Abstract
Preterm parturition is a syndrome caused by several mechanisms of disease, including intrauterine infection/inflammation, uteroplacental ischemia, uterine overdistension, cervical disease, maternal/fetal stress, abnormal allogeneic responses, allergic reactions, and unknown insults. An allergic-like mechanism was proposed as a potential etiology for the preterm parturition syndrome, based on the observation that eosinophils were present in the amniotic fluid in a fraction of women with preterm labor and a history of allergy, coupled with the observation that conditioned media from degranulated mast cells (the effector cells of type 1 hypersensitivity) induced contractility of human myometrial strips. This communication describes a case of a pregnant woman who had an allergic reaction and regular uterine contractions after the ingestion of lobster meat, to which she was known to be allergic. Preterm labor subsided after the treatment of antihistamines and steroids. The patient subsequently delivered at term. At follow-up, the child was diagnosed with atopy and asthma, and required frequent use of inhaled corticosteroids and beta-2 adrenergic agents.
Keywords: Allergy, preterm delivery, preterm birth, eosinophils, uterine allergy, pregnancy, preterm parturition, seafood, hypersensitivity, atopy, gestation, parturition, labor, allergic reaction
DISCUSSION
We report this observation to call attention to clinicians of the association between an allergic reaction to a food allergen and the onset of preterm labor, as well as the response of the clinical manifestations of allergy (skin rash) and uterine contractions to an antihistamine and betamethasone.
Hypersensitivity, Allergy, and Atopy
The general view of the immune system is that it evolved to protect the host against invading microorganisms. Therefore, after the primary invasion, re-exposure to the insult leads to a lesser injury because of protective immunity (largely due to adaptive immunity which has memory). The notion that the immune system could cause damage to the host (e.g. autoimmune disease, allergy, etc.) developed in 1894 after a simple set of observations in which guinea pigs immunized with diphtheria toxin became ill or even died after re-exposure to a second dose of the toxin, even if the doses of the toxin were so low that it would not have an effect in non-immunized animals. Emil von Behring called this phenomenon “hypersensitivity” and proposed that it was caused by the toxin.[1] A similar observation was reported in 1898 by Charles Richet and Jules Hericourt[2] after re-exposure of animals to eel toxin. However, the effect was also attributed to the effect of the toxin in the animals. The seminal breakthrough occurred in experiments conducted by Charles Richet and Paul Portier in 1902.[3,4] When attempting to immunize dogs against marine toxins, a laboratory assistant at the Sorbonne, France noticed that if the toxin was injected into surviving dogs, even the smallest dose of the toxin caused a systemic reaction and clinical shock. This time, Richet concluded that the phenomenon was due to a hypersensitive state caused by a immune system primed to the toxin. Richet named the phenomenon “anaphylaxis,” which means “against protection” (from the Greek “ana: opposite” and “phylaxis: protection”). This discovery was recognized with a Nobel Prize in Physiology and Medicine in 1913 (for a detailed account of the history of anaphylaxis and Charles Richet, the reader is referred to excellent reviews by Kroker[5] and Löwy[6]).
The origins of the concept of “allergy” has an equally fascinating history. In 1903, Maurice Arthus[7] at the Institute Pasteur demonstrated that repeated injections of horse serum into the dermis of rabbits resulted in a local reaction at the injection site characterized by neutrophil infiltration, hemorrhage, and tissue death. He called this phenomenon “the Arthus reaction.” Two years later two pediatricians in Vienna, Austria, Bela Schick and Clemens von Pirquet,[8] revisited earlier reports that some patients receiving anti-diphtheria and anti-tetanum serum as part of passive immunization became ill with local and systemic symptoms, and termed this phenomenon “serum sickness.” The intellectual debate at the time was centered on the controversy of why the immune system would protect the host in some cases, and harm it in others (e.g. hypersensitivity). Von Pirquet coined the term “allergy” to refer to any immunological response, regardless of its clinical outcome (allergy from “allos: other” and “ergon: work”). The purpose of von Pirquet was to emphasize the change in reactivity that occurred in response to a wide variety of biologic stimuli. His view was that immunity and hypersensitivity were closely related manifestations of allergy. Some objected at the time to the term “allergy”.[9] Importantly, von Pirquet believed that an antibody-antigen reaction was the basis for allergy. However, the antibody was not demonstrated until Prausnitz and Küstner described this in Germany in 1921.[10] The key experiment was that an antibody was demonstrated to be present in the serum of allergic individuals and capable of transferring immediate hypersensitivity to the skin of normal recipients. This test was known as the Prausnitz-Küstner reaction. The precise nature of the antibody responsible for the allergic reaction was not known.
The concept of atopy was coined in 1923 by Coca and Cooke[11] to describe the clinical manifestations of hypersensitivity in humans. The word “atopy”, which means “out of place”, was adapted from the Greek. It appears that Coca and Cooke were not aware of the work of Prausnitz and Küstner. Soon after, Coca and Grove[12] introduced the term “atopic reagins” as the specific substances present in the serum of atopic individuals responsible for the clinical manifestations of atopy, such as hay fever and bronchial asthma.
