Abstract
The chemo-preventative effects of dithiolethione compounds are attributed to their activation of anti-oxidant response elements (ARE) by reacting with the Nrf2/Keap1 protein complex. In this study, we demonstrate anti-proliferative effects of the dithiolethione compound ACS-1 in human cancer cell lines (A549 and MDA-MB-231) by increasing the activity of the tumor suppressor PP2A. ACS-1 inhibited EGF-induced cellular proliferation in a concentration and time-dependent manner. Akt activation, as determined by serine-473 phosphorylation, was inhibited by ACS-1 in cells stimulated with either EGF or fibronectin. Furthermore, ACS-1 inhibited mTOR signaling and decreased c-myc protein levels. ACS-1 did not proximally alter EGFR or integrin signaling, but caused a concentration-dependent increase in PP2A activity. The effect of ACS-1 on Akt activation was not observed in the presence of the PP2A inhibitor okadaic acid. ACS-1 effects on PP2A activity were independent of ARE activation and cAMP formation. In addition to ACS-1, other dithiolethione compounds showed similar effects in reducing Akt activation, suggesting that this class of compounds may have other effects beyond chemoprevention.
Keywords: Dithiolethione, PP2A, Akt, cancer, breast, lung
Introduction
Dithiolethione containing compounds have demonstrated cytoprotective and chemopreventive properties in animal models as well as clinical trials (Zhang and Munday, 2008). While the clinical application of the dithiolethione oltipraz in smokers have revealed adverse side-effects that discouraged the therapeutic use of this compound, (Zhang and Munday, 2008) anethole dithiolethione (ADT) has been administered safely without complications and is clinically used for xerostomia (Hamada et al., 1999). Bronchial dysplasia is one of the best surrogate end point markers available for the assessment of novel chemopreventive drugs. The application of ADT in a phase IIb clinical study demonstrated chemopreventive effects of ADT in smokers with bronchial dysplasia as characterized by statistically significant reductions in the appearance of new lesions, the progression rate of pre-existing lesions, as well as overall disease progression of the patient (Lam et al., 2002). The chemopreventive and cytoprotective effects of dithiolethiones arise from their ability to activate the anti-oxidant response element (ARE) via direct interaction with the KEAP1/Nrf2 complex (Kwak et al., 2003; Lee and Surh, 2005). Genes that are positively regulated by ARE include Phase II enzymes such as HO-1, GST and NQO1, and the overall net effect is to defend the cell from oxidative or xenobiotic stress (Kwak et al., 2001).
The PI3K/Akt signaling pathway is a major contributor to cellular proliferation, and mutations in this pathway are common in human cancers (Yuan and Cantley, 2008). PI3K is activated by a number of extracellular stimuli and results in the formation of PIP3 that is required for Akt activation. Akt kinase activity has multiple effects in cancer progression including an increase in tumor angiogenesis, cancer cell invasion and metastatic potential (Dillon et al., 2007; Qiao et al., 2008). Akt activity is negatively regulated by protein phoshatase 2A (PP2A) (Andjelkovic et al., 1996; Resjö et al., 2002). PP2A is comprised of three families of subunits whose combined activity and cellular localization depends upon the exact subunit composition and selected post-translational modifications (Eichhorn et al., 2009). PP2A is considered to have tumor suppressor activities that also function on other proteins involved in cancer progression such as RalA and c-myc (Arnold and Sears, 2008; Sablina et al., 2007).
In this report, we investigate the anti-proliferative effects of dithiolethiones in aggressive human breast and lung cancer cell lines. ACS-1, a prototypical dithiolethione, inhibited Akt and mTOR activation from both epidermal growth factor (EGF) or fibronectin stimulation and inhibited cell proliferation in a dose-dependent manner. We show here that ACS-1 increases the activity of the tumor suppressor PP2A and that the inhibition of proliferation is sensitive to PP2A inhibition. The anti-proliferative effects were independent of ARE activation and suggests a novel mechanism for this class of compounds beyond interacting with KEAP1/Nrf2 complex. Therefore these compounds may have utility in the treatment of human cancers by activating a known tumor suppressor as well as cancer prevention.
Results
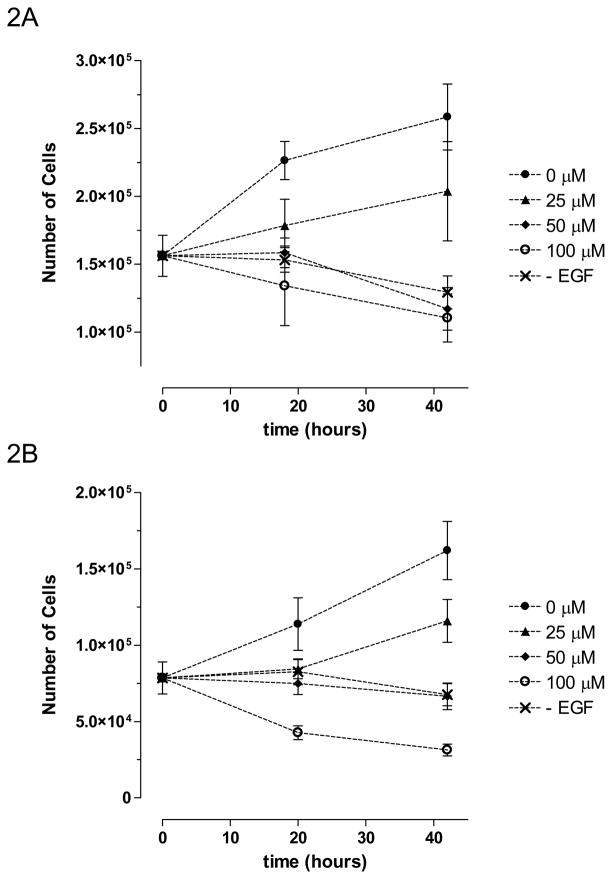
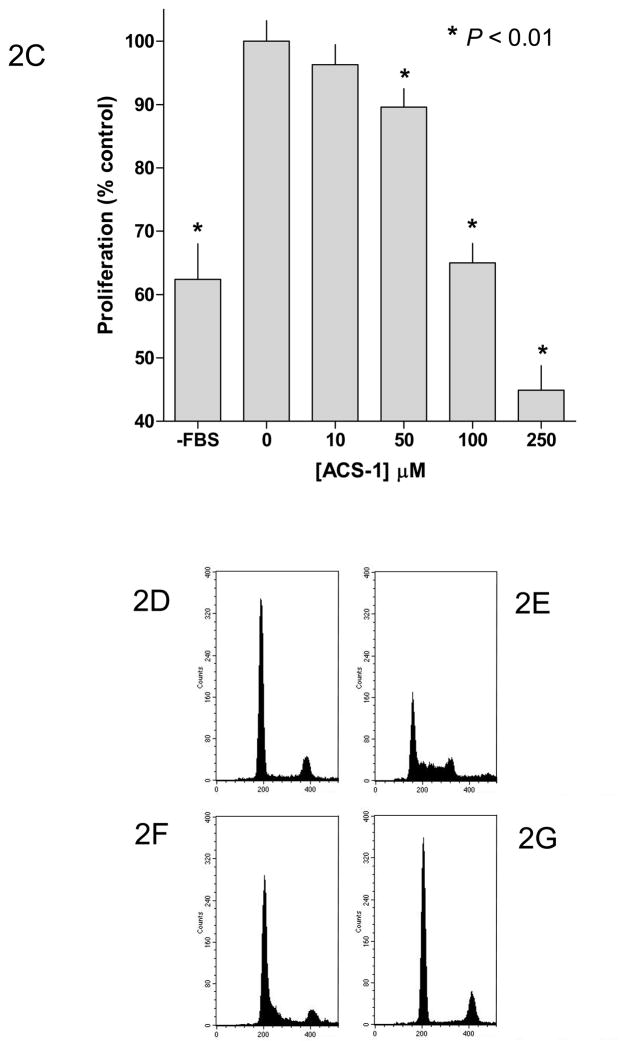
Our previous work has suggested that the ACS-1 component, a dithiolethione with a similar structure to ADT (Figure 1), of S-NSAIDs causes a decrease in cancer cell proliferation (Moody, submitted). EGF has been shown to mediate cancer cell proliferation by activating the PI3K/Akt/mTOR pathway, which may provide therapeutic targets (Carnero et al., 2008; Dillon et al., 2007). Toward this end, we tested the potential therapeutic effect of ACS-1 on EGF-induced cell proliferation. Adherent A549 or MDA-MB-231 (MB231) cells were grown in serum-free media supplemented with EGF +/− ACS-1 and cells were counted at the indicated times. ACS-1 inhibited both A549 (Figure 2A) and MB231 (Figure 2B) proliferation in a concentration-dependent manner. In addition, ACS-1 inhibited cell proliferation in A549 (Figure 2C) and MB231 (data not shown) cells grown in media containing 10% fetal bovine serum compared to untreated controls. Cell cycle analysis of MB231 cells show that ACS-1 causes a G0/G1 block and inhibits progression into S phase as compared to serum controls (Figure 2D and Table 1), while showing no indication of dead or dying cells.
