Abstract
Several recent studies suggest that predegenerated nerves (PDNs) or dissociated PDNs (dPDNs) can improve behavioral and histological outcomes following transplantation into the injured rat spinal cord. In the current study we tested the efficacy of dPDN transplantation by grafting cells isolated from the sciatic nerve 7 days after crush. We did not replicate one study, but rather assessed what appeared, based on five published reports, to be a reported robust effect of dPDN grafts on corticospinal tract (CST) regeneration and locomotor recovery. Using a standardized rodent spinal cord injury model (200 kD IH contusion) and transplantation procedure (injection of GFP+ cells 7 days post-SCI), we demonstrate that dPDN grafts survive within the injured spinal cord and promote the ingrowth of axons to a similar extent as purified Schwann cell (SC) grafts. We also demonstrate for the first time that while both dPDN and SC grafts promote the ingrowth of CGRP axons, neither graft results in mechanical or thermal hyperalgesia. Unlike previous studies, dPDN grafts did not promote long-distance axonal growth of CST axons, brainstem spinal axons, or ascending dorsal column sensory axons. Moreover, using a battery of locomotor tests (Basso Beattie Bresnahan [BBB] score, BBB subscore, inked footprint, Catwalk, and ladderwalk), we failed to detect any beneficial effects of dPDN transplantation on the recovery of locomotor function after SCI. We conclude that dPDN transplants are not sufficient to promote CST regeneration or locomotor recovery after SCI.
Key words: axon regeneration, Basso Beattie Bresnahan score, Catwalk, Hargreaves, ladderwalk, peripheral nerve, Schwann cells, spinal cord injury, transplant, von Frey
Introduction
Numerous studies have demonstrated that axons regenerate into both peripheral nerve (PN) grafts and Schwann cell (SC) grafts (Chen et al., 1996; David and Aguayo, 1981; Richardson et al., 1982; Xu et al., 1995b,1997,1999). At present, however, there is no evidence that corticospinal tract (CST) axons enter PN grafts (David and Aguayo, 1981; Richardson et al., 1982; Hiebert et al., 2002), or SC bridges (Chen et al., 1996; Meijs et al., 2004; Menei et al., 1998; Xu et al., 1995a,1997,1999), with or without the addition of growth factors, except when acidic fibroblast growth factor (aFGF) is present (Cheng et al., 1996). In contrast, there have been several reports of CST regeneration following transplantation of predegenerated nerves (PDNs; Dinh et al., 2007; Rasouli et al., 2006), or cells isolated from them (Ban et al., 2009; Feng et al., 2008; Ferguson et al., 2001) in rodent spinal cord injury (SCI) models. This CST response is augmented when neurotrophins are present (Feng et al., 2008). Transplantation of intact or dissociated PDNs (dPDNs) was also reported to enhance open-field locomotion as assessed by the Basso Beattie Bresnahan (BBB) score after both dorsal hemisection (Dinh et al., 2007), and contusive SCI (Ban et al., 2009; Feng et al., 2008; Rasouli et al., 2006).
This reported efficacy of intact and dPDN transplants led us to initiate a study to compare the transplantation of PDN cells with our usual technique of SC transplantation into a clinically relevant, standardized contusion model. The five studies reporting beneficial effects of PDN cells used differing SCI models (e.g., contusion and dorsal hemisection), different injury levels (e.g., T10 and T11), and various times post-injury for transplantation (e.g., 1, 2, 3, and 25 weeks). Also, different protocols were employed for the preparation of cells (intact, dissociated or cultured PDN cells), different transplantation media (with or without collagen and growth factors), and different placements of the transplants (into the lesion or on top of the lesion). Because all of the studies demonstrated efficacy despite these differences, we tested the effectiveness of dPDN transplants using an injury model (moderate thoracic contusion at T9), transplant procedure (injection into the lesion epicenter 7 days post-SCI), and behavioral testing procedures (BBB, ladderwalk, Catwalk, inked footprint, von Frey and Hargreave tests), with which we have extensive experience. Thus, our study is not a replication of any one of the five earlier investigations cited.
We sought to improve upon previous reports in several ways. One, we expressed green fluorescent protein (GFP) in the transplanted cells to conclusively identify them and assess the size of the transplants, whereas previous studies used unlabeled cells. Two, we assessed ingrowth of multiple axonal populations using immunohistochemistry (neurofilament, calcitonin gene-related peptide [CGRP], and serotonin [5-HT] antibody staining), and anterograde tracing (CST and ascending sensory axons), instead of examining only CST regeneration. Three, we quantified the number of transplanted cells prior to transplantation; no effort was made previously to determine this number. Four, because our focus was to assess functional recovery, it was not possible to use sural nerve autografts, which can induce deficits in hindlimb function (Houle et al., 2006). Instead, highly inbred Fischer 344 rats were used as donors and recipients, to avoid the need for immunosuppression. Therefore, it was our goal to compare dPDN transplants with changes we thought salutary with implants of SCs by our previous methods. No attempt was made to use a combination strategy such as adding neurotrophins, which we have found in other studies to increase efficacy.
Using a comprehensive histological and behavioral approach, we demonstrate that PDN cell transplants promote the ingrowth of axons. We were unable, however, to demonstrate CST regeneration or any beneficial effects on locomotor recovery following transplantation of PDN cells into the injured spinal cord.
Methods
Schwann cell cultures
SCs were extracted from the sciatic nerves of adult female Fischer 344 rats (Harlan Sprague Dawley, Indianapolis, IN). The nerves were isolated and placed in D10 medium (DMEM with 10% fetal bovine serum [FBS]). After 2 weeks, explants were treated with dispase (Roche, Indianapolis, IN) and collagenase (Worthington, Lakewood, NJ), dissociated, and the SCs were centrifuged (1500 rpm/370 g at 4°C). The SCs were then resuspended in D10-3M (D10 containing pituitary extract, 20 μg/mL, Biomedical Technologies, Stoughton, MA; forskolin, 2 μM, Sigma-Aldrich, St. Louis, MO; and heregulin, 2.5 nM, Genentech, San Francisco, CA). Once confluent, fibroblasts were eliminated using Thy1.1 and rabbit complement (MP Biomedicals, Solon, OH) treatment. The resulting cultures were 95–99% SCs. At passage 2, SCs were infected with a lentiviral vector carrying the GFP gene. A multiplicity of infection (MOI) of 30 was used. This resulted in >99% of the SCs expressing GFP. Following lentiviral infection, the SCs were frozen at −80°C in D10 medium with 8% dimethyl sulfoxide (DMSO) and 20% FBS, and subsequently stored in liquid nitrogen until ready for use. For transplantation, the cells were thawed, plated onto poly-L-lysine-coated culture plates (PLL; Sigma-Aldrich), expanded to confluence, trypsinized, and resuspended in DMEM-F12 medium. A total of 2×106 cells in 6 μL were transplanted per animal.
Cells isolated from predegenerated nerves
The animals were anesthetized with isoflurane in 2% oxygen. The sciatic nerves of adult, female, Fischer 344 rats (Harlan) were isolated bilaterally by separating the muscle along the fascia following a skin incision along the thigh. Each nerve was crushed twice, as close to the hip as possible, with fine forceps for 10 seconds. The skin was then closed with wound clips. The animals were given buprenorphine (0.05 mg/kg, twice a day for 2 days), and gentamicin (5 mg/kg daily for 7 days).
Seven days after sciatic nerve crush, the animals were sacrificed by administration of CO2 followed by cervical dislocation. The entire sciatic nerve distal to the crush site, including the sural nerve, was isolated and placed in L15 medium (Gibco, Carlsbad, CA). The nerves were pooled, cleaned, stripped of the perineurium from the major fascicles, minced, and rinsed with DMEM+gentamicin. The minced nerves were then treated with 1.67 mg/mL collagenase type XI (Sigma-Aldrich) and 0.67 mg/mL dispase (Gibco) for 30 min at 37°C, before adding 8.3 μg/mL DNAase (Sigma-Aldrich). The nerves were triturated with a fine pipette, incubated for an additional 30 min at 37°C, and triturated. D10 medium was added and the cell suspension was centrifuged for 10 min at 1000 rpm (164 g). The pellet was resuspended in fresh D10 two additional times and centrifuged for 5 min at 1500 rpm (370 g). After the final centrifugation the pellet was resuspended in 1 mL of D10, and lentiviral GFP (30 MOI) was added, and the dissociated tissue was incubated overnight at 37°C and 6% CO2. The following day, the cells were rinsed with DMEM-F12 and centrifuged (1500 rpm/370 g for 5 min) twice, before resuspending in sufficient DMEM-F12 to allow for passage of the mixture through the injection pipette. Due to extensive myelin debris, a total of 1.7×105 cells in 6 μL was transplanted. Preliminary in vitro experiments tested a range of MOIs (10, 30, 50, and 100). An MOI of 30 resulted in most of the cells expressing GFP after 3 days. Higher MOIs did not result in an obvious increase in the number of GFP-expressing cells upon microscopic visualization.
Spinal cord injury
Sixty-three adult female Fischer 344 rats received an SCI (Table 1; Experiment 1, n=24; Experiment 2, n=39). Their mean weight±SEM for Experiment 1 was 190±1.4 g, and for Experiment 2 was 196±1 g. The animals were anesthetized with isoflurane and 2% O2, the skin on the back was incised, and the T8–T10 vertebrae were isolated. A laminectomy was performed at T9 to expose the underlying spinal cord. The lateral processes of T8 and T10 were clamped, and the rats received a 200 kD injury (mean±SEM: Experiment 1=205±0.8 kD; Experiment 2=206.5±1.1 kD) with the Infinite Horizons impactor. The muscles were sutured in anatomical layers and the skin was closed with wound clips. The animals received 10 ml of lactated Ringer's solution, buprenorphine (0.05 mg/kg twice a day for 2 days), and gentamicin (5 mg/kg daily for 7 days). The bladders were manually expressed twice a day until reflex urination returned.
Table 1.
Numbers of Rats Assigned to Treatment Groups and Included in Behavioral and Histological Analyses
| Total contused | Excluded prior to TP | Assigned to TP group | Died at/after surgery | Removed for 3 week histology | Behavioral analysis | CST analysis | CTβ analysis | Histological analysis | Plastic sections | |
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | 24 | 3 | 21 | 2 | 2 | 17 | 13 | 4 | ||
| SCI+dPDN | 12 | 2 | 1 | 9 | 7 | 7 | 2 | |||
| SCI | 9 | 1 | 8 | 6 | 6 | 2 | ||||
| Experiment 2 | 39 | 9 | 30 | 1 | 29 | 10 | 16 | 26 | 3 | |
| SCI+SC | 9 | 1 | 8 | 2 | 5 | 7 | 1 | |||
| SCI+dPDN | 10 | 0 | 10 | 4 | 5 | 9 | 1 | |||
| SCI | 11 | 0 | 11 | 4 | 6 | 10 | 1 |
SCI, spinal cord injury; dPDN, dissociated predegenerated nerve; SC, Schwann cell; CST, corticospinal tract; CTβ, cholera-toxin beta; TP; transplantation.
