
While it may seem counter-productive for a virus to encode proteins that both evade and activate the immune system (see Table 5), in the context of chronic inflammation it makes sense. Inflammation is primarily mediated by hyperactivation of the humoral arm of the immune system (Th2-mediated responses) and is often accompanied by a decrease in cellular immunity (Th1-mediated responses), which is the more effective antiviral response. Having finished our discussion of mechanisms used by KSHV to downregulate Th1-mediated responses such as suppression of IFNs and MHC-1 downregulation, we now focus on mechanisms that the virus uses to activate Th2 responses, leading to continued inflammation and KS tumor progression.
Pro-inflammatory cytokines
The type of immune response, be it Th1 or Th2, is regulated in part by the predominant chemokines present at the site of injury. KSHV encodes three chemokine ligands, vCCL-1 (K6), vCCL-2 (K4) and vCCL-3 (K4.1) that share homology with the cellular macrophage inflammatory protein MIP-1α (reviewed in [65]). These proteins bind to and signal through the G-coupled protein receptors CCR8, CCR3 and CCR4, respectively. These receptors are expressed on the surface of Th2 cells, and their activation leads to the preferential chemotaxis of Th2 lymphocytes to the site of infection. vCCL-2 can additionally act as an antagonist of CCR1 and CCR5, which are expressed on Th1 lymphocytes, thereby contributing to the evasion of the antiviral response [66, 67].
Another KSHV protein that stimulates the proinflammatory immune response is vGPCR (ORF74). The constitutive activity of vGPCR induces myriad signaling pathways, including JNK/SAPK, PLC/PKC, MAPK, and PI3K/Akt/mTor (reviewed in [68]. This leads to the activation of NF-κB, which has become increasingly linked to chronic inflammation and cancer progression, primarily via the prevention of apoptosis [69], but also via the induction of angiogenesis [70]. The activation of multiple pathways by vGPCR in monocytes and T cells leads to the production of several proinflammatory cytokines including IL-1β, TNFα, IL-6, IL-2, IL-4 [71]. vGPCR-induced activation of NF-κB in endothelial cells results in expression of RANTES, IL-8 and GM-CSF as well as the adhesion molecules VCAM-1, ICAM-1 and E-selectin [72]. Since vGPCR is a lytic gene product, any effect that its signaling has on tumor formation or progression probably occurs via a paracrine mechanism. KSHV also induces NF-κB via the expression of vFLIP, as described above. Because vFLIP is latent and therefore widely expressed in KS and PEL tissues, the activation of NF-κB is constitutive, not transient. This constant signaling from the tumor tissue could be a major stimulus for both proliferation and chronic infiltration by leukocytes.
Another source of KSHV-induced proinflammatory cytokines may come from the action of Kaposin B. Kaposin B is expressed from a CUG upstream of the K12/Kaposin A ORF, but is read from a different reading frame, such that Kaposin A is not expressed [73]. The mRNA that encodes the entire kaposin locus is abundant in KS tumor tissue, but the relative expression levels of the three kaposin isoforms is not clear [73]. A screen for cellular Kaposin B binding partners revealed that the MAPK-associated protein kinase MK2 was both bound and activated by Kaposin B. MK2 is normally activated by p38, leading to the stabilization of transcripts that harbor AU-rich elements (AREs) at their 3prime; untranslated regions. There are thousands of such transcripts, including those that encode cytokines. Kaposin B was then demonstrated to stabilize the ARE-containing IL-6 and GM-CSF messages in KSHV-infected cells [74], suggesting that Kaposin B may contribute to the high levels of cytokines and potentially other chemotactic and angioproliferative factors observed in KS lesions.
Cellular IL-6 has long been associated with KS and MCD tumor development. It has been shown to enhance proliferation of KS cells in culture [36] and was found in high levels in blood samples from MCD patients, which correlated with disease progression [75]. The signaling pathways activated by IL-6 and vIL-6 that mediate an inflammatory response include JAK/STAT, MAPK, and PI3K/AKT/mTor [76–79]. Cyclooxygenase-2 (Cox-2) is another pro-inflammatory, pro-angiogenic molecule upregulated by KSHV, expressed in KS tissue and likely involved in KS pathogenesis [80].
The importance of this pro-inflammatory component to KS tumorigenesis is highlighted by the success of the PI3K/AKT/mTor pathway inhibitor rapamycin (sirolimus) in clinical trials for posttransplant KS [81–83].
Angiogenesis
Angiogenesis is the formation of new blood vessels to increase blood supply to a site of injury, and is a normal inflammatory response that, when aberrantly stimulated, enhances tumor pathogenesis [84]. Two of the major cellular cytokines involved in angiogenesis are vascular endothelial growth factor (VEGF) and IL-6, which interact with their respective receptors to induce endothelial cell proliferation. IL-6 can also indirectly induce angiogenesis by upregulating VEGF [85], and KSHV vIL-6-expressing rodent cells and implanted tumors also induce VEGF [86]. KSHV encodes several other proteins that can induce VEGF, including all three KSHV-encoded chemokines (vCCLs). These have been shown to induce angiogenesis in experimental models [87, 88], and the mechanism for this activity is likely the induction of VEGF [89]. Similarly, vGPCR upregulates both VEGF and its receptor VEGF-R2 in KSHV-infected endothelial cells [90]. It has been postulated that this may create a paracrine feedback loop for continued cellular proliferation and angiogenesis [91]. Finally, K1 has also been demonstrated to induce VEGF expression and secretion [92].
