Abstract
The hippocampus and the medial temporal lobe cortex (MTLC) both contribute to long-term memory. While their contributions are thought to be dissociable, the nature of the representations that each region supports remains unclear. The Complementary Learning Systems (CLS) modeling approach suggests that hippocampus represents overlapping information in a sparser and therefore more separated fashion than MTLC. We tested this prediction using a collaborative referencing paradigm whereby hippocampal amnesic patients and a partner work together to develop and use unique labels for a set of abstract visual stimuli (tangrams) across extended interactions. Previously, we reported that amnesic patients demonstrate intact learning when the tangrams are conceptually dissimilar. Here, we manipulated the degree of visual similarity; half of the stimuli were dissimilar to one another (e.g., camel, giraffe), and half were similar (e.g., birds). We hypothesized that while patients would have little difficulty with the dissimiliar tangrams (quickly arriving at unique and concise labels) they would be unable to rapidly form distinct representations of highly similar visual patterns. Consistent with this prediction, patients and both healthy and brain-damaged comparison participants showed similar rates of learning for dissimilar tangrams, but the similar tangrams proved more difficult for hippocampal patients as reflected in the greater number of words they used to describe each similar card. This result supports the CLS model’s central claim of hippocampal specialization for pattern separation, and suggests that our collaborative referencing paradigm may be a useful tool for observing extended encoding of complex representations.
The medial temporal lobes (MTL) are widely acknowledged to play a necessary role in memory (Scoville & Milner, 1957), and there is strong evidence that different MTL components make dissociable contributions to memory performance (Aggleton & Brown, 1999; Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001). Theories of MTL function typically divide the region into at least two distinct components: the hippocampus; and the adjacent neocortex, including perirhinal, entorhinal, and parahippocampal cortices (i.e., medial temporal lobe cortex or MTLC). Different theorists have assigned different functions to these components, but a broadly-shared assumption is that the hippocampus is a higher-order mnemonic processor than the MTLC and therefore necessary for relational memory (Eichenbaum & Cohen, 2001), recollection (Aggleton & Brown, 1999), or complex (visuo)spatial representations (Cowell, Bussey, & Saksida, 2010). Although these characterizations vary, the nature of the system’s underlying representations must be shaped and limited by the neural connectivity of hippocampus and MTLC. Following from that premise, one particularly promising theoretical approach has been to model the unique neural connectivity of MTLC and hippocampus and to make inferences based on model performance under different conditions (McClelland, McNaughton, & O'Reilly, 1995; Norman & O'Reilly, 2003; Norman, 2010). A straightforward prediction of such models is that focal hippocampal damage should make pattern separation (and completion) more difficult. We sought to evaluate this claim by testing the ability of patients with bilateral hippocampal lesions to learn about a set of items in which the degree of visual similarity was manipulated.
Mnemonic contributions of the hippocampus have been described by a variety of mechanistic models, including the Complementary Learning Systems (CLS) framework (McClelland et al., 1995; Norman & O'Reilly, 2003). According to the CLS model, patterns of connectivity within the hippocampus make it uniquely well-suited both to rapidly separate novel inputs into distinct neural representations and to complete degraded, previously-experienced inputs into previously-learned patterns. Meanwhile, the generic neocortical connections within MTLC slowly sharpen many overlapping representations simultaneously; the highly separated representations of hippocampus are much less likely to overlap and can therefore be distinguished quickly. Hippocampal damage should therefore impair the ability to distinguish old from new items when test items are presented one at a time and lures resemble the target (e.g., in a Yes/No recognition task). Absent hippocampus, the overlapping features of a lure resembling the target will activate many features of the target in an MTLC representation and could result in a false alarm. Meanwhile, MTLC alone is thought to be sufficient to discriminate lures from targets so long as both are presented simultaneously, as in a forced-choice test format. In this case, the similarity of target’s stored representation all test items can be compared simultaneously and directly, and should be diagnostic. Notably, MTLC does not possess patterns of intraregional connectivity that differ from most neocortex, and presumably any cortical region with appropriate inputs could support similar representations. Hence, MTLC will be used here as shorthand for non-hippocampal neocortical regions, and our methods attempted to dissociate hippocampal representation from other cortical representations.