Transplantation
Seven days after SCI, the animals underwent transplantation. To be included in the study, the rats needed to have a BBB score less than 4 at 1 day, and of 7 to 10.5 at 7 days post-SCI. Animals with scores outside of this range (Experiment 1, n=3; Experiment 2, n=9) were removed and used for a different study not involving behavioral outcome measures. Two additional rats died at the time of transplantation in Experiment 1. The remaining rats in Experiment 1 were divided into two groups: transplantation of dPDNs (n=9) or SCI controls (n=10). One rat from each group in Experiment 1 was sacrificed early (3 weeks) to examine early transplant histology. In Experiment 2, the rats were randomly assigned to one of three treatment groups: transplantation of dPDNs (n=10), transplantation of SCs (n=9), or injection of medium (n=11). On the day of transplantation, the rats were randomly assigned to the treatment groups based on the number of cells available. BBB scores at days 1 and 7 were examined to ensure that the scores were similar for each of the groups. In instances when the BBB scores were not balanced between the groups, individual rats were reassigned to ensure that the mean BBB scores at days 1 and 7 were similar for all groups at the time of transplantation.
Rats were anesthetized with ketamine and xylazine, the laminectomy site was re-exposed and 6 μL of GFP-SCs, GFP-dPDN cells, or DMEM-F12 were injected using a pulled glass capillary tube attached to a Hamilton syringe. The dorsal process of T8 was clamped to stabilize the vertebral column and the injections were made stereotaxically using the Hamilton syringe affixed to a motorized nano-injector (Stoelting, Wood Dale, IL). Injections occurred at a rate of 1 μL/min into the injury site at a depth of 1.5 mm. The needle was left in place for an additional 2 min before removal, and the muscles were sutured closed in anatomical layers, then the skin was closed with wound clips. The animals received lactated Ringer's, buprenorphine, and gentamicin as above.
For SC transplants, 2×106 SCs in 6 μL were injected. For dPDN transplants, a total of 1.7×105 cells (SCs, macrophages, and fibroblasts) interspersed with myelin debris were resuspended in 6 μL and injected. Although the number of cells in dPDN transplants was ∼1/10 that of purified SC transplants, a similar volume of tissue was injected in both treatment groups. The large volume of myelin debris accounts for the difference in the number of cells that was injected. Previous studies with dPDN have not reported the number of cells transplanted (Feng et. al., 2008; Ferguson et al., 2001; Tinsley et al., 2006); they reported the volume of cells injected instead.
Axonal tracing
Corticospinal tract (CST)
Four weeks prior to sacrifice, 11 animals in Experiment 2 (vehicle, n=4; GFP-SCs, n=3; GFP-PDN, n=4) were reanesthetized with isoflurane and 2% O2. The hindlimb cortex was exposed and six 500 nL injections of 10% biotinylated-dextran amine 3000 MW (BDA-3000; Invitrogen) in PBS were made stereotaxically with a pump (Stoelting). The tracer was slowly injected at 100 nL/min through a pulled pipette attached to a Hamilton syringe at the following coordinates: depth, 1.5 mm; AP, −0.7, −1.4, −2.5 mm; ML,±2.3 mm). One GFP-SC rat died after tracing.
Sensory tracing
Three days prior to sacrifice, all 17 remaining animals in Experiment 1 and the remaining 19 animals in Experiment 2 that did not receive CST tracing, were reanesthetized with isoflurane and 2% O2, and the sciatic nerve was isolated, crushed, and injected with 5 μL of cholera-toxin beta (CTβ; ListBiological Laboratories, Inc., Campbell, CA). The needle was left in place briefly before the skin was closed with wound clips. The animals were given buprenorphine and gentamicin as above.
Behavioral testing
BBB score and subscore
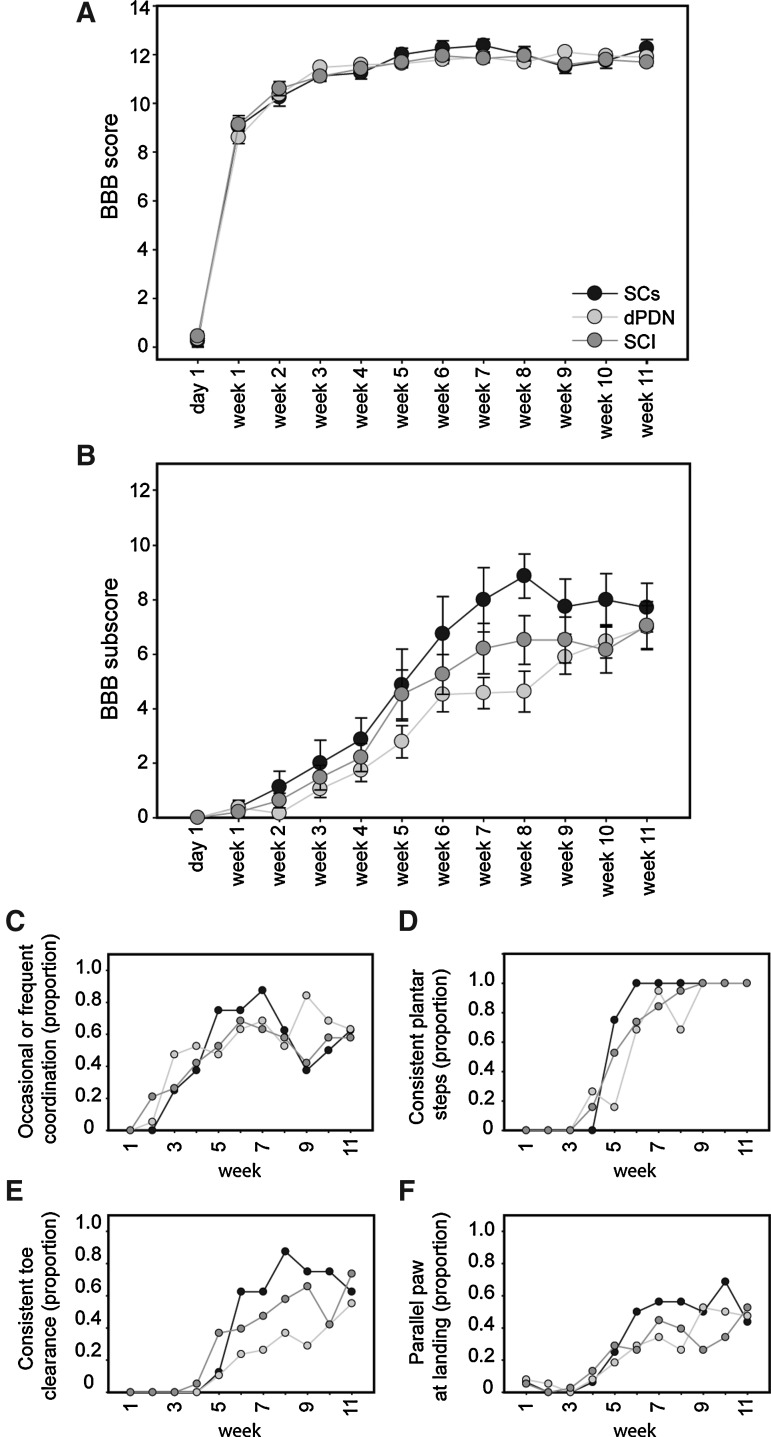
For Experiments 1 and 2 all animals were pretrained for 2 weeks prior to injury by exposing them to the testing pool. On the final pretraining day all animals were scored for their open-field locomotor ability using the 21-point BBB score (Basso et al., 1995), and the 13-point BBB subscore (Popovich et al., 1999). Animals not obtaining a score of 21 and 13, respectively, due to excessive hindpaw rotation, which occasionally is observed in Fischer rats (n=3), were removed and used as PDN donors. After SCI, the animals were tested on days 1 and 7 (prior to transplantation) and weekly thereafter for the remainder of the study (11 weeks total). All BBB testing was done live by two independent observers who were unaware of the treatment groups. Scores are reported as mean±SEM. The proportion of rats in each group that obtained specific BBB measurements is also reported. There are no error bars associated with the proportions.
Footprint analysis
For Experiments 1 and 2 footprints were taken 11 weeks post-injury by inking the rats' forepaws and hindpaws and having them run across a sheet of paper. The base of support (BOS, defined as the distance between the hindpaws), the stride length (SL, defined as the distance between the middle of the hindpaws on subsequent steps), and the angle of rotation (R, defined as the angle of rotation from parallel of the middle hindpaw digit), were measured for three consecutive steps for each hindlimb.
Catwalk
For Experiment 2, additional footprints were taken using the Catwalk gait analysis system (Hamers et al., 2001). The animals were pretrained on the Catwalk for 2 weeks prior to injury by daily exposure to the Catwalk runway, where they were required to make three passes across the Catwalk. The rat's home cage was placed at the end of the Catwalk to encourage the rat to cross the walkway. After each pass the rat was allowed to return to its home cage before the next run. After three successful passes the training session was ended and the rat was given banana-flavored food pellets. On the last day of training the footprints were recorded from at least three passes when the rat walked across the Catwalk without stopping for at least 3 steps. Animals that failed to learn the task and cross the Catwalk consistently before injury were removed from the study (n=2) and used as dPDN donors. At 5 and 11 weeks after injury the footprints were recorded as above. For the final Catwalk analysis, the rats were reacclimatized to the Catwalk for 5 days prior to data collection. Catwalk files were analyzed for mean stride length, mean base of support, difference between forelimb and hindlimb print intensity, and degree and type of coordination.
Ladderwalk
In Experiment 2, 5 and 10 weeks after injury the animals were videotaped while walking across an elevated ladder with rungs spaced 16 mm apart. The animals ran across the walkway and back and were scored using the ladderwalk score developed by Metz and Whishaw (2002), and the number of footfalls for each rat was determined at each time point by an observer unaware of the treatment groups.
Sensory testing
Mechanical allodynia
In Experiment 2, the animals were tested for mechanical allodynia using an electronic von Frey filament (electro von Frey anesthesiometer; IITC Life Science, Woodland Hills, CA), following the methods outlined in the Ohio State University SCI training program. Prior to collecting baseline data, the animals were acclimatized to the testing apparatus (four sessions of 10 min each). During the first two sessions, the animals were acclimatized to the cage by placing them in the small acrylic glass testing apparatus. The rats were observed while eating banana-flavored food pellets (with exploration, grooming, frequency of urination/defecation, and the number of pellets consumed being noted). During the third session the animals were acclimatized to the testing procedure by having the experimenter move her hands underneath the cages. During the fourth session the plantar surface of the animal's foot was poked with the von Frey filament, as would be done during actual testing. For data collection, the von Frey filament was placed on the plantar surface of the paw while the rats were either grooming or eating banana pellets, and the force (g) required to elicit a response of the hindpaw associated with discomfort due to the filament (i.e., quick withdrawal reflex, grabbing or licking the foot being tested, and/or the cessation of grooming and eating) was noted. Data from a total of five trials/hindlimb were collected at each time point (prior to injury, and 3, 5, 7, 9, and 11 weeks post-injury). The high and low scores for each hindlimb were removed from each session's data. As no differences were observed between the right and left hindpaws, the data are the combined means±SEM of both hindpaws.