Matrix metalloproteinases (MMP) are key enzymes that remodel the extracellular matrix during tumor invasion, metastasis, and angiogenesis [93]. MMP-1, -2, -3, -9, and -19 are expressed in KS tumors [94, 95], and their importance to KS pathology has been demonstrated by the efficacy of an MMP inhibitor in treating AIDS-KS [96]. The KSHV protein K1 has been shown to induce the expression and activation of MMP-9, an MMP that is critical for the angiogenic switch in tumor progression [92, 97]. In vitro studies with KSHV-infected endothelial cells suggest that the virus induces MMP-1, -2 and -9 secretion and activation, thus allowing infected cells to invade an extracellular matrix [98]. A recent study indicates that KSHV, specifically LANA-1, may increase the levels of the extracellular matrix metalloproteinase inducer (emmprin) as a means to activate MMPs [99].
Angiopoietins are another family of vascular growth factors important for angiogenesis. Angiopoietin-1 (Ang-1) binds and activates the receptor tyrosine kinase Tie-2, which synergizes with VEGF to promote endothelial cell proliferation and stabilization of blood vessels. In contrast, angiopoietin-2 (Ang-2) is an antagonist of Tie-2 that destabilizes existing vessels. Ang-2 is often upregulated at sites of vascular remodeling, while Ang-1 is ubiquitously expressed in endothelial cells. While both Ang-1 and Ang-2 are found in KS tumors, Ang-2 is expressed at higher levels [27, 100] and is induced in KSHV-infected endothelial cells [101].
Tumor cells in environments that are low in oxygen induce angiogenesis via the stabilization of hypoxia-induced factors (HIF), which are transcription factors that interact with promoters containing hypoxia response elements (HRE). Two key regulators of angiogenesis, VEGF and VEGFR1, contain HREs [102]. Interestingly, KSHV encodes several means by which to upregulate HIFs. For example, vGPCR-dependent activation of both MAPK and p38 kinases leads to the subsequent phosphorylation and activation of HIF1α, and this is the likely mechanism for vGPCR’s induction of VEGF [103]. In addition, KSHV latent gene expression induces the transcriptional upregulation of both HIF1α and HIF1β [104]. Other important angiogenic proteins expressed in KS lesions are IL-1β, FGF-2 and PDGF-Rβ (reviewed in [105]). The mechanisms for their upregulation are not yet known.
Most of the pro-inflammatory cytokines and angiogenic factors produced or induced by KSHV have likely evolved to create a highly proliferative environment that favors viral genome maintenance as well as the consistent, yet low level of reactivation from latency. Both lytic and latent genes are responsible, suggesting that both autocrine and paracrine mechanisms contribute to the microenvironment. In addition to virally induced factors, the persistent viral infection attracts a chronic leukocyte infiltrate, which in turn secretes cytokines, chemokines, enzymes, and growth factors that favor the growth of infected cells and contribute to KS progression.
Treatment
Treatment of KS is aimed at symptom palliation, tumor shrinkage, and prevention of disease progression [106]. Therapeutic decisions depend upon the presence and extent of symptomatic and extracutaneous KS, the HIV status of the patient and their corresponding HIV viral load, and the host immune status (e.g. CD4 cell count and co-morbid disease). The advent of HAART has lead to a marked reduction in the incidence, morbidity and mortality associated with AIDS-related KS [107]. Similar advances in chemotherapy and supportive care protocols have also allowed for KS to be more effectively managed. The recent identification of several molecular targets has provided several new potential therapeutic strategies [108][109].
Most if not all patients with AIDS-related KS should receive antiretroviral therapy, assuming access to such therapy is available. Effective antiretroviral regimens are associated with both a reduction in the incidence of AIDS-related KS and a regression in size and number of existing lesions. The effects of HAART on KS are multifactorial and include inhibition of HIV replication, diminished production of the HIV-1 transactivating protein Tat, amelioration of the immune response against KSHV and perhaps some direct antiangiogenic activity of protease inhibitors. The dramatic impact of HAART on KS is highlighted by a large Swiss cohort study of HIV-infected patients [110]. In this cohort, the relative risk (hazard ratio) of KS development between 1997 and 1998 (HAART-era) compared with a pre-HAART time period between 1992 and 1994 was 0.08 (95% confidence interval, 0.03–0.22), representing a dramatic reduction in this AIDS-defining malignancy. Furthermore, it has been demonstrated that both protease inhibitor- and non-nucleoside reverse transcriptase inhibitor (NNRTI)-based HAART regimens are equally effective in protecting against KS. Immune reconstitution inflammatory syndrome, with paradoxical worsening of stable opportunistic infections and KS (KS flare), can occur in the setting of marked HAART-induced recovery of the immune system.
Systemic chemotherapy is given to patients with more advanced or rapidly progressive KS. Typical indications for such systemic therapy include widespread skin involvement, extensive KS of the oral cavity, symptomatic pedal or scrotal edema, symptomatic visceral involvement, and immune reconstitution inflammatory syndrome-induced KS flare. Although several chemotherapeutic agents (bleomycin, vinblastine, vincristine, hydroxydaunomycin, adriamycin, and etoposide) were shown to be active against KS in the past, liposomal anthracyclines (pegylated liposomal doxorubicin and liposomal daunorubicin) and taxanes (paclitaxel) constitute the backbone of current systemic cytotoxic therapy against KS.
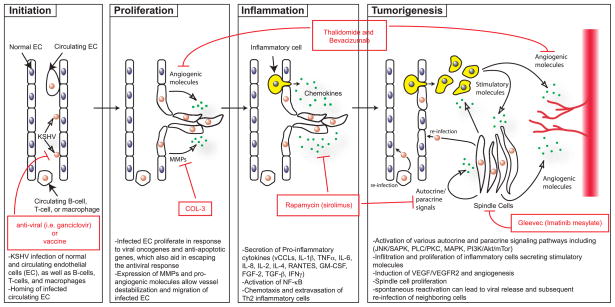
Novel agents used to treat KS include angiogeneisis inhibitors, tyrosine kinase inhibitors, and matrix metalloproteinase inhibitors (see Figure 3). Thalidomide, which has significant antiangiogenic activity partly through inhibition of basic fibroblast growth factor-induced angiogenesis, has been shown to provide a partial response in treating KS [111]. Bevacizumab, a monoclonal antibody against VEGF, may provide similar potential activity in the treatment of KS. Imatinib mesylate, a PDGF-R and c-Kit inhibitor, has been found to result in marked clinical and histologic regression of KS [112]. The activity of other tyrosine kinase inhibitors (e.g. sorafenib and sunitinib) requires further investigation. Several trials have shown that COL-3, a chemically modified tetracycline that is a matrix metalloproteinase inhibitor, is beneficial in the treatment of AIDS-related KS [113]. Rapamycin (sirolimus), an inhibitor of the PI3K/Akt/mTor pathway, has been very effective in treating posttransplant [81–83] as well as classic [114] [115] KS and are currently being investigated for HIV-related KS.
