Abstract
Objectives
Psychotropic medications, including the atypical antipsychotics, have historically been scrutinized for cardiac effects and risk of sudden death. Aripiprazole is an atypical antipsychotic approved for pediatric use in schizophrenia, bipolar I disorder, and autistic disorder. Adult studies have evaluated aripiprazole's effects on electrocardiograms, but no pediatric studies have been published to date.
Methods
Electrocardiographic data were collected from children and adolescents participating in a 14-week, prospective, open-label study (n=25) of aripiprazole for irritability in pervasive developmental disorder not otherwise specified and Asperger's disorder. A 12-lead electrocardiogram was obtained at the baseline and end point visits. The electrocardiograms were evaluated for abnormal findings, and the PR, QRS, QTc, and RR intervals were recorded. The QT interval was corrected using Bazett's, United States Food and Drug Administration (FDA) Pharmacology Division, and Fridericia's formulas.
Results
Twenty-four subjects received both baseline and posttreatment electrocardiograms. The mean age was 8.6 years (range 5–17 years). The average final aripiprazole dose was 7.8 mg/day (range 2.5–15 mg/day). There were no significant differences noted with the PR, QRS, RR, and QTc intervals after aripiprazole therapy. Also, there was no significant correlation between the dose given and the percent change in the QTc. No post-treatment QTc exceeded 440 ms.
Conclusions
To our knowledge, this is the first systematic evaluation of the cardiac effects of aripiprazole in children and adolescents. The results are consistent with previously published literature in adults that aripiprazole has no significant cardiac effects and can be deemed a low risk for causing sudden death. It will be important to confirm these findings in a randomized controlled trial.
Introduction
Aripiprazole is one of the newer second-generation antipsychotics and was first approved for use in the United States in 2002 for treatment of schizophrenia in adults. Second-generation antipsychotics are called atypical because of their affinity for neuroreceptors other than dopamine (Muench and Hamer 2010). Aripiprazole acts as a partial agonist at the dopaminergic D2 receptor at lower doses, an antagonist at the dopaminergic D2 receptor at higher doses, a partial agonist at the serotonergic 5-HT1A receptor, and an antagonist at the 5-HT2A receptor, thus uniquely functioning as a dopamine–serotonin system stabilizer (Burris et al. 2002; Jordan et al. 2002; Winans 2003). Aripiprazole has an affinity for dopaminergic, serotonergic, histaminic, and α-adrenergic brain receptors (Shapiro et al. 2003).
The indications for aripiprazole in adults have been expanded to include bipolar I disorder and major depressive disorder. Pediatric indications include schizophrenia (ages 13–17 years), manic or mixed episodes related to bipolar I disorder (ages 10–17 years), and irritability associated with autistic disorder (ages 6–17 years). In addition, a recent prospective, open-label study suggests that aripiprazole may help reduce irritability in pediatric patients with pervasive developmental disorder not otherwise specified (PDD-NOS) and Asperger's disorder (Stigler et al. 2009).
Reports of sudden death from antipsychotic drugs have historically caused concern about possible cardiac effects. Antipsychotics including thioridazine, sertindole, and clozapine have been reported in the literature to be linked to sudden death (Kelly et al. 1963; Barnett 1996; Hoehns et al. 2001). Accordingly, antipsychotic medications were subsequently evaluated to determine their cardiac effects and risk for causing sudden death. Changes on the electrocardiogram (ECG)—specifically QTc prolongation—were studied to evaluate if a medication would be more likely to cause sudden death. Another surrogate marker that has been prominently studied is the affinity for a medication to bind and inhibit certain cardiac ion channels that result in QTc prolongation.
The QT interval on an ECG represents cardiac ventricular depolarization and repolarization to baseline. The QT shortens with faster heart rates and thus is standardized by “correcting” the QT with respect to the heart rate, producing the corrected QT interval (QTc). There are three methods reported in the literature as commonly seen to correct the QT interval: Bazett's (QTcB), United States Food and Drug Administration (FDA) Neuropharmacology Division (QTcN), and Fridericia's (QTcF) formulas (Bazett 1920; Casey et al. 2003; Witchel et al. 2003). Bazett's is the most prevalent, but has been shown to be inaccurate at low and high heart rates (Crumb and Cavero 1999).
Blockade of particular potassium (K+) currents will delay cardiac repolarization and cause a prolongation in the QT interval. Abnormally long QT prolongation has been associated with an increased risk of arrhythmia development—specifically an arrhythmia known as torsades de pointes (TdP)—and related sudden death. TdP is a polymorphic, multifocal ventricular arrhythmia that is triggered by early afterdepolarizations and subsequent premature ventricular contractions that occur during delayed repolarization (Vieweg 2003). If TdP progresses to ventricular fibrillation, then sudden death can occur. Adult studies have found a 10–17% mortality rate with TdP (Shah 2004).
Certain individuals are more susceptible to QT prolongation from drugs than others (Roden 2004). These individuals often have certain traits including the following: female gender, bradycardia, prolonged baseline QT, electrolyte disturbances, diuretic use, high dosing of medication, rapid intravenous drug administration, use of drugs interfering with cytochrome P450 metabolism, cardiac hypertrophy, and genetic risk factors (Lindström et al. 2005).