Research directly testing CLS predictions in humans with hippocampal damage has been rare and equivocal. While two patients with focal hippocampal damage (Holdstock et al., 2002; Holdstock, Mayes, Gong, Roberts, & Kapur, 2005; Mayes, Holdstock, Isaac, Hunkin, & Roberts, 2002) and others with MCI (Westerberg et al., 2006) behaved as predicted by the CLS by exhibiting better forced-choice than Yes/No performance, other hippocampal lesion patients have not (Bayley, Wixted, Hopkins, & Squire, 2008). While the predictions of CLS in forced choice and Yes/No paradigms are clear, recognition has long been a disputatious arena for memory researchers in part because the data are relatively sparse. Mnemonic representations are evaluated often only once in a test phase using either binary or confidence-graded measures of recognition, leaving the underlying qualities of the representations and the encoding-time representations largely unexplored. At the same time, thorough study of amnesic patients’ representations and encoding is challenging given their memory impairment. The manipulation of tasks that can produce apparently normal representations despite hippocampal damage may provide an avenue of investigation.
Patients with hippocampal damage can exhibit normal learning in some circumstances. In our previous work using a collaborative referencing task, we examined the ability of patients with hippocampal amnesia to acquire referential labels for a set of cards displaying Chinese tangrams across a series of communicative interactions with familiar partners (Duff, Hengst, Tranel, & Cohen, 2006). To complete the task, participant pairs (patient and familiar partner) sat at a table facing each other with identical boards with 12 numbered spaces and a set of 12 identical cards on the table in front of each participant. A low barrier was placed between the partners, obscuring each person’s view of the other’s stimulus cards. Amnesic patients (directors) communicated to their partner (matchers) how to fill the numbered spaces so that at the end of the trial the two boards looked alike. The task was completed across 24 trials (6 trials per session, 2 sessions on 2 consecutive days). We found that despite profound declarative memory impairments the patients developed and used unique references for each card (e.g., siesta man), which across trials they produced in an increasingly concise and simplified form, requiring fewer words and less time to complete the task. In fact, the patients demonstrated a rate of learning comparable to that of healthy comparison participants. Given that the observed learning occurred gradually, and at a normal rate, we have argued that the learning was achieved through the tuning of cortical processors associated with non-relational memory (e.g., MTLC).
In the current experiment, we acquired similarly rich data about learned (or tuned) representations by modifying the collaborative referencing task to use stimuli that would differentially benefit from normal pattern separation. Instead of heterogeneous stimuli, items in the current set belonged to two conditions: half were visually similar (see bottom row of Figure 1) to one another; while the other half were dissimilar (see top row of Figure 1) to one another and to the items in the similar seti. Although tangrams are abstract and interpreted idiosyncratically, items in our similar set resembled stylized birds as reflected in the labels assigned to them (see Results). Put another way, the overlapping visual features of the similar set were intended to yield conceptual overlap which would in turn require greater attention to distinguishing features of individual items. We hypothesized that MTLC representations would be poorly suited to this because of substantial visual similarity (i.e., overlap) in the initial representations. Meanwhile, hippocampal representations are well separated immediately, potentially allowing rapid unique identification. Healthy participants with highly separated hippocampal representations were therefore expected to show no difference in learning rates between similar and dissimilar items, while amnesic patients relying on MTLC representations alone were expected to represent similar items less efficiently than dissimilar items, with differences evident in longer descriptions of similar items and slower learning for those items.
Figure 1.
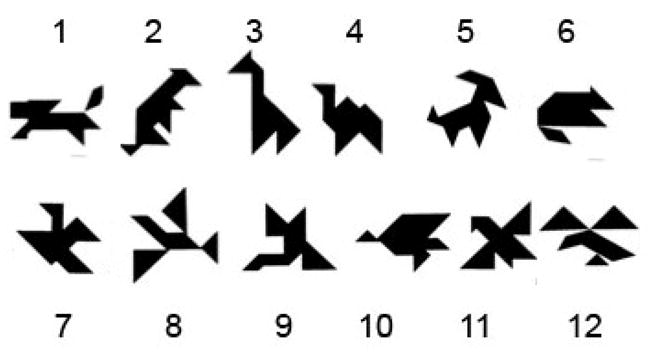
The set of tangrams for which participants and their partners collaboratively generated labels. (Top row: dissimiliar cards; Bottom row: similar cards).
Participants included two amnesic pairs (amnesic patients and their familiar partners (e.g., spouse, sibling) and six comparison pairs (healthy participants and their familiar partners) (see Table 1)ii. Healthy comparison participants included 5 men and 1 woman and were on average 62.6±8.5 years old with 16.4±2.6 years of education.
Table 1.