Figure 3.
Model of KS tumorigenesis with sites for therapeutic intervention
There are no vaccines available for KSHV and research in this area has not been very active likely due to the lack of a suitable animal model and to the efficacy of HAART in dramatically lowering the incidence of KS. However, the prospects for a new non-human primate model that exhibits persistent KSHV infection with a robust anti-KSHV antibody response are promising [116]. A recent study using a recombinant murine herpesvirus 68 (MHV68) engineered to constitutively express the viral transcription activator, RTA protected mice from subsequent wildtype MHV68 challenge, potentially providing a novel vaccine approach for KSHV [117].
Conclusion
After initial KSHV infection, the development of KS depends upon the generation of a unique local microenvironment, as illustrated in Figure 3. This model, which is based on the data described in this review, depicts a potential pathway of KS development involving four stages of progression. These include the initiation of virus infection, proliferation and migration of infected endothelial cells, induction of a pro-inflammatory response and finally tumor formation. Key viral and cellular factors thought to play a role at each of these stages are indicated. In addition, current therapeutics and their targets are highlighted.
Thus far, much of the data regarding KS progression have come from analyses of the contribution made by individual KSHV genes. While such studies have provided invaluable insights into the tools available to the virus, the data have yet to definitively establish a hierarchy of events required for tumor progression. A more comprehensive view of the oncogenic process will greatly benefit from an animal model that can successfully recapitulate the disease and allow for the testing of defined viral mutants, in addition to rigorous clinical evaluation of asymptomatic and KS-afflicted KSHV-seropositive individuals. Only then will we gain a more complete understanding of the complex interplay between viral-encoded proteins and the host immune system, and how this interaction leads to KS. Fortunately, this interpretation of the disease as a convergence of immune evasion, oncogenesis, inflammation, and angiogenesis has broadened the potential targets for treatment. Ongoing clinical trials are evaluating inhibitors of VEGF, MMPs, and cytokine signaling pathways. As the role of additional molecular mechanisms involved in KS tumorigenesis, such as the Akt/mammalian target of rapamycin pathway and NF-kB, become apparent we can anticipate the development of new therapeutic agents to target these pathogenetic mechanisms. These mechanism-based agents are a superb example of therapeutic approaches bridging basic research with clinical practice.
Acknowledgments
The authors would like to thank the following agencies for their support. Ashlee Moses’ laboratory was supported by NIH grants CA 099906 and RR00163. Janet Douglas would like to acknowledge the Collins Medical Trust.
References
- 1.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, et al. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338(14):948–54. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 2.Kestens L, Melbye M, Biggar RJ, Stevens WJ, Piot P, et al. Endemic African Kaposi’s sarcoma is not associated with immunodeficiency. Int J Cancer. 1985;36(1):49–54. doi: 10.1002/ijc.2910360109. [DOI] [PubMed] [Google Scholar]
- 3.Rubin AI, Stiller MJ. A listing of skin conditions exhibiting the koebner and pseudo-koebner phenomena with eliciting stimuli. J Cutan Med Surg. 2002;6(1):29–34. doi: 10.1007/s10227-001-0029-6. [DOI] [PubMed] [Google Scholar]
- 4.Leidner RS, Aboulafia DM. Recrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS. 2005;19(10):635–44. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler J, Dorfman RF. Overview of kaposi’s sarcoma: History, epidemiology, and biomedical features. In: Ziegler JL, Dorfman RF, editors. Kaposi’s sarcoma: Pathophysiology and clinical management. Marcel Dekker, Inc; New York: 1988. pp. 1–22. [Google Scholar]
- 6.Hayward GS. Initiation of angiogenic Kaposi’s sarcoma lesions. Cancer Cell. 2003;3(1):1–3. doi: 10.1016/s1535-6108(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 7.Casper C, Redman M, Huang ML, Pauk J, Lampinen TM, et al. HIV infection and human herpesvirus-8 oral shedding among men who have sex with men. J Acquir Immune Defic Syndr. 2004;35(3):233–8. doi: 10.1097/00126334-200403010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chatlynne LG, Ablashi DV. Seroepidemiology of Kaposi’s sarcoma-associated herpesvirus (KSHV) Semin Cancer Biol. 1999;9(3):175–85. doi: 10.1006/scbi.1998.0089. [DOI] [PubMed] [Google Scholar]
- 9.Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31. doi: 10.1186/1746-1596-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donnell PJ, Pantanowitz L, Grayson W. Unique histologic variants of cutaneous Kaposi sarcoma. Am J Dermatopathol. 2010;32(3):244–50. doi: 10.1097/DAD.0b013e3181b7f6a7. [DOI] [PubMed] [Google Scholar]
- 11.Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in-situ Kaposi’s sarcoma. BMC Clin Pathol. 2006;6:7. doi: 10.1186/1472-6890-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruocco V, Schwartz RA, Ruocco E. Lymphedema: an immunologically vulnerable site for development of neoplasms. J Am Acad Dermatol. 2002;47(1):124–7. doi: 10.1067/mjd.2002.120909. [DOI] [PubMed] [Google Scholar]
- 13.Pantanowitz L, Fruh K, Marconi S, Moses AV, Dezube BJ. Pathology of rituximab-induced Kaposi sarcoma flare. BMC Clin Pathol. 2008;8:7. doi: 10.1186/1472-6890-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pantanowitz L, Dezube BJ, Pinkus GS, Tahan SR. Histological characterization of regression in acquired immunodeficiency syndrome-related Kaposi’s sarcoma. J Cutan Pathol. 2004;31(1):26–34. doi: 10.1046/j.0303-6987.2004.0132.x. [DOI] [PubMed] [Google Scholar]