The next development was the discovery of immunoglobulin E (IgE) in 1968.[13] Bennich and Johansson[14] at Uppsala University discovered that the serum of a patient with multiple myeloma had high concentrations of a para-protein, which lacked antigenic determinants for the four then known immunoglobulin heavy chains (γ,μ,α, and δ), which were IgG, IgM, IgA and IgD. Subsequently, Stanworth and Humphrey[15] asked the question of whether this new protein was capable of blocking the sensitization of a normal person’s skin induced by serum in a Prausnitz-Küstner reaction. The experiment was completed within 5 days (Humphrey volunteered for the experiment), and a positive result laid the ground for the definition of the molecular basis of allergy. Ishizaka et al[16] had previously demonstrated that a normal human β2A globulin was capable of blocking the Prausnitz-Küstner reaction.[17] At a meeting in Lausanne, Switzerland, three groups working in the field agreed on naming this new immunoglobulin “IgE”.[18]
What is The Difference Between Atopy and Allergy?
It has been proposed that these widely used terms should be used in the context of IgE-mediated allergy. “Atopy” refers to an exaggerated tendency to mount an IgE response to several common environmental allergens, and implies a predisposition to allergic diseases. “Allergy”, in contrast, refers to the clinical expression of atopic IgE-mediated disease.[19] Atopic individuals may or may not have clinical symptoms. Allergic diseases are expressed as hyper-responsiveness of a particular organ (often called “target organ”), which includes the skin (urticaria), nose (rhinitis), eye (conjunctivitis), tracheobronchial tree (asthma), gastrointestinal tract (food allergies), and the uterus (uterine contractions, preterm labor, and possibly fetal distress). When atopy is expressed as a systemic response, it is called anaphylaxis, and involves the entire body. It is worth noting that not all hyper-responsiveness is mediated by IgE. For example, some patients have atopic asthma, which is airway inflammation mediated by IgE, but not all cases of asthma are the result of atopy. The former is often referred to as “intrinsic asthma”, and the latter as “extrinsic asthma”.[2]
Classification of Hypersensitivity Reactions
In 1963, Phillip Gell and Robin Coombs[20] classified hypersensitivity into four types:
Type I, IgE-mediated hypersensitivity (e.g. allergic rhinitis, asthma, systemic anaphylaxis).
Type II, Direct antibody-mediated cytolytic hypersensitivity (e.g. drug allergies such as penicillin or chronic urticaria).
Type III, Immune complex-mediated hypersensitivity (e.g. serum sickness or Arthus reaction).
Type IV, Delayed type cell-mediated hypersensitivity (e.g. contact dermatitis or chronic asthma).
These hypersensitivities develop in two stages: the sensitization stage, and the effector or elicitation stage. The sensitization stage is basically a primary immune response, while the effector stage is a secondary immune response that has an early and late component. In this context, hypersensitivity is defined as any excessive or abnormal secondary immune response to a sensitizing agent. The precise reasons why one individual may be sensitized to an antigen (and thus will experience a hypersensitivity reaction upon a secondary exposure), and another primed by the antigen (will mount a normal secondary immune response) remain obscure.[2]
Type I Hypersensitivity
This reaction is mediated by IgE directed against common environmental allergens. It is also known as immediate hypersensitivity, because the response is rapid and occurs within 30 minutes of exposure to the allergen. Most individuals generate IgG, IgM, or IgA antibodies against common environmental antigens. These antibodies help in clearing the allergens, and the antigen-antibody reaction produces no adverse events to the host. However, among individuals producing IgE antibodies, Type I hypersensitivity reactions have a broad clinical spectrum, ranging from itching and swelling to breathing difficulties, and even shock or death. It is unknown why some individuals produce IgE after exposure to the same allergen, and others do not. Similarly, it is not known why some allergens induce a localized reaction (e.g. allergic rhinitis) and others co-induce a systemic reaction (anaphylaxis).[2]
Sensitization is initiated when antigens penetrate the skin or a mucosal barrier, and is identified by immature dendritic cells or other antigen-presenting cells located in the area of penetration. The allergen is then presented to the draining lymph nodes, where the now mature dendritic cells present the processed allergen to a naive Th-cell. The question of why allergens induce dendritic cells to mature and activate Th-cells is a puzzling one, if one assumes that allergens are innocuous, and therefore, do not supply a “danger signal”. Some have argued that allergens induce either cellular stress or damage in every subject. However, in non-atopic individuals, the response is mediated by IgG antibodies, and would be asymptomatic.[2]
Allergen-activated B-cells and Th-2 cells in the lymph nodes draining from the point of entry express tissue-specific homing receptors that allow them to mobilize back to the target tissue, in which the allergens enter. Presumably, the cooperation of allergen-specific B- and Th-2 cells is designed to eliminate the allergen. Free IgE antibodies bind to high-affinity FcεR1 on mast cells in tissues and basophils in blood. Such cells are now considered to be armed or sensitized. An important step in the development of a Type I hypersensitivity reaction is the differentiation of naive allergen-specific T-cells into Th-2 cells. This is favored by cytokines such as interleukin (IL)-4 and IL-13. These Th-2 cells producing IL-4 and IL-13 stimulate allergen-specific plasma cells to produce IgE. [2]
After sensitization is accomplished (arming of mast cells and basophils), the re-exposition to an allergen elicits the effector stage of Type I hypersensitivity. The early phase is mediated primarily by the degranulation of mast cells and basophils. The primary mediators of allergic symptoms are histamine, serotonin, and other preformed molecules. The high speed of Type I hypersensitivity is attributed to the release of preformed mediators. After the initial release of mediators, mast cells start to break down and generate arachidonic acid from membrane phospholipids, which leads to the production of prostaglandins and lipooxygenase products such as leukotrienes. These mediators have been implicated in sustaining the allergic response for several hours. Histamine is a key mediator and induces vasodilatation, increased vessel permeability, bronchial smooth muscle contraction, mucous production, and sensory nerve stimulation. An interesting feature of mast cells is that they contain stored large intracellular pools of tumor necrosis factor (TNF)-α, which is released during the course of a Type I hypersensitivity reaction.[2,21]
