Abstract
Hypotension after traumatic brain injury (TBI) worsens outcome. We published the first report of TBI plus hemorrhagic shock (HS) in mice using a volume-controlled approach and noted increased neuronal death. To rigorously control blood pressure during HS, a pressure-controlled HS model is required. Our hypothesis was that a brief, severe period of pressure-controlled HS after TBI in mice will exacerbate functional deficits and neuropathology versus TBI or HS alone. C57BL6 male mice were randomized into four groups (n=10/group): sham, HS, controlled cortical impact (CCI), and CCI+HS. We used a pressure-controlled shock phase (mean arterial pressure [MAP]=25–27 mm Hg for 35 min) and its treatment after mild to moderate CCI including, a 90 min pre-hospital phase, during which lactated Ringer's solution was given to maintain MAP >70 mm Hg, and a hospital phase, when the shed blood was re-infused. On days 14–20, the mice were evaluated in the Morris water maze (MWM, hidden platform paradigm). On day 21, the lesion and hemispheric volumes were quantified. Neuropathology and hippocampal neuron counts (hematoxylin and eosin [H&E], Fluoro-Jade B, and NeuN) were evaluated in the mice (n=60) at 24 h, 7 days, or 21 days (n=5/group/time point). HS reduced MAP during the shock phase in the HS and CCI+HS groups (p<0.05). Fluid requirements during the pre-hospital phase were greatest in the CCI+HS group (p<0.05), and were increased in HS versus sham and CCI animals (p<0.05). MWM latency was increased on days 14 and 15 after CCI+HS (p<0.05). Swim speed and visible platform latency were impaired in the CCI+HS group (p<0.05). CCI+HS animals had increased contusion volume versus the CCI group (p<0.05). Hemispheric volume loss was increased 33.3% in the CCI+HS versus CCI group (p<0.05). CA1 cell loss was seen in CCI+HS and CCI animals at 24 h and 7 days (p<0.05). CA3 cell loss was seen after CCI+HS (p<0.05 at 24 h and 7 days). CA1 cell loss at 21 days was seen only in CCI+HS animals (p<0.05). Brief, severe, pressure-controlled HS after CCI produces robust functional deficits and exacerbates neuropathology versus CCI or HS alone.
Key words: blast injury, controlled cortical impact, head injury, head trauma, Morris water maze, polytrauma, secondary insult
Introduction
Traumatic brain injury (TBI) is a major cause of morbidity and mortality in both the civilian and military settings. Following an assessment of the National Institutes of Health (NIH) Traumatic Coma Data Bank, Chesnut and associates (1993) reported that the occurrence of hypotension and/or hypoxemia after TBI was associated with a doubling of mortality rates and increased morbidity in humans, thus identifying secondary insults as a critical factor in determining outcomes. Similarly, blast polytrauma victims, such as armed servicemen and victims of terrorist attacks, often experience TBI plus hemorrhagic shock (HS). Nelson and colleagues (2006) reported highly detrimental effects of secondary insults in blast TBI, namely a 100% mortality rate when blast TBI was associated with hypotension. Indeed, because of these poor outcomes, patients with secondary insults have been routinely excluded from clinical TBI trials. It is, however, unclear if combined insults are less or more amenable to novel therapies than TBI alone. Thus, combined TBI plus HS (TBI+HS) is potentially an important new therapy for use in civilian and blast polytrauma.
DeWitt and associates (1992) showed that in cats, blood pressure autoregulation of cerebral blood flow (CBF) was disturbed after TBI, suggesting that the injured animals could tolerate only small variations in mean arterial pressure (MAP) while maintaining constant CBF. Clinically, it has long been suggested that even mild TBI can sometimes produce marked disturbances or even complete loss of blood pressure autoregulation of CBF (Strebel et al., 1997). Glass and colleagues (1999), using a pig model of TBI, reported that even mild hemorrhage exacerbated lesion volume versus TBI alone. These and other studies have shown that the pathophysiology of TBI is unique in the setting of superimposed HS. Experimental studies have focused on large animals such as pigs or dogs, targeted the assessment of acute cerebrovascular physiology such as intracranial pressure (ICP) or CBF, and tested almost exclusively novel fluids or other strategies to optimize blood pressure in this setting.