- 15.Gill PS, Tsai YC, Rao AP, Spruck CH, 3rd, Zheng T, et al. Evidence for multiclonality in multicentric Kaposi’s sarcoma. Proc Natl Acad Sci U S A. 1998;95(14):8257–61. doi: 10.1073/pnas.95.14.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duprez R, Lacoste V, Briere J, Couppie P, Frances C, et al. Evidence for a multiclonal origin of multicentric advanced lesions of Kaposi sarcoma. J Natl Cancer Inst. 2007;99(14):1086–94. doi: 10.1093/jnci/djm045. [DOI] [PubMed] [Google Scholar]
- 17.Rabkin CS, Janz S, Lash A, Coleman AE, Musaba E, et al. Monoclonal origin of multicentric Kaposi’s sarcoma lesions. N Engl J Med. 1997;336(14):988–93. doi: 10.1056/NEJM199704033361403. [DOI] [PubMed] [Google Scholar]
- 18.Nickoloff BJ. PECAM-1 (CD31) is expressed on proliferating endothelial cells, stromal spindle-shaped cells, and dermal dendrocytes in Kaposi’s sarcoma. Arch Dermatol. 1993;129(2):250–1. [PubMed] [Google Scholar]
- 19.Kaaya EE, Parravicini C, Ordonez C, Gendelman R, Berti E, et al. Heterogeneity of spindle cells in Kaposi’s sarcoma: comparison of cells in lesions and in culture. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(3):295–305. [PubMed] [Google Scholar]
- 20.Regezi JA, MacPhail LA, Daniels TE, DeSouza YG, Greenspan JS, et al. Human immunodeficiency virus-associated oral Kaposi’s sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am J Pathol. 1993;143(1):240–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Beckstead JH, Wood GS, Fletcher V. Evidence for the origin of Kaposi’s sarcoma from lymphatic endothelium. Am J Pathol. 1985;119(2):294–300. [PMC free article] [PubMed] [Google Scholar]
- 22.Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, et al. Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58(8):1599–604. [PubMed] [Google Scholar]
- 23.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15(4):434–40. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 24.Nadimi H, Saatee S, Armin A, Toto PD. Expression of endothelial cell markers PAL-E and EN-4 and Ia-antigens in Kaposi’s sarcoma. J Oral Pathol. 1988;17(8):416–20. doi: 10.1111/j.1600-0714.1988.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Edwards JR, Espinosa O, Banerji S, Jackson DG, et al. Expression of a lymphatic endothelial cell marker in benign and malignant vascular tumors. Hum Pathol. 2004;35(7):857–61. doi: 10.1016/j.humpath.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Herndier B, Ganem D. The biology of Kaposi’s sarcoma. Cancer Treat Res. 2001;104:89–126. doi: 10.1007/978-1-4615-1601-9_4. [DOI] [PubMed] [Google Scholar]
- 27.Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, et al. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet. 2004;36(7):687–93. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- 28.Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, et al. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36(7):683–5. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 29.Sivakumar R, Sharma-Walia N, Raghu H, Veettil MV, Sadagopan S, et al. Kaposi’s sarcoma-associated herpesvirus induces sustained levels of vascular endothelial growth factors A and C early during in vitro infection of human microvascular dermal endothelial cells: biological implications. J Virol. 2008;82(4):1759–76. doi: 10.1128/JVI.00873-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen A, Henderson S, Lagos D, Nikitenko L, Coulter E, et al. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev. 2010;24(2):195–205. doi: 10.1101/gad.553410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pyakurel P, Pak F, Mwakigonja AR, Kaaya E, Heiden T, et al. Lymphatic and vascular origin of Kaposi’s sarcoma spindle cells during tumor development. Int J Cancer. 2006;119(6):1262–7. doi: 10.1002/ijc.21969. [DOI] [PubMed] [Google Scholar]
- 32.Taddeo A, Presicce P, Brambilla L, Bellinvia M, Villa ML, et al. Circulating Endothelial Progenitor Cells Are Increased in Patients with Classic Kaposi’s Sarcoma. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.23. [DOI] [PubMed] [Google Scholar]
- 33.Pellet C, Kerob D, Dupuy A, Carmagnat MV, Mourah S, et al. Kaposi’s sarcoma-associated herpesvirus viremia is associated with the progression of classic and endemic Kaposi’s sarcoma. J Invest Dermatol. 2006;126(3):621–7. doi: 10.1038/sj.jid.5700083. [DOI] [PubMed] [Google Scholar]
- 34.Della Bella S, Taddeo A, Calabro ML, Brambilla L, Bellinvia M, et al. Peripheral Blood Endothelial Progenitors as Potential Reservoirs of Kaposi’s Sarcoma-Associated Herpesvirus. PLoS ONE. 2008;3(1):e1520. doi: 10.1371/journal.pone.0001520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry M, Uthman A, Geusau A, Rieger A, Furci L, et al. Infection of circulating CD34+ cells by HHV-8 in patients with Kaposi’s sarcoma. J Invest Dermatol. 1999;113(4):613–6. doi: 10.1046/j.1523-1747.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- 36.Miles SA, Rezai AR, Salazar-Gonzalez JF, Vander Meyden M, Stevens RH, et al. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1990;87(11):4068–72. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delli Bovi P, Donti E, Knowles DM, 2nd, Friedman-Kien A, Luciw PA, et al. Presence of chromosomal abnormalities and lack of AIDS retrovirus DNA sequences in AIDS-associated Kaposi’s sarcoma. Cancer Res. 1986;46(12 Pt 1):6333–8. [PubMed] [Google Scholar]
- 38.Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, et al. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394(6693):588–92. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 39.Delgado T, Carroll PA, Punjabi AS, Margineantu D, Hockenbery DM, et al. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mocroft A, Youle M, Gazzard B, Morcinek J, Halai R, et al. Anti-herpesvirus treatment and risk of Kaposi’s sarcoma in HIV infection. Royal Free/Chelsea and Westminster Hospitals Collaborative Group. Aids. 1996;10(10):1101–5. [PubMed] [Google Scholar]
- 41.Glesby MJ, Hoover DR, Weng S, Graham NM, Phair JP, et al. Use of antiherpes drugs and the risk of Kaposi’s sarcoma: data from the Multicenter AIDS Cohort Study. J Infect Dis. 1996;173(6):1477–80. doi: 10.1093/infdis/173.6.1477. [DOI] [PubMed] [Google Scholar]