Four to six hours after the initiation of the Type I reaction, chemotactic factors locally released at the site of allergen exposure bring other immune cells including neutrophils, eosinophils, Th-2 lymphocytes, mast cells, macrophages and basophils. The chemokines involved include IL-8 (which attracts neutrophils), IL-5, IL-3, and GM-CSF. The latter three chemokines induce activation and differentiation of eosinophils. Eosinophils are the key cells implicated in the tissue damage occurring in the late phase of a Type I hypersensitivity reaction. Eosinophils can degranulate and release leukotrienes, platelet activating factor, IL-4, IL-10, major basic protein, eosinophilic cationic protein and eosinophilic derived neurotoxin. In addition, eosinophils can release and/or activate proteases including collagenases and peroxidases, setting the stage for more tissue damage. Natural killer T-cells appear to have a role in the late phase of a Type I hypersensitivity reaction. Mice lacking NKT-cells have reduced allergen-induced airway inflammation, and the administration of NKT-cells from wild mice allows the development of airway damage. Of interest is that this deleterious effect is not observed with the transfer of NKT-cells from IL-4 and IL-3 knockout mice. This suggests that NKT-cells supply part of the IL-4 required to support the Th-2 differentiation of allergen-specific memory T-cells.[2]
The reasons for the appearance of IgE in mammals are unclear. While some believe that IgE is involved in the protection against helminthes,[22,23] others have argued that the primary function of IgE is defense against environmental toxins and venoms.[24] It is unknown why Type I hypersensitivity reactions occur in response to environmental inert antigens and not to microbial pathogens. Thus, humans can be allergic to pollen and dust mites but not allergic to group B streptococcus. The IgE class of antibodies is thought to have evolved in vertebrates within the last 300 million years,[25] before the evolution of modern mammals, and there is evidence that an allergic-like response exists in some known mammalian species.[24]
Clinical Manifestations of Type I Hypersensitivity Reactions
The clinical manifestations of allergy occur at the site at which mast cell degranulations occur. If the allergen enters the bloodstream or is absorbed from the gut, systemic anaphylaxis can occur. If the port of entry is the respiratory tract, this can result in allergic rhinitis or allergic asthma, depending upon whether mast cell degranulation occurs in the nose or in the tracheobronchial tree. The skin is also a place for immediate and delayed reactions. Examples are atopic dermatitis or eczema, and urticaria (hives).[21] The uterus is a rich source of mast cells that can be found in the myometrium and endometrium. Thus, degranulation of mast cells can lead to increased uterine contractility and labor.[26]
Evidence that Allergy is a Mechanism of Disease for Premature Labor
The term “Great Obstetrical Syndromes”[27] was coined to propose that obstetrical disorders responsible for maternal death and perinatal morbidity and mortality are conditions characterized by multiple etiologies, a long preclinical stage, frequent fetal involvement, clinical manifestations that are often adaptive in nature, and predisposition to a particular syndrome influenced by gene-environment interactions. Several mechanisms of disease have been proposed for premature labor,[28,29] such as intra-amniotic infection/inflammation,[30–49] cervical disease,[50,51] uterine overdistension,[52] endocrine disorders,[51,53–55] vascular insults,[56–59] abnormal allograft reaction,[56–60] and allergy.[61] Indeed, we have suggested that a Type I hypersensitivity reaction may be associated with preterm labor.[61] The evidence in support of this proposal is the following: 1) the uterus is a rich source of mast cells the – effector cells of allergic-like immunologic reactions;[62] 2) several products of mast cell degranulation can induce myometrial contractility (i.e., histamine and prostaglandins);[63,64] 3) pharmacologic degranulation of mast cells with a compound called “48/80” induces myometrial and cervical contractility;[65–67] 4) incubation of myometrial strips from sensitized and non-sensitized animals with an anti-IgE antibody increases myometrial contractility;[65] 5) human myometrial strips obtained from women known to be allergic to ragweed demonstrate increased myometrial contractility when challenged in vitro by the allergen.[26] Moreover, sensitivity of the myometrial strips of non-allergic women can be transferred passively by preincubation of the strips with human serum;[26] 6) non-pregnant guinea pigs sensitized with ovalbumin and then challenged with this antigen demonstrate increased uterine tone;[65,68] 7) traditional descriptions of animals dying of anaphylactic shock demonstrate enhanced uterine contractility when autopsy was performed immediately after death;[69,70] 8) we have identified a sub-group of patients with preterm labor who have eosinophils in the amniotic fluid as the predominant white blood cell.[61] Under normal circumstances white blood cells are not present in amniotic fluid. The presence of eosinophils, therefore, suggests an abnormal immune response and may represent a marker for an allergic-like response in preterm labor; 9) we have been able to induce preterm labor and delivery in a guinea pig model of Type I hypersensitivity. Animals sensitized with ovalbumin who were challenged during pregnancy with the same protein were more likely to deliver a preterm neonate than those challenged with saline. Moreover, preterm labor and delivery was prevented with treatment with antihistamines. Sensitization with ovalbumin by itself without a challenge during pregnancy did not change the duration of gestation;[71] 10) preterm labor and delivery induced by a Type I hypersensitivity reaction could be prevented by pretreatment with a histamine H1 receptor antagonist (ketotifen);[71] 11) patients with asthma are at increased risk for preterm delivery;[72,73] and 12) case reports have documented that exposure to allergens can induce uterine contractions.[74–76] Collectively, this evidence is persuasive that Type I hypersensitivity reaction can induce preterm labor and delivery.