Thermal analgesia
In Experiment 2, the rats were tested for thermal analgesia using the Hargreaves apparatus (paw/tail stimulator analgesia meter; IITC Life Science), which was set to an active intensity (AI) of 39. The rats were tested prior to injury as well as 3, 5, 7, 9, and 11 weeks post-injury, with a 48-h interval between von Frey and Hargreaves testing. Two animals were tested at a time and only the animals that were currently being tested were present in the room. For data collection, the light source was placed underneath the rat's foot while it was grooming or eating banana pellets. Only trials successfully eliciting a response from the rat that indicated the experience of pain such as sudden foot withdrawal, licking and/or grabbing the foot, and/or the cessation of eating or grooming behavior within 25 sec were included. A total of five trials each were collected from the left and right hind foot, with the high and low responses from each session removed. As no differences were detected between the right and left hindpaw responses, the data are represented as means±SEM latency for withdrawal.
Tissue collection and histology
Three weeks (n=2) or 12 weeks (n=46) after injury, the rats were terminally anesthetized with ketamine and xylazine and transcardially perfused with heparin and saline followed by 4% paraformaldehyde. The brain and spinal cord were removed and post-fixed overnight in 4% paraformaldehyde. Seven spinal cords were processed for plastic sections (Experiment 1: n=4; Experiment 2: n=3). These were representative cases for each of the groups, as determined by impact measurements, BBB scores, and notes taken at the time of transplantation. The remaining tissue was cryopreserved with 30% sucrose-PBS. Horizontal cryostat sections 20 μm thick were collected onto eight serial sets of slides. This resulted in 160 μm between the adjacent tissue sections.
For plastic tissue processing, a 15 mm segment of spinal cord was removed and blocked into 1-mm coronal blocks. Tissue blocks containing GFP+ SC transplants were identified by GFP expression prior to embedding. The tissue blocks were post-fixed overnight in 2% glutaraldehyde in 0.1 M phosphate buffer to aid in myelin preservation, treated with OsO4, and processed and embedded in Epon/Araldite as previously described (Hill et al., 2007).
Immunohistochemistry was performed to identify axons. Entire sets of slides from Experiment 1 or 2 were stained at one time. Neurofilaments were identified with anti-SMI 311 and 312 (1:1000 each; Covance, Emeryville, CA), sensory axons with anti-CGRP (1:1000; Sigma-Aldrich), and brainstem spinal serotonergic fibers with anti-5-HT (1:2000; Immunostar Inc., Hudson, WI). Species-specific Alexa 594 and/or 660 (1:200; Invitrogen, Carlsbad, CA) were used for detection. Nuclei were identified using Hoechst (1:1000; Sigma-Aldrich), and/or Toto (1:1000; Invitrogen). For all staining the tissue was washed three times in PBS, and blocked for 1 h with 5% normal goat serum and 0.1% Tween in PBS before the antibodies were applied at room temperature overnight. The following day the primary antibody was removed with three rinses of PBS, and the secondary antibody and nuclear stain were applied for 2 h at room temperature before they were washed off (3× with PBS). The tissue was then briefly rinsed in water and the slides were allowed to dry before cover-slipping with Vectashield (Vector Laboratories, Burlingame, CA).
CTβ-labeled sensory axons originating from L3–L5 were identified by immunohistochemistry for CTβ followed by ABC staining. An entire set of slides containing all cases from Experiments 1 and 2 that received injections of CTβ were processed at the same time. Tissue sections were washed with Tris-buffered saline with 2% Triton (TBS-T), treated with 2:1 chloroform:methanol to remove lipids for 1 h, rewashed, and treated with 10% methanol, then rinsed with 30% H2O2 in distilled H2O for 1 h to quench endogenous peroxidase activity. The tissue was then washed with TBS-T and blocked overnight with 5% normal rabbit serum. This was followed by incubation with goat anti-CTβ (1:20:000; List Biological Laboratories, Campbell, CA) for 60 h, washes with TBS-T, incubation with biotinylated anti-goat IgG (1:200; Vector Laboratories) for 2 h, and then ABC staining per the manufacturer's instructions (Vectastain ABC Elite Kit, 5 μL/mL each of component A and B; Vector Laboratories). The sections were then reacted for 15 min with DAB, counterstained with cresyl violet, dehydrated, and cover-slipped with Sub-X (Surgipath, Richmon, IL). To assess CTβ axonal ingrowth, 29 cases from Experiments 1 and 2 were examined (SCs: n=5; dPDNs: n=12; SCI: n=12).
CST axons were identified by BDA staining using the protocol established by Herzog and Brosamle (1997), and described in Maier and associates (2008). The tissue was incubated overnight with avidin-biotin complex (1:100 each of component A and B), and nickel-DAB was used as a chromagen to identify the labeled axons before the tissue was dehydrated and cover-slipped with Sub-X. To assess CST axonal ingrowth, two sets of slides from the 10 cases in Experiment 2 receiving tracer injection were examined (SCs: n=2; dPDNs: n=4; SCI: n=4). For one set, the tissue was counterstained with cresyl violet prior to dehydration and coverslipping. No counterstaining was used for the second set. By examining two sets of slides the separation between sections examined for CST regeneration was 40–80 μm.
Quantification of transplant volume and axonal ingrowth
Axonal ingrowth and transplant volume were quantified in cases receiving transplants in Experiment 2 (SCs: n=7; dPDNs: n=9). Cavity length and lesion volume were measured for Experiment 2 (SCs: n=7; dPDNs: n=9; SCI: n=10). All quantification was done using Stereoinvestigator® (MBF Bioscience, Williston, VT), using every eighth tissue section (every section on a given set of slides) containing the region of interest. Transplant and cyst volumes were quantified using the Cavalieri function (200×200 μm grid). For transplant volume the GFP+ transplant site was outlined at 10×, and marks touching GFP+ cells were identified. For cyst volume, the lesion was outlined at 10×, and marks overlying the cavity were identified. The lesion site was defined as the region of spinal cord containing cysts, transplant, and/or damaged tissue containing macrophages or irregularly distributed nuclei. It was distinguished from spared tissue by the appearance and distribution of the Hoechst-positive nuclei. Total lesion volume was determined from the lesion outline areas (lesion area×section thickness×section interval). Axonal length of neurofilament (NF-), CGRP-, or 5-HT-positive axons within the GFP+ transplant was quantified using the spaceball function in Stereoinvestigator. Separate slides were used for each axonal marker. The spaceball probe creates a user-defined sphere or hemisphere relative to the tissue section thickness. At each site of measurement a probe is produced; we used a hemisphere. At each focal point in the tissue the hemisphere is represented by a circle. The circles increase in size through the depth (z) of the tissue section. By marking the axons crossing the probe distributed through the region of interest, an estimate of total axonal length is achieved. For all axonal length measurements, the GFP+ transplant was outlined at 10×, and labeled axons crossing the hemispherical spaceball probe were marked at 63×(grid: 200×300 μm; spaceball 16 μm diameter). Axon density was calculated by dividing the axon length in mm of NF+, CGRP+, or 5-HT+ axons by the transplant volume in mm3. The percent CGRP+and 5-HT+ axons were calculated as [(CGRP+ or 5-HT+ axonal length)/(NF+ axonal length)]×100.
Results
Identification of transplants and transplant morphology
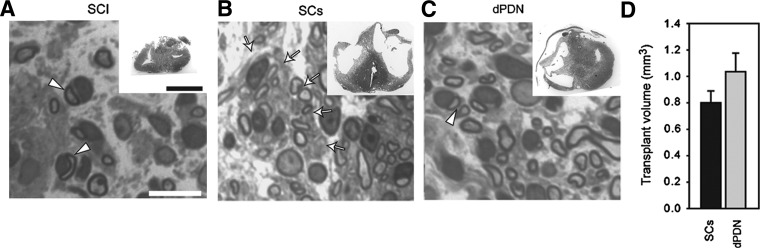
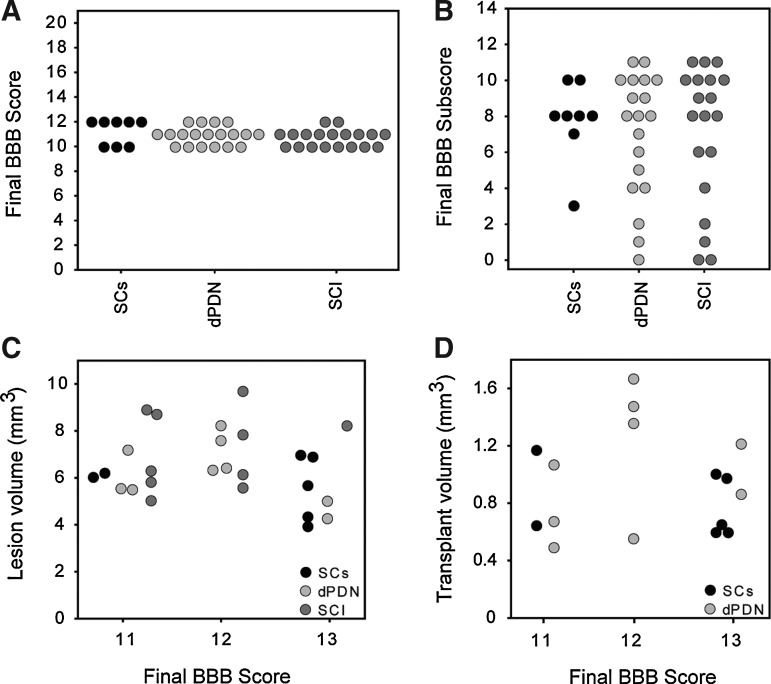
The 200 kD IH contusion resulted in the formation of a cystic cavity. At the level of the central canal, the mean cyst length was 4.30±0.26 mm 12 weeks post-SCI, similar to the 4.25±0.17 mm previously reported for a 12.5 mm NYU injury at 14 weeks post-SCI (Hill et al., 2001). Transplants, confined to the injury site, were identified by regions of dense SC myelination in plastic sections (Fig. 1B and C), and by GFP expression in frozen sections (Fig. 2A and B). Transplants were observed in all cases receiving either dPDN cells or purified SCs. With injection of similar volumes, no differences in transplant volumes were detected between GFP+ SC and GFP+ dPDN cell transplants (Fig. 1D). Both dPDN and SC transplants significantly reduced the volume of the cystic cavity (analysis of variance [ANOVA]: F(2,23)=5.3, p<0.02; Dunnett's post-hoc test: SCs, p=0.01; dPND, p=0.01), although transplants only partially filled the lesion site and cysts persisted (cyst volume: dPDN, 1.57±0.20 mm3; SC, 1.56±0.21 mm3; SCI, 2.75±0.39 mm3). The lesion volumes for each of the groups were 6.20±0.42 mm3 for dPDN cell transplants, 5.69±0.44 mm3 for SC transplants, and 7.24±0.51 mm3 for injury controls. SC transplants showed a trend towards smaller lesion volumes compared to injury controls (ANOVA: F(2,23)=2.74, p=0.086; Dunnett's post-hoc test: SC, p=0.03; dPDN, p=0.1).
FIG. 1.