Few studies have reported on the use of rodents to explore the effect of HS on traditional end-points such as neuronal damage or functional outcome after TBI, and conflicting results have been found. Matsushita and co-workers (2001) reported that mild hemorrhagic hypotension to a mean of 60 mm Hg for 30 min after fluid percussion injury (FPI) in rats produced a 48% reduction in CBF at 20 min, and doubled contusion volume at 72 h. In contrast, Schütz and associates (2006) studied the effect of a similar level of hemorrhagic hypotension (MAP 50–60 mm Hg) for 30 min after FPI in rats and reported no expansion of either brain edema or lesion volume, but exacerbation of cognitive task performance deficits.
There has, however, been little study of the effect of HS on outcomes after experimental TBI in mice. The ability to use mutant strains or small volumes of highly novel test fluids such as blood substitutes could have important advantages. However, the technical challenges of inducing and appropriately monitoring and managing HS in mice are obvious. We recently published a study showing that 90 min but not 60 min of moderate volume-controlled HS (MAP 35–45 mm Hg) after controlled cortical impact (CCI) in mice increased hippocampal neuronal death in brain tissue at 7 days post-injury. Additional reports from our group demonstrated exacerbation of CBF reductions after CCI by HS in this model (Dennis et al., 2006), and its use to evaluate novel neuroprotective therapies (Exo et al., 2009; Shellington et al., 2011). In those studies we used a mild CCI followed by a mild to moderate level of HS for a period of 90 min using a volume-controlled approach. However, since cerebral perfusion pressure (CPP) is a critical determinant of CBF, there is a theoretical advantage to using a pressure-controlled rather than volume-controlled HS model to induce a secondary insult after TBI. Unlike volume-controlled HS models, in which a set volume of blood is removed and MAP is an uncontrolled parameter that is observed, in pressure-controlled HS models, MAP is rigorously maintained at a fixed target throughout the HS phase. This approach should lead to a more reproducible model of a secondary insult to the brain after TBI, and provide relevant information about the effect of specific perfusion pressures on the degree of secondary damage. We hypothesized that a brief period of severe pressure-controlled HS after CCI in mice would exacerbate both functional deficits and long-term neuropathology versus sham, HS, or CCI alone.
Methods
Controlled cortical impact model
The institutional animal care and use committees of both the University of Pittsburgh School of Medicine and the United States Army approved the experiments used in this study. C57/BL6 mice (n=133; Jackson Laboratories, Bar Harbor, ME), 12–15 weeks of age, weighing 27.92±0.96 g, were given ad libitum food and water and were housed in controlled environmental conditions until the study began. Four groups were created: sham (craniotomy, catheters, and anesthesia, but no CCI or HS), HS only, CCI, or CCI+HS. In order to complete the injury protocol, all mice were anesthetized using a nose cone with 4% isoflurane in oxygen. Anesthesia was maintained with 1.5% isoflurane in a 2:1 N2O/oxygen mixture for surgical preparation, and under sterile prep and drape, an inguinal cut-down was done, and femoral venous and arterial catheters (PE-50 tubing) were inserted. The mice were then placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and using a dental drill, a 5-mm craniotomy was performed over the left parietal cortex and the bone flap was removed. A microprobe (Physitemp, Clifton, NJ) was inserted through a burr hole into the left frontal cortex to monitor brain temperature, and body temperature was monitored via rectal probe. Immediately following the craniotomy, the inhalational anesthesia was changed to 1% isoflurane in room air for 10 min before beginning the injury protocols. While maintaining the brain temperature at 37±0.5°C, a mild to moderate CCI was performed with a pneumatic impactor (Bimba, Monee, IL) using a 3-mm flat-tip impounder that impacted the left parietal cortex at 5 m/sec at a depth of 1.0 mm. After the impact, the craniotomy defect was immediately replaced and sealed with dental cement and the scalp was closed.