Figure 1.
Chemical structure of ADT and ACS-1.
Figure 2.
ACS-1 inhibits cellular proliferation in EGF-stimulated A549 and MB231 cells. (A) A549 and (B) MB231 cells were incubated with ACS-1 in serum free DMEM +0.1% BSA + EGF (100 ng/mL) for the indicated times are counted. Values represent the mean ± SD (n=6). (C) MTS assay showing ACS-1 mediated (24 hour exposure) inhibition of A549 cellular proliferation in complete growth media. (D–G) Cell cycle analysis of synchronized MB231 cells incubated for 24 hours in serum free media (D), serum replete media with ACS-1 (0, 25 and 100 μM) (E, F and G, respectively).
Table 1.
Cell cycle analysis of MB231 cells in response to 24 hour exposure to ACS-1 ± serum
| ACS-1 (μM) | Serum | % G0/G1 | % S | % G2/M |
|---|---|---|---|---|
| 0 | − | 75.4 | 9.1 | 15.5 |
| 0 | + | 36.8 | 54.6 | 8.6 |
| 25 | + | 76.8 | 18.9 | 4.4 |
| 100 | + | 81.7 | 6.3 | 12.0 |
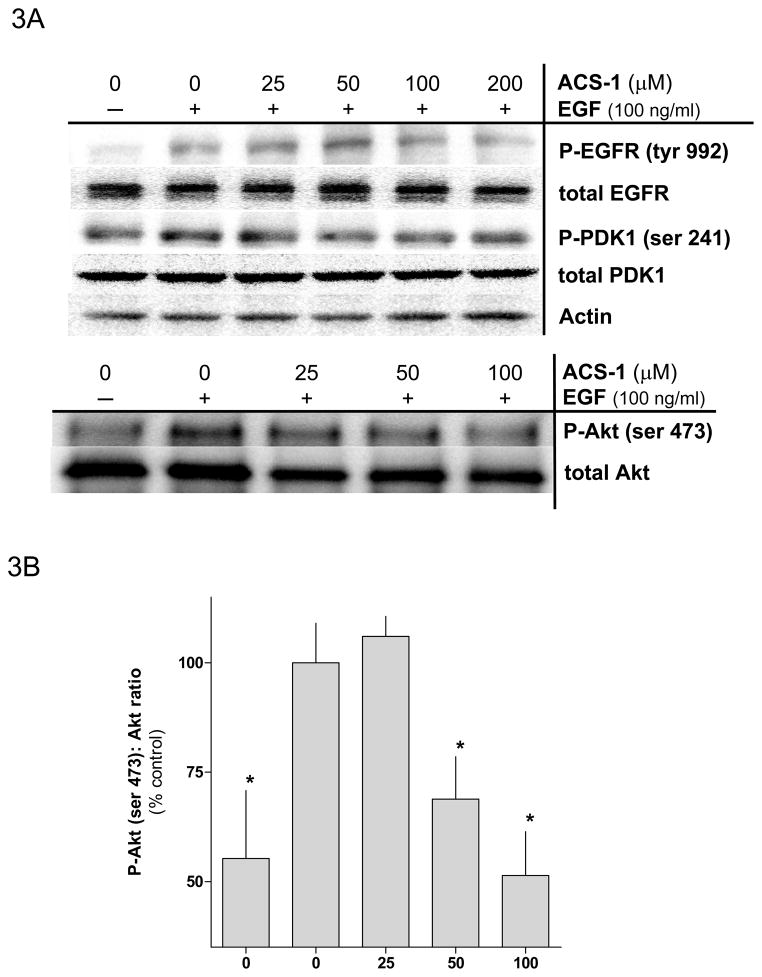
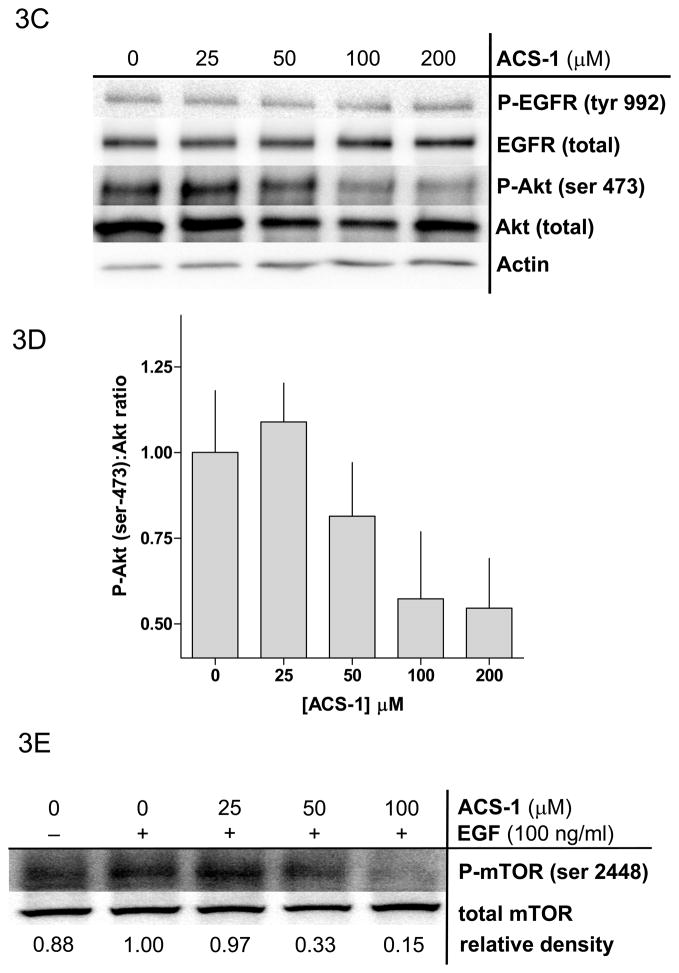
Because ACS-1 inhibited cellular proliferation in response to EGF stimulation, the effect of ACS-1 on epidermal growth factor receptor (EGFR) signaling was examined. Serum starved MB231 cells were treated with ACS-1 for 2 hr at 37°C followed by EGF stimulation for 2 minutes. ACS-1 did not effect EGFR activation as represented by tyrosine-992 phosphorylation when normalized to total EGFR (Figure 3A). EGFR phosphorylation mediates activation of the PI3K pathway and PIP3 formation. While enhanced PIP3 levels mediate increases in pPDK1-(serine 241) as well as pAkt-(thr 308), complete activation of Akt is dependent upon phosphorylation at serine 473, which is mediated by mTOR/RICTOR (Hresko and Mueckler, 2005). ACS-1 significantly suppressed EGF mediated Akt-(ser 473) phosphorylation (Figures 3A and 3B) at concentrations of 50 μM and higher but had little to no effect on pPDK1-(serine 241) (Figure 3A) or pAkt-(thr 308) (data not shown). Similar results were observed in the A549 cell line (Figure 3C). At concentrations consistent with the inhibition of Akt-(ser 273) phosphorylation, ACS-1 also inhibited EGF-induced mTOR activation in MB231 cells (Figure 3E), a downstream substrate of active Akt.