Transplant morphology and myelination. Appearance of the injury/transplant site (insets in A–C) and SC myelinated axons (A–C). Myelinated axons within SC transplants (B) are largely dispersed, whereas axons within dPDN grafts (C) are predominantly fasciculated. Transplant volumes were not significantly different between SC and dPDN transplants (D; t-test: p=0.15; scale bars=10 μm in A–C, 1 mm in the insets in A–C; arrowheads indicate myelinating SCs; arrows indicate axon fascicles.
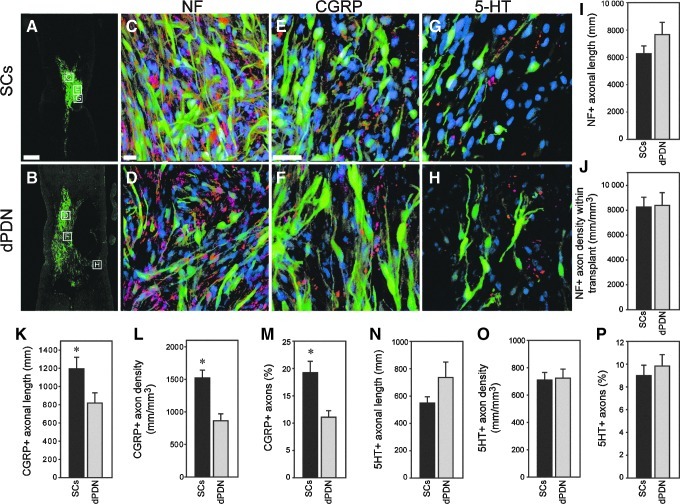
FIG. 2.
Axonal growth into SC and dPDN transplants. Appearance of transplants (SCs: green in A, C, and E; dPDN cells: green in B, D, and F) and appearance and quantification of the ingrowth of axons. NF+ axons (red in C and D) grow into both SC (C) and dPDN (D) cell grafts. No significant differences were detected in axonal length (I) or density (J) between SC and dPDN transplants. A proportion of the axons within SC (E) and dPDN cell (F) transplants are CGRP+ (red in E and F). The total CGRP axonal length (K), the density of CGRP fibers (L), and the percentage of total NF+ axons that are CGRP+ (M), are greater in SC transplants compared to dPDN transplants (t-test: p≤0.05). A small proportion of axons within the SC and dPDN cell transplants are 5-HT+ and are primarily confined to the transplant margins (red in G and H). The total 5-HT axonal length (N), density of 5-HT+ axons (O), and the percentage of NF+ axons that are 5-HT+ (P) are similar in SC and dPDN transplants. Boxed regions in A and B depict the approximate locations of the images shown in C–H taken from adjacent tissue sections. NF+ and CGRP+ axon images are from the transplant center. 5-HT+ axon images are from the transplant periphery (scale bars=500 μm in A and B; 20 μm in C–H; *p≤0.05.
Expression of GFP in PDN cells was confirmed initially at 3 weeks post-transplantation and subsequently detected and examined at 11 weeks. The majority of GFP-labeled dPDN cells were SCs (confirmed by p75 staining, data not shown), although some macrophages were also labeled (confirmed by OX-42 staining, data not shown). In frozen sections 11 weeks post-transplantation, dPDN cell transplants were more dispersed and fasciculated than SC transplants (Fig. 2B). In plastic sections, this corresponded to a more fibrotic transplant and the presence of bundles of myelinated axons (Fig. 1C); in SC transplants, myelinated axons were dispersed with only occasional fascicles (Fig. 1B).
In SC transplants, GFP+ SCs (Fig. 2A) appeared similar to those previously illustrated (i.e., bipolar and confined to the lesion site; Hill et al., 2007, 2010). Myelinated axons were detected throughout the lesion site (Fig. 1B), in contrast to injury-only controls (Fig. 1A), in which axons myelinated by SCs were confined primarily to the dorsal column region.
Axonal growth into dPDN and SC transplants
Neurofilament-positive (NF+) axons grew into both dPDN and SC transplants (Fig. 2C and D). Their total lengths within GFP+ dPDN and SC transplants were similar (Fig. 2I), and resulted in comparable NF+ axonal densities (axonal length/transplant volume) within the transplants (Fig. 2J).
Growth of sensory axons into dPDN and SC transplants was examined by immunohistochemistry for CGRP, and anterograde tracing of ascending sensory fibers from L3–L5 following injection of CTβ into the sciatic nerve. Similar to previous reports (Martin et al., 1996; Takami et al., 2002; Xu et al., 1995b), CGRP+ axons penetrated SC transplants (Fig. 2E). CGRP+ axons also entered dPDN cell transplants (Fig. 2F). The total length of CGRP axons was significantly greater in SC transplants than in dPDN cell transplants (Fig. 2K). This resulted in a significantly greater density of CGRP+axons within SC transplants (Fig. 2L). CGRP axons (c-fibers and aδ-fibers) represented 19.2±2.1% of axons entering SC transplants, and 11.1±1.2% of the axons entering dPDN cell transplants (Fig. 2M). Only a few ascending sensory proprioceptive axons originating from distal lumbar DRGs entered the transplant/injury site. Anterogradely-traced CTβ-labeled axons were occasionally detected scattered within the cresyl violet–stained transplant, as well as within trabeculae in injury-only controls (data not shown). In all groups, the labeled axons were predominantly found in the dorsal spinal cord close to the caudal lesion margin; occasionally axons were observed at the lesion midpoint. Only rarely were CTβ-labeled axons found to bypass the lesion in the spared dorsal tissue in any of the SC (n=5), dPDN (n=11), or SCI (n=13) cases examined.
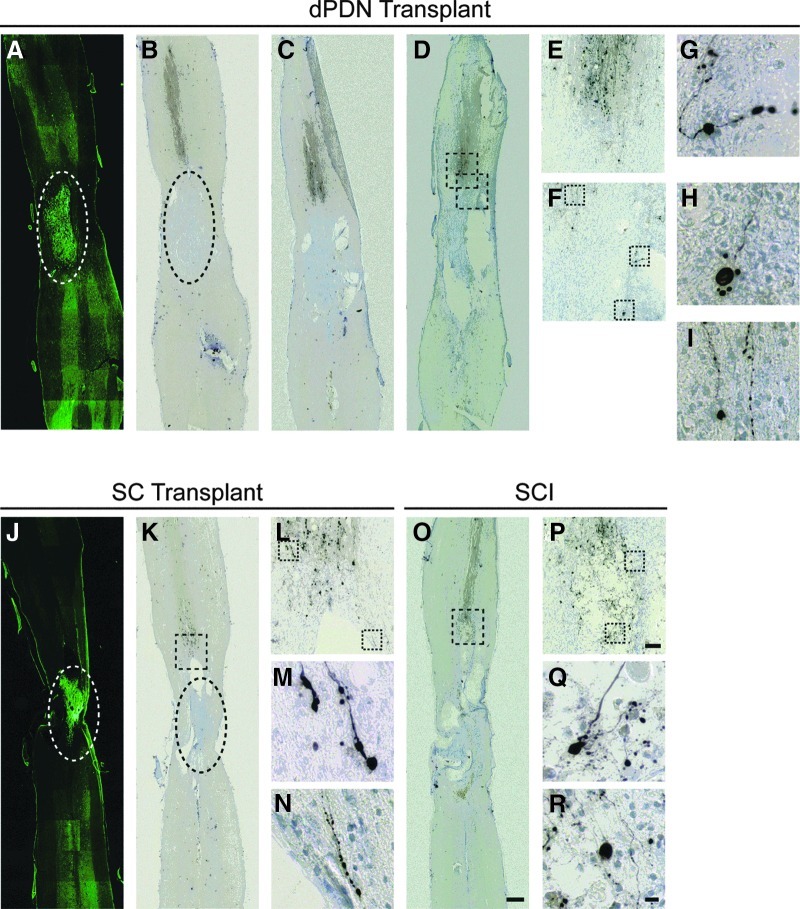
To determine if dPDN cell grafts were able to promote the regeneration of CST axons as previously reported (Ban et al., 2009; Dinh et al., 2007; Feng et al., 2008; Ferguson et al., 2001; Rasouli et al., 2006), anterograde tracing of BDA injected into the hindlimb cortex was performed. Similar to previous reports (Brosamle and Schwab, 1997; Hill et al., 2001), anterograde tracing of the CST primarily labeled axons within the dorsal columns. In injury-only rats (n=4), labeled axons extended to the lesion margin (Fig. 3O and P). They were observed to end in retraction bulbs (Fig. 3Q and R). Occasionally axons were observed in the dorsal lateral and ventral CST extending past the rostral lesion margin (data not shown). Following transplantation of SCs (n=2) or dPDNs (n=4), the labeled CST axons appeared similar to those in injury-only controls (Fig. 3A–N). Despite our ability to detect very fine CST fibers (Fig. 3G–I, M, and N), we did not detect labeled axons penetrating the injury site or within the GFP+ transplant (compare Fig. 3B with A for dPDNs, and Fig. 3K with J for SCs).
FIG. 3.
Corticospinal tract (CST) tracing. Biotinylated-dextran amine (BDA)-traced CST axons (B–D, K, and O) approach the lesion/transplant but do not extend into or around dissociated predegenerated nerve (dPDN) (A) or Schwann cell (J) transplants. Dashed ovals demarcate similar regions in adjacent sections with green fluorescent protein-labeled transplants (A and J) and BDA traced axons with cresyl violet counterstained nuclei (B and K). For a dPDN transplant case (A–I), CST axons in three adjacent sections separated by 160 μm are depicted (B–D). Single sections through the dorsal CST are shown for the SC (K) and SCI (O) groups. E, F, L, and P are enlargements of the boxed regions in D, K, and O, of the main termination of the dorsal CST rostral to the injury/transplant. Fine CST axons were detected and terminated in retraction bulbs in all cases (dPDN, G–I; SCs, M and N; SCI, Q and R). G–I, M, N, Q, and R are enlargements of the boxed regions in F, L, and P (scale bars=500 μm in A–D, J, K, and O;=50 μm in E, F, L, and P;=10 μm in G–I, M, N, and P–R; green, GFP-labeled transplanted cells; brown, BDA-traced CST axons; blue, cresyl violet counterstained nuclei).
To determine if grafts promoted the ingrowth of brainstem spinal axons, immunohistochemistry for serotonin (5-HT) was used. 5-HT axons entered both SC and dPDN cell grafts, and were primarily located on the edges of the transplant where the transplant density was low (Fig. 2G and H). No differences were detected in axonal length (Fig. 2N: SC, 550±55 mm; dPDN, 735±115 mm), density of 5-HT axons (Fig. 2O), or the percentage of 5-HT+ axons (Fig. 2P) within SC and dPDN cell transplants.