Questions that require further research are what is the nature of the allergen that induces this reaction, and why uterine mast cells should be preferentially degranulated. It is known that the human fetus is exposed to common allergens (i.e., house or dust mites), as this has been detected in amniotic fluid in the midtrimester of pregnancy and in fetal blood. Moreover, the concentrations of the allergen are higher in fetal than maternal blood.[77] However, it is not clear yet whether the fetus is participating in a Type I hypersensitivity reaction. It is noteworthy that allergen-specific reactivity has been demonstrated in umbilical cord blood at birth and as early as 22 weeks of gestation, indicating that the human fetus can recognize allergens in early pregnancy.[78]
Pregnancy is considered a state in which there is preponderance of a Th-2 cytokine response which favors the differentiation of naïve CD4+ T cells to the Th-2 phenotype with increased capacity for cytokine secretion in the IL-4 gene cluster and predisposes to a switch to IgE production by B cells. Human decidua contains immune cells capable of identifying local foreign antigens, including macrophages and dendritic cells, as well as B and T cells. [79–81]
Food Allergy
Food allergies affect approximately 3.7% of the population,[82] and is more frequent in children than in adults,[83] and in women than in men.[84] It may be expected that the clinical manifestation of food allergies would be largely restricted to the gastrointestinal tract, but this is not the case: acute asthma, laryngeal angioedema, and cardiovascular collapse have all been reported.[85,86] It is noteworthy that food allergy is the most common cause of life-threatening anaphylaxis[87] and carries a fatality risk of 1%.[88,89] Hence, the broad clinical spectrum of food allergy ranges from a skin reaction to life-threatening anaphylaxis. In the current case report, the skin and the uterus were the target organs.
It is estimated that IgE mediated food allergy affects nearly 4% of the US population,[82] with fish and shellfish being the two most common causes of food allergic reactions. Many allergens have been identified in fish and shellfish, with cross-reactivity between shellfish, arachnids, and insects. Shellfish is a non-taxonomic term that includes crustaceans (shrimp, crab, crawfish, lobster) and mollusks which, unlike fish, are invertebrates. The major antigen responsible for cross-reactivity among distinct species of fish and amphibians are parvalbumins, a group of proteins resistant to thermal and enzymatic degradation which controls calcium flow in the sarcoplasm of white meat.[90] The major shellfish antigen is tropomyosin, a protein which is essential for muscle contraction in both vertebrates and invertebrates. Tropomyosins are the pan-allergens responsible for cross-reactivity between crustaceans, arachnids, and mollusks.[92] Cross-reactivity is the recognition of distinct antigens by the same IgE antibody which results in a clinical manifestation caused by antigens which are homologous to different species. Although 50% of individuals allergic to some type of fish are at risk of reacting to a second species, those allergic to some types of crustaceans carry a 75% risk of cross-reactivity due to the greater similarity among tropomyosin compared to parvalbumins.[92]
Tropomyosin is the major antigen responsible for cross-reaction between shrimp and crab,[93] lobster,[94] insects such as cockroaches,[95] arachnids such as house dust mites,[96] and mollusks such as squid,[97] oyster,[98] snail,[99] mussels, clams, and scallops.[100] More than 80% of individuals with shrimp allergy are positive on skin prick test to crab, crawfish, and lobster,[101] and those with immediate reaction to shrimp ingestion have reaction rates of 50–100% to lobster, crab and crawfish.[102] This degree of cross-reactivity can cause risk and embarrassment for fish and shellfish individuals, since many of the antigens may be hidden (e.g. Worcestershire sauce contains anchovies). A population of shrimp-naive orthodox Jews, who obeyed strict Kosher dietary laws prohibiting the ingestion of shellfish and who suffered perennial allergic rhinitis and house dust mite/cockroach hypersensitivity, were found to be prick skin test positive for shrimp antigen.[103] A comprehensive list of allergens and the species from which they derive their name is shown in an excellent review by Wild and Lehrer.[90]
Type I Hypersensitivity Reactions and Uterine Contractions in Humans
We have identified three reports demonstrating that an allergic reaction can lead to increased uterine contractility. In one report, a woman who, following ingestion of shellfish during pregnancy, developed erythema of the palms and urticarial areas of the face and abdomen, uterine contractions every three to four minutes, and hypotension (90/60). The patient was treated with ephedrine (5 mg intravenously). A second dose of ephedrine was administered, because there was no improvement in the maternal blood pressure. After 10 mg of ephedrine, the fetal heart rate became 180–185 bpm, and subsequently developed late decelerations. Two hours later, the patient recovered, and the fetal heart rate abnormalities gradually disappeared. The patient eventually delivered a term newborn appropriate for gestational age, after a cesarean section.[74] The second case is a report of anaphylactic shock in response to a wasp sting during pregnancy.[75] The third case is a patient with a history of atopic dermatitis who developed a severe anaphylactic reaction after a vaginal examination with a latex glove. Within two hours, the clinical condition improved, but the patient developed regular uterine contractions.[76]
Conclusion
We present a case in which ingestion of shellfish led to hives and the onset of regular preterm uterine contractions. Both abated after treatment with antihistamines and steroids. We propose that this case, along with others previously reported and a body of experimental literature, supports the view that Type I hypersensitivity reaction with degranulation of uterine mast cells during pregnancy may lead to premature labor.