Pressure-controlled hemorrhagic shock
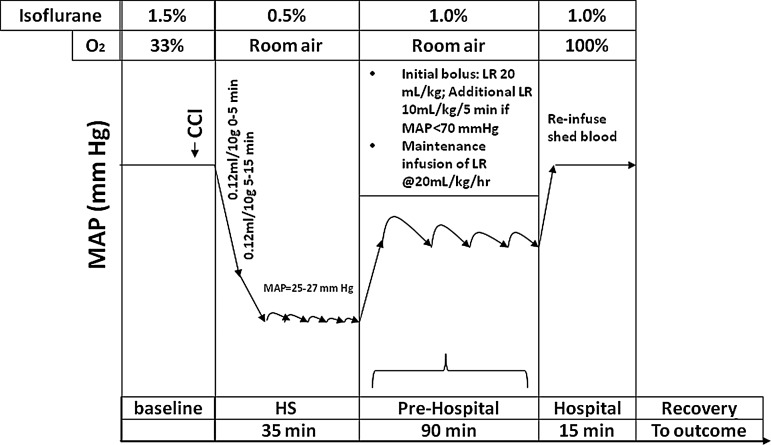
A clinically-relevant three-phase paradigm was used to produce HS and resuscitation (Fig. 1). This included shock, pre-hospital, and hospital phases. To initiate HS, beginning 5 min after CCI or at an identical anesthesia time point in mice in the HS-only group, 0.12 mL of blood per 10 g of body weight was removed over 5 min via the venous catheter into a 1-mL tuberculin syringe containing 0.07 mL of citrate anticoagulant (Cardian BCT, Lakewood, CO). An additional 0.12 mL of blood per 10 g of body weight was then withdrawn from the venous catheter. This represents ∼30% of the blood volume of the mouse (Riches et al., 1973). MAP of 25–27 mm Hg was achieved and subsequently rigorously maintained by continued removal or re-infusion of autologous citrated blood from the venous catheter in ∼0.05-mL aliquots using the same syringe. The mice remained at this level of HS to complete a 35-min shock phase. Longer durations of HS after CCI at this MAP level resulted in acute mortality in some mice in pilot studies, and thus the 35-min HS duration was selected as the longest duration reproducibly achievable with a low mortality rate. Following shock, mice entered a pre-hospital phase, and were resuscitated with lactated Ringer's (LR) solution. An initial 20-mL/kg bolus of LR was administered followed by additional 10-mL/kg boluses every 5 min if the MAP was <70 mm Hg. For reference, the normal MAP in isoflurane-anesthetized mice is ∼85 mm Hg in our preparation; a value of 70 mm Hg was selected based on the generally accepted goal of attempting to maintain CPP of at least ∼60 mm Hg. ICP in our model during the HS phase generally ranges between 2 and 10 mm Hg (unpublished data). In addition, in all groups, a maintenance infusion of LR was given at a rate of 4 mL/kg/h. After 90 min of resuscitation, the mice entered a hospital phase, during which their shed blood was re-infused over 15 min, which restored MAP to near baseline levels. This simulated definitive care in a hospital setting. During HS, anesthesia was reduced from 1% isoflurane in room air to 0.5% isoflurane in room air (Fig. 1). During the pre-hospital phase, room air was used to mimic field resuscitation in combat casualty care, where oxygen is not readily available. During the hospital phase, 1% isoflurane in pure oxygen was administered, again mimicking clinical care in a combat support hospital or emergency department.
FIG. 1.