- 42.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, et al. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340(14):1063–70. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 43.Glaunsinger B, Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol Cell. 2004;13(5):713–23. doi: 10.1016/s1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- 44.Moses AV, Jarvis MA, Raggo C, Bell YC, Ruhl R, et al. Kaposi’s sarcoma-associated herpesvirus-induced upregulation of the c-kit proto-oncogene, as identified by gene expression profiling, is essential for the transformation of endothelial cells. J Virol. 2002;76(16):8383–99. doi: 10.1128/JVI.76.16.8383-8399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raggo C, Ruhl R, McAllister S, Koon H, Dezube BJ, et al. Novel cellular genes essential for transformation of endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2005;65(12):5084–95. doi: 10.1158/0008-5472.CAN-04-2822. [DOI] [PubMed] [Google Scholar]
- 46.Poole LJ, Yu Y, Kim PS, Zheng QZ, Pevsner J, et al. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi’s sarcoma-associated herpesvirus. J Virol. 2002;76(7):3395–420. doi: 10.1128/JVI.76.7.3395-3420.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornelissen M, van der Kuyl AC, van den Burg R, Zorgdrager F, van Noesel CJ, et al. Gene expression profile of AIDS-related Kaposi’s sarcoma. BMC Cancer. 2003;3:7. doi: 10.1186/1471-2407-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAllister SC, Hansen SG, Ruhl RA, Raggo CM, DeFilippis VR, et al. Kaposi sarcoma-associated herpesvirus (KSHV) induces heme oxygenase-1 expression and activity in KSHV-infected endothelial cells. Blood. 2004;103(9):3465–73. doi: 10.1182/blood-2003-08-2781. [DOI] [PubMed] [Google Scholar]
- 49.Rossi G, Sartori G, Rusev BC, Sgambato A. Expression and molecular analysis of c-kit and PDGFRs in Kaposi’s sarcoma of different stages and epidemiological settings. Histopathology. 2009;54(5):619–22. doi: 10.1111/j.1365-2559.2009.03270.x. [DOI] [PubMed] [Google Scholar]
- 50.Ramirez-Amador V, Martinez-Mata G, Gonzalez-Ramirez I, Anaya-Saavedra G, de Almeida OP. Clinical, histological and immunohistochemical findings in oral Kaposi’s sarcoma in a series of Mexican AIDS patients. Comparative study. J Oral Pathol Med. 2009;38(4):328–33. doi: 10.1111/j.1600-0714.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 51.Douglas JL, Whitford JG, Moses AV. Characterization of c-Kit expression and activation in KSHV-infected endothelial cells. Virology. 2009;390(2):174–85. doi: 10.1016/j.virol.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lennartsson J, Ronnstrand L. The stem cell factor receptor/c-Kit as a drug target in cancer. Curr Cancer Drug Targets. 2006;6(1):65–75. doi: 10.2174/156800906775471725. [DOI] [PubMed] [Google Scholar]
- 53.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fonsato V, Buttiglieri S, Deregibus MC, Bussolati B, Caselli E, et al. PAX2 expression by HHV-8-infected endothelial cells induced a pro-angiogenic and pro-invasive phenotype. Blood. 2007 doi: 10.1182/blood-2007-04-085555. [DOI] [PubMed] [Google Scholar]
- 55.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, et al. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190(7):1025–32. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Wang S, Maeng H, Young DP, Prakash O, et al. K1 protein of human herpesvirus 8 suppresses lymphoma cell Fas-mediated apoptosis. Blood. 2007;109(5):2174–82. doi: 10.1182/blood-2006-02-003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwack Y, Hwang S, Byun H, Lim C, Kim JW, et al. Kaposi’s sarcoma-associated herpesvirus open reading frame 50 represses p53-induced transcriptional activity and apoptosis. J Virol. 2001;75(13):6245–8. doi: 10.1128/JVI.75.13.6245-6248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J, Seo T, Hwang S, Lee D, Gwack Y, et al. The K-bZIP protein from Kaposi’s sarcoma-associated herpesvirus interacts with p53 and represses its transcriptional activity. J Virol. 2000;74(24):11977–82. doi: 10.1128/jvi.74.24.11977-11982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi’s sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. Embo J. 2002;21(11):2602–15. doi: 10.1093/emboj/21.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng P, Scott CW, Cho NH, Nakamura H, Chung YH, et al. Kaposi’s sarcoma-associated herpesvirus K7 protein targets a ubiquitin-like/ubiquitin-associated domain-containing protein to promote protein degradation. Mol Cell Biol. 2004;24(9):3938–48. doi: 10.1128/MCB.24.9.3938-3948.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HR, Toth Z, Shin YC, Lee JS, Chang H, et al. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 4 targets MDM2 to deregulate the p53 tumor suppressor pathway. J Virol. 2009;83(13):6739–47. doi: 10.1128/JVI.02353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat Med. 1997;3(3):293–8. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, et al. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi’s sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol. 2004;78(7):3601–20. doi: 10.1128/JVI.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16(3):241–8. doi: 10.1046/j.1473-2165.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 65.Nicholas J. Human gammaherpesvirus cytokines and chemokine receptors. J Interferon Cytokine Res. 2005;25(7):373–83. doi: 10.1089/jir.2005.25.373. [DOI] [PubMed] [Google Scholar]
- 66.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, et al. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277(5332):1656–9. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 67.Weber KS, Grone HJ, Rocken M, Klier C, Gu S, et al. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur J Immunol. 2001;31(8):2458–66. doi: 10.1002/1521-4141(200108)31:8<2458::aid-immu2458>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 68.Cannon M. The KSHV and other human herpesviral G protein-coupled receptors. Curr Top Microbiol Immunol. 2007;312:137–56. doi: 10.1007/978-3-540-34344-8_5. [DOI] [PubMed] [Google Scholar]