Figure 1.
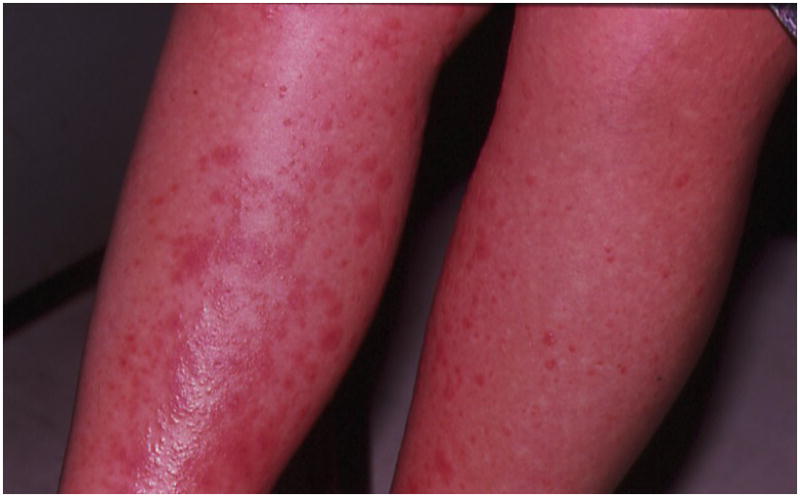
Extensive exanthemous lesions in the lower extremities in a pregnant woman at 31 weeks of gestation, which developed shortly after eating lobster meat.
CASE REPORT.
A 28 year-old Hispanic woman was admitted at 31 weeks of gestation with an episode of spontaneous preterm labor and a generalized pruritic maculo-papular rash (Figure 1), both of which developed shortly before admission following ingestion of lobster meat. Her medical and surgical histories, as well as her prenatal care, had been unremarkable. On admission, she had regular uterine contractions every three minutes which were subjectively strong as assessed by the obstetrician and patient. An anti-histamine agent (clorphenamine) and betamethasone were administered orally, following which both the rash and uterine contractions subsided. The pregnancy progressed satisfactorily and at 40 weeks gestation, the woman was admitted in spontaneous labor. The patient underwent a cesarean section because of failure to progress in labor (arrest of dilation). A viable male infant weighing 3780 g, with Apgar scores of 9 and 9was delivered. The only significant finding at delivery was multiple loops of nuchal cord. Her postoperative course was uneventful and the mother and baby were discharged home on the 4th postoperative day. Follow-up of the child revealed the development of atopic disorders which included repeated rashes as well as asthma, requiring frequent administration of inhaled corticosteroids and beta-2 adrenergic agents.
Acknowledgments
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Jackson M. Introduction: Allergy and History. Stud Hist Phil Biol & Biomed Sci. 2003;34:383–398. [Google Scholar]
- 2.Allergy and Hypersensitivity. 1. 2006. pp. 923–962. [Google Scholar]
- 3.Portier P, Richet C. De l’action anaphylactique de certains venins. CR Société de Biologie. 1902;54:170–172. [Google Scholar]
- 4.Portier P, Richet C. Nouveux faits de l’anaphylaxie ou sensibilisation aux toxins par des doses reitérées. CR Société de Biologie. 1902;54:548–551. [Google Scholar]
- 5.Kroker K. Immunity and Its Other: The Anaphylactic Selves of Charles Richet. Stud Hist Phil Biol & Biomed Sci. 1999;30:273–296. [Google Scholar]
- 6.Löwy I. On guinea pigs, dogs and men: anaphylaxis and the study of biological individuality, 1902–1939. Stud Hist Phil Biol & Biomed Sci. 2003;34:399–423. [Google Scholar]
- 7.Arthus M. Injections répétées du sérum de cheval chez le lapin. CR Société de Biologie. 1903;55:817–820. [Google Scholar]
- 8.Von Pirquet C, Schick B. Die Serumkrankheit. 1905. [Google Scholar]
- 9.Schick B. Pediatrics in Vienna at the beginning of the century. J Pediatr. 1957;50:114–124. doi: 10.1016/s0022-3476(57)80019-5. [DOI] [PubMed] [Google Scholar]
- 10.Prausnitz C, Küstner H. Studien über die Überempfindlichkeith. Zbl Bakt Parasits Infect I Abt Orig. 1921;86:160–169. [Google Scholar]
- 11.Coca AF, Cooke RA. On the classification of the phenomena of hypersensitiviness. J Immunol. 1923;8:163–182. [Google Scholar]
- 12.Coca AF, Grove EF. Studies in hypersensitiveness: XIII. A study of atopic reagins. J Immunol. 1925;10:445–464. [Google Scholar]
- 13.Bennich HH, Ishizaka K, Johansson SG, Rowe DS, Stanworth DR, Terry WD. Immunoglobulin E. A new class of human immunoglobulin. Immunochemistry. 1968;5:327–328. doi: 10.1016/0019-2791(68)90128-6. [DOI] [PubMed] [Google Scholar]
- 14.Johansson SG, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13:381–394. [PMC free article] [PubMed] [Google Scholar]
- 15.Stanworth DR, Humphrey JH, Bennich H, Johansson SG. Specific inhibition of the Prausnitz-Kustner reaction by an atypical human myeloma protein. Lancet. 1967;2:330–332. doi: 10.1016/s0140-6736(67)90171-7. [DOI] [PubMed] [Google Scholar]
- 16.Ishizaka K, Ishizaka T, Hornbrook MM. Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J Immunol. 1966;97:75–85. [PubMed] [Google Scholar]
- 17.Ishizaka K, Ishizaka T, Hornbrook MM. BLOCKING OF PRAUSNITZ-KUESTNER SENSITIZATION WITH REAGIN BY NORMAL HUMAN BETA-2A GLOBULIN. J Allergy Clin Immunol. 1963;34:395–403. doi: 10.1016/0021-8707(63)90002-9. [DOI] [PubMed] [Google Scholar]
- 18.Stanworth DR. The discovery of IgE. Allergy. 1993;48:67–71. doi: 10.1111/j.1398-9995.1993.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 19.Durham SR, Church MK. Principles of Allergy Diagnosis. 2. 2001. pp. 3–16. [Google Scholar]