Schematic diagram of the protocol used to study the effect of severe pressure-controlled hemorrhagic shock (HS) on functional and neuropathological outcomes after controlled cortical impact (CCI) in mice. Controls included sham, HS only, and CCI-only groups. A clinically-relevant three-phase model was used that included a 35-min shock phase, during which time the mean arterial pressure (MAP) target was 25–27 mm Hg—a severe HS level. After the shock phase, mice in the HS and CCI+HS groups were resuscitated during a pre-hospital phase, simulating field resuscitation for 90 min using lactated Ringer's (LR) solution. An initial bolus of 20 mL/kg was followed by boluses every 5 min to maintain MAP >70 mm Hg. During the pre-hospital phase, maintenance LR was also infused at 4 mL/kg/h. After the pre-hospital phase, a hospital phase followed, which included reinfusion of the shed blood, simulating transfusion in the trauma bay or emergency department.
Monitoring protocol
Via the femoral arterial catheter, MAP was continuously monitored and recorded at baseline, after CCI, and every 5 min during HS and resuscitation; heart rate was similarly monitored and recorded. Laboratory evaluation with arterial blood gases, lactate, glucose, hematocrit (HCT), hemoglobin (Hb), sodium, potassium, calcium, magnesium, blood urea nitrogen (BUN), and osmolarity was obtained at baseline (Stat Profile; Nova Biomedical, Waltham, MA), 30 min into the shock phase, and at the end of the pre-hospital and hospital phases.
At the completion of this protocol, the catheters and probes were removed, anesthesia was discontinued, and the mice were returned to their cages with food and water ad libitum. To ensure that anesthetic differences between groups did not produce differential neuroprotective effects (Statler et al., 2000, 2006), the mice in the sham and CCI-only groups were exposed to an identical duration of anesthesia as those in the HS and CCI+HS groups. We carried out studies until each group had 10 mice for the entire 21-day outcome protocol.
Morris water maze testing
Morris water maze (MWM) performance was evaluated as previously described (Sinz et al., 1999; Whalen et al., 1999b) with modifications. Prior to surgery, mice underwent a pre-training protocol in the MWM. Mice completed a hidden platform test four times a day for 5 days, and those able to reach a pre-defined minimum criterion (finding the platform in <40 sec) were allowed to continue to randomization into the functional outcome portion of the protocol. On days 14–18 post-injury (n=10 per group), the hidden-platform version of the MWM was used. Both pre- and post-injury testing was performed using a white pool (83 cm diameter, 60 cm deep) filled with 24°C water to 29 cm depth was situated in a room with several extra-maze cues located on the walls. Contained within the pool was a 10-cm round goal platform, located 1 cm below the surface of the water ∼15 cm from the southwest wall. A video-tracking system mounted above the pool (AnyMaze; Stoelting Co., Wood Dale, IL) recorded the swim speeds of the mice. Each mouse was subjected to a series of four trials per day. For each trial, the mice were randomized to one of four starting locations and were placed in the pool. The mice were given a maximum of 120 sec to find the submerged platform, with latency recorded. The mice were placed in a 37°C incubator for 4 min between trials. To test for potential nonspecific deficits in visual and motor function, a visible platform task was performed on days 19 and 20. The platform was raised 2 cm above the water surface with mice randomized to starting locations. A single probe trial to determine memory retention was performed on day 18, in which the platform was removed and the percentage time in the platform quadrant was measured by a video-tracking system. Swim speed was also assessed. Average latency to find the hidden platform was calculated for each mouse on each trial day. In addition, overall mean latency was quantified for the entire period of hidden platform testing for each mouse.
Volumetric assessments
At 21 days after the insult, upon completion of the MWM testing, the mice were re-anesthetized with 4% isoflurane and sacrificed by transcardial perfusion with 50 mL of ice-cold saline with heparin, followed by 50 mL of 10% buffered formalin. The brains were allowed to remain in situ for 24 h, and then were carefully removed. The brains were cryoprotected in 15% and 30% sucrose and frozen in liquid nitrogen. Serial coronal sections 10 μm thick were taken every 0.5 mm through the entire brain, mounted on slides, and stained with cresyl violet. Image analysis software (MCID; Imaging Research, Saint Catherines, Ontario, Canada) was used by a blinded observer (J.N.H.) to analyze lesion and hemispheric volumes as previously described (Whalen et al., 1999a).