- 69.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 70.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99(8):1501–6. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwarz M, Murphy PM. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001;167(1):505–13. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- 72.Pati S, Cavrois M, Guo HG, Foulke JS, Jr, Kim J, et al. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi’s sarcoma pathogenesis. J Virol. 2001;75(18):8660–73. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadler R, Wu L, Forghani B, Renne R, Zhong W, et al. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma-associated herpesvirus. J Virol. 1999;73(7):5722–30. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCormick C, Ganem D. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science. 2005;307(5710):739–41. doi: 10.1126/science.1105779. [DOI] [PubMed] [Google Scholar]
- 75.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74(4):1360–7. [PubMed] [Google Scholar]
- 76.Stahl N, Boulton TG, Farruggella T, Ip NY, Davis S, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263(5143):92–5. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 77.Lutticken C, Wegenka UM, Yuan J, Buschmann J, Schindler C, et al. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- 78.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, et al. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5(5):449–60. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 79.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, et al. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273(16):9703–10. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- 80.Sharma-Walia N, Paul AG, Bottero V, Sadagopan S, Veettil MV, et al. Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010;6(2):e1000777. doi: 10.1371/journal.ppat.1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352(13):1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 82.Gutierrez-Dalmau A, Sanchez-Fructuoso A, Sanz-Guajardo A, Mazuecos A, Franco A, et al. Efficacy of conversion to sirolimus in posttransplantation Kaposi’s sarcoma. Transplant Proc. 2005;37(9):3836–8. doi: 10.1016/j.transproceed.2005.10.076. [DOI] [PubMed] [Google Scholar]
- 83.Lebbe C, Euvrard S, Barrou B, Pouteil-Noble C, Garnier JL, et al. Sirolimus conversion for patients with posttransplant Kaposi’s sarcoma. Am J Transplant. 2006;6(9):2164–8. doi: 10.1111/j.1600-6143.2006.01412.x. [DOI] [PubMed] [Google Scholar]
- 84.O’Byrne KJ, Dalgleish AG, Browning MJ, Steward WP, Harris AL. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000;36(2):151–69. doi: 10.1016/s0959-8049(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 85.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271(2):736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 86.Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, et al. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93(12):4034–43. [PubMed] [Google Scholar]
- 87.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278(5336):290–4. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 88.Stine JT, Wood C, Hill M, Epp A, Raport CJ, et al. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95(4):1151–7. [PubMed] [Google Scholar]
- 89.Liu C, Okruzhnov Y, Li H, Nicholas J. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J Virol. 2001;75(22):10933–40. doi: 10.1128/JVI.75.22.10933-10940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, et al. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391(6662):86–9. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 91.Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, et al. Kaposi’s sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell. 2003;3(2):131–43. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 92.Wang L, Wakisaka N, Tomlinson CC, DeWire SM, Krall S, et al. The Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 2004;64(8):2774–81. doi: 10.1158/0008-5472.can-03-3653. [DOI] [PubMed] [Google Scholar]
- 93.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 94.Impola U, Cuccuru MA, Masala MV, Jeskanen L, Cottoni F, et al. Preliminary communication: matrix metalloproteinases in Kaposi’s sarcoma. Br J Dermatol. 2003;149(4):905–7. doi: 10.1046/j.1365-2133.2003.05561.x. [DOI] [PubMed] [Google Scholar]
- 95.Meade-Tollin LC, Way D, Witte MH. Expression of multiple matrix metalloproteinases and urokinase type plasminogen activator in cultured Kaposi sarcoma cells. Acta Histochem. 1999;101(3):305–16. doi: 10.1016/S0065-1281(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 96.Cianfrocca M, Cooley TP, Lee JY, Rudek MA, Scadden DT, et al. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi’s sarcoma: a phase I AIDS malignancy consortium study. J Clin Oncol. 2002;20(1):153–9. doi: 10.1200/JCO.2002.20.1.153. [DOI] [PubMed] [Google Scholar]
- 97.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian LW, Xie J, Ye F, Gao SJ. Kaposi’s Sarcoma-Associated Herpesvirus Infection Promotes Invasion of Primary Human Umbilical Vein Endothelial Cells by Inducing Matrix Metalloproteinases. J Virol. 2007 doi: 10.1128/JVI.00016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin Z, Dai L, Slomiany MG, Toole BP, Parsons C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 2010;70(10):3884–9. doi: 10.1158/0008-5472.CAN-09-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD. Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol. 2000;156(6):2179–83. doi: 10.1016/S0002-9440(10)65088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye FC, Blackbourn DJ, Mengel M, Xie JP, Qian LW, et al. Kaposi’s sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J Virol. 2007;81(8):3980–91. doi: 10.1128/JVI.02089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, et al. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res. 2004;95(2):146–53. doi: 10.1161/01.RES.0000134920.10128.b4. [DOI] [PubMed] [Google Scholar]
- 103.Sodhi A, Montaner S, Patel V, Zohar M, Bais C, et al. The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60(17):4873–80. [PubMed] [Google Scholar]