Participants’ Demographic, Neuropsychological, and Anatomical Characteristics
| Patient | Sex | Ed | Year of Birth | WAIS-III FSIQ | WMS-III GMI | Etiology; Damage | HC Volume |
|---|---|---|---|---|---|---|---|
| 2363 | Male | 16 | 1956 | 98 | 73 | Anoxia; Bilateral HC damage | −2.64 |
| 2563 | Male | 16 | 1955 | 102 | 75 | Anoxia; Bilateral HC damage | N/A |
Note. Ed = Education; WAIS-III = Wechsler Adult Intelligence Scale; FSIQ = Full Scale Intelligence Quotient, WMS-III GMI = Wechsler Memory Scale-III; GMI = General Memory Index; HC = hippocampus. Hippocampal z-score represent the combined (left and right hemisphere) studentised residuals of hippocampal volume relative to a group of comparison subjects (Allen et al., 2006). Patient 2563 wears a pacemaker and was unable to undergo the MRI examination. Only damage to the HC was visible on computerised tomography (see Duff et al., 2006, 2009; Hannula et al., 2007 for more extensive description of patients).
Although placement accuracy was high, both amnesic and comparison pairs found the similar tangrams (birds) more challenging; 77% (34/44) of placement errors were made on the similar tangrams. On average, the two amnesic pairs made more errors at placing the cards than comparison pairs (average=11.0 errors across the 24 trials (96.1 % accuracy), while on average only 3.6 errors were made by the comparison pairs across the 24 trials (98.7% accuracy).
The main dependent variable of interest was the initial description word count: the first attempt by the director (amnesic patient) at describing each of the 12 cards during each trial, before the matcher provided substantive input (see Duff et al., 2006 Supplementary Methods). We analyzed the number of words in the initial description of the similar and dissimilar tangrams separately across trials to assess learning across card types.
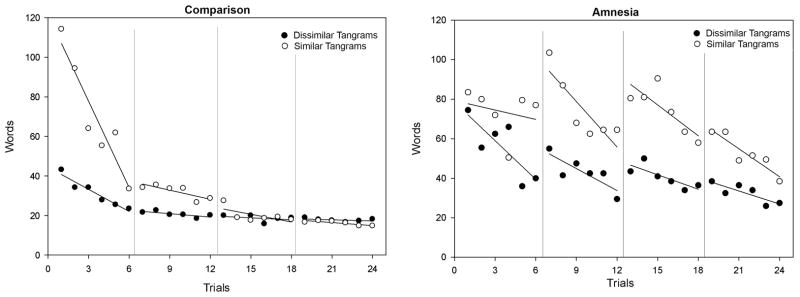
Across the entire task, we found that amnesic (AM) and healthy comparison (NC) directors demonstrated a comparable reduction in the number of words used to describe the dissimilar cards (average NC Trial 1=43.2 words; Trial 24=18.2 words; 70.8% reduction; AM Trial 1=74.5 words; Trial 24=27.5 words; 57.8% reduction; Mann-Whitney U test: Z=0; p=1.0). The critical comparison here was how healthy and amnesic directors described the highly similar tangrams (birds). All directors required more words to describe the similar cards than they did for the dissimilar cards, particularly on the initial trials (e.g., on Trial 2, NCs used on average 94.0 words for the similar cards and only 34.0 words for the dissimilar cards, while AMs averaged 80.0 and 55.5 words, respectively), again suggesting that generating unique representations and references for these cards was challenging. However, the comparison directors’ displayed significantly larger reduction in the average number of words required to describe the similar cards across trials (average NC Trial 1=114.2 words; Trial 24=14.8 words; 87.0% reduction) than the amnesic directors, who were particularly challenged on the similar cards and required more than twice as many words to reference those cards on the final trial as healthy comparison participants (AM Trial 1= 83.5 words; Trial 24=38.5 words; 53.9% reduction; Mann-Whitney U test: Z=−2.0; p=0.04; see Figure 2).
Figure 2.
Mean word counts (words required for referencing the cards) for healthy comparison (left panel) and amnesic patients (right panel) to reference all 12 tangrams on each of the trials; shown with session-by-session linear trends. Note that while the comparison participants rapidly reduce their word counts for both similar and dissimilar items, the amnesic participants show much slower reductions in word counts for the similar tangrams.