- 20.Gell PGH, Coombs RRA. The classification of allergic reactions underlying disease. 1963. First. [Google Scholar]
- 21.Murphy K, Travers P, Walport M. Allergy and Hypersensitivity. 7. 2008. pp. 555–598. [Google Scholar]
- 22.Thornton CA, Holloway JA, Popplewell EJ, Shute JK, Boughton J, Warner JO. Fetal exposure to intact immunoglobulin E occurs via the gastrointestinal tract. Clin Exp Allergy. 2003;33:306–311. doi: 10.1046/j.1365-2222.2003.01614.x. [DOI] [PubMed] [Google Scholar]
- 23.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 24.Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 25.Hadge D, Ambrosius H. Evolution of low molecular weight immunoglobulins--IV. IgY-like immunoglobulins of birds, reptiles and amphibians, precursors of mammalian IgA. Mol Immunol. 1984;21:699–707. doi: 10.1016/0161-5890(84)90022-1. [DOI] [PubMed] [Google Scholar]
- 26.Garfield RE, Irani AM, Schwartz LB, Bytautiene E, Romero R. Structural and functional comparison of mast cells in the pregnant versus nonpregnant human uterus. Am J Obstet Gynecol. 2006;194:261–267. doi: 10.1016/j.ajog.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Romero R. Prenatal medicine: The child is the father of the man. J Mat Fet Neonat Med. 2009 doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin Obstet Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 31.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 32.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 34.Ledger WJ. Infection and premature labor. Am J Perinatol. 1989;6:234–236. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 36.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 37.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 38.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 39.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 40.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, Khalek N, Camacho N, Hendler I, Mittal P, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, Kim CJ, Khalek N, Mittal P, Goncalves LF, et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet. Gynecol. 2007;30:793–798. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449–461. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, Vaisbuch E, Than NG, Mazaki-Tovi S, Chaiworapongsa T, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, Mittal P, Kim CJ, Kim MJ, Espinoza J, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763–775. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero R, Espinoza J, Rogers WT, Moser A, Nien JK, Kusanovic JP, Gotsch F, Erez O, Gomez R, Edwin S, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med. 2008;21:367–388. doi: 10.1080/14767050802045848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, Nhan-Chang CL, Erez O, Kim CJ, Espinoza J, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–1103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 51.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet. Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol. 1990;10:347–350. [PubMed] [Google Scholar]
- 53.Csapo AI, Pohanka O, Kaihola HL. Progesterone deficiency and premature labour. Br Med J. 1974;1:137–140. doi: 10.1136/bmj.1.5899.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazor M, Hershkovitz R, Chaim W, Levy J, Sharony Y, Leiberman JR, Glezerman M. Human preterm birth is associated with systemic and local changes in progesterone/17 beta-estradiol ratios. Am J Obstet Gynecol. 1994;171:231–236. doi: 10.1016/0002-9378(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 55.Fidel PI, Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med. 1998;7:222–226. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 56.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 57.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89:265–271. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 58.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 59.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Sepulveda W, Baumann P, Yoon BH, Brandt F, Gomez R, Mazor M, Sorokin Y, Cotton D. The preterm labor syndrome: Biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease. Am J Obstet Gynecol. 1993;168:288. [Google Scholar]
- 61.Romero R, Mazor M, Avila C, Quintero R, Munoz H. Uterine “allergy”: A novel mechanism for preterm labor. Am J Obstet Gynecol. 1991;164:375. [Google Scholar]