Assessment of hippocampal neuron counts and neuropathology
In addition to the studies of long-term functional and brain volumetric outcomes, a total of 60 additional mice that did not undergo post-injury functional outcome testing were randomized to each of the four groups (sham, HS, CCI, and CCI+HS), to evaluate the time course of neuropathology at 24 h, 7 days, and 21 days after the insult. For these studies, at the pre-determined time points, the mice were anesthetized with 4% isofluorane and killed by transcardial perfusion with ice-cold saline followed by 10% buffered formalin as described above. Brain tissue was fixed in 10% buffered formalin and embedded in paraffin. Multiple 5-μm sections, 200 μm apart from the bregma at −1.86 to −2.26 mm, were prepared from each brain. The sections were stained with H&E (Thermo Scientific, Pittsburgh, PA), and Fluoro-Jade C (FJC; Schmued et al., 2005), and NeuN (Millipore, Temecula, CA), with sections from three of the five mice in the CCI and CCI+HS groups necropsied at 7 days and 21 days also having been stained for glial fibrillary acidic protein (GFAP). Hippocampal neuronal survival and degeneration were quantified per 0.1 mm in CA1 and CA3 in sections stained with H&E and FJC, respectively, by an evaluator blinded to treatment group (M.X.), using a Nikon Eclipse E600 microscope (Melville, NY), and ImageJ software (NIH), as previously reported (Exo et al., 2009; Shellington et al., 2011). In addition, a neuropathologist (R.H.G.) qualitatively assessed injury in coronal brain sections from mice from the paraffin-embedded brain sections described above (coronal sections at the level of the dorsal hippocampus), as well as sections from a subgroup of 25 mice stained with the amino cupric silver (CuAg) technique. The heads from these additional 25 mice (1 naïve, 6 sham, and 6 mice from each of the CCI, HS, and CCI+HS groups) had been sent to NeuroScience Associates (Knoxville, TN), where the brains were removed and processed in a single multi-brain block. Sixty coronal step sections from this block (extending from the olfactory bulbs to the medulla oblongata) were stained with the CuAg technique for degenerating cells and processes. For the sham, CCI, HS, and CCI+HS groups, there were two mice from each time point (24 h, 72 h, and 21 d) in this block.
Statistical analysis
Physiologic parameters were analyzed using generalized estimating equations and a mixed effects model (group, time, and group×time interaction). When group effect differences were identified, post-hoc testing was performed using the Bonferroni correction for multiple comparisons. Volumetric analyses, total swim latencies, swim speeds, probe trial latencies, and cell counts were compared between groups using analysis of variance (ANOVA), and Student-Newman-Keuls testing for post-hoc multiple comparisons. MWM latencies at each time point were compared using a two-way ANOVA for repeated measures. Total latencies were compared using one-way ANOVA. Pearson correlation coefficients were calculated to assess for significant correlations between parameters. All data are provided as mean±standard error of the mean (SEM). Significance was set at p≤0.05.