Sensory testing
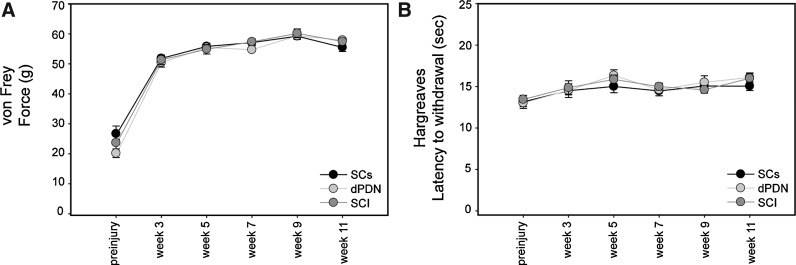
To date, the effects of dPDN or SC transplants on sensory function have not been reported. To assess whether these transplants resulted in altered sensation and/or pain, the force required to elicit hindlimb withdrawal (von Frey testing), and the latency of hindlimb withdrawal to heat (Hargreaves testing) were measured. Following injury there was a significant increase in both the force (ANOVA: F(5,130)=344.6, p<0.001) and latency (ANOVA: F(5,130)=7.2, p<0.001) required to elicit hindlimb withdrawal (Fig. 4A and B). The increase in force needed to elicit hindlimb withdrawal post-SCI persisted for the 11 weeks examined, and did not differ between the SCI, SC, and dPDN groups (p>0.05 by ANOVAs). The increase in latency to withdraw the hindlimb was significant only for the dPDN group at 5, 9, and 11 weeks, and the SCI group at 5 and 11 weeks (ANOVAs: p<0.05; Dunnett's post hoc test: p<0.05). Importantly, neither SC nor dPDN transplants resulted in a decrease in the force required to elicit hindlimb withdrawal, or in a decrease in latency to hindlimb withdrawal, which are measures of induction of neuropathic pain.
FIG. 4.
Sensory testing. Following SCI there is an increase both in the force necessary to elicit hindlimb withdrawal (von Frey testing; A), and the latency of hindlimb withdrawal to heat (Hargreaves testing; B). No differences were detected between the treatment groups.
Motor testing
Several tests were performed to assess locomotor recovery. BBB open field locomotor testing was used to assess gross locomotor recovery. The ladderwalk was used to quantify fine motor control of the hindlimbs. Gait was measured using both the Catwalk gait analysis system and inked footprints. Neither SC nor dPDN transplants resulted in any statistically significant improvements over injury alone.
BBB
All groups recovered to a BBB score of ∼11 by 3 weeks post-injury (Fig. 5A; dPDN, 11.5±0.1; SC, 11.1±0.2; SCI, 11.1±0.2). Scores remained similar after 11 weeks (dPDN, 11.9±0.2; SC, 12.3±0.4; SCI, 11.7±0.2). Between 3 and 11 weeks the rats in all groups improved their locomotor performance on the parameters detected in the subscore (Fig. 5B). No statistically significant differences were detected between groups (ANOVA: F(11,473)=2.0, p=0.15). The distribution of final BBB and BBB subscores for individual rats were evenly distributed for each of the three groups (Fig. 6A and B). Final BBB scores did not correlate with either lesion volume (Fig. 6C), or transplant volume (Fig. 6D). Figure 5C–F depicts the proportion of rats in each group over time that achieved occasional to frequent coordination (Fig. 5C), consistent plantar stepping (Fig. 5D), consistent toe clearance (Fig. 5E), and parallel paw position at landing (Fig. 5F). Whereas the proportion of animals meeting the criteria was similar at 11 weeks, a greater proportion of rats receiving SC transplants recovered consistent plantar steps, toe clearance, and parallel paw position 2–3 weeks earlier than either dPDN transplants or SCI alone.
FIG. 5.
Motor testing by Basso Beattie Bresnahan (BBB) score. Neither dPDN nor SC transplants enhanced open field locomotion as reflected by the BBB score (A) and subscore (B). Data points show mean±SEM. All animals recovered occasional to frequent coordination to a similar extent (C). A greater proportion of rats with SC transplants showed earlier recovery of consistent plantar stepping (D), enhanced toe clearance (E), and parallel paw position at landing (F). There is no error associated with the proportion.
FIG. 6.
Distribution of BBB scores, BBB subscores, lesion volumes and transplant volumes for individual rats. Depicted are the final individual BBB (A) and BBB subscores (B) for rats in which lesion and transplant volumes were quantified. Neither lesion volume (C) nor transplant volume (D) correlated with final BBB score.
Ladderwalk
The percentage of steps that were missteps (scores of 0–2 on the Metz and Whishaw scale) did not significantly differ between the three groups (ANOVA: F(2,26)=0.4, p=0.68; Fig. 7). There was wide variability in the number of missteps between the different rats. Combined, all groups showed a significant improvement on the ladderwalk between 5 and 10 weeks (ANOVA: F(1,26)=6.6, p<0.05), as detected by a decrease in the percentage of steps that were missteps. No significant differences, however, were found in any of the individual treatment groups following post-hoc analyses.
FIG. 7.
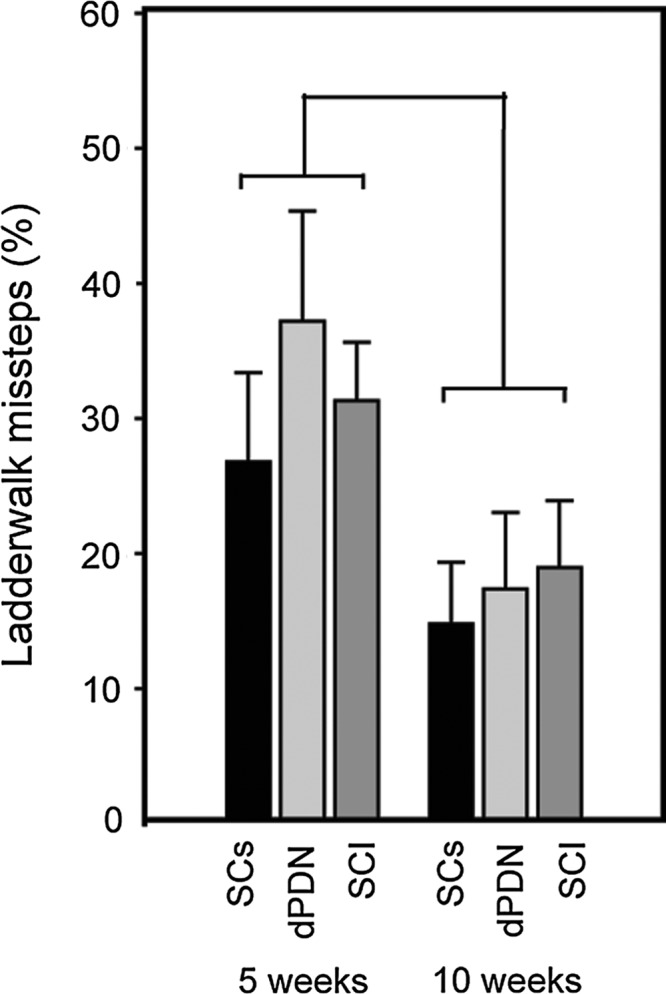
Motor testing on the ladderwalk test. All groups improved on the ladderwalk between 5 and 10 weeks. No significant differences were detected between the groups.
Catwalk and inked footprints
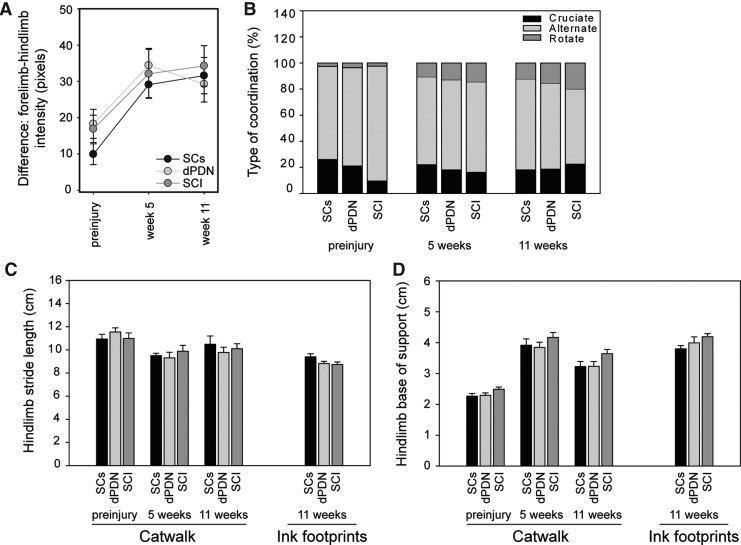
To assess the pressure that rats were exerting on their hindlimbs versus forelimbs while walking, the difference in pixel intensity between the forelimbs and hindlimbs was examined (Fig. 8A). All groups showed a shift toward placing more weight on their forelimbs than their hindlimbs after injury. There was no difference between the treatment groups (ANOVA: F(2,25)=0.4, p=0.66). As the animals recovered after injury they altered their gait (Fig. 8B). Prior to injury the footfall pattern during stepping primarily used by the rats was to alternate between a forelimb and a hindlimb. This step pattern is termed alternate coordination (i.e., right forelimb [RF], right hindlimb [RH], left forelimb [LF], left hindlimb [LH], or LF, RH, RF, LH). After SCI, the rats increased the percentage of steps in which they placed each forelimb in succession, before subsequently moving each hindlimb in succession, starting with the hindpaw on the same side as the last forepaw placed. This is termed rotate coordination (i.e., RF, LF, LH, RH, or LF, RF, RH, LH). Cruciate coordination is similar to rotate coordination, except the hindpaw opposite to the last forepaw placed moves after the forepaws are moved (i.e., RF, LF, RH, LH, or LF, RF, LH, RH). No differences were found between the treatment groups in the type of coordinated stepping used to cross the Catwalk. The Catwalk was also used to measure stride length (Fig. 8C), and base of support (Fig. 8D) over the course of the study. After injury the rats significantly increased their base of support (ANOVA: F(2,50)=117.6, p<0.001), and significantly decreased their stride length (ANOVA: F(2,50)=8.1, p<0.001). No differences in base of support or stride length were observed between the treatment groups (ANOVA: base of support, F(2,25)=0.56, p=0.69; stride length, F(2,25)=0.05, p=0.95; Fig. 6C and D). Inked footprints taken at the end of the study resulted in similar measurements to those determined with the Catwalk system (right bars in Fig. 8C and D).
FIG. 8.
Motor testing by Catwalk and inked footprint. No significant differences were detected between control rats and those receiving SC or dPDN grafts following gait analysis. In all groups, rats placed greater weight on their forelimbs after SCI; A. Rats use a different pattern of stepping following injury (B). Catwalk and inked footprint analysis resulted in similar results for stride length (C) and base of support (D).
Discussion
Several reports indicate that transplantation of intact or dPDNs are effective for improving axonal regeneration and locomotor function after SCI (Ban et al., 2009; Dinh et al., 2007; Feng et al., 2008; Ferguson et al., 2001; Rasouli et al., 2006). Given that the methods differ significantly across the five studies, we did not set out to precisely replicate the methods of any one study. Instead, we tested whether the reported beneficial effects of dPDN cells were robust and reproducible in a standardized injury and transplantation model used in our laboratory for years. We did not observe the previously reported beneficial effects of PDN transplants. We will first outline the similarities and differences between all the studies (summarized in Table 2), and then discuss why dPDNs may have failed in our hands.
Table 2.