Measurement of the QTc has been associated with predicting an individual's risk for abnormal arrhythmias and subsequent sudden death. Normal QTc values have historically been set at 450 ms for males and 460 ms for females. A higher risk of TdP development occurs if the QTc is >500 ms or increases at least 60 ms above baseline (Schwartz et al. 1993; Haddad and Anderson 2002). There is also a higher risk of sudden death when the QTc surpasses 500 ms (Drici and Priori 2007).
Certain antipsychotics are known to block K+ currents. The incidence of QT prolongation with typical antipsychotics is ∼10%, with TdP developing in 1 in 10,000 users (Titier et al. 2005). The incidence of sudden cardiac death from individuals taking antipsychotics is twice that of the general population (Glassman and Bigger 2001; Ray et al. 2009). Antipsychotics have also been shown to cause PR prolongation, ST depression, and blunting of the T wave on ECGs (Drici and Priori 2007). Consequently, multiple studies have been published to evaluate ECG changes for atypical antipsychotics. Clozapine has been shown both to prolong the QTc interval and have an increased risk of causing sudden death with overdose (Trenton et al. 2003; Lin et al. 2004). Ziprasidone also can result in a significant increase in QTc (Blair et al. 2005; Correll et al. 2011). Quetiapine does not appear to significantly prolong the QT interval with normal dosing, but there is evidence that it can prolong the QT interval with overdosing (Posey et al. 1999; Harrigan et al. 2004). Conflicting reports exist concerning QT prolongation with both risperidone and olanzapine (Yerrabolu et al. 2000; Cohen et al. 2001; Czekalla et al. 2001; Chiu et al. 2005).
Regarding aripiprazole, there have been multiple published reports in the adult literature showing no evidence of clinically significant QTc prolongation. To date, there have been no studies focused on the effects of aripiprazole on electrocardiographic measures in pediatric patients. This study is based on electrocardiographic data collected during a 14-week, prospective, open-label study of aripiprazole for irritability in youth with PDD-NOS or Asperger's disorder (Stigler et al. 2009). Our original report found that 22 (88%) of 25 pediatric subjects with severe irritability responded to aripiprazole, based on Clinical Global Impressions-Improvement (CGI-I) scores of 1 or 2 and a 25% or greater improvement on the Irritability subscale of the Aberrant Behavior Checklist (ABC-I) (Stigler et al. 2009). The purpose of this current analysis is to evaluate the risk of aripiprazole for sudden cardiac events in pediatric patients by evaluating the effects of aripiprazole on pediatric ECGs before and after 14 weeks of therapy. The hypothesis of this study was that no significant change would be seen on ECG, including PR, QRS, and QTc intervals.
Methods
Subject selection
The Institutional Review Board at our institution reviewed and approved this prospective study. The Methods section for this study is based on a 14-week, prospective, open-label study performed at our institution (Stigler et al. 2009).
Twenty-five children and adolescents, aged 5–17 years, were enrolled in a 14-week, prospective, open-label study to determine the effectiveness and tolerability of aripiprazole for irritability in PDD-NOS and Asperger's disorder. Written informed consent was obtained from the participant's legal guardian, and subjects provided assent when able. The 25 subjects were all diagnosed with PDD-NOS or Asperger's disorder according to the criteria from the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) by a board-certified child and adolescent psychiatrist experienced in the assessment and diagnosis of PDDs.
Subjects were required to have a mental age of at least 18 months, as determined by the Wechsler Intelligence Scales or Leiter International Test of Intelligence-Revised (Roid and Miller 1997; Wechsler 1999). Subjects were required to be physically healthy and free of all psychotropic medications for at least 2 weeks (4 weeks for fluoxetine). Additional inclusion criteria included a CGI-Severity (S) scale score of at least 4 (“Moderately Ill”) focused specifically on target symptoms of irritability (aggression, self-injury, tantrums); and a score ≥ 18 on the Aberrant Behavior Checklist-Irritability subscale (ABC-I) (Guy 1976; Aman et al. 1985; Aman and Singh 1994).
Exclusion criteria included a comorbid DSM-IV-TR diagnosis of another PDD or other primary psychiatric disorder, active seizure disorder, significant medical condition, positive urine pregnancy test, or history of neuroleptic malignant syndrome. In addition, any subject who did not receive both a baseline and posttreatment ECG was excluded from this study.
Study design and monitoring
The subjects were enrolled in the prospective, open-label study to gather pilot data on aripiprazole in children and adolescents with PDD-NOS or Asperger's disorder. All subjects underwent a medical history and full psychiatric examination prior to entry into the study. After enrollment, all subjects were seen for a baseline visit with follow-up visits scheduled every 2 weeks. An end point visit was also scheduled at the conclusion of the 14-week study period. The baseline and end point visits consisted of a physical examination and a 12-lead ECG with rhythm strip. Vital signs, height, and weight were obtained at each visit.