Figure 3.
ACS-1 inhibits EGF activation of Akt and mTOR in MB231 and A549 cells. Serum starved cells were treated with ACS-1 for 2 hours and stimulated with EGF. (A) Western blot analysis of EGF signaling pathway activation in response to EGF and/or ACS-1. (B) Graphical representation of Akt activation in MB231 cells; ratios of P-Akt-(ser 473):total Akt are presented as percent of EGF control (mean ± SD). (C) Western blot analysis of ACS-1 effects on EGF signaling in A549 cells. (D) Graphical representation of Akt activation in A549 cells; ratios of P-Akt-(ser 473):total Akt are presented as percent of EGF control (mean ± SD). (E) Representative western blot analysis of P-mTOR-(ser 2448) in MB231 cells treated with ACS-1 and stimulated with EGF.
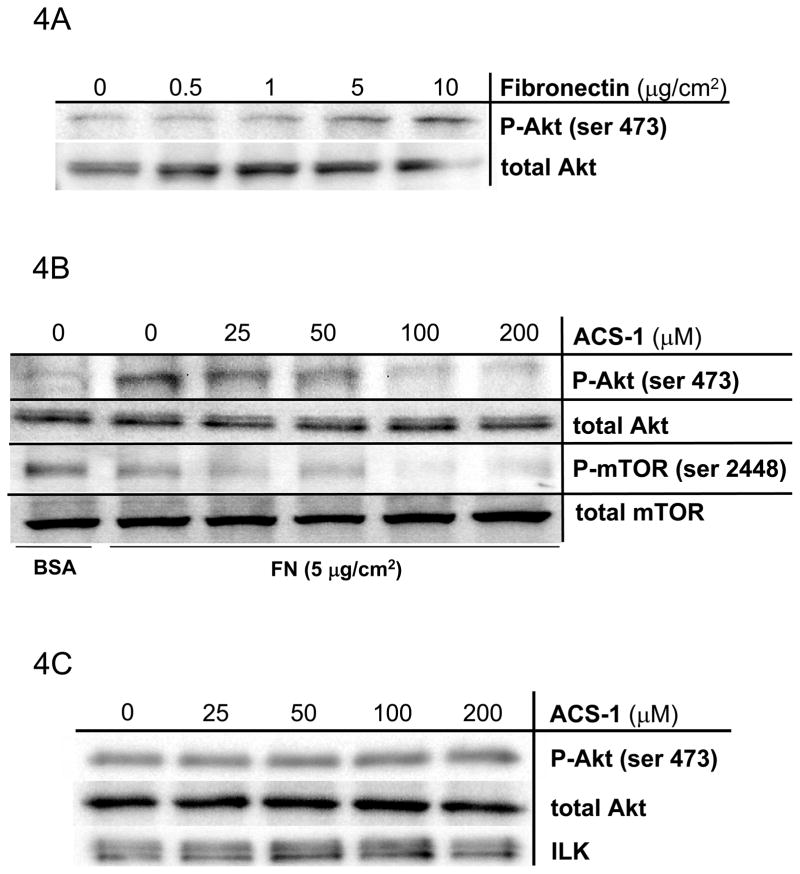
Mechanisms other than growth factor receptor activation mediate Akt signaling in human cancers. Extracellular matrix proteins (e.g. fibronectin, laminin) activate extracellular integrin complexes to initiate intracellular signaling via integrin linked kinase (ILK) activity, which directly activates Akt (Danen and Yamada, 2001; Persad and Dedhar, 2003). To address the effects of ACS-1 on Akt stimulation by integrin activation, A549 cells were plated on increasing concentrations of fibronectin (FN). Figure 4A demonstrates a FN-dependent increase in pAkt-(ser 473), which is concentration dependently suppressed by ACS-1 (Figure 4B). Moreover, ACS-1 also suppressed FN-mediated mTOR (ser 2448) activation (Figure 4B). ACS-1 had no effect on integrin signaling at the level of ILK activity (Figure 4C), which phosphorylates Akt ser473. ILK activity was unchanged with respect to ACS-1 concentration as determined by phosphorylation of recombinant Akt suggesting that ACS-1 targets are downstream of ILK.
Figure 4.
ACS-1 inhibits Akt activation in FN-stimulated A549 cells. (A) FN, in a concentration-dependent manner, activates Akt in A549 cells. Serum starved A549 cells were plated into Petri dishes coated with differing amounts of FN and incubated for 2 hours. (B) ACS-1 inhibits FN-induced Akt and mTOR activation. ACS-1 treated A549 cells seeded onto FN-coated dishes and incubated for 2 hours. (C) ILK was immunoprecipitated from cells plated on FN in the presence of ACS-1 and ILK kinase activity was determined by recombinant Akt phosphorylation (ser 473).
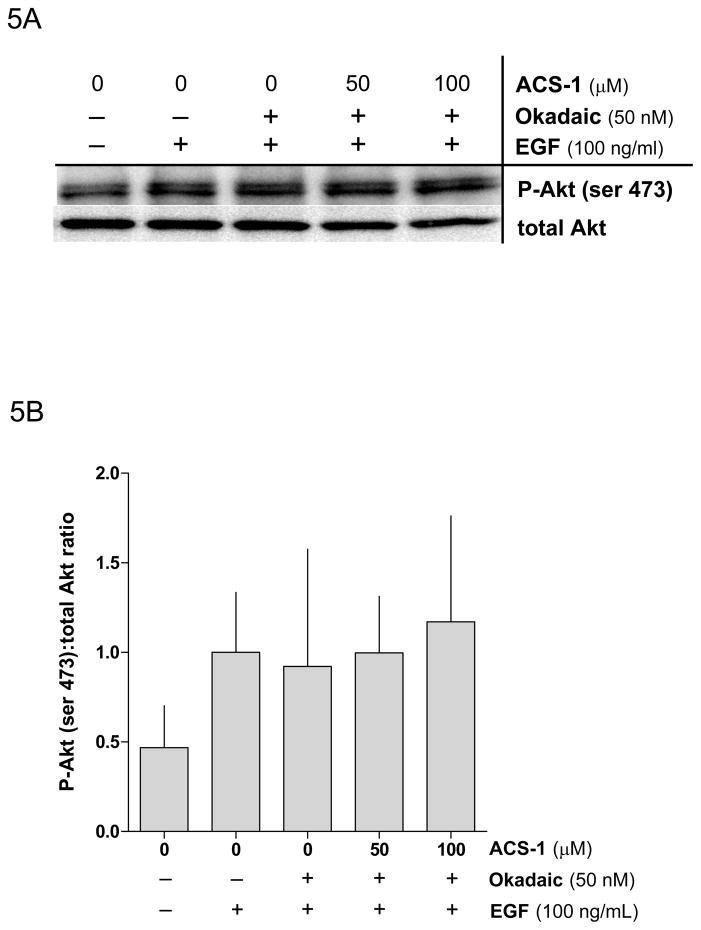
Since ACS-1 suppressed Akt phosphorylation without proximally affecting EGFR or integrin signaling, we surmised that ACS-1 might impact a downstream target specific to Akt regulation. A major endogenous negative regulator of Akt signaling is PP2A (Andjelkovic et al., 1996; Resjö et al., 2002; Rocher et al., 2007; Sato et al., 2000; Ugi et al., 2004). To examine a potential role of ACS-1 in targeting PP2A, serum starved A549 or MB231 cells were treated with ACS-1 and okadaic acid (50 nM), an inhibitor of PP2A, for 2 hr and then stimulated with EGF. Figures 5A and 5B demonstrate inhibitory effects of okadaic acid on ACS-1 mediated suppression of pAkt-(ser 473) in EGF stimulated cells, indicating that PP2A may be a critical target of the anti-proliferative properties of ACS-1.
Figure 5.