Results
Acute physiology
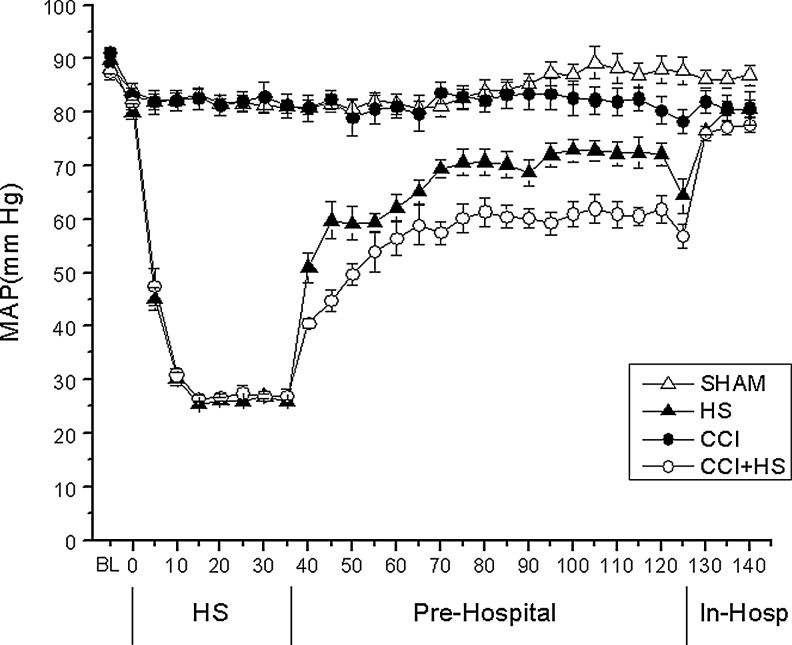
HS produced the anticipated reductions in MAP during the HS phase (p<0.05 group×time effect; Fig. 2), for both the HS and CCI+HS groups versus the sham or CCI-only groups. Recovery of MAP was also slowest in the CCI+HS group versus the HS, CCI, and sham groups (group×time effect p<0.05). Hemorrhage produced a divergent heart rate response in the HS and CCI+HS groups (Fig. 3). There were no group or time differences for brain or rectal temperatures across the shock and resuscitation periods (Table 1).
FIG. 2.
Mean arterial pressure (MAP, mm Hg) versus time (min) in mice subjected to sham, hemorrhagic shock (HS), controlled cortical impact (CCI) traumatic brain injury, or combined CCI+HS. As outlined in Figure 1, a three-phase model was used that included a shock phase, a pre-hospital resuscitation phase, and a hospital phase (In-Hosp). The HS and CCI+HS groups showed the anticipated marked reduction in MAP during the 35-min shock phase (p<0.05 for group×time interaction), since only these two groups were exposed to hemorrhage. In addition, recovery of MAP was slowest in the CCI+HS group during the pre-hospital phase (p<0.05 for group×time interaction).
FIG. 3.
Heart rate (HR, beats per minute) versus time (min) in mice subjected to sham, hemorrhagic shock (HS), controlled cortical impact (CCI) traumatic brain injury, or combined CCI+HS. As outlined in Figure 1, a three-phase model was used that included a shock phase, a pre-hospital resuscitation phase, and a hospital phase (In-Hosp). Hemorrhage appeared to produce a divergent HR response in the HS and CCI+HS groups, with a depressor response during the first 25 min of the shock phase in the HS-alone group, but tachycardia in the combined CCI+HS group (p<0.05 for the effect of both time and group×time interaction).
Table 1.
Physiological Parameters
| |
Time |
|||
|---|---|---|---|---|
| Parameter | Baseline | During shock phase | End of pre-hospital phase | End of hospital phase |
| Group: Sham | ||||
| Tbrain (°C) | 37.02±0.15 | 37.17±0.13 | 37.10±0.17 | 37.08±0.20 |
| Trectal (°C) | 37.78±0.55 | 37.94±0.22 | 37.60±0.33 | 37.89±0.22 |
| pH | 7.40±0.03 | 7.43±0.02 | 7.42±0.03 | 7.37±0.03 |
| Pao2 (torr) | 152.04±6.58 | 85.41±7.08 | 90.04±9.00 | 446.84±14.46 |
| Paco2 (torr) | 28.40±3.63 | 25.18±1.95 | 27.20±3.80 | 30.70±3.76 |
| Base excess (mEq/L) | −5.19±0.64 | −4.99±0.68 | −5.69±1.24 | −5.90±0.76 |
| Lactate (mmol/L) | 2.25±0.53 | 1.92±0.39 | 1.86±0.65 | 1.41±0.31 |