Comparison of Methods and Results from SCI Studies Using PDN Transplants
| |
Spinal cord injury experimental conditions |
PDN experimental conditions |
Experimental treatments |
Experimental results |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Exp't | Rat strain | Injury model | Injury level | Time of transplant (weeks post-SCI) | Length of study (weeks post-SCI) | PDN nerve source | Autologous transplant (Y/N) | Time to nerve collection (weeks post-injury) | Nerve preparation | Method of transplantation | Treatment groups | Other manipulations | Axon tracing | CST regeneration (Y/N) | Final BBB score |
| Ferguson et al., 2001 | Exp't 1 | Adult female | dHx | T10 | 2(?) | 2, 56 | Sural | Y | 1 | Intact PDN (3–5 mm) | Sutured into lesion | PDN+NTF | 1% collagen+BDNF, NT-3, GDNF | Dried WGA-HRP or dried BDA | Y | ND |
| Injury alone | N | ND | ||||||||||||||
| Exp't 2 | Adult female | dHx | T10 | 25 | 27 | Sural | Y | 1 | Mechanically minced | Injection (1–3 μL) | dPDN+NTF | 1% collagen+BDNF, NT-3, GDNF | Dried WGA-HRP or dried BDA | Y | ND | |
| Exp't 3 | Adult female | dHx | T10 | 3 | 5 | Sural | Y | 1 | Mechanically minced | Injection (1–3 μL) | dPDN+NTF | NT-3 via minipump 1 week pre-TP | Dried WGA-HRP or dried BDA | Y | ND | |
| dPDN+vehicle | DMEM via minipump 1 week pre-TP | ? | ND | |||||||||||||
| Dinh et al., 2007 | Adult female Sprague-Dawley | dHx | T10 | 1 | 6 | Sciatic | N | 2 | Intact PDN (2 mm) | Sutured over dHx (after glial scar resection) | PDN | 10% BDA (4 μL×8 sites) | Y | ∼14 | ||
| PN | Y | ∼13.5 | ||||||||||||||
| Rasouli et al., 2006 | Adult female Sprague-Dawley | Contusion; 175 kD IH | T10 | 1 | 8 | Sciatic | N | 2 | Intact PDNs (2 mm) | Nerve placed dorsally over injury | PDN | Glial resection | 10% BDA (4 μL×8 sites) | Y | ∼11 | |
| PN | Glial resection | Y | ∼11 | |||||||||||||
| SCI | None | N | ∼9 | |||||||||||||
| SCI | Glial resection | N | ∼6 | |||||||||||||
| Feng et al., 2008 | Exp't 1 | Female Sprague-Dawley | Contusion; 12.5 NYU impactor-type device | T11 | 3 | 11 | Sural | Y | 1 | Mechanically minced (1.5 cm) | Injection | dPDN+NTF | 1% collagen+BDNF, NT-3, GDNF | Dried BDA | Y | ∼14 |
| dPDN | 1% collagen | Y | ∼10.5 | |||||||||||||
| Vehicle | none | Y | ∼8 | |||||||||||||
| Exp't 2 | Female Sprague-Dawley | Contusion; 12.5 NYU impactor-type device | T11 | 3 | 26 | Sural | Y | 1 | Mechanically minced (1.5 cm) | Injection | dPDN+NTF | 1% collagen mixed with BDNF, NT-3, GDNF | Dried BDA | Y | ∼17 | |
| Vehicle | None | Y | ∼11 | |||||||||||||
| Ban et al., 2009 | Adult female Wistar | Contusion; 50 mm NYU | T10 | 1 | 13 | Saphenous | Y | 1 | Mechanically and enzymatically minced and cultured 1 week | Injection | dPDN IV (tail vein) | 10% BDA (0.5 μL×2 depths×10 sites) | Y | 6.89±0.74 | ||
| dPDN intrathecal (L1–L2) | Y | 10.05±1.05 | ||||||||||||||
| dPDN intraspinal | Y | 13.17±0.71 | ||||||||||||||
| Hill et al., 2010 | Adult female Fischer | Contusion; 200 kD IH | T9 | 1 | 12 | Sciatic | N | 1 | Mechanically and enzymatically minced | Injection | dPDN | 10% BDA (0.5 μL×6 sites) | N | 11.9±0.1 | ||
| SCs | N | 12.3±0.4 | ||||||||||||||
| SCI | N | 11.7±0.2 | ||||||||||||||
PDN, predegenerated nerve; SCI, spinal cord injury; CST, corticospinal tract; Exp't, experiment; NYU, New York University; dHx, dorsal hemisection; dPDN, dissociated predegenerated nerve; NTF, neurotrophic factors; PN, peripheral nerve; BDNF, brain-derived neurotrophic factor; BDA, biotinylated-dextran amine; GDNF, glial cell line–derived growth factor; NT-3, neurotrophin-3; WGA-HRP, wheat germ agglutin-horseredish-peroxidas; TP, transplant; DMEM, Dulbecco's modified Eagle's medium; BBB, Basso-Beattie-Bresnahan scale; IH, infinite horizon impactor.
Methodological differences between PDN studies
All the studies used rat SCI models, damaged the sciatic nerve or its branches 1–2 weeks before nerve isolation, and reported successful CST regeneration. The four studies that assessed recovery of function using an open field locomotion test reported beneficial improvements. None of the studies, including those with allografts, used immunosuppression or reliably identified the transplanted cells. Each differed with respect to injury model and severity; methods of isolating, processing, and transplanting the nerves; and the delay between SCI and transplantation. Several rat strains were used.
PDN grafts were successful in both contusion and dorsal hemisection (dHx) models of SCI. Injury severity and delay between injury and transplantation differed between contusion studies. Feng and associates (2008) transplanted minced PDNs 3 weeks after a T11 injury with a NYU-type device. Rasouli and colleagues (2006) transplanted PDN grafts 1 week after a 175 kD IH contusion injury at T10. Ban and co-workers (2009) transplanted PDN cells 7 days after a 50 mm NYU contusion injury. dHx studies also used different delays between injury and transplantation. Dinh and associates (2007) transplanted PDNs 1 week after a dHx at T10 and Ferguson and colleagues (2001) transplanted intact or minced PDNs at either 2 or 25 weeks after injury. These differences suggest that the beneficial effects of PDNs are not dependent on injury severity or the delay between injury and transplantation.
We used a 200 kD IH injury at T9 with a delay of 7 days between injury and transplantation. This injury severity is similar to the moderate 12.5 mm NYU contusion reported by the Feng group (2008), and ranks between the more moderate and severe contusion injuries used in the other two contusion studies (Ban et al., 2009; Rasouli et al., 2006). Our delay between injury and transplantation was similar to that used in the Ban (2009), Dinh (2007), and Rasouli (2006) studies. The delay was also similar to the 7 to 10 day delay frequently used to assess transplants in the injured spinal cord. This delay is, however, shorter than the 2, 3, or 25 week delay examined by Ferguson and associates (2001) and Feng and colleagues (2008). We do not think the difference in transplant time accounts for the differences seen in axonal growth and behavioral outcomes between our study and that of Feng and co-workers (2008). Previous studies with purified SC transplants showed similar results when the cells were transplanted after a delay of 7 days (Takami et al., 2002), or 8 weeks (Barakat et al., 2005).
The previous studies also used several different methods to prepare and transplant the PDNs. Intact or mechanically dissociated PDNs as well as enzymatically dissociated PDNs subsequently proliferated in vitro were all reported to be beneficial. Intact PDNs were held in place by either sutures (Dinh et al., 2007; Rasouli et al., 2006), or by using a collagen solution (Ferguson et al., 2001). Mechanically dissociated PDNs were resuspended in collagen with or without neurotrophic factors prior to injection into the injury site (Feng et al., 2008; Ferguson et al., 2001). Dissociated PDNs were kept in vitro for 1 week prior to collection and injection into the lesion epicenter (Ban et al., 2009). The variety of methods used to isolate and transplant the cells suggests that their effects are not dependent on cell processing.
We combined the methods of the groups of Ferguson (2001), Feng (2008), and Ban (2009) to prepare our cells. We used both mechanically and enzymatically dissociated PDNs; enzymes were necessary to more fully dissociate the cells so that they could pass through the syringe and the fine-pulled glass capillary injection pipette. Our previous experience with labeled cells indicated that cells leak out of the cord when the syringe alone is used, because of the long bevel of the Hamilton syringe needle tip. This does not occur when Hamilton syringes are fitted with a smaller pulled glass pipette tip.
One critical difference between our study and those of Feng and associates (2008) and Ferguson and colleagues (2001) is that we did not add collagen or neurotrophic factors to the PDN transplants. Given the claim that PDNs and PDN cells promote regeneration, we did not want to confound our results by adding these factors. Moreover, three of the studies reporting beneficial effects of PDN grafts on regeneration and recovery of function did not use collagen or neurotrophic factors (Table 2). Injection of collagen after SCI is sufficient to promote CST regeneration in animal models (Joosten et al., 1995), and the addition of the neurotrophin NT-3 further augments this response and results in improved function on the gridwalk test (Houweling et al., 1998). Neither collagen alone nor collagen plus neurotrophin controls were used in the studies by Feng and associates (2008) and Ferguson and colleagues (2001).
Several rat strains were used, including Wistar (Ban et al., 2009) and Sprague-Dawley (Dinh et al., 2007; Rasouli et al., 2006). Two studies did not report the strain (Feng et al., 2008; Ferguson et al., 2001). The differences in rat strain suggest that the effects of PDN grafts are independent of rat strain, and should have been effective in Fischer rats, which do not require immunosuppression for allograft survival. But it should be mentioned that there are reports of differences in outcomes depending upon the rat strain used. Moreover, after contusion, lesion size can vary markedly within the same strain of Fischer rats reared in different facilities of the same company (Bunge and Pearse, 2012).
The previous success of PDN grafts using a variety of methods suggest that their effects are robust and not dependent on the injury model, time between injury and transplantation, method of cell preparation, or rat strain. It is unlikely that the differences in the methods used between this study and previous studies account for the failure to detect differences in CST regeneration and recovery of function. And yet there are numerous examples of replication studies that do not find the same improvements that were reported in the initial publications (Bunge and Pearse, 2012; Sharp et al., 2012).
Consistency of the injuries
Consistent injuries are essential for assessing the success of preclinical treatments in SCI animal models in both original and replication studies. In the current study, we used the IH impactor to produce the injuries and confirmed that the mean injury severity and impact curves were similar between treatment groups. The injuries resulted in cystic cavities of a length comparable to those previously produced following moderate contusion with the NYU device (Hill et al., 2001). Not only was the histology consistent between our previous work with the NYU device and the current study using the IH impactor, but the recovery curve of our rats was also similar to that of Basso and associates (1996) for rats with a moderate 12.5 NYU contusion (an injury equivalent to the 200 kD IH injury used in the current study). Moreover, our BBB recovery curves were identical for Experiments 1 and 2. Together, this demonstrates that we were able to produce consistent and reproducible SCIs.