All of the subjects initially received 1.25 mg/day of aripiprazole for 3 days. The dosage was then increased to 2.5 mg/day and continued until the end of week 2. The dosage was then titrated to a maximum of 15 mg/day over the next 4 weeks, if optimal clinical response had not occurred and intolerable adverse effects had not emerged. The dosage maintenance phase lasted 8 weeks at the optimal dosage.
All of the ECGs obtained by our clinical research nurse were of clinical diagnostic quality and free of artifact. In addition, no sedation was used in obtaining the ECGs. A board-certified pediatric cardiologist formally read every ECG and recorded any abnormal findings, the ventricular rate, PR interval, QRS interval, and QT interval. The QT interval was then corrected using Bazett's correction (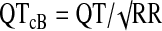 ), the FDA correction factor (QTcN=QT/RR0.37), and Fridericia's correction (
), the FDA correction factor (QTcN=QT/RR0.37), and Fridericia's correction (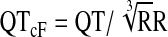 ). All intervals are listed in ms except for RR, which is listed in seconds.
). All intervals are listed in ms except for RR, which is listed in seconds.
Statistical analysis
The baseline and posttreatment ECG data for the various measurements were collected and analyzed using Microsoft Office Excel 2007 SP2 (Microsoft, Redmond, WA). Paired t tests were used to determine the significance of any ECG changes from baseline to the end of aripiprazole treatment. The data were presented as a mean with standard deviation (SD). Ranges were also presented for the respective measurements. Results were considered statistically significant when p≤0.05 (two-tailed).
The change in ECG measurements from baseline to posttreatment was analyzed. The mean, SD, range of the actual changes in value, and the percent difference represented were also calculated. In addition, the correlation coefficient of the percent change in QTc intervals to aripiprazole dose was calculated for each of the correction factors.
Results
Subject selection
Twenty-five subjects were included in the study. This group consisted of 19 males and 6 females with a mean age of 8.6 years (range 5–17 years). Twenty-one subjects were diagnosed with PDD-NOS, and four subjects were diagnosed with Asperger's disorder. Twenty-two (88%) subjects were Caucasian, two were African-American (8%), and one was Asian (4%). Eighteen (72%) subjects had a prior history of treatment with one or more psychotropic medications, primarily targeting aggression or hyperactivity. These medications included atomoxetine, carbamazepine, clonidine, dextroamphetamine, guanfacine, haloperidol, imipramine, methylphenidate, mixed amphetamine salts, paroxetine, quetiapine, risperidone, sertraline, and valproic acid.
Subjects received a mean final aripiprazole dosage of 7.8 mg/day (range 2.5–15 mg/day). Twenty-two (88%) of the 25 subjects completed all 14 weeks of the study. The remaining three subjects were included in the analysis according to the intent-to-treat principle (last observation carried forward). They completed 6 weeks (n=1) or 8 weeks (n=2) of aripiprazole prior to leaving the study, and all three had posttreatment ECGs at their exit visit. Two of the subjects withdrew because of parental request, and the other subject withdrew because of possible seizure activity, which was later determined to be unrelated to the study drug. No clinically significant changes in heart rate or blood pressure were recorded during the study.
All 25 subjects had an ECG obtained at baseline. Twenty-one (84%) had an ECG performed at the end of the 14-week period. Three subjects (12%) had their posttreatment ECGs performed at their respective early termination visits at 6 and 8 weeks of therapy. One subject (4%) did not have a posttreatment ECG available for analysis (missing data) and was excluded from the results of this study.
Electrocardiographic changes
There were no abnormal findings seen on ECG as a result of aripiprazole, including any changes in voltages, axes, or morphology. One subject had a leftward deviation of the QRS axis that was noted both on the baseline and posttreatment ECGs. Another subject had sinus tachycardia in the posttreatment ECG with baseline and posttreatment heart rates of 80 and 117 beats per minute, respectively. Of note, that subject terminated the study early at 8 weeks for a non-cardiac-related reason.
There was no significant difference noted on any of the ECG parameters from the baseline to posttreatment periods. There was a very minimal increase in the mean of the PR, QRS, RR, and QT intervals. The corrected QT interval was calculated using the three different corrections. In each case, the mean QTc was minimally decreased after treatment from the baseline measurement. The mean, SD, ranges, and p values of the baseline and posttreatment ECG measurements are shown in Table 1.
Table 1.
Comparison of Baseline and Posttreatment Electrocardiographic Measurements for Subjects Completing the Study (n=24)
| |
Baseline |
Posttreatment |
|
||
|---|---|---|---|---|---|
| Interval | Mean (SD) | Range | Mean (SD) | Range | p value |
| PR | 130 (19) | 98–170 | 133 (19) | 100–174 | 0.16 |
| QRS | 82 (13) | 56–106 | 82 (8.9) | 60–92 | 0.92 |
| RR | 0.73 (0.18) | 0.48–1.43 | 0.75 (0.16) | 0.52–1.3 | 0.42 |
| QT | 352 (33) | 290–440 | 353 (33) | 304–446 | 0.70 |
| QTcB | 416 (21) | 368–468 | 412 (16) | 385–439 | 0.23 |
| QTcN | 398 (18) | 375–443 | 395 (15) | 368–419 | 0.39 |
| QTcF | 393 (18) | 368–436 | 391 (16) | 363–414 | 0.49 |
QTcB=Bazett's correction (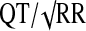 ); QTcN=FDA Neuropharmacology Division's correction (QT/RR0.37); QTcF=Fridericia's correction (
); QTcN=FDA Neuropharmacology Division's correction (QT/RR0.37); QTcF=Fridericia's correction (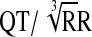 ).