- 62.Rudolph MI, Reinicke K, Cruz MA, Gallardo V, Gonzalez C, Bardisa L. Distribution of mast cells and the effect of their mediators on contractility in human myometrium. Br J Obstet Gynaecol. 1993;100:1125–1130. doi: 10.1111/j.1471-0528.1993.tb15178.x. [DOI] [PubMed] [Google Scholar]
- 63.Padilla L, Reinicke K, Montesino H, Villena F, Asencio H, Cruz M, Rudolph MI. Histamine content and mast cells distribution in mouse uterus: the effect of sexual hormones, gestation and labor. Cell Mol Biol. 1990;36:93–100. [PubMed] [Google Scholar]
- 64.Rudolph MI, Bardisa L, Cruz MA, Reinicke K. Mast cells mediators evoke contractility and potentiate each other in mouse uterine horns. Gen Pharmacol. 1992;23:833–836. doi: 10.1016/0306-3623(92)90233-a. [DOI] [PubMed] [Google Scholar]
- 65.Garfield RE, Bytautiene E, Vedernikov YP, Marshall JS, Romero R. Modulation of rat uterine contractility by mast cells and their mediators. Am J Obstet Gynecol. 2000;183:118–125. doi: 10.1067/mob.2000.105741. [DOI] [PubMed] [Google Scholar]
- 66.Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. Endogenous mast cell degranulation modulates cervical contractility in the guinea pig. Am J Obstet Gynecol. 2002;186:438–445. doi: 10.1067/mob.2002.120488. [DOI] [PubMed] [Google Scholar]
- 67.Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. IgE-independent mast cell activation augments contractility of nonpregnant and pregnant guinea pig myometrium. Int Arch Allergy Immunol. 2008;147:140–146. doi: 10.1159/000135701. [DOI] [PubMed] [Google Scholar]
- 68.Bytautiene E, Vedernikov YP, Maner WL, Saade GR, Romero R, Garfield RE. Challenge with ovalbumin antigen increases uterine and cervical contractile activity in sensitized guinea pigs. Am J Obstet Gynecol. 2008;199:658–6. doi: 10.1016/j.ajog.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 69.Schultz WH. Physiological studies in anaphylaxis: the reaction of smooth muscle of the guinea pig sensitized with horse serum. J Pharmacol Exp Ther. 1910;1:549–567. [Google Scholar]
- 70.Dale H. The anaphylactic reaction of plain muscle in the guinea-pig. J Pharmacol Exp Ther. 1913;4:167–223. [Google Scholar]
- 71.Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191:1356–1361. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 72.Kramer MS, Coates AL, Michoud MC, Dagenais S, Moshonas D, Davis GM, Hamilton EF, Nuwayhid B, Joshi AK, Papageorgiou A, et al. Maternal asthma and idiopathic preterm labor. Am J Epidemiol. 1995;142:1078–1088. doi: 10.1093/oxfordjournals.aje.a117561. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Wen SW, Demissie K, Marcoux S, Kramer MS. Maternal asthma and pregnancy outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2001;184:90–96. doi: 10.1067/mob.2001.108073. [DOI] [PubMed] [Google Scholar]
- 74.Klein VR, Harris AP, Abraham RA, Niebyl JR. Fetal distress during a maternal systemic allergic reaction. Obstet Gynecol. 1984;64:15S–17S. doi: 10.1097/00006250-198409001-00004. [DOI] [PubMed] [Google Scholar]
- 75.Habek D, Cerkez-Habek J, Jalsovec D. Anaphylactic shock in response to wasp sting in pregnancy. Zentralbl Gynakol. 2000;122:393–394. [PubMed] [Google Scholar]
- 76.Shingai Y, Nakagawa K, Kato T, Fujioka T, Matsumoto T, Kihana T, Noda K, Mori T. Severe allergy in a pregnant woman after vaginal examination with a latex glove. Gynecol Obstet Invest. 2002;54:183–184. doi: 10.1159/000067888. [DOI] [PubMed] [Google Scholar]
- 77.Holloway JA, Warner JO, Vance GH, Diaper ND, Warner JA, Jones CA. Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet. 2000;356:1900–1902. doi: 10.1016/S0140-6736(00)03265-7. [DOI] [PubMed] [Google Scholar]
- 78.Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol. 1996;7:109–116. doi: 10.1111/j.1399-3038.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 79.Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. doi: 10.1051/rnd:19881006. [DOI] [PubMed] [Google Scholar]
- 80.Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol. 1996;156:4027–4034. [PubMed] [Google Scholar]
- 81.Kammerer U, Schoppet M, McLellan AD, Kapp M, Huppertz HI, Kampgen E, Dietl J. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol. 2000;157:159–169. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–819. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 83.Moneret-Vautrin DA, Morisset M. Adult food allergy. Curr Allergy Asthma Rep. 2005;5:80–85. doi: 10.1007/s11882-005-0060-6. [DOI] [PubMed] [Google Scholar]