In previous studies, the groups were relatively new to using spinal cord contusion models, and either used established injury devices or developed their own (Ban et al., 2009, NYU, T10, 50 mm; Feng et al., 2008, NYU-like, T11, 12.5 mm; Rasouli et al., 2006, IH, T10, 175 kD). Injury parameters for the other groups were not reported. The lesion sizes, based on the figures, are smaller than expected for moderate-to-severe spinal cord contusion injuries in all three reports. The BBB recovery curves differed from expected as well (Basso et al., 1996). Together, this suggests variable and/or atypical contusion injuries. Milder injuries result in more CST sparing and improvement in locomotor function, and this could account, in part, for the differences seen between this study and previous findings.
Identification of the transplant
Previous studies did not reliably identify the PDN transplants, which is essential for determining their consistency and for understanding whether the beneficial effects are mediated by the transplanted cells. Because host SCs are present within the spinal cord after injury (Beattie et al., 1997; Hill et al., 2007; Takami et al., 2002; Zawadzka et al., 2010), reliable markers are necessary. In the current study, GFP was used to identify transplanted cells within the lesion; transplants were detected in all cases. Volumes of dPDN and SC transplants were consistent with our previous studies (Golden et al., 2007; Hill et al., 2007). By identifying the transplant we were able to demonstrate that dPDN grafts (and SC grafts) significantly reduced the volume of cystic cavities. Cystic cavities that persisted were similar in volume to those previously quantified (Pearse et al., 2004). These measurements further demonstrated the consistency of our methods.
Assessment of axonal growth
The previous studies focused solely on anterogradely-labeled CST axons. In addition to CST axons, we examined NF+ axons, sensory axons (CGRP+ axons and CTβ-traced axons), and brainstem spinal axons (5-HT+ axons). PDN transplants promoted the ingrowth of NF+ axons to a similar extent as SC grafts. Based on axon quantification within similar SC transplants (Golden et al., 2007), we estimate that the total number of axons (myelinated and non-myelinated) entering dPDN transplants was approximately 30,000–45,000. Sensory axons from the anterolateral system, but not ascending dorsal columns, grew into dPDN transplants, as indicated by the presence of CGRP+ axons and the absence of CTβ-traced L3–L5 proprioceptive axons, similar to SC grafts (Moon et al., 2006; Xu et al., 1997). The inability of the grafts to promote proprioceptive axonal growth likely contributes to the failure of animals to regain coordination (Metz and Whishaw, 2002). 5-HT+ axons, from the raphe in the brainstem (Bowker et al., 1981), were restricted to the margins of the transplant. CST axons approached the lesion and splayed around the rostral lesion margin, as did those in SCI and SC controls.
There are several potential reasons for the discrepancies between our CST results and those of previous reports (Ban et al., 2009; Dinh et al., 2007; Feng et al., 2008; Ferguson et al., 2001; Rasouli et al., 2006). The first is the amount of tracer used. Previous studies used much larger volumes of tracer (5–24 μL of BDA: Ban et al., 2009; Dinh et al., 2007; Rasouli et al., 2006), or placement of dry WGA-HRP or BDA (Feng et al., 2008). Leakage of tracer into the CSF can result in a labeling artifact at the site of SCI (Steward et al., 2008). Indeed, HRP staining was located on the meninges and within the central canal in the Ferguson (2001) study, suggesting this may have occurred. We used small injections of anterograde tracer (1.2 μL total), as in our previous work documenting the response of CST axons to contusive SCI (Hill et al., 2001).
A second reason could be variations in the sparing of CST fibers. The failure to distinguish between spared and regenerating fibers is a known complication of using partial lesions (Steward et al., 2003). In our hands, a moderate spinal cord contusion results in a complete loss of the dorsal CST, but with occasional sparing of fibers in the dorsolateral or ventral CST (Hill et al., 2001). In the work of Feng and associates (2008), numerous labeled axons were detected laterally and caudally in the only transplant case depicted (PDNs+neurotrophins). Given the long, straight nature of the CST labeling in the work of both Feng (2008) and Rasouli (2006), it is possible that at least some of the labeled CST axons were spared.
A third possibility is that our results are a false-negative arising from the small number of rats that received CST tracing. However, if this is true, then our results would indicate that the effect of dPDNs on CST regeneration is not robust.
It is not surprising that dPDN grafts did not promote CST axonal growth. The major cellular constituent of dPDN grafts is SCs. SC and PN grafts do not promote CST growth (Chen et al., 1996; David and Aguayo, 1981; Meijs et al., 2004; Menei et al., 1998; Richardson et al., 1982; Xu et al., 1995b,1997,1999), even when combination strategies are used (Fouad et al., 2005; Golden et al., 2007; Guest et al., 1997; Hiebert et al., 2002; Xu et al., 1995a), except for the addition of aFGF (Cheng et al., 1996).
Assessment of locomotor function in dPDN grafts
We did not detect any improvement in motor function following dPDN transplantation by multiple locomotor tests (BBB, BBB subscore, inked footprint, Catwalk, and ladderwalk). The validity of these results is supported by both the consistency of our injuries (as indicated by similar impact parameters between groups), and our ability to replicate our BBB results between Experiments 1 and 2. This differs from the work by Feng and associates (2008), in which the BBB scores and recovery curves differed dramatically between their two studies. Unlike some studies that had small sample sizes per group (Feng et al., 2008), and high BBB score variability within groups (Feng et al., 2008; Rasouli et al., 2006), the current study had a large number of animals per group and very little variability (small SEMs). Importantly, the number of animals reached sufficient power to be able to detect a one point difference in the BBB scores had it been present. We used stringent inclusion criteria for our rats. By using the BBB scores at days 1 and 7 prior to transplantation, we excluded rats showing early recovery and ensured equivalent BBB scores between the groups at the time of transplantation. This is in contrast to at least one study, in which the BBB scores at the time of transplantation appeared to differ between the groups (Dinh et al., 2007). In our study, BBB testing was done following established protocols by two observers with years of testing experience. This is in contrast to the study by Feng and colleagues (2008), in which only a single observer performed BBB testing and in which the BBB recovery curves were atypical. The BBB recovery seen in previous studies occurred slowly over several weeks in control animals and did not plateau as expected in graded, reproducible injuries (Basso et al., 1996).
The rats in Experiment 2 underwent ladderwalk and Catwalk locomotor tests and inked footprint analysis. No behavioral improvements were detected following dPDN transplantation, further confirming that dPDN transplants failed to improve function. Previously, differences in hindlimb exorotation were noted in two studies (Ban et al., 2009; Dinh et al., 2007), but footprint parameters were not recorded prior to injury and the differences detected immediately after transplantation were maintained throughout the study (Dinh et al., 2007), suggesting non-transplant-mediated effects.
None of the previous studies used the Catwalk or ladderwalk tests. Our results on the Catwalk test, measuring differences in forelimb-hindlimb intensity (a measurement of hindlimb weight support), were consistent with those previously reported after SCI (Liebscher et al., 2005). Overall, the results from our comprehensive assessment of locomotor function failed to detect any beneficial effect of transplanting cells from dPDNs. Given the lack of CST, 5-HT, and ascending sensory axonal growth, it is not surprising that we were unable to replicate the previously reported improvements in locomotor function.
Assessment of locomotor function in rats receiving SC grafts
Whereas the focus here was on the effects of dPDN transplants, SC transplants were included in one of the two studies. Similar to the dPDN transplants, SC transplants did not result in improvement in locomotor function. Although there were no significant improvements in the BBB subscore, in part because the low number of rats in the SC group resulted in the group being statistically under-powered, an increased proportion of SC transplant rats showed earlier recovery of some aspects of function (Fig. 5D–F: consistent plantar stepping, consistent toe clearance, and parallel paw position). This is consistent with SC transplants having modest effects on some aspects of locomotor function when used alone (Takami et al., 2002).
Pain testing
Transplantation of stem cells can result in allodynia (Hofstetter et al., 2005). Enhanced CGRP sprouting after SCI results in abnormal innervation of the grey matter, and CGRP is also associated with neuropathic pain, including allodynia (Bennett et al., 2000; Oku et al., 1987). Although CGRP fibers grew into both dPDN and SC transplants, for the first time we demonstrate that neither dPDN nor SC grafts result in thermal or mechanical hyperalgesia (Fig. 4).
SCs versus dPDNs
The results using dPDN grafts were similar to those testing purified SCs despite the number of dPDN cells being less. Because it was not possible to increase the number of dPDN SCs, we do not know if they would have been more effective if more numerous. Similar to SC transplants, combination strategies may be required to enhance regeneration and recovery of function with dissociated or intact PDN transplants. Whereas, augmentation of PDN grafts with neurotrophins has been reported to further enhance axonal regeneration and recovery of function (Feng et al., 2008; Ferguson et al., 2001; Tinsley et al., 2006) we know for more about combination strategies with SCs. Additionally, isolation of PDNs requires crushing of the nerve 1 week prior to collection; in essence this produces a conditioning lesion, a consequence of which is the upregulation of regenerative genes and enhanced sensory axon regeneration (Belyantseva and Lewin, 1999; Oudega et al., 1994) including sprouting of CGRP axons which are associated with neuropathic pain (Bennett et al., 2000). Given that neuropathic pain is already a problem after SCI (Anderson, 2004; Widerstrom-Noga and Turk, 2004), further aberrant sprouting of pain fibers into the dorsal horn is not desirable. Also, dPDN grafts are a mixed cell populations containing SCs, fibroblasts, and activated macrophages. Although activated macrophages have been tested in a Phase I clinical trial because of their ability to promote axonal regeneration and recovery of function (Knoller et al., 2005; Schwartz and Yoles, 2005), there is substantial controversy about their use given the dichotomous role of inflammation in recovery after SCI (Popovich and McTigue, 2009; Schwartz and Yoles, 2005), with the type of macrophage affecting recovery (Kigerl et al., 2009). Because of these potential concerns, it is our opinion that purified SCs, not dPDNs, should be used to develop combination strategies.
Conclusion
In the current study, we tested the efficacy of transplantation of cells isolated from predegenerated sciatic nerve. Using a standardized injury model and transplantation procedure, we demonstrate that dPDN grafts survive within the injured spinal cord and promote the ingrowth of axons. dPDN grafts did not promote long-distance axonal growth from corticospinal axons, brainstem spinal axons, or ascending dorsal column sensory axons. Moreover, using a battery of locomotor tests (BBB, BBB subscore, inked footprint, Catwalk, and ladderwalk), dPDN grafts failed to improve locomotor function after SCI. We conclude from our work that dPDN grafts do not provide sufficient improvement in SCI repair.
Acknowledgments
We gratefully acknowledge the Miami Project Viral Vector Core for production of lentiviral vectors; the Miami Project Animal Care Core for animal care, BBB, and ladderwalk testing; and Shivraj Bhosle for assistance with Catwalk analysis. We would also like to thank Yelena Pressman for assistance with SCs, Margaret Bates and Raisa Puzis for EM/plastic section processing, and Dianna Willis (Burke Medical Research Institute/Weill Medical College of Cornell University) for assistance with editing and Adobe Illustrator.