).
There were no significant changes in any of the electrocardiographic parameters after the treatment phase of aripiprazole. All of the numbers measured were in the normal range for their respective intervals. All values are expressed in ms, except RR which is expressed in seconds. Statistical significance is defined as p≤0.05 using a paired, two-tailed t test.
Focusing on the difference and percent change of the various ECG parameters, none of the parameters changed significantly in either direction, as seen in Table 2. All three corrected QTc means decreased after aripiprazole treatment with individual differences ranging from −54 to +23 ms. This represented individual percent changes ranging from −11.5% to +6.1%. With respect to the means, QTc percent changes were all < −1%.
Table 2.
Effect of Aripiprazole on Electrocardiographic Measurements for Subjects Completing the Study (n=24)
| |
Change |
% Change |
||
|---|---|---|---|---|
| Interval | Mean (SD) | Range | Mean (SD) | Range |
| PR | 3 (11) | −22–22 | 2.8 (8.3) | −15.3–18 |
| QRS | 0 (9) | −19–16 | 1.0 (10.4) | −21.3–21.6 |
| RR | 0.02 (0.12) | −0.28–0.17 | 4.3 (15.7) | −35.3–22.8 |
| QT | 2 (24) | −72–32 | 0.8 (6.6) | −18.8–9.4 |
| QTcB | −4 (16) | −54–23 | −0.9 (3.8) | −11.5–6.1 |
| QTcN | −2 (13) | −41–19 | −0.5 (3.2) | −9.3–4.8 |
| QTcF | −2 (13) | −38–19 | −0.4 (3.2) | −8.7–4.9 |
QTcB=Bazett's correction (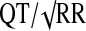 ); QTcN=FDA Neuropharmacology Division's correction (QT/RR0.37); QTcF=Fridericia's correction (
); QTcN=FDA Neuropharmacology Division's correction (QT/RR0.37); QTcF=Fridericia's correction (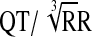 ).
).
Aripiprazole had a very minor effect on the electrocardiographic parameters measured. The absolute change is expressed in ms. The % change is reported in percentages.
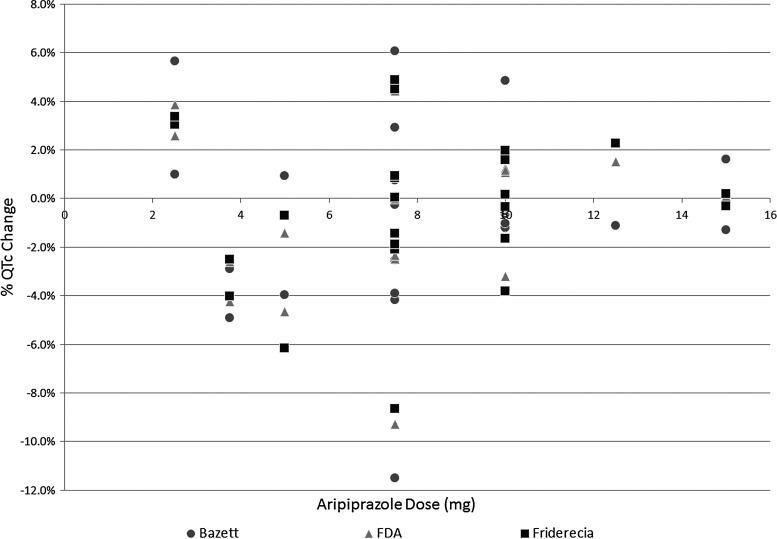
In addition, no clear correlation could be found between the aripiprazole dose given (range 2.5–15 mg) and the percent change in the respective QTc values as seen in Figure 1. The correlation coefficients between aripiprazole dose and the Bazett's, FDA, and Fridericia's QTc corrections were 0.04, 0.07, and 0.08, respectively.
FIG 1.
Correlation between aripiprazole dose and percent QTc change. A scatter plot showing the relationship of aripiprazole dose in mg to the resulting percent change among QTc values. No significant correlation was found for any of the listed correction factors.
Finally, evaluating individual results, 3 subjects out of the 24 had a baseline QTc value>440 ms. One subject had a respective baseline QTcB value of 468 ms, the second subject had a QTcB of 448 ms, and the third subject had a QTcB of 445 ms. In all three cases, the subjects' QTc values decreased after receiving aripiprazole. The first subject's posttreatment QTcB was 414 ms, showing a decrease of 54 ms (-11.5%). In fact, no posttreatment QTc value across the three corrections in any subject exceeded 440 ms.