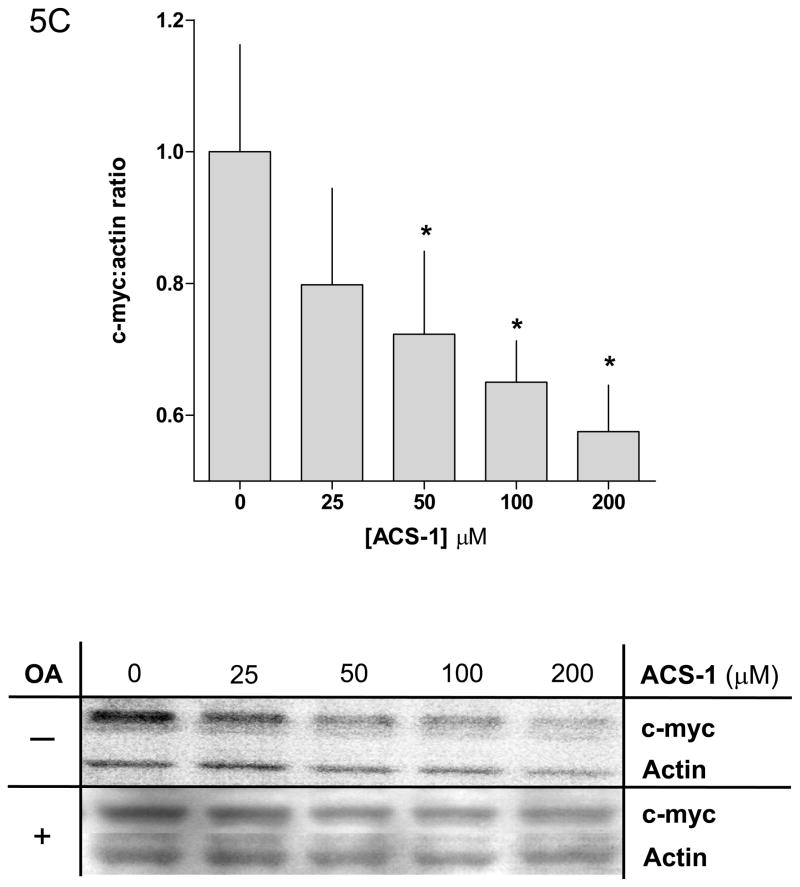
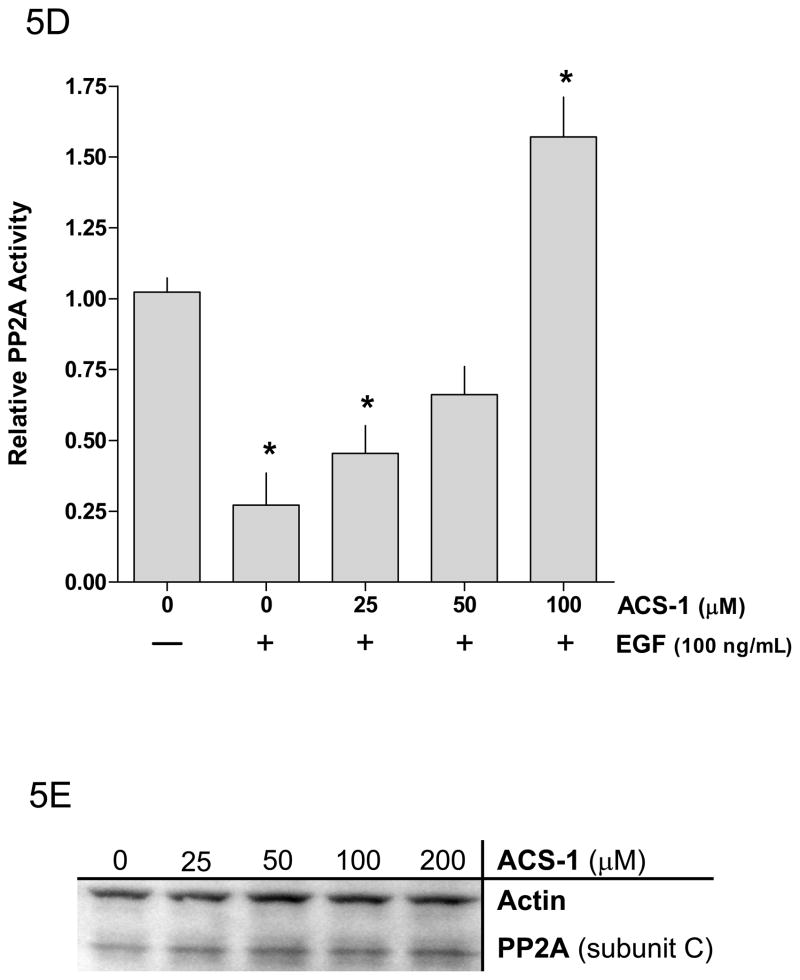
Okadaic acid abates the effect of ACS-1 on Akt activation. (A) Western blot and (B) densitometry analysis of Akt-(ser 473) phosphorylation in A549 cells incubated with ACS-1 and/or Okadaic acid for 2 hours and stimulated with EGF for 30 minutes. (C) ACS-1 increases PP2A activity as determined by total c-myc protein expression in MB231 cells treated with ACS-1 for 2 hours. C-myc protein levels are not altered in the presence of okadaic acid (OA). (D) Serum starved MB231 cells were treated with ACS-1 for 2 hours stimulated with EGF and lysed. PP2A (subunit C) was immunoprecipitated from whole cell extracts, washed with assay buffer and PP2A phosphatase activity measured. (E) ACS-1 does not affect the level of PP2A expression. Serum starved MB231 cells were treated with ACS-1 for 2 hours and stimulated with EGF for 30 minutes. PP2A and actin protein levels were determined by Western blot analysis.
The proto-oncogene c-myc is an endogenous substrate for PP2A and dephosphorylation of c-myc by PP2A promotes the ubiquitination and subsequent proteasomal degradation of c-myc (Arnold and Sears, 2008; Arnold and Sears, 2006). A549 and MB231 cells were incubated with ACS-1 for 2 hr and then stimulated with EGF for 30 minutes. ACS-1 caused a concentration-dependent decrease in total c-myc protein levels in MB231 cells (Figure 5C) that is not observed in the presence of okadaic acid. To directly measure cellular PP2A activity, the PP2A catalytic subunit was immunoprecipitated from whole cell lysates and assayed for phosphatase activity using a PP2A-specific synthetic phospho-peptide substrate. PP2A activity was significantly decreased in cells treated with EGF compared to untreated cells. However, the ACS-1 pretreatment of EGF stimulated cells caused a dose-dependent increase in PP2A activity in both MB231 (Figure 5D) and A549 cells (data not shown). In both cell lines, pretreatment with 50 μM ACS-1 returned PP2A activity to near basal levels, while 100 μM ACS-1 caused a 1.5-fold increase in activity beyond basal levels. Moreover, the time of ACS-1 exposure is sufficiently short to preclude increased PP2A translation (Figure 5E). Taken together, these results suggest a role of ACS-1 in post-translationally targeting PP2A at the level of activity.