- 104.Carroll PA, Kenerson HL, Yeung RS, Lagunoff M. Latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J Virol. 2006;80(21):10802–12. doi: 10.1128/JVI.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koon HB, Pantanowitz L, Dezube BJ. Antiangiogenic Therapy for Kaposi’s sarcoma. In: Davis D, Herbst RS, Abbruzzese JL, editors. Anti-angiogenic cancer therapy. CRC; 2007. pp. 753–781. [Google Scholar]
- 106.Dezube BJ, Pantanowitz L, Aboulafia DM. Management of AIDS-related Kaposi sarcoma: advances in target discovery and treatment. AIDS Read. 2004;14(5):236–8. 243–4, 251–3. [PubMed] [Google Scholar]
- 107.Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, Di Trolio R, De Placido S, et al. Management of AIDS-related Kaposi’s sarcoma. Lancet Oncol. 2007;8(2):167–76. doi: 10.1016/S1470-2045(07)70036-0. [DOI] [PubMed] [Google Scholar]
- 108.Sullivan RJ, Pantanowitz L, Dezube BJ. Targeted therapy for Kaposi sarcoma. BioDrugs. 2009;23(2):69–75. doi: 10.2165/00063030-200923020-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arav-Boger R. Treatment for Kaposi sarcoma herpesvirus: great challenges with promising accomplishments. Virus Genes. 2009;38(2):195–203. doi: 10.1007/s11262-008-0325-y. [DOI] [PubMed] [Google Scholar]
- 110.Ledergerber B, Telenti A, Egger M. Risk of HIV related Kaposi’s sarcoma and non-Hodgkin’s lymphoma with potent antiretroviral therapy: prospective cohort study. Swiss HIV Cohort Study. Bmj. 1999;319(7201):23–4. doi: 10.1136/bmj.319.7201.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Little RF, Wyvill KM, Pluda JM, Welles L, Marshall V, et al. Activity of thalidomide in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2000;18(13):2593–602. doi: 10.1200/JCO.2000.18.13.2593. [DOI] [PubMed] [Google Scholar]
- 112.Koon HB, Bubley GJ, Pantanowitz L, Masiello D, Smith B, et al. Imatinib-induced regression of AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2005;23(5):982–9. doi: 10.1200/JCO.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 113.Dezube BJ, Krown SE, Lee JY, Bauer KS, Aboulafia DM. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi’s sarcoma: an AIDS Malignancy Consortium Study. J Clin Oncol. 2006;24(9):1389–94. doi: 10.1200/JCO.2005.04.2614. [DOI] [PubMed] [Google Scholar]
- 114.Merimsky O, Jiveliouk I, Sagi-Eisenberg R. Targeting mTOR in HIV-Negative Classic Kaposi’s Sarcoma. Sarcoma. 2008:825093. doi: 10.1155/2008/825093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guenova E, Metzler G, Hoetzenecker W, Berneburg M, Rocken M. Classic Mediterranean Kaposi’s sarcoma regression with sirolimus treatment. Arch Dermatol. 2008;144(5):692–3. doi: 10.1001/archderm.144.5.692. [DOI] [PubMed] [Google Scholar]
- 116.Chang H, Wachtman LM, Pearson CB, Lee JS, Lee HR, et al. Non-human primate model of Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog. 2009;5(10):e1000606. doi: 10.1371/journal.ppat.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia Q, Freeman ML, Yager EJ, McHardy I, Tong L, et al. Induction of protective immunity against murine gammaherpesvirus 68 infection in the absence of viral latency. J Virol. 2010;84(5):2453–65. doi: 10.1128/JVI.01543-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pantanowitz L, Dezube BJ. Advances in the pathobiology and treatment of Kaposi sarcoma. Curr Opin Oncol. 2004;16(5):443–9. doi: 10.1097/00001622-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 119.Dourmishev LA, Dourmishev AL, Palmeri D, Schwartz RA, Lukac DM. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol Mol Biol Rev. 2003;67(2):175–212. doi: 10.1128/MMBR.67.2.175-212.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chandriani S, Ganem D. Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi’s sarcoma-associated herpesvirus. J Virol. 2010;84(11):5565–73. doi: 10.1128/JVI.02723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ganem D. KSHV infection and the pathogenesis of Kaposi’s sarcoma. Annu Rev Pathol. 2006;1:273–96. doi: 10.1146/annurev.pathol.1.110304.100133. [DOI] [PubMed] [Google Scholar]
- 122.Di Bartolo DL, Cannon M, Liu YF, Renne R, Chadburn A, et al. KSHV LANA inhibits TGF-{beta} signaling through epigenetic silencing of the TGF-{beta} type II receptor. Blood. 2008 doi: 10.1182/blood-2007-09-110544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laman H, Coverley D, Krude T, Laskey R, Jones N. Viral cyclin-cyclin-dependent kinase 6 complexes initiate nuclear DNA replication. Mol Cell Biol. 2001;21(2):624–35. doi: 10.1128/MCB.21.2.624-635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verschuren EW, Hodgson JG, Gray JW, Kogan S, Jones N, et al. The role of p53 in suppression of KSHV cyclin-induced lymphomagenesis. Cancer Res. 2004;64(2):581–9. doi: 10.1158/0008-5472.can-03-1863. [DOI] [PubMed] [Google Scholar]
- 125.Sun Q, Zachariah S, Chaudhary PM. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-kappaB activation. J Biol Chem. 2003;278(52):52437–45. doi: 10.1074/jbc.M304199200. [DOI] [PubMed] [Google Scholar]
- 126.Chugh P, Matta H, Schamus S, Zachariah S, Kumar A, et al. Constitutive NF-kappaB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc Natl Acad Sci U S A. 2005;102(36):12885–90. doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199(7):993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128.Efklidou S, Bailey R, Field N, Noursadeghi M, Collins MK. vFLIP from KSHV inhibits anoikis of primary endothelial cells. J Cell Sci. 2008 doi: 10.1242/jcs.022343. [DOI] [PubMed] [Google Scholar]