Discussion
Aripiprazole
This is the first known study to evaluate prospectively the effects of aripiprazole on pediatric ECGs before and after a specified treatment period. The results confirmed the hypothesis that no significant change would occur on the posttreatment ECGs. Specifically, there were no significant differences in the PR, QRS, RR, QT, or QTc intervals in this study. These results agree with previously published reports in adults, which have shown that aripiprazole is one of the safest atypical antipsychotics with respect to cardiac adverse events.
Because aripiprazole is relatively new to the market compared to other atypical antipsychotics, existing data are relatively limited concerning adverse events. From the adult data that has been published, no evidence has been shown that aripiprazole causes any significant QTc prolongation as defined by a QTc ≥450 ms or a ≥10% increase from baseline. The studies reviewed followed subjects for 4–52 weeks using various dosages, and only Kasper et al. had subjects with a QTc ≥500 ms, which was found to be an isolated finding in 2 out of 810 individuals (Casey et al. 2003; Kasper et al. 2003; Potkin et al. 2003; Chrzanowski et al. 2006; McEvoy et al. 2007; Zimbroff et al. 2007). In addition, a meta-analysis was performed on various randomized-controlled trials to determine the effects of atypical antipsychotics on the QTc. Aripiprazole was found to be the only atypical antipsychotic out of seven that demonstrated both a statistically significant lesser mean change in QTcB and a statistically significant lower risk of causing QTcB prolongation (Chung and Chua 2011).
The proper evaluation of the cardiac effects of a medication includes both its effects during typical usage and during overdoses. Young et al. (2009) evaluated 286 cases of aripiprazole toxicity reported to a poison control center. These cases included 157 subjects who were < 18 years of age. None of the reported subjects had dysrhythmias or abnormal QRS or QTc intervals on ECG (Young et al. 2009). The main symptoms of aripiprazole toxicity include somnolence, nausea, vomiting, ataxia, and tremulousness (Melhem et al. 2009).
Risk of QTc prolongation
Drug-related prolongation of QT intervals occurs as certain medications bind and create malfunction of particular K+ currents during repolarization in cardiac myocytes. The particular current involved is the rapidly activating delayed rectifier potassium current (IKr) encoded by the human Ether-a-go-go Related Gene (hERG). Studies have found that almost all versions of drug-induced long QT syndrome and TdP are related to blockade of this K+ current and gene (Yap and Camm 2000; Glassman and Bigger 2001; Kannankeril et al. 2010). Unlike congenital long QT syndrome, drug-related prolongation in QTc does not always directly correlate with risk of arrhythmia production (Taylor 2003).
To evaluate the hERG blockade capabilities of various antipsychotics, Silvestre and Prous used a whole-cell patch clamp technique to determine the half-maximal inhibitory concentration (IC50) of the medications on human embryonic kidney cells. Higher potency for blocking hERG would theoretically result in an increased risk of prolonging the QTc interval and producing TdP. In this study, antipsychotics with high potency for blocking hERG produced IC50 values in the nanomolar (nM) range, indicating that smaller concentrations were required to reach IC50. Aripiprazole's hERG binding was found to be low in potency and was in the micromolar (μM) range. To illustrate this point further, Silvestre and Prous found that the IC50 value for pimozide—an antipsychotic known to cause QT prolongation and TdP—was 6.49 nM compared to aripiprazole's IC50 value of 1,096 nM (MHRA/CSM 1995). Antipsychotics with IC50 values for hERG blockade < 600 nM should be considered as having a high arrhythmogenic risk (Silvestre and Prous 2007).
Electrocardiography
The values in the ECG parameters in this study were obtained by the reviewing pediatric cardiologist. There was no comparison performed in this study between automated and manual measurements, as this difference has already been established. There is a significant amount of correlation between manual and automated readings (r=0.52), but poor agreement between the two in identifying long QTc intervals (κ=0.25). Compared with manual measurements as the gold standard, automated measurements had a sensitivity of 25% and a specificity of 95% (Blair et al. 2005).
Clinical practice
Although the results of this study and evidence in the literature point to aripiprazole having little risk of QTc prolongation and TdP development, certain aspects should be understood when using atypical antipsychotics in clinical practice. First, a detailed personal, medical, and family history and physical examination is paramount to determine any underlying risk factors an individual may have, before starting treatment. Multiple interactions can increase the risk of drug-related long QT syndrome and sudden death. Specific patient populations are at higher risk of sudden death from antipsychotics. These include individuals with congenital long QT syndrome, structural heart disease, or predisposition for electrolyte abnormalities. In addition, certain combinations of medications may also result in dangerous interactions, including multiple medications that can prolong the QT interval or medications that interfere with the cytochrome P450 metabolism of certain QT-prolonging medications, thus possibly increasing the medication's blood levels (Glassman and Bigger 2001; Titier et al. 2005; McNally et al. 2007). Web sites have been created containing databases of medications that are known to prolong the QT interval (www.azcert.org) or interfere with the cytochrome P450 metabolism of QT prolonging medications (www.drug-interactions.com).