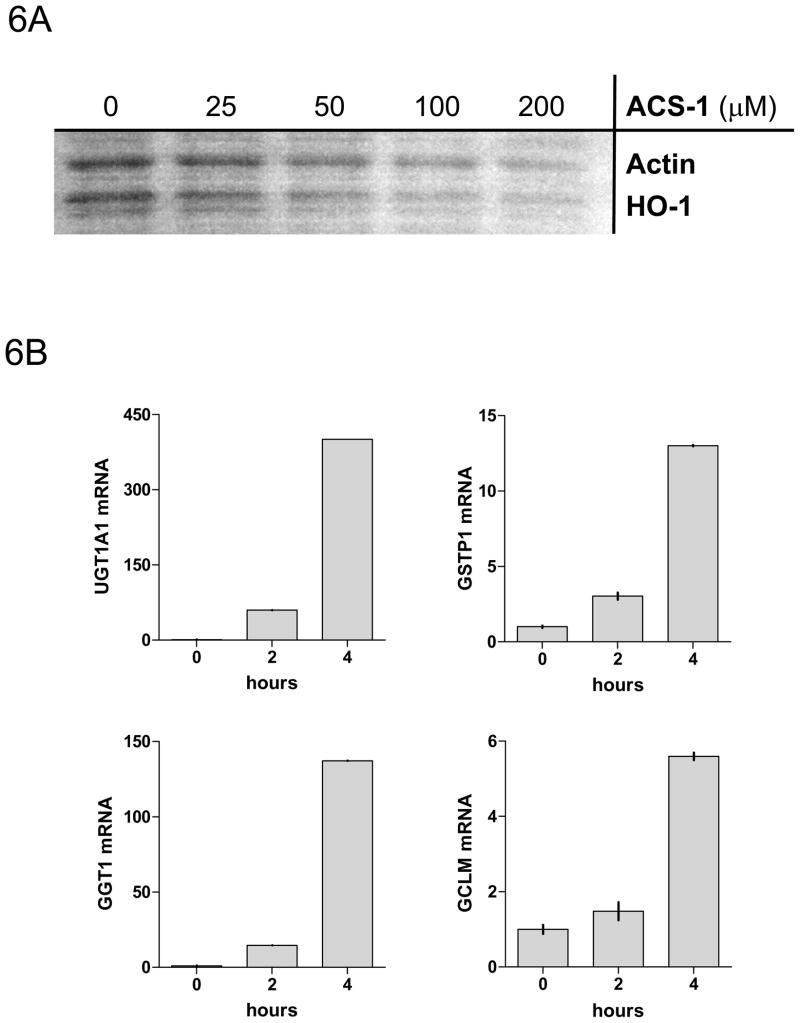
Heme oxygenase-1 (HO-1), a gene product that is induced by dithiolethiones via Nrf2/ARE, is not induced within the exposure time of ACS-1 that elicited the increase in PP2A activity (Figure 6A). Additionally, the inhibition of pAkt-(ser473) by ACS-1 is not effected by the presence of the protein synthesis inhibitor G418 (data not shown). Furthermore, phase II genes are not fully activated by ACS-1 within the two hours of PP2A activation, but are subsequently activated at later time points as determined by RT-PCR of MB231 cells treated with 50 μM ACS-1 (Figure 6B). Therefore it appears that ACS-1 activates the tumor suppressor PP2A independent of de novo protein synthesis and PP2A functions to inhibit Akt signaling independently of Nrf2/ARE activation.
Figure 6.
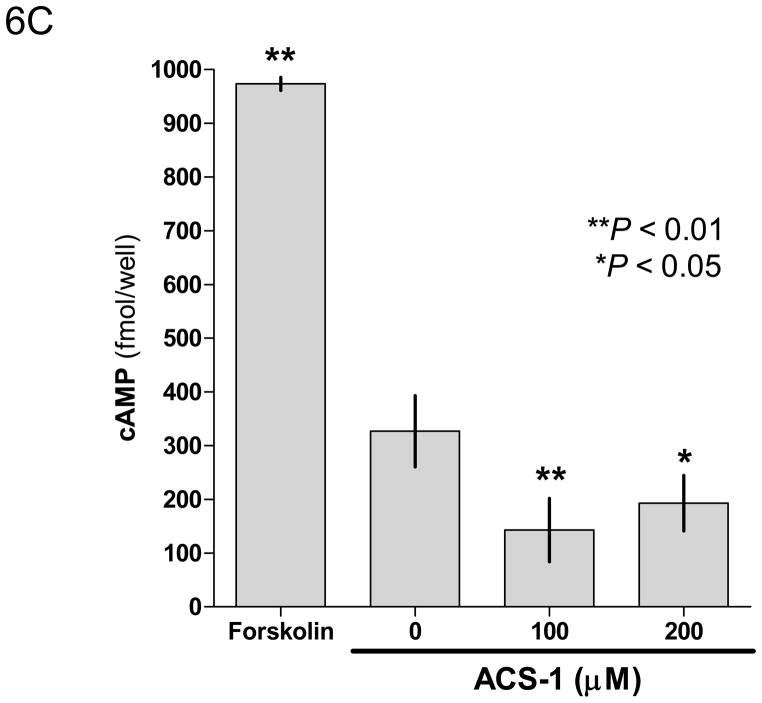
ACS-1 effects PP2A activity independent of protein production or phase II activation. Serum starved MB231 cells were treated with ACS-1 for 2 hours. (A) Western blot analysis of HO-1 and actin indicate that ARE controlled genes are not upregulated within the time of PP2A activation by ACS-1. (B) MB231 cells were exposed to 50 μM ACS-1 for indicated times and the induction of phase II genes (UGTA1, GSTP1, GCT1, and GCLM) were measured by RT-PCR. (C) Effect of ACS-1 on cellular cAMP levels. MB231 cells were exposed to forskolin or ACS-1 for 2 hours and cAMP was measured via ELISA.
PP2A activity is increased by forskolin via increasing intracellular cAMP levels (Feschenko et al., 2002). To determine if ACS-1 induced PP2A activity by a similar mechanism, serum starved MB231 cells were treated with a phosphodiesterase inhibitor, IBMX, and either forskolin or ACS-1 for 2 hr and total intracellular cAMP levels were determined by ELISA. Forskolin caused a significant increase in cAMP compared to untreated control, while ACS-1 caused a significant decrease in cAMP (Figure 6C). These results suggest that ACS-1 increases PP2A activity in a manner that is independent of cAMP.
Discussion
Dithiolethiones are known to exert cellular chemo-preventive effects by activating the KEAP1/Nrf2/ARE pathway, however the major findings of this report are that dithiolethiones interact with chemical motif targets other than the Keap1/Nrf2 complex and that interaction with one of these target chemical motifs results in the activation of the tumor suppressor PP2A. Dithiolethione activation of PP2A has anti-proliferative effects in aggressive breast and lung cancer cell lines, as PP2A acts as a negative regulator of Akt and mTOR signaling. These results indicate that in addition to the chemopreventive properties, which have previously been shown, this class of compounds demonstrates anti-mitogenic effects that may also impact cancer progression and metastasis.
ACS-1 caused a dose and time-dependent decrease in cellular proliferation in both A549 and MB231 cell lines stimulated with either fetal bovine serum or recombinant EGF. This observation is a novel function for the dithiolethione class of compounds, as previous reports have identified properties associated with chemoprevention and radioprotection (Kim et al., 1998; Kwak et al., 2001; Zhang and Munday, 2008). Stimulation of A549 and MB231 cells with EGF or fibronectin resulted in the activation of the PI3K/Akt/mTOR pathway, which is a dominant signaling pathway that controls cell proliferation in many cancers (Carnero et al., 2008; Dillon et al., 2007). Multiple mutations in the PI3K/Akt/mTOR pathway as well as the over expression of endogenous activators of this pathway are common in human tumors (Yuan and Cantley, 2008). ACS-1 caused a dose-dependent inhibition of Akt (ser 473) phosphorylation in A549 and MB231 cell lines stimulated with either EGF or FN with an approximate IC50 of 50 μM. Furthermore, ACS-1 inhibited the activation of mTOR, a downstream target of active Akt kinase with a similar IC50 concentration.