- 84.Kanny G, Moneret-Vautrin DA, Flabbee J, Beaudouin E, Morisset M, Thevenin F. Population study of food allergy in France. J Allergy Clin Immunol. 2001;108:133–140. doi: 10.1067/mai.2001.116427. [DOI] [PubMed] [Google Scholar]
- 85.Bischoff S, Crowe SE. Food allergy and the gastrointestinal tract. Curr Opin Gastroenterol. 2004;20:156–161. doi: 10.1097/00001574-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 86.Bischoff SC. Food allergies. Curr Gastroenterol Rep. 2006;8:374–382. doi: 10.1007/s11894-006-0022-8. [DOI] [PubMed] [Google Scholar]
- 87.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 88.Helbling A, Hurni T, Mueller UR, Pichler WJ. Incidence of anaphylaxis with circulatory symptoms: a study over a 3-year period comprising 940,000 inhabitants of the Swiss Canton Bern. Clin Exp Allergy. 2004;34:285–290. doi: 10.1111/j.1365-2222.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 89.Moneret-Vautrin DA, Kanny G, Morisset M, Rance F, Fardeau MF, Beaudouin E. Severe food anaphylaxis: 107 cases registered in 2002 by the Allergy Vigilance Network. Eur Ann Allergy Clin Immunol. 2004;36:46–51. [PubMed] [Google Scholar]
- 90.Wild LG, Lehrer SB. Fish and shellfish allergy. Curr Allergy Asthma Rep. 2005;5:74–79. doi: 10.1007/s11882-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 91.Leung PS, Chow WK, Duffey S, Kwan HS, Gershwin ME, Chu KH. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: evidence for tropomyosin as the common allergen. J Allergy Clin Immunol. 1996;98:954–961. doi: 10.1016/s0091-6749(96)80012-1. [DOI] [PubMed] [Google Scholar]
- 92.Torres Borrego J, Martinez Cuevas JF, Tejero Garcia J. Cross reactivity between fish and shellfish. Allergol Immunopathol (Madr) 2003;31:146–151. doi: 10.1016/s0301-0546(03)79282-9. [DOI] [PubMed] [Google Scholar]
- 93.Leung PS, Chen YC, Gershwin ME, Wong SH, Kwan HS, Chu KH. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J Allergy Clin Immunol. 1998;102:847–852. doi: 10.1016/s0091-6749(98)70027-2. [DOI] [PubMed] [Google Scholar]
- 94.Leung PS, Chen YC, Mykles DL, Chow WK, Li CP, Chu KH. Molecular identification of the lobster muscle protein tropomyosin as a seafood allergen. Mol Mar Biol Biotechnol. 1998;7:12–20. [PubMed] [Google Scholar]
- 95.Santos AB, Chapman MD, Aalberse RC, Vailes LD, Ferriani VP, Oliver C, Rizzo MC, Naspitz CK, Arruda LK. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104:329–337. doi: 10.1016/s0091-6749(99)70375-1. [DOI] [PubMed] [Google Scholar]
- 96.Aki T, Kodama T, Fujikawa A, Miura K, Shigeta S, Wada T, Jyo T, Murooka Y, Oka S, Ono K. Immunochemical characterization of recombinant and native tropomyosins as a new allergen from the house dust mite, Dermatophagoides farinae. J Allergy Clin Immunol. 1995;96:74–83. doi: 10.1016/s0091-6749(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 97.Miyazawa H, Fukamachi H, Inagaki Y, Reese G, Daul CB, Lehrer SB, Inouye S, Sakaguchi M. Identification of the first major allergen of a squid (Todarodes pacificus) J Allergy Clin Immunol. 1996;98:948–953. doi: 10.1016/s0091-6749(96)80011-x. [DOI] [PubMed] [Google Scholar]
- 98.Ishikawa M, Ishida M, Shimakura K, Nagashima Y, Shiomi K. Tropomyosin, the Major Oyster Crassostrea gigas Allergen and its IgE-binding Epitopes. Jouranal of Food Science. 1998;63:44–47. [Google Scholar]
- 99.Ishikawa M, Ishida M, Shimakura K, Nagashima Y, Shiomi K. Purification and IgE-binding epitopes of a major allergen in the gastropod Turbo cornutus. Biosci Biotechnol Biochem. 1998;62:1337–1343. doi: 10.1271/bbb.62.1337. [DOI] [PubMed] [Google Scholar]
- 100.Chu KH, Wong SH, Leung PS. Tropomyosin Is the Major Mollusk Allergen: Reverse Transcriptase Polymerase Chain Reaction, Expression and IgE Reactivity. Mar Biotechnol(NY) 2000;2:499–509. doi: 10.1007/s101260000035. [DOI] [PubMed] [Google Scholar]
- 101.Daul CB, Morgan JE, Waring NP, McCants ML, Hughes J, Lehrer SB. Immunologic evaluation of shrimp-allergic individuals. J Allergy Clin Immunol. 1987;80:716–722. doi: 10.1016/0091-6749(87)90293-4. [DOI] [PubMed] [Google Scholar]
- 102.Waring NP, Daul CB, deShazo RD, McCants ML, Lehrer SB. Hypersensitivity reactions to ingested crustacea: clinical evaluation and diagnostic studies in shrimp-sensitive individuals. J Allergy Clin Immunol. 1985;76:440–445. doi: 10.1016/0091-6749(85)90724-9. [DOI] [PubMed] [Google Scholar]
- 103.Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33:956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]