Aripiprazole is metabolized by the cytochrome P450 2D6 (CYP2D6) and cytochrome P450 3A4 (CYP3A4) enzymes and therefore is subject to medications that interact with these enzymes. Medications that induce those enzymes—such as carbamazepine with CYP3A4—can result in increased clearance of aripiprazole and decreased blood levels. On the other hand, medications that inhibit those enzymes—such as fluoxetine and paroxetine with CYP2D6—can increase blood levels by inhibiting clearance of aripiprazole. Aripiprazole itself is unlikely to cause pharmacokinetic interactions with other medications metabolized by cytochrome P450.
Universal screening ECGs are not likely to be cost effective except in patients with certain baseline risk factors as described previously (Roden 2004). However, the data for aripiprazole have shown no evidence of QTc prolongation in high-dose animal, adult, or pediatric studies; therefore, the current recommendation for aripiprazole is that no ECG is needed for monitoring with or without risk factors (McNally et al. 2007).
Nevertheless, varying recommendations exist in the literature concerning the importance of screening ECGs when starting certain psychotropic medications. To determine the most at-risk individuals, an evaluation prior to starting the medication should be performed. Histories should focus on screening for any clinical symptoms including syncope, palpitations, or unexplained shortness of breath with exercise or exercise intolerance, as well as reviewing past medical history for any evidence of congenital or acquired heart disease. Questions concerning family history should determine the presence of sudden or unexplained death, familial arrhythmias, heart failure, or conduction abnormalities in any first- or second-degree relatives. Finally, a physical examination should evaluate for the presence of hypertension, murmurs, gallops, clicks, or other pathologic cardiovascular findings (Perrin et al. 2008; Warren et al. 2009; Hammerness et al. 2011).
Limitations
As occurs many times in the pediatric literature with prospective studies, one of the limitations of this study is the relatively small number of subjects enrolled. In addition, this study was based on an open-label trial. As such, these results should be confirmed in the future with a double-blind, placebo-controlled study, to further evaluate this topic and reduce the risk for type II errors.
Conclusions
To our knowledge, this is the first direct evaluation of the effects of aripiprazole on ECGs in pediatric subjects. This study's results agree with previously published data that aripiprazole has no significant effects on the QTc interval and therefore can be deemed a low risk for TdP development and causing sudden death. These preliminary findings suggest that for pediatric patients requiring atypical antipsychotics, aripiprazole should be considered in those who have higher risks for a prolonged QT interval compared with the general population. Large-scale prospective studies are needed to confirm these findings.
Clinical Significance
This is an original, prospective clinical research study focusing systematically on the effects of aripiprazole on the ECGs of pediatric patients. This association has been extensively reviewed in studies with adult subjects. In pediatrics, no study has focused primarily on the cardiac effects of aripiprazole. Previous discussion on the topic has been limited to part of the adverse events section in studies focusing on the efficacy of aripiprazole. This study shows that aripiprazole has no significant effects on ECGs.
Disclosures
Dr. McDougle has affiliations with Bristol-Myers Squibb Co., F. Hoffmann-LaRoche Ltd., and Forest Research Institute. Dr. Erickson has affiliations with Bristol-Myers Squibb Co., F. Hoffmann-LaRoche Ltd., and Seaside Therapeutics. Dr. Posey has affiliations with Bristol-Myers Squibb Co., Eli Lilly and Co., Forest Research Institute, Novartis, and Shire. Dr. Stigler has affiliations with Bristol-Myers Squibb Co., Eli Lilly and Co., Forest Research Institute, Ortho-McNeil Janssen, and Seaside Therapeutics. Dr. Ho, Dr. Caldwell, and Ms. Orsagh-Yentis have no disclosures.