| Glucose | 82.60±28.90 | 77.20±24.00 | 67.30±35.94 | 48.44±16.85 |
| Hematocrit (%) | 40.40±1.96 | 38.80±2.30 | 39.80±32.15 | 37.89±1.45 |
| Hemoglobin (g/dL) | 13.46±0.68 | 12.87±0.77 | 13.28±0.66 | 12.61±0.55 |
| Group: HS | ||||
| Tbrain (°C) | 37.04±0.14 | 37.03±0.31 | 36.99±0.25 | 37.18±0.22 |
| Trectal (°C) | 37.72±0.58 | 38.16±0.17 | 37.66±0.40 | 37.82±0.23 |
| pH | 7.41±0.02 | 7.44±0.03 | 7.41±0.04 | 7.33±0.06 |
| PaO2 (torr) | 158.38±46.52 | 114.54±18.85 | 86.87±12.42 | 447.60±12.79 |
| PaCO2 (torr) | 28.22±3.30 | 18.90±2.72 | 27.32±5.25 | 36.20±9.22 |
| Base excess (mEq/L) | −4.75±1.50 | −8.72±1.11 | −5.80±1.44 | −5.62±1.04 |
| Lactate (mmol/L) | 2.49±0.54 | 5.24±1.10 | 1.78±0.72 | 1.40±0.42 |
| Glucose | 80.40±40.68 | 139.80±42.47 | 63.25±31.17 | 53.90±15.31 |
| Hematocrit (%) | 40.90±1.29 | 28.00±1.25 | 22.00±3.50 | 31.00±2.21 |
| Hemoglobin (g/dL) | 13.60±0.44 | 9.38±0.42 | 7.37±1.15 | 10.41±0.72 |
| Group: CCI | ||||
| Tbrain (°C) | 37.29±0.72 | 36.98±0.29 | 37.03±0.23 | 37.00±0.11 |
| Trectal (°C) | 37.51±0.62 | 38.24±0.13 | 37.96±0.23 | 37.94±0.26 |
| pH | 7.39±0.02 | 7.41±0.01 | 7.42±0.03 | 7.38±0.03 |
| Pao2 (torr) | 162.22±5.71 | 84.62±4.89 | 90.16±9.63 | 456.37±20.47 |
| Paco2 (torr) | 28.50±1.70 | 25.83±2.34 | 25.59±2.32 | 29.81±6.78 |
| Base excess (mEq/L) | −5.49±1.06 | −5.51±1.16 | −5.48±1.43 | −5.73±2.32 |
| Lactate (mmol/L) | 2.62±0.60 | 2.15±0.49 | 1.99±0.45 | 1.57±0.64 |
| Glucose | 92.22±40.41 | 106.44±53.07 | 65.22±16.32 | 56.06±33.57 |
| Hematocrit (%) | 39.56±1.42 | 37.33±2.45 | 38.30±2.36 | 37.20±1.75 |
| Hemoglobin (g/dL) | 13.19±0.45 | 12.39±0.78 | 12.83±0.82 | 12.42±0.64 |
| Group: CCI+HS | ||||
| Tbrain (°C) | 37.15±0.21 | 36.88±0.26 | 37.17±0.32 | 37.12±0.14 |
| Trectal (°C) | 37.94±0.74 | 38.22±0.36 | 37.74±0.30 | 37.74±0.20 |
| pH | 7.42±0.05 | 7.38±0.04 | 7.42±0.03 | 7.34±0.04 |
| Pao2 (torr) | 159.99±9.81 | 98.49±12.24 | 87.39±13.53 | 461.42±18.14 |
| Paco2 (torr) | 25.51±3.27 | 20.37±3.77 | 26.52±2.82 | 33.15±5.00 |
| Base excess (mEq/L) | −5.61±1.05 | −10.64±1.45 | −6.12±1.05 | −6.21±1.14 |
| Lactate (mmol/L) | 2.29±0.86 | 5.96±2.22 | 2.60±0.81 | 1.81±0.43 |
| Glucose | 59.89±15.70 | 150.80±61.34 | 52.50±15.83 | 49.50±10.88 |
| Hematocrit (%) | 39.11±2.76 | 25.30±1.57 | 17.70±1.57 | 29.50±2.92 |
| Hemoglobin (g/dL) | 12.98±0.96 | 8.44±0.54 | 5.90±0.56 | 9.83±1.02 |
All values are mean±standard error of the mean. Values of sodium, potassium, calcium, magnesium, blood urea nitrogen, and osmolarity did not differ between groups and are not shown.