Finally, by the end of the task, comparison directors were consistently using concise, unique, and even somewhat arbitrary, labels for the similar tangrams (e.g., hummingbird, eagle, goose, phoenix). Strikingly, amnesic directors’ were less concise and more generic (e.g., “bird with uneven wings and the triangle head,” “bird in flight with the triangle head,” “bird flying, looking straight left.”). One amnesic director expressed awareness of the difficultly in generating unique labels for the similar tangrams (e.g., “I just can’t get names for the birds”).
In order to tie our result more closely to hippocampal damage and not to the presence of brain damage more generally, we collected data using an identical protocol from one braindamaged comparison participant (BDC participant) and his healthy partner. The BDC participant was a 56-year-old male with low-average intelligence (WAIS-III FSIQ of 85) and no declarative memory impairment (WMS-III GMI=95). Structural MRI data revealed a lesion in the dorsal aspects of the left parietal cortex, but there was no damage to hippocampus or any MTLC structure. Like healthy comparison directors, and in striking contrast to amnesic patients, the BDC director showed comparable rates of reduction in the words used to describe both the dissimilar (Trial 1=34 words; Trial 24=12 words; 64.7% reduction) and similar tangrams (Trial 1=63 words; Trial 24=23 words; 63.5% reduction). Likewise, by the end of the task the BDC pair was able to develop concise, unique labels for the similar tangrams, including goose, buzzard, and two-tailed hawk.
Our results show a clear influence of hippocampal pattern separation as indexed by rates of learning and by the nature and length of references. As expected based on previous work, all participants showed a similar rate of learning for the dissimilar items especially during the first 6 trials (Trial 1–6 mean (SD) slope of regression lines for NC=−3.76 (4.02), i.e., 3.76 fewer words used per trial, range −7.40 to 3.40; AM mean=−6.50, range −10.43 to −2.57, i.e., within or less than the normal range), but amnesic patients were selectively impaired when confronted with similar items (Trial 1–6 mean (SD) slope of regression line NC=−14.37 (8.57), range −28.66 to −6.25 ; AM mean =−1.56, range −4.25 to 1.09, i.e., outside and greater than the normal range). We tested the reliability of these differences using a modified statistical technique appropriate for small sample sizes that has been reported elsewhere (Konkel et al., 2008; Berryhill et al., 2007; Olson et al., 2006)iii, and while the groups were indistinguishable for dissimilar items (observed T(1.28)= −0.74 was < 2.87, the 95% level of the permuted set), amnesic patients showed reliably slower reductions in word counts for similar items (observed T(5.69)=2.11 was > 1.83, the 95% level of the permuted set). Importantly, the dissociation was evident both within the amnesic patient group itself and between comparison groups and the amnesic patients. Over the course of 24 trials, the patients consistently used more words to describe similar items despite their normal learning patterns for the dissimilar items. This gross deficit appeared to be specific to hippocampal damage, as the BDC pair mirrored healthy performance with similar and dissimilar items while the amnesic pairs did not.
Impairments in pattern separation after hippocampal damage provide a compelling explanation for these results. Described using the terminology of CLS (Norman & O'Reilly, 2003; Norman, 2010), without the highly separated representations unique to the hippocampus, the amnesic patients would be forced to rely on slower sharpening within MTLC to support distinct representations of the knowledge being developed about each item. While this tuning would be sufficient to equal normal learning when the set of learned items was heterogeneous, increased homogeneity within the set would cause learning to be generalized to the features common to items in the similar set. This kind of destructive retuning of connections between previously-encountered features could be construed as a form of catastrophic interference (McCloskey & Cohen, 1989) that the hippocampus normally prevents. Overlapping, interfering representations would not preserve useful knowledge that could improve performance on later trials.
Although explicit tests of CLS in human amnesic patients have been rare, other researchers have examined the effects of item similarity on learning after MTL lesions. Conditional discrimination learning tasks requiring participants to learn which of two items is correct have suggested that greater similarity may impair performance in MTL-lesion patients (Barense et al., 2005; Graham et al., 2006; Lee et al., 2005; Lee, Barense, & Graham, 2005), but with the caveat that hippocampal damage affected only scene representations, while leaving object representations undisturbed. Our results clearly suggest that sufficiently similar object representations may indeed benefit from hippocampal processing, and the rapid but measurable learning evident in NCs indicates that hippocampal influence is more likely mnemonic than perceptual in nature. This aligns with recent neuroimaging work that has identified regionally specific hippocampal contributions to object-based pattern separation and pattern completion (Bakker, Kirwan, Miller, & Stark, 2008; Carr, Rissman, & Wagner, 2010; Kirwan, Jones, Miller, & Stark, 2007; Stark, Yassa, & Stark, 2010; Yassa et al., 2010).