References
- Aman MG. Singh NN. Supplement to Aberrant Behavior Checklist Manual. East Aurora, NY: Slosson Educational Publications; 1994. [Google Scholar]
- Aman MG. Singh NN. Stewart AW. Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–91. [PubMed] [Google Scholar]
- Barnett AA. Safety concerns over antipsychotic drug, sertindole. Lancet. 1996;348:256–257. [Google Scholar]
- Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- Blair J. Scahill L. State M. Martin A. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44:73–79. doi: 10.1097/01.chi.0000145372.61239.bb. [DOI] [PubMed] [Google Scholar]
- Burris KD. Molski TF. Xu C. Ryan E. Tottori K. Kikuchi T. Yocca FD. Molinoff PB. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human D2 dopamine receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- Casey DE. Carson WH. Saha AR. Liebeskind A. Ali MW. Jody D. Ingenitoon GG. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology. 2003;166:391–399. doi: 10.1007/s00213-002-1344-3. [DOI] [PubMed] [Google Scholar]
- Chiu CC. Chang WH. Huang MC. Chiu YW. Lane HY. Regular-dose risperidone on QTc intervals. J Clin Psychopharmacol. 2005;25:391–393. doi: 10.1097/01.jcp.0000170032.78060.cd. [DOI] [PubMed] [Google Scholar]
- Chrzanowski WK. Marcus RN. Torbeyns A. Nyilas M. McQuade RD. Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: A 52-week, open-label comparison with olanzapine. Psychopharmacology (Berl) 2006;189:259–266. doi: 10.1007/s00213-006-0564-3. [DOI] [PubMed] [Google Scholar]
- Chung AK. Chua SE. Effects on prolongation of Bazett's corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: A meta-analysis. J Psychopharmacol. 2011;25:646–666. doi: 10.1177/0269881110376685. [DOI] [PubMed] [Google Scholar]
- Cohen H. Loewenthal U. Matar M. Kotler M. Association of autonomic dysfunction and clozapine. Heart rate variability and risk for sudden death in patients with schizophrenia on long-term psychotropic medication. Br J Psychiatr. 2001;179:167–171. doi: 10.1192/bjp.179.2.167. [DOI] [PubMed] [Google Scholar]
- Correll CU. Lops JD. Figen V. Malhotra AK. Kane JM. Manu P. QT interval duration and dispersion in children and adolescents treated with ziprasidone. J Clin Psychiatry. 2011;72:854–860. doi: 10.4088/JCP.10m05990yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumb W. Cavero I. QT interval prolongation by non-cardiovascular drugs: Issues and solutions for novel drug development. Pharm Sci Technol Today. 1999;2:270–280. doi: 10.1016/s1461-5347(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Czekalla J. Beasley CM., Jr Dellva MA. Berg PH. Grundy S. Analysis of the QTc interval during olanzapine treatment of patients with schizophrenia and related psychosis. J Clin Psychiatry. 2001;62:191–198. doi: 10.4088/jcp.v62n0310. [DOI] [PubMed] [Google Scholar]
- Drici M. Priori S. Cardiovascular risks of atypical antipsychotic drug treatment. Pharmacoepidemiol Drug Saf. 2007;16:882–890. doi: 10.1002/pds.1424. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Bigger JT., Jr Antipsychotic drugs: Prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology (NIMH Publication No. 76-338) Washington, DC: Department of Health, Education, and Welfare, National Institute of Mental Health; 1976. [Google Scholar]
- Haddad PM. Anderson IM. Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs. 2002;62:1649–1671. doi: 10.2165/00003495-200262110-00006. [DOI] [PubMed] [Google Scholar]
- Hammerness PG. Perrin JM. Shelley–Abrahamson R. Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50:978–990. doi: 10.1016/j.jaac.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Harrigan EP. Miceli JJ. Anziano R. Watsky E. Reeves KR. Cutler NR. Sramek J. Shiovitz T. Middle M. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–69. doi: 10.1097/01.jcp.0000104913.75206.62. [DOI] [PubMed] [Google Scholar]
- Hoehns JD. Fouts MM. Kelly MW. Tu KB. Sudden cardiac death with clozapine and sertraline combination. Ann Pharmacother. 2001;35:862–866. doi: 10.1345/aph.16185. [DOI] [PubMed] [Google Scholar]
- Jordan S. Koprivica V. Chen R. Tottori K. Kikuchi T. Altar CA. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441:137–140. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- Kasper S. Lerman MN. McQuade RD. Saha A. Carson WH. Ali M. Archibald D. Ingenito G. Marcus R. Pigott T. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol. 2003;6:325–337. doi: 10.1017/S1461145703003651. [DOI] [PubMed] [Google Scholar]
- Kannankeril P. Roden DM. Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly HG. Fay JE. Laverty SG. Thioridazine hydrochloride (Mellaril): Its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J. 1963;89:546–554. [PMC free article] [PubMed] [Google Scholar]
- Lin CH. Chen MC. Wang SY. Lin CY. Predictive factors for QTc prolongation in schizophrenic patients taking antipsychotics. J Formos Med Assoc. 2004;103:437–441. [PubMed] [Google Scholar]
- Lindström E. Farde L. Eberhard J. Haverkamp W. QTc interval prolongation and antipsychotic drug treatments: Focus on sertindole. Int J Neuropsychopharmacol. 2005;8:615–629. doi: 10.1017/S1461145705005250. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. Daniel DG. Carson WH., Jr McQuade RD. Marcus RN. A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res. 2007;41:895–905. doi: 10.1016/j.jpsychires.2007.05.002. [DOI] [PubMed] [Google Scholar]