HS, hemorrhagic shock; CCI, controlled cortical impact; CCI+HS, controlled cortical impact plus hemorrhagic shock; Tbrain, brain temperature; Trectal, rectal temperature; Pao2, partial arterial oxygen pressure; Paco2, partial arterial carbon dioxide pressure.
There was a difference between groups for blood lactate, which was increased at the end of the shock phase in the HS and TBI+HS groups (p<0.05 for HS and CCI+HS versus sham or CCI only; Table 1). Similarly, there was a group effect for magnesium level (p<0.05), which was modestly reduced at the end of the hospital phase in the HS and TBI+HS groups, presumably related to the use of citrate anticoagulant in the shed blood that was re-infused. There were also no group effect differences for pH, partial arterial carbon dioxide pressure (Paco2), partial arterial oxygen pressure (Pao2), HCT, Hb, or glucose (Table 1), or sodium, potassium, calcium, magnesium, BUN, or osmolarity (data not shown). There were significant effects of time on Pao2, HCT, and glucose, but no group×time interactions.
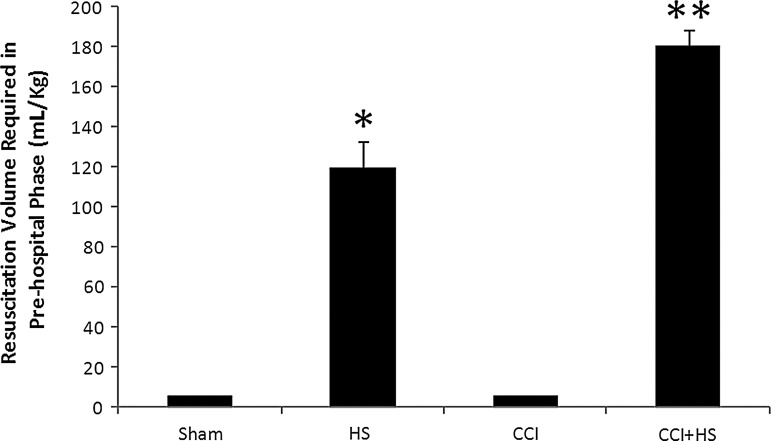
As anticipated, fluid requirements to maintain MAP at the desired target of >70 mm Hg during the pre-hospital resuscitation phase were markedly increased in the HS and CCI+HS groups (p<0.05 versus the sham and CCI groups). Specifically, fluid requirements were 6±0, 120±12.5, 6±0, and 181±7.9 mL/kg in the sham, HS, CCI, and CCI+HS groups, respectively (Fig. 4). In addition, the fluid requirements in the CCI+HS group were greater than those in the HS only group (p<0.05 by ANOVA and Student-Neuman-Keuls testing).
FIG. 4.
Fluid requirements during the pre-hospital resuscitation phase to maintain mean arterial pressure (MAP) >70 mm Hg were greatest in the CCI+HS group (**p<0.05 versus all other groups), and were also increased in the HS group (*p<0.05 versus sham and CCI only). Fluid administered to the sham and CCI-only groups represented a maintenance infusion of lactated Ringer's solution at 4 mL/kg/h, thus totaling 6 mL/kg in each of these groups over the 60-min pre-hospital phase (HS, hemorrhagic shock; CCI, controlled cortical impact).
To achieve 10 mice completing the entire protocol, a total of 52 mice were studied in the sham, HS, CCI, and CCI+HS groups, respectively. Differences between groups in mortality rate were not statistically significant.
Functional outcome