This research was sponsored by funding to M.B.B. by National Institute for Neurological Disorders and Stroke grants 09923 and 38665, the Christine E. Lynn Distinguished Professorship, The Miami Project to Cure Paralysis, the Helen Wilshire Walsh Foundation, and the State of Florida; and to C.E.H. by the Burke Foundation. Funding was restricted to financial contributions.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Ban D.X. Kong X.H. Feng S.Q. Ning G.Z. Chen J.T. Guo S.F. Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat's spinal cord injury. Brain Res. 2009;1256:149–161. doi: 10.1016/j.brainres.2008.11.098. [DOI] [PubMed] [Google Scholar]
- Barakat D.J. Gaglani S.M. Neravetla S.R. Sanchez A.R. Andrade C.M. Pressman Y. Puzis R. Garg M.S. Bunge M.B. Pearse D.D. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Beattie M.S. Bresnahan J.C. Komon J. Tovar C.A. Van M. Anderson D.K. Faden A.I. Hsu C.Y. Noble L.J. Salzman S. Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp. Neurol. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- Belyantseva I.A. Lewin G.R. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. Eur. J. Neurosci. 1999;11:457–468. doi: 10.1046/j.1460-9568.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Bennett A.D. Chastain K.M. Hulsebosch C.E. Alleviation of mechanical and thermal allodynia by CGRP (8-37) in a rodent model of chronic central pain. Pain. 2000;86:163–175. doi: 10.1016/s0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Bowker R.M. Westlund K.N. Coulter J.D. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res. 1981;226:187–199. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Brosamle C. Schwab M.E. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J. Comp. Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bunge M.B. Pearse D.D. Response to the report, “A re-assessment of a combinatorial treatment involving Schwann cell transplants and elevation of cyclic AMP on recovery of motor function following thoracic spinal cord injury in rats” by Sharp et al. Exp. Neurol. 2012;233:645–648. doi: 10.1016/j.expneurol.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Chen A. Xu X.M. Kleitman N. Bunge M.B. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp. Neurol. 1996;138:261–276. doi: 10.1006/exnr.1996.0065. [DOI] [PubMed] [Google Scholar]
- Cheng H. Cao Y. Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- David S. Aguayo A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Dinh P. Bhatia N. Rasouli A. Suryadevara S. Cahill K. Gupta R. Transplantation of preconditioned Schwann cells following hemisection spinal cord injury. Spine. 2007;32:943–949. doi: 10.1097/01.brs.0000261408.61303.77. [DOI] [PubMed] [Google Scholar]
- Feng S.Q. Zhou X.F. Rush R.A. Ferguson I.A. Graft of pre-injured sural nerve promotes regeneration of corticospinal tract and functional recovery in rats with chronic spinal cord injury. Brain Res. 2008;1209:40–48. doi: 10.1016/j.brainres.2008.02.075. [DOI] [PubMed] [Google Scholar]
- Ferguson I.A. Koide T. Rush R.A. Stimulation of corticospinal tract regeneration in the chronically injured spinal cord. Eur. J. Neurosci. 2001;13:1059–1064. doi: 10.1046/j.1460-9568.2001.01482.x. [DOI] [PubMed] [Google Scholar]
- Fouad K. Schnell L. Bunge M.B. Schwab M.E. Liebscher T. Pearse D.D. Combining Schwann cell bridges and olfactory ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden K.L. Pearse D.D. Blits B. Garg M.S. Oudega M. Wood P.M. Bunge M.B. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp. Neurol. 2007;207:203–217. doi: 10.1016/j.expneurol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J.D. Hesse D. Schnell L. Schwab M.E. Bunge M.B. Bunge R.P. Influence of IN-1 antibody and acidic FGF-fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J. Neurosci. Res. 1997;50:888–905. doi: 10.1002/(SICI)1097-4547(19971201)50:5<888::AID-JNR24>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hamers F.P. Lankhorst A.J. van Laar T.J. Veldhuis W.B. Gispen W.H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- Herzog A. Brosamle C. ‘Semifree-floating’ treatment: a simple and fast method to process consecutive sections for immunohistochemistry and neuronal tracing. J. Neurosci. Methods. 1997;72:57–63. doi: 10.1016/s0165-0270(96)00156-2. [DOI] [PubMed] [Google Scholar]
- Hiebert G.W. Khodarahmi K. McGraw J. Steeves J.D. Tetzlaff W. Brain-derived neurotrophic factor applied to the motor cortex promotes sprouting of corticospinal fibers but not regeneration into a peripheral nerve transplant. J. Neurosci. Res. 2002;69:160–168. doi: 10.1002/jnr.10275. [DOI] [PubMed] [Google Scholar]
- Hill C.E. Beattie M.S. Bresnahan J.C. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp. Neurol. 2001;171:153–169. doi: 10.1006/exnr.2001.7734. [DOI] [PubMed] [Google Scholar]
- Hill C.E. Guller Y. Raffa S.J. Hurtado A. Bunge M.B. A calpain inhibitor enhances the survival of Schwann cells in vitro and after transplantation into the injured spinal cord. J. Neurotrauma. 2010;27:1685–1695. doi: 10.1089/neu.2010.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C.E. Hurtado A. Blits B. Bahr B.A. Wood P.M. Bunge M.B. Oudega M. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur. J. Neurosci. 2007;26:1433–1445. doi: 10.1111/j.1460-9568.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- Hofstetter C.P. Holmstrom N.A. Lilja J.A. Schweinhardt P. Hao J. Spenger C. Wiesenfeld-Hallin Z. Kurpad S.N. Frisen J. Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Houle J.D. Tom V.J. Mayes D. Wagoner G. Phillips N. Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J. Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling D.A. Lankhorst A.J. Gispen W.H. Bar P.R. Joosten E.A. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp. Neurol. 1998;153:49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- Joosten E.A. Bar P.R. Gispen W.H. Collagen implants and cortico-spinal axonal growth after mid-thoracic spinal cord lesion in the adult rat. J. Neurosci. Res. 1995;41:481–490. doi: 10.1002/jnr.490410407. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A. Gensel J.C. Ankeny D.P. Alexander J.K. Donnelly D.J. Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoller N. Auerbach G. Fulga V. Zelig G. Attias J. Bakimer R. Marder J.B. Yoles E. Belkin M. Schwartz M. Hadani M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J. Neurosurg. Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- Liebscher T. Schnell L. Schnell D. Scholl J. Schneider R. Gullo M. Fouad K. Mir A. Rausch M. Kindler D. Hamers F.P. Schwab M.E. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Maier I.C. Baumann K. Thallmair M. Weinmann O. Scholl J. Schwab M.E. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J. Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. Robe P. Franzen R. Delree P. Schoenen J. Stevenaert A. Moonen N. Effects of Schwann cell transplantation in a contusion model of rat spinal cord injury. J. Neurosci. Res. 1996;45:588–597. doi: 10.1002/(SICI)1097-4547(19960901)45:5<588::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Meijs M.F. Timmers L. Pearse D.D. Tresco P.A. Bates M.L. Joosten E.A. Bunge M.B. Oudega M. Basic fibroblast growth factor promotes neuronal survival but not behavioral recovery in the transected and Schwann cell implanted rat thoracic spinal cord. J. Neurotrauma. 2004;21:1415–1430. doi: 10.1089/neu.2004.21.1415. [DOI] [PubMed] [Google Scholar]
- Menei P. Montero-Menei C. Whittemore S.R. Bunge R.P. Bunge M.B. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci. 1998;10:607–621. doi: 10.1046/j.1460-9568.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- Metz G.A. Whishaw I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and coordination. J. Neurosci. Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Moon L.D. Leasure J.L. Gage F.H. Bunge M.B. Motor enrichment sustains hindlimb movement recovered after spinal cord injury and glial transplantation. Restor. Neurol. Neurosci. 2006;24:147–161. [PubMed] [Google Scholar]
- Oku R. Satoh M. Fujii N. Otaka A. Yajima H. Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res. 1987;403:350–354. doi: 10.1016/0006-8993(87)90074-6. [DOI] [PubMed] [Google Scholar]
- Oudega M. Varon S. Hagg T. Regeneration of adult rat sensory axons into intraspinal nerve grafts: promoting effects of conditioning lesion and graft predegeneration. Exp. Neurol. 1994;129:194–206. doi: 10.1006/exnr.1994.1161. [DOI] [PubMed] [Google Scholar]
- Pearse D.D. Marcillo A.E. Oudega M. Lynch M.P. Wood P.M. Bunge M.B. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J. Neurotrauma. 2004;21:1223–1239. doi: 10.1089/neu.2004.21.1223. [DOI] [PubMed] [Google Scholar]
- Popovich P. McTigue D. Damage control in the nervous system: beware the immune system in spinal cord injury. Nat. Med. 2009;15:736–737. doi: 10.1038/nm0709-736. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Guan Z. Wei P. Huitinga I. van Rooijen N. Stokes B.T. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Rasouli A. Bhatia N. Suryadevara S. Cahill K. Gupta R. Transplantation of preconditioned Schwann cells in peripheral nerve grafts after contusion in the adult spinal cord. Improvement of recovery in a rat model. J. Bone Joint Surg. Am. 2006;88:2400–2410. doi: 10.2106/JBJS.E.01424. [DOI] [PubMed] [Google Scholar]
- Richardson P.M. McGuinness U.M. Aguayo A.J. Peripheral nerve autografts to the rat spinal cord: studies with axonal tracing methods. Brain Res. 1982;237:147–162. doi: 10.1016/0006-8993(82)90563-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Yoles E. Macrophages and dendritic cells treatment of spinal cord injury: from the bench to the clinic. Acta Neurochir. Suppl. 2005;93:147–150. doi: 10.1007/3-211-27577-0_25. [DOI] [PubMed] [Google Scholar]
- Sharp K.G. Flanagan L.A. Yee K.M. Steward O. A re-assessment of a combinatorial treatment involving Schwann cell transplants and elevation of cyclic AMP on recovery of motor function following thoracic spinal cord injury in rats. Exp. Neurol. 2012;233:625–644. doi: 10.1016/j.expneurol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Sharp K. Yee K.M. Hofstadter M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp. Neurol. 2008;209:446–468. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Zheng B. Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J. Comp. Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- Takami T. Oudega M. Bates M.L. Wood P.M. Kleitman N. Bunge M.B. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley R.B. Zhang S.H. Feng S.Q. Rush R.A. Ferguson I.A. Use of engineered peripheral nerve autografts for spinal cord repair. Neuroreport. 2006;17:261–265. doi: 10.1097/01.wnr.0000199462.09165.12. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga E.G. Turk D.C. Exacerbation of chronic pain following spinal cord injury. J. Neurotrauma. 2004;21:1384–1395. doi: 10.1089/neu.2004.21.1384. [DOI] [PubMed] [Google Scholar]
- Xu X.M. Chen A. Guenard V. Kleitman N. Bunge M.B. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Xu X.M. Guenard V. Kleitman N. Aebischer P. Bunge M.B. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 1995a;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Xu X.M. Guenard V. Kleitman N. Bunge M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 1995b;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Xu X.M. Zhang S.X. Li H. Aebischer P. Bunge M.B. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur. J. Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Zawadzka M. Rivers L.E. Fancy S.P. Zhao C. Tripathi R. Jamen F. Young K. Goncharevich A. Pohl H. Rizzi M. Rowitch D.H. Kessaris N. Suter U. Richardson W.D. Franklin R.J. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]