Focal hippocampal lesions impaired the ability of patients to learn about similar, but not dissimilar, items, and this finding is congruent with existing theories positing a unique hippocampal contribution to pattern separation. Use of our version of a collaborative referencing task (see Duff et al., 2008) provided new insight into the formation of and changes in mnemonic representations over time, and may be a useful tool for subsequent investigations.
Acknowledgments
Grant support from NIH F32 DC008825, NIH R01 MH67681, and NIH P01 NS19632. Special thanks to Elizabeth Rainville, Samantha Shune, and Lauren Steinmetz in the Duff Communication and Memory Lab for transcribing and coding the sessions.
Footnotes
In pilot work, 7 healthy individuals rated the distinctiveness of each tangram in comparison to the others. Each distinct tangram (those on the top row of Figure 1) was rated as being distinct from the other 11. The similar tangrams (those on the bottom row) were each rated as being distinct from each of the top 6, but were rated as highly similar to the other tangrams on the bottom row.
The two amnesic pairs participated in the Duff et al. (2006) study while the healthy pairs were recruited for this study.
Group-level differences in learning rates, characterized as regression slopes fitted to initial word counts for each pair across Trials 1–6, were analyzed using permutation tests. First, we calculated T values for observed group differences in learning rates (T tests did not assume equal variance between groups, and therefore used the Welch modification to degrees of freedom). Then, the eight (two amnesic and six normal comparison) learning rate values were randomized, and the T value reflecting the difference between the first two randomized observations and the remaining six was recorded. This procedure was repeated 1000 times, yielding a sample of all possible T values that could have arisen from the observed learning rates. The sample was then sorted, and the T value at the 95th percentile of the sorted sample was used as our lower bound for reliability. We conducted a similar permutation analysis using the nonparametric two-sample Wilcox test, and the same pattern of results was obtained.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal– anterior thalamic axis. Behavioral and Brain Sciences. 1999;22(3):425–489. doi: 10.1017/S0140525X99002034. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science (New York, NY) 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee ACH, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. Journal of Neuroscience. 2005;25(44):10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Wixted JT, Hopkins RO, Squire LR. Yes/no recognition, forcedchoice recognition, and the human hippocampus. Journal of Cognitive Neuroscience. 2008;20(3):505–512. doi: 10.1162/jocn.2008.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(52):14415–23. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65(3):298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HH, Wilkes-Gibbs D. Referring as a collaborative process. Cognition. 1986;22(1):1–39. doi: 10.1016/0010-0277(86)90010-7. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Components of recognition memory: Dissociable cognitive processes or just differences in representational complexity? Hippocampus. 2010;20(11):1245–1262. doi: 10.1002/hipo.20865. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Development of shared information in communication despite hippocampal amnesia. Nature Neuroscience. 2006;9(1):140–146. doi: 10.1038/nn1601. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Collaborative discourse facilitates efficient communication and new learning in amnesia. Brain and Language. 2008;106(1):41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain 2001 [Google Scholar]
- Graham KS, Scahill VL, Hornberger M, Barense MD, Lee ACH, Bussey TJ, Saksida LM. Abnormal categorization and perceptual learning in patients with hippocampal damage. Journal of Neuroscience. 2006;26(29):7547–7554. doi: 10.1523/JNEUROSCI.1535-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15(2):203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O'Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12(3):341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CEL. High-resolution fMRI investigation of the medial temporal lobe. Human Brain Mapping. 2007;28:959–966. doi: 10.1002/hbm.20331 ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Frontiers in human neuroscience. Vol. 2. Frontiers Research Foundation; 2008. Hippocampal amnesia impairs all manner of relational memory; p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Barense MD, Graham KS. The contribution of the human medial temporal lobe to perception: Bridging the gap between animal and human studies. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 2005;58(3–4):300–325. doi: 10.1080/02724990444000168. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Hodges JR, Graham KS. Perceptual deficits in amnesia: Challenging the medial temporal lobe 'mnemonic' view. Neuropsychologia. 2005;43(1):1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12(3):325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning-systems in the hippocampus and neocortex - insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: Revisiting the complementary learning systems model. Hippocampus. 2010;20(11):1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. Journal of cognitive neuroscience. 2006;18(7):1087–97. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning & Memory (Cold Spring Harbor, NY) 2010;17(6):284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and alzheimer's disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage. 2010;51(3):1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]