- 129.Guasparri I, Wu H, Cesarman E. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signalling. EMBO Rep. 2006;7(1):114–9. doi: 10.1038/sj.embor.7400580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79(14):9301–5. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cai X, Cullen BR. Transcriptional origin of Kaposi’s sarcoma-associated herpesvirus microRNAs. J Virol. 2006;80(5):2234–42. doi: 10.1128/JVI.80.5.2234-2242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, et al. Identification of microRNAs of the herpesvirus family. Nat Methods. 2005;2(4):269–76. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 133.Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. Rna. 2006;12(5):733–50. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450(7172):1096–9. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, et al. Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol. 2007;81(23):12836–45. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Calame K. MicroRNA-155 function in B Cells. Immunity. 2007;27(6):825–7. doi: 10.1016/j.immuni.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 137.Ziegelbauer JM, Sullivan CS, Ganem D. Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nat Genet. 2009;41(1):130–4. doi: 10.1038/ng.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5(4):376–85. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 139.Samols MA, Skalsky RL, Maldonado AM, Riva A, Lopez MC, et al. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007;3(5):e65. doi: 10.1371/journal.ppat.0030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bellare P, Ganem D. Regulation of KSHV lytic switch protein expression by a virus-encoded microRNA: an evolutionary adaptation that fine-tunes lytic reactivation. Cell Host Microbe. 2009;6(6):570–5. doi: 10.1016/j.chom.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic regulation of Kaposi’s sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. J Virol. 2010;84(6):2697–706. doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lei X, Bai Z, Ye F, Xie J, Kim CG, et al. Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol. 2010;12(2):193–9. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gottwein E, Cullen BR. A human herpesvirus microRNA inhibits p21 expression and attenuates p21-mediated cell cycle arrest. J Virol. 2010;84(10):5229–37. doi: 10.1128/JVI.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A. 1999;96(10):5704–9. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee H, Guo J, Li M, Choi JK, DeMaria M, et al. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi’s sarcoma-associated herpesvirus. Mol Cell Biol. 1998;18(9):5219–28. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee H, Veazey R, Williams K, Li M, Guo J, et al. Deregulation of cell growth by the K1 gene of Kaposi’s sarcoma-associated herpesvirus. Nat Med. 1998;4(4):435–40. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- 147.Prakash O, Tang ZY, Peng X, Coleman R, Gill J, et al. Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J Natl Cancer Inst. 2002;94(12):926–35. doi: 10.1093/jnci/94.12.926. [DOI] [PubMed] [Google Scholar]
- 148.Prakash O, Swamy OR, Peng X, Tang ZY, Li L, et al. Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood. 2005;105(10):3987–94. doi: 10.1182/blood-2004-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B. Immortalization of primary endothelial cells by the K1 protein of Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2006;66(7):3658–66. doi: 10.1158/0008-5472.CAN-05-3680. [DOI] [PubMed] [Google Scholar]
- 150.Offermann MK. Kaposi sarcoma herpesvirus-encoded interferon regulator factors. Curr Top Microbiol Immunol. 2007;312:185–209. doi: 10.1007/978-3-540-34344-8_7. [DOI] [PubMed] [Google Scholar]
- 151.Jayachandra S, Low KG, Thlick AE, Yu J, Ling PD, et al. Three unrelated viral transforming proteins (vIRF, EBNA2, and E1A) induce the MYC oncogene through the interferon-responsive PRF element by using different transcription coadaptors. Proc Natl Acad Sci U S A. 1999;96(20):11566–71. doi: 10.1073/pnas.96.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152.Areste C, Blackbourn DJ. Modulation of the immune system by Kaposi’s sarcoma-associated herpesvirus. Trends Microbiol. 2009;17(3):119–29. doi: 10.1016/j.tim.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 153.Kanno T, Sato Y, Sata T, Katano H. Expression of Kaposi’s sarcoma-associated herpesvirus-encoded K10/10.1 protein in tissues and its interaction with poly(A)-binding protein. Virology. 2006;352(1):100–9. doi: 10.1016/j.virol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 154.Yu Y, Wang SE, Hayward GS. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity. 2005;22(1):59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 155.Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298(5597):1432–5. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- 156.Cloutier N, Flamand L. Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen inhibits interferon (IFN) beta expression by competing with IFN regulatory factor-3 for binding to IFNB promoter. J Biol Chem. 2010;285(10):7208–21. doi: 10.1074/jbc.M109.018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, et al. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. 2010;12(5):513–9. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- 158.Mullick J, Bernet J, Singh AK, Lambris JD, Sahu A. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) open reading frame 4 protein (kaposica) is a functional homolog of complement control proteins. J Virol. 2003;77(6):3878–81. doi: 10.1128/JVI.77.6.3878-3881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spiller OB, Robinson M, O’Donnell E, Milligan S, Morgan BP, et al. Complement regulation by Kaposi’s sarcoma-associated herpesvirus ORF4 protein. J Virol. 2003;77(1):592–9. doi: 10.1128/JVI.77.1.592-599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J Clin Invest. 2001;107(12):1599–606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115(5):1369–78. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mansouri M, Douglas J, Rose PP, Gouveia K, Thomas G, et al. Kaposi sarcoma herpesvirus K5 removes CD31/PECAM from endothelial cells. Blood. 2006;108(6):1932–40. doi: 10.1182/blood-2005-11-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bartee E, McCormack A, Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2(10):e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, et al. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2009;83(19):9672–81. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lagos D, Trotter MW, Vart RJ, Wang HW, Matthews NC, et al. Kaposi sarcoma herpesvirus-encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood. 2007;109(4):1550–8. doi: 10.1182/blood-2006-05-024034. [DOI] [PubMed] [Google Scholar]