Abstract
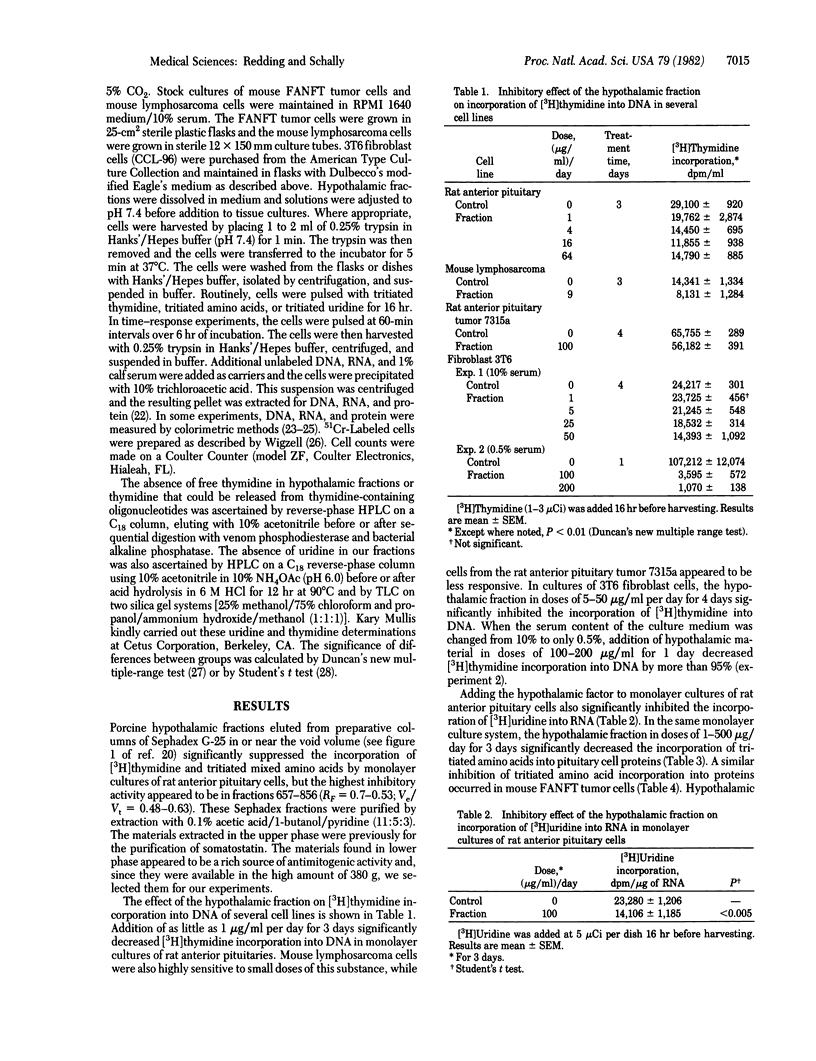
A fraction purified from acetic acid extracts of porcine hypothalami was found to contain significant antimitogenic activity when tested in normal and neoplastic cell lines. Addition of this hypothalamic material (1-100 micrograms/ml) to culture media significantly inhibited [3H]thymidine incorporation into cellular DNA in several cell lines. Amino acid incorporation into pituitary proteins and uridine incorporation into RNA were also significantly reduced by this factor(s). Addition to the culture media of this hypothalamic material at 5 micrograms/ml and 50 micrograms/ml per day decreased by 17% and 36%, respectively, cell numbers of 3T6 fibroblast cell cultures. Time-response curves showed that the inhibition of [3H]thymidine incorporation into DNA in 3T6 fibroblast cells begins within 2 hr after adding this fraction to the culture medium. The inhibitory action cannot be explained by a direct cytotoxic effect since 3T6 cells labeled with 51Cr and incubated for 6 hr in the presence of this hypothalamic fraction fail to show an increase in the release of 51Cr into the medium as compared with controls. Incubation with trypsin and chymotrypsin completely abolished the antimitogenic activity of this material and pepsin decreased it. This strongly suggests that the antimitogenic activity exhibited by this fraction is due to a polypeptide(s). These observations provide evidence for the presence in the mammalian hypothalamus of an antimitogenic peptide(s) that may be involved in the regulation of cell proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall P. T., Szopa B., Burzynski S. R., Hazlewood C. F. Polypeptides that inhibit human breast cancer cell division. Cancer Biochem Biophys. 1979;3(2):93–96. [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Grieshaber C. K., Hymer W. C. Effect of hypothalamic extracts on protein synthesis in rat anterior pituitary tissue. Proc Soc Exp Biol Med. 1968 Jun;128(2):459–463. doi: 10.3181/00379727-128-33037. [DOI] [PubMed] [Google Scholar]
- Holley R. W. Evidence that a rat liver "inhibitor" of the synthesis of DNA in cultured mammalian cells is arginase. Biochim Biophys Acta. 1967 Sep 26;145(2):525–527. doi: 10.1016/0005-2787(67)90076-7. [DOI] [PubMed] [Google Scholar]
- Khar A., Debeljuk L., Jutisz M. Effect of hypothalamic extracts on the incorporation of [3H]-thymidine by pituitary cells in culture. Proc Soc Exp Biol Med. 1978 Jul;158(3):471–474. doi: 10.3181/00379727-158-40228. [DOI] [PubMed] [Google Scholar]
- Klein K., Coetzee M. L., Madhav R., Ove P. Inhibition of tritiated thymidine incorporation in cultured cells by rat kidney extract. J Natl Cancer Inst. 1979 Jun;62(6):1557–1564. [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I., OVE P. Inhibition of growth of cultured mammalian cells by liver extracts. Biochim Biophys Acta. 1960 Feb 12;38:153–153. doi: 10.1016/0006-3002(60)91208-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Letnansky K. Inhibition of thymidine incorporation into the DNA of normal and neoplastic cells by a factor from bovine maternal placenta: interaction of the inhibitor with cell membranes. Biosci Rep. 1982 Jan;2(1):39–45. doi: 10.1007/BF01142197. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Terayama H. Nature of rat liver cell sap factors inhibiting the DNA synthesis in tumour cells. Biochim Biophys Acta. 1971 Jan 28;228(2):324–330. doi: 10.1016/0005-2787(71)90040-2. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Coy D. H., Schally A. V. Prostate carcinoma tumor size in rats decreases after administration of antagonists of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1273–1276. doi: 10.1073/pnas.79.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V. Inhibition of prostate tumor growth in two rat models by chronic administration of D-Trp6 analogue of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6509–6512. doi: 10.1073/pnas.78.10.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- Schally A. V., Dupont A., Arimura A., Redding T. W., Nishi N., Linthicum G. L., Schlesinger D. H. Isolation and structure of somatostatin from porcine hypothalami. Biochemistry. 1976 Feb 10;15(3):509–514. doi: 10.1021/bi00648a009. [DOI] [PubMed] [Google Scholar]
- Tolis G., Ackman D., Stellos A., Mehta A., Labrie F., Fazekas A. T., Comaru-Schally A. M., Schally A. V. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1658–1662. doi: 10.1073/pnas.79.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]