- McNally P. McNiholas F. Oslizlok P. The QT interval and psychotropic medications in children: Recommendations for clinicians. Eur Child Adolesc Psychiatry. 2007;16:33–46. doi: 10.1007/s00787-006-0573-0. [DOI] [PubMed] [Google Scholar]
- Melhem S. Katz K. Jameson A. Shellenbarger D. Akhtar J. Prolonged toxicity in a 2-year-old after accidental ingestion of aripiprazole. Pediatr Emerg Care. 2009;25:105–106. doi: 10.1097/PEC.0b013e318196faaf. [DOI] [PubMed] [Google Scholar]
- MHRA/CSM: Cardiac arrhythmias with pimozide (Orap) Curr Probl Pharmacovigilance. 1995;21:1. [Google Scholar]
- Muench J. Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81:617–622. [PubMed] [Google Scholar]
- Perrin JM. Friedman RA. Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451–453. doi: 10.1542/peds.2008-1573. [DOI] [PubMed] [Google Scholar]
- Posey DJ. Walsh KH. Wilson GA. McDougle CJ. Risperidone in the treatment of two very young children with autism. J Child Adolesc Psychopharmacol. 1999;9:273–276. doi: 10.1089/cap.1999.9.273. [DOI] [PubMed] [Google Scholar]
- Potkin SG. Saha AR. Kujawa MJ. Carson WH. Ali M. Stock E. Stringfellow J. Ingenito G. Marder SR. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60:681–690. doi: 10.1001/archpsyc.60.7.681. [DOI] [PubMed] [Google Scholar]
- Ray WA. Chung CP. Murray KT. Hall K. Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death [Erratum N Engl J Med 361:1814, 2009] N Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- Roid GH. Miller LJ. Leiter International Performance Scale–Revised. Wood Dale, IL: Stoelting Company; 1997. [Google Scholar]
- Schwartz PJ. Moss AJ. Vincent GM. Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88:782–784. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- Shah RR. Drug-induced QT interval prolongation: regulatory perspectives, drug development. Ann Med. 2004;36(Suppl):47–52. doi: 10.1080/17431380410032445. [DOI] [PubMed] [Google Scholar]
- Shapiro DA. Renock S. Arrington E. Chiodo LA. Liu LX. Sibley DR. Roth BL. Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Silvestre JS. Prous JR. Comparative evaluation of hERG potassium channel blockade by antipsychotics. Methods Find Exp Clin Pharmacol. 2007;29:457–465. doi: 10.1358/mf.2007.29.7.1119172. [DOI] [PubMed] [Google Scholar]
- Stigler KA. Diener JT. Kohn AE. Li L. Erickson CA. Posey DJ. McDougle CJ. Aripiprazole in pervasive developmental disorder not otherwise specified and Asperger's disorder: A 14-week, prospective, open-label study. J Child Adolesc Psychopharmacol. 2009;19:265–274. doi: 10.1089/cap.2008.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107:85–95. doi: 10.1034/j.1600-0447.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- Titier K. Girodet P-O. Verdoux H. Molimard M. Bégaud B. Haverkamp W. Lader M. Moore N. Atypical antipsychotics: From potassium channels to Torsade de Pointes and sudden death. Drug Saf. 2005;28:35–51. doi: 10.2165/00002018-200528010-00003. [DOI] [PubMed] [Google Scholar]
- Trenton A. Currier G. Zwemer F. Fatalities associated with therapeutic use and overdose of atypical antipsychotics. CNS Drugs. 2003;17:307–324. doi: 10.2165/00023210-200317050-00002. [DOI] [PubMed] [Google Scholar]
- Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. J Clin Psychiatry. 2003;5:205–215. doi: 10.4088/pcc.v05n0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren AE. Hamilton RM. Bélanger SA. Gray C. Gow RM. Sanatani S. Côté JM. Lougheed J. LeBlanc J. Martin S. Miles B. Mitchell C. Gorman DA. Weiss M. Schachar R. Cardiac risk assessment before the use of stimulant medications in children and youth: A joint position statement by the Canadian Paediatric Society, the Canadian Cardiovascular Society, and the Canadian Academy of Child and Adolescent Psychiatry. Can J Cardiol. 2009;25:625–630. doi: 10.1016/s0828-282x(09)70157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans E. Aripiprazole. Am J Health Syst Pharm. 2003;60:2437–2445. doi: 10.1093/ajhp/60.23.2437. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Witchel HJ. Hancox JC. Nutt DJ. Psychotropic drugs, cardiac arrhythmia, and sudden death. J Clin Psychopharmacol. 2003;23:58–77. doi: 10.1097/00004714-200302000-00010. [DOI] [PubMed] [Google Scholar]
- Yap YG. Camm J. Risk of torsades de pointes with non-cardiac drugs. Br Med J. 2000;320:1158–1159. doi: 10.1136/bmj.320.7243.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerrabolu M. Prabhudesai S. Tawam M. Winter L. Kamalesh M. Effect of risperidone on QT interval and QT dispersion in the elderly. Heart Dis. 2000;2:10–12. [PubMed] [Google Scholar]
- Young MC. Shah N. Cantrell FL. Clark RF. Risk assessment of isolated aripiprazole exposures and toxicities: a retrospective study. Clin Toxicol (Phila) 2009;47:580–583. doi: 10.1080/15563650902980027. [DOI] [PubMed] [Google Scholar]
- Zimbroff D. Warrington L. Loebel A. Yang R. Siu C. Comparison of ziprasidone and aripiprazole in acutely ill patients with schizophrenia or schizoaffective disorder: A randomized, double-blind, 4-week study. Int Clin Psychopharmacol. 2007;22:363–370. doi: 10.1097/YIC.0b013e32816f7779. [DOI] [PubMed] [Google Scholar]