Since ACS-1 inhibited Akt signaling in both EGF and integrin stimulated cells, but did not cause a change in PDK1 phosphorylation, EGFR activation or ILK activity, we concluded that ACS-1 operates on a common downstream target. A major negative regulator of Akt signaling is the ubiquitous protein phosphatase PP2A, a tumor suppressor, which functions to dephosphorylate and thus reduce Akt kinase activity (Andjelkovic et al., 1996; Resjö et al., 2002). ACS-1 failed to inhibit Akt signaling in cells treated with the PP2A inhibitor okadaic acid, which suggests that PP2A or a related phosphatase activity is required for the inhibition of Akt signaling by ACS-1. Furthermore, PP2A immunoprecipitated from ACS-1 treated cells verified that ACS-1 did indeed increase total PP2A activity when compared to untreated control. Moreover, EGF stimulation of cells caused a significant decrease in PP2A activity compared to unstimulated serum starved cells, and is consistent with previous reports (Chen et al., 1992; Ugi et al., 2002). However, EGF stimulated cells treated with ACS-1 showed a dose-dependent increase in PP2A activity. PP2A activity returned to levels comparable to unstimulated serum starved cells with 50 μM ACS-1 and exceeded basal levels at 100 μM. These results suggest that increased PP2A activity mediates Akt inhibition by ACS-1 in EGF stimulated cells. In addition to altering Akt signaling, c-myc is a substrate for PP2A phosphatase activity and negatively regulates c-myc protein levels by the induction of ubiquitin dependent proteolysis (Arnold and Sears, 2008; Arnold and Sears, 2006; Trotta et al., 2007). ACS-1 treatment resulted in a dose-dependent decrease in c-myc protein that is not observed with okadaic acid, further verifying that PP2A is activated by ACS-1 and may contribute to the anti-proliferative effects observed by ACS-1.
The PP2A holoenzyme is comprised of three subunits: a catalytic subunit (PP2AC), a scaffolding subunit (PP2AA) and a regulatory subunit (PP2AB). Although PP2A is a ubiquitous protein and has phosphatase activity with a diverse array of substrates, the activity is tightly regulated. There are approximately 26 known regulatory subunits (PP2AB) and the identity of the B subunit regulates multiple enzymatic parameters such as substrate specificity, cellular localization and specific activity (Eichhorn et al., 2009; Janssens and Goris, 2001). In addition to the array of B subunits that can potentially control phosphatase activity, there are also binding proteins such as SET and alpha4 that can either inhibit or increase PP2A activity, respectively (Li et al., 1995; Prickett and Brautigan, 2006; Trotta et al., 2007). Additionally, altering the phosphorylation and methylation status of the PP2A subunits can further modulate PP2A activity (Chen et al., 1992; Longin et al., 2007; Xing et al., 2008).
In addition to the multiple endogenous regulators of PP2A activity, pharmacological agents have been described that modulate PP2A activity. Forskolin induces PP2A activity by increasing intracellular cAMP levels (Feschenko et al., 2002). However ACS-1 did not cause an increase in cAMP suggesting that cAMP is not a viable mechanism for ACS-1 activation of PP2A. FTY720, a synthetic analog of myriocin, has been shown to inhibit Akt activation by increasing PP2A activity in human leukemia cells (Matsuoka et al., 2003). FTY720 also functions as a cannabinoid receptor type 1 (CB1) antagonist (Paugh et al., 2006). Interestingly, another CB1 antagonist, SR141716, has anti-proliferative effects in MB231 mouse xenografts and increases p27KIP1 expression (Sarnataro et al., 2006). These results are consistent with impaired Akt activity since Akt signaling has proliferative effects and negatively regulates p27KIP1 (Liang et al., 2002). However, CB1 receptor antagonists can affect adenylate cyclase activity, and it is possible that FTY720 and other CB1 antagonists activate PP2A via an increase in cellular cAMP levels similar to forskolin. Endostatin, an anti-angiogenic peptide, decreases ERK1/2 activity by increasing PP2A activity (Schmidt et al., 2006). ACS-1 and similar compounds have also been shown to be anti-angiogenic and we show here that these compounds activate PP2A (Isenberg et al., 2007). The observed effects of endostatin on Akt/PP2A activity are similar to the effects observed with ACS-1 in this report, yet the mechanism of PP2A activation by endostatin is not known.
ACS-1 exerts anti-proliferative effects independent of ARE activation as HO-1 protein expression did not change with respect to ACS-1 concentration. The effect of ACS-1 on Akt activation was not altered in the presence of a protein synthesis inhibitor suggesting that the effects of ACS-1 are independent of de novo ARE gene products. Furthermore, phase II genes are not fully upregulated within the two hours of ACS-1 treatment that results in PP2A activation, consistent with an alternate molecular target for dithiolethiones. S-NSAIDs that release ACS-1 have been shown to be anti-angiogenic which is contradictory to ARE activation as HO-1 activity is associated with a pro-angiogenic response (Dulak et al., 2008). Additionally, HO-1 activates Akt in colon cancer cells and the Nrf2 activator carnosol upregulates HO-1 expression via ARE binding but also activates PI3K pathways, including Akt (Martin et al., 2004) (Busserolles et al., 2006). Taken together, these observations suggest that ACS-1 activates PP2A, which mediates Akt inhibition in an Nrf2/ARE independent manner, and identifies another chemical motif in addition to the KEAP1/Nrf2 complex targeted by dithiolethiones.
We also examined whether pAkt-(ser 473) suppression is mediated by other dithiolthione compounds. EGF stimulated MB231 cells exposed to ADT, Oltipraz, and D3T showed similar inhibitory effects on Akt-(ser 473) phosphorylation (data not shown). Another unique aspect of this class of compounds is that they release H2S (Li et al., 2007). However, H2S had no Akt inhibitory affects as demonstrated by time course and dose response experiments performed on MB231 cells exposed to authentic H2S or NaSH (data not shown). The clinical applications of dithiolethione compounds may have unwanted Akt inhibition and associated pathologies such as diabetes; however dithiolethione compounds release H2S upon decomposition, which is known to activate Akt (Cai et al., 2007). This feature of H2S release may alleviate systemic Akt inhibition by dithiolethiones. Furthermore, dithiolethiones prevent insulin resistance by inhibiting TNF-α induced S6 kinase-1 phsophorylation, consistent with mTOR inhibition presented here (Bae et al., 2007). Therefore, the clinical use of dithiolethiones is not expected to develop pathological conditions of decreased Akt activity (i.e. diabetes).
A novel class of NSAIDs (S-NSAIDs) have recently been described, which incorporates the cytoprotective effects of dithiolethiones with the anti-inflammatory effects of COX inhibition by covalently linking a NSAID with ACS-1 (Li et al., 2007). NSAIDs are useful cancer chemoprevention or chemotherapeutic agents due to the role of inflammation in the initiation, progression and metastasis of many human cancers; however chronic NSAID administration is associated with gastrointestinal and cardiovascular toxicity (Guadagni et al., 2007). S-NSAIDs dramatically reduce gut ulceration compared to the parent NSAID (Li et al., 2007). Additionally, S-NSAIDs exhibit anti-angiogenic properties by increasing HSP-27 phosphorylation in endothelial cells (Isenberg et al., 2007) and potently inhibit some cytochrome P450 enzymes (Bass, in press). We have shown that S-NSAIDs have anti-proliferative effects in lung cancer non-small cell lung carcinoma (NSCLC) line both in vitro and in vivo (Moody, in press). Also, we have found that S-NSAIDs increase the radiation induced tumor growth delay in a mouse xenograft model (unpublished results). Interestingly, S-NSAIDs, but not NSAIDs, cause an increase in E-cadherin expression in human cancer cells, suggesting a mesenchymal to epithelial transition in the phenotype of the transformed cell (Moody, in press). Taken together, these observations indicate that, while S-NSAIDs behave as NSAIDs with reduced toxicity, the dithiolethione moiety of S-NSAIDs has unique anti-cancer properties that are not attributable to either NSAID function or to ARE activation. The results of this report confirm that ACS-1 has anti-proliferative effects on human cancer cell lines and establish the tumor suppressor PP2A as a novel effector molecule of dithiolethiones.
The above data demonstrates that dithiolethione compounds increase the activity of the tumor suppressor PP2A, and thus down regulates Akt and c-myc signaling. PP2A has recently emerged as a tumor suppressor and pharmacological activation of PP2A appears to be a viable target in cancer chemotherapy (Perrotti and Neviani, 2008). Few compounds have been demonstrated to increase PP2A activity; however we show here that dithiolethiones, usually associated with chemoprevention, can increase PP2A tumor suppressor activity. Moreover, ACS-1 is closely related to ADT, which is currently in clinical use. The findings reported here show another potential indication for this drug and suggest that other compounds with similar reactivity may have anti-proliferative effects in human cancers.
Materials and Methods
Cell culture and Reagents
A549 human lung carcinoma and MDA-MB-231 human breast adenocarcinoma cells (ATCC, Manassas, VA) were cultured in DMEM (InVitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO) and passaged two to three times per week. ACS-1 was obtained from (Sulfidris, Milan, Italy). Human recombinant EGF, Akt and human fibronectin were purchased from R&D Systems (Minneapolis, MN). G418 was purchased from InVitrogen. Okadaic acid was purchased from Millipore (Billerica, MA). P-Akt (serine 473), pan Akt, P-EGFR (tyrosine 992), EGFR, P-mTOR (serine 2448), anti-rabbit HRP antibodies were purchased from Cell Signaling (Danvers, MA) and PP2A, Actin, and anti-mouse HRP were from Santa Cruz Biotechnology (Santa Cruz, CA).
Western Blot analysis
Serum starved cells were treated for 2 hours with ACS-1 and stimulated with EGF for either 2 or 30 minutes at 37°C. The cells were placed on ice and the media was aspirated and the cells were washed with cold PBS. Cells were harvested in cold 2X RIPA lysis buffer supplemented with Protease Inhibitor Cocktail Set I (EMD Chemicals Gibbstown, NJ) and PMSF (Sigma-Aldrich St. Louis, MO), and stored at −80°C. The whole cell lysates were cleared by centrifugation (14,000 × g for 5 minutes at 4°C) and the protein content was determined by BCA assay (Thermo Fisher Scientific Rockford, IL). Equal amounts of protein were loaded onto Novex 4–20% Tris-Glycine gradient gels (InVitrogen Carlsbad, CA), separated by electrophoresis and were transferred to PVDF membranes using the iBlot transfer system (InVitrogen Carlsbad, CA). After blocking with 0.5% BSA, the membranes were probed with rabbit polyclonal or mouse monoclonal antibodies and HRP-conjugated secondary antibodies using the SNAP i.d. protein detection system (Millipore Billerica, MA). Membranes were imaged using ECF Western blotting kit (GE Healthcare Piscataway, NJ) and recorded on a FluoroChem™ SP imaging system using AlphaEase® FC software (Alpha Innotech San Leandro, CA). Images were further analyzed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Proliferation assays
Cells (1 × 105; A549 and MB231) were seeded in DMEM + 10 % FBS and incubated for 3 hours to allow the cells adhere. The cells are washed (3X) with serum free DMEM and incubated for the appropriate times with DMEM supplemented with 0.1 % BSA, EGF (100 ng/ml) and the indicated concentration of ACS-1. After the indicated time of treatment, the media was removed, cells washed with PBS, trypsinized and counted with a Beckman Coulter Z2 particle counter and size analyzer (Fullerton, CA). Proliferation in the presence of 10% FBS was also assessed by the MTS assay using the CellTiter96® AQueous assay (Promega, Madison, WI).
Cell cycle analysis
MB231 cells were synchronized by overnight serum starvation and then incubated for 24 hours in the presence of serum free RPMI, RPMI + 10% FBS, or RPMI + 10% FBS containing ACS-1. Cells were fixed in ethanol and treated with RNase A and Triton X-100 for 30 minutes at 37°C. Cells were then stained with propidium iodide and analyzed by flow cytometry.
Fibronectin activation
Petri dishes (60 mm) were coated with fibronectin overnight at 4°C, aspirated and blocked with PBS + 1% BSA for 30 minutes at room temperature. The coated plates were then washed with PBS and used immediately. Cultured A549 cells were trypsinized, washed in DMEM + 10% FBS and then washed twice in serum free DMEM. The cells were treated with ACS-1 for 30 minutes prior to plating on fibronectin-coated plates. Cells were incubated on FN-coated plates for 2 hours, placed on ice, washed with cold PBS and lysed as described for Western blot analysis.
ILK kinase assay
ILK kinase assay was performed as previously described with modification (Sawai et al., 2006). A549 cells were incubated with ACS-1 for 30 minutes at 37°C and plated on FN coated (5 μg/cm2) dishes for 2 hours. Cells were lysed with lysis buffer and cleared lysate was immunoprecipitated overnight with anti-ILK antibody at 4°C. Antibody complexes were isolated with Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 3 hours at 4°C and washed three times in lysis buffer. ILK activity was assayed by incubating the immunoprecipitated protein with GST-Akt (2 μg; recombinant human, R&D Systems, Minneapolis, MN) in kinase reaction buffer (50 mM HEPES, pH 7.0, 200 μM ATP, 2 mM MgCl2, 2 mM MnCl2, 200 mM NaVO4, 200 mM NaF) for 30 min at 37°C. Proteins were heat denatured in 4x SDS-loading buffer at 90°C for 5 min. ILK and Phospho-Akt (ser 473) were detected by western blot analysis.
Phosphatase activity assay
A549 or MB231 cells were treated with the same ACS-1/EGF regime as above and whole cell lysates were collected. PP2A was immunoprecipitated by incubating the cleared lysate with anti-PP2A subunit C overnight at 4°C and then conjugated with Protein A/G PLUS-Agarose beads for 3 hours at 4°C. The beads were washed three times in PP2A assay buffer (100 mM Tris-HCl pH 8.0, 2 mM DTT, 1 mM MnCl2 and 0.01% Brij 35) and then incubated for 30 minutes at 30°C in 100 μl of the same buffer containing 2 μM of the PP2A phospho-peptide substrate Arg-ArgAla-pThr-Val-Ala (BIOMOL International, Plymouth Meeting, PA). The reaction was terminated by placing the samples on ice and the amount of phosphate released from the peptide substrate was determined using the Malachite green assay (Echelon Bioscience, Salt Lake City, UT).
Statistical Analysis
Data represented are mean of at least three independent experiments ± S.D. Statistical comparisons were performed by one-way ANOVA with Dunnett post-test analysis and significance was indicated by P < 0.05.
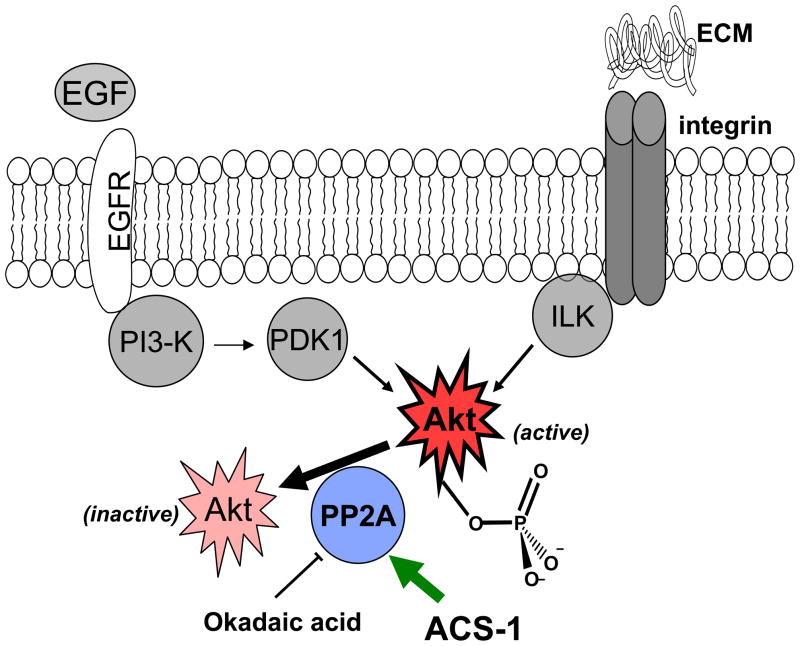
Figure 7.
Scheme of protein interactions. Akt is activated by convergent pathways in human cancers. ACS-1 inhibits Akt activation by increasing the activity of PP2A and not by altering EGFR or integrin signaling.
Footnotes
Conflict of interest
Piero Del Soldato and Anna Sparatore are shareholders of Sulfidris, Milan, Italy. This company has patent rights on some reagents used in this study.
References
- Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold H, Sears R. A tumor suppressor role for PP2A-B56α through negative regulation of c-Myc and other key oncoproteins. Cancer and Metastasis Reviews. 2008;27:147–158. doi: 10.1007/s10555-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold HK, Sears RC. Protein Phosphatase 2A Regulatory Subunit B56{alpha} Associates with c-Myc and Negatively Regulates c-Myc Accumulation. Mol Cell Biol. 2006;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EJ, Yang Yoon Mee, Kim Jin Wan, Kim Sang Geon. Identification of a novel class of dithiolethiones that prevent hepatic insulin resistance via the adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway. Hepatology. 2007;46:730–739. doi: 10.1002/hep.21769. [DOI] [PubMed] [Google Scholar]
- Busserolles J, Megías J, Terencio MC, Alcaraz MJ. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. The International Journal of Biochemistry & Cell Biology. 2006;38:1510–1517. doi: 10.1016/j.biocel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Cai W-J, Wang M-J, Moore PK, Jin H-M, Yao T, Zhu Y-C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–98. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–4. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- Danen EHJ, Yamada KM. Fibronectin, integrins, and growth control. Journal of Cellular Physiology. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme Oxygenase-1 and Carbon Monoxide in Vascular Pathobiology: Focus on Angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn PJA, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ. A Novel cAMP-Stimulated Pathway in Protein Phosphatase 2A Activation. J Pharmacol Exp Ther. 2002;302:111–118. doi: 10.1124/jpet.302.1.111. [DOI] [PubMed] [Google Scholar]
- Guadagni F, Ferroni P, Palmirotta R, Del Monte G, Formica V, Roselli M. Non-steroidal anti-inflammatory drugs in cancer prevention and therapy. Anticancer Res. 2007;27:3147–62. [PubMed] [Google Scholar]
- Hamada T, Nakane T, Kimura T, Arisawa K, Yoneda K, Yamamoto T, et al. Treatment of xerostomia with the bile secretion-stimulating drug anethole trithione: a clinical trial. Am J Med Sci. 1999;318:146–51. doi: 10.1097/00000441-199909000-00009. [DOI] [PubMed] [Google Scholar]
- Hresko RC, Mueckler M. mTOR/RICTOR Is the Ser473 Kinase for Akt/Protein Kinase B in 3T3-L1 Adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Jia Y, Field L, Ridnour LA, Sparatore A, Del Soldato P, et al. Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid. Br J Pharmacol. 2007;151:142–151. doi: 10.1038/sj.bjp.0707194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Nam SY, Kim CW. In Vivo Radioprotective Effects of Oltipraz in [gamma]-Irradiated Mice. Biochemical Pharmacology. 1998;55:1585–1590. doi: 10.1016/s0006-2952(97)00669-2. [DOI] [PubMed] [Google Scholar]
- Kwak M-K, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, et al. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2001;480–481:305–315. doi: 10.1016/s0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- Kwak M-K, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of Gene Expression by Cancer Chemopreventive Dithiolethiones through the Keap1-Nrf2 Pathway. IDENTIFICATION OF NOVEL GENE CLUSTERS FOR CELL SURVIVAL. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Lam S, MacAulay C, le Riche JC, Dyachkova Y, Coldman A, Guillaud M, et al. A Randomized Phase IIb Trial of Anethole Dithiolethione in Smokers with Bronchial Dysplasia. J Natl Cancer Inst. 2002;94:1001–1009. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- Lee J-S, Surh Y-J. Nrf2 as a novel molecular target for chemoprevention. Cancer Letters. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radical Biology and Medicine. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Li M, Guo H, Damuni Z. Purification and Characterization of Two Potent Heat-Stable Protein Inhibitors of Protein Phosphatase 2A from Bovine Kidney. Biochemistry. 1995;34:1988–1996. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- Longin S, Zwaenepoel K, Louis JV, Dilworth S, Goris J, Janssens V. Selection of Protein Phosphatase 2A Regulatory Subunits Is Mediated by the C Terminus of the Catalytic Subunit. J Biol Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, et al. Regulation of Heme Oxygenase-1 Expression through the Phosphatidylinositol 3-Kinase/Akt Pathway and the Nrf2 Transcription Factor in Response to the Antioxidant Phytochemical Carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol. 2003;138:1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Cassidy MP, He H, Milstien S, Sim-Selley LJ, Spiegel S, et al. Sphingosine and Its Analog, the Immunosuppressant 2-Amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, Interact with the CB1 Cannabinoid Receptor. Mol Pharmacol. 2006;70:41–50. doi: 10.1124/mol.105.020552. [DOI] [PubMed] [Google Scholar]
- Perrotti D, Neviani P. Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer and Metastasis Reviews. 2008;27:159–168. doi: 10.1007/s10555-008-9119-x. [DOI] [PubMed] [Google Scholar]
- Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer and Metastasis Reviews. 2003;22:375–384. doi: 10.1023/a:1023777013659. [DOI] [PubMed] [Google Scholar]
- Prickett TD, Brautigan DL. The {alpha}4 Regulatory Subunit Exerts Opposing Allosteric Effects on Protein Phosphatases PP6 and PP2A. J Biol Chem. 2006;281:30503–30511. doi: 10.1074/jbc.M601054200. [DOI] [PubMed] [Google Scholar]
- Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2008;7:2991–6. doi: 10.4161/cc.7.19.6784. [DOI] [PubMed] [Google Scholar]
- Resjö S, Göransson O, Härndahl L, Zolnierowicz S, Manganiello V, Degerman E. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cellular Signalling. 2002;14:231–238. doi: 10.1016/s0898-6568(01)00238-8. [DOI] [PubMed] [Google Scholar]
- Rocher G, Letourneux C, Lenormand P, Porteu F. Inhibition of B56-containing Protein Phosphatase 2As by the Early Response Gene IEX-1 Leads to Control of Akt Activity. J Biol Chem. 2007;282:5468–5477. doi: 10.1074/jbc.M609712200. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, et al. The Tumor Suppressor PP2A A[beta] Regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnataro D, Pisanti S, Santoro A, Gazzerro P, Malfitano AM, Laezza C, et al. The Cannabinoid CB1 Receptor Antagonist Rimonabant (SR141716) Inhibits Human Breast Cancer Cell Proliferation through a Lipid Raft-Mediated Mechanism. Mol Pharmacol. 2006;70:1298–1306. doi: 10.1124/mol.106.025601. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, et al. Integrin-linked kinase activity is associated with interleukin-1[alpha]-induced progressive behavior of pancreatic cancer and poor patient survival. Oncogene. 2006;25:3237–3246. doi: 10.1038/sj.onc.1209356. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Wenzel D, Thorey I, Sasaki T, Hescheler J, Timpl R, et al. Endostatin influences endothelial morphology via the activated ERK1/2-kinase endothelial morphology and signal transduction. Microvascular Research. 2006;71:152–162. doi: 10.1016/j.mvr.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Trotta R, Ciarlariello D, Col JD, Allard J, II, Neviani P, Santhanam R, et al. The PP2A inhibitor SET regulates natural killer cell IFN-{gamma} production. J Exp Med. 2007;204:2397–2405. doi: 10.1084/jem.20070419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, et al. Protein Phosphatase 2A Negatively Regulates Insulin’s Metabolic Signaling Pathway by Inhibiting Akt (Protein Kinase B) Activity in 3T3-L1 Adipocytes. Mol Cell Biol. 2004;24:8778–8789. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugi S, Imamura T, Ricketts W, Olefsky JM. Protein Phosphatase 2A Forms a Molecular Complex with Shc and Regulates Shc Tyrosine Phosphorylation and Downstream Mitogenic Signaling. Mol Cell Biol. 2002;22:2375–2387. doi: 10.1128/MCB.22.7.2375-2387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Li Z, Chen Y, Stock JB, Jeffrey PD, Shi Y. Structural Mechanism of Demethylation and Inactivation of Protein Phosphatase 2A. 2008;133:154–163. doi: 10.1016/j.cell.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Mol Cancer Ther. 2008;7:3470–3479. doi: 10.1158/1535-7163.MCT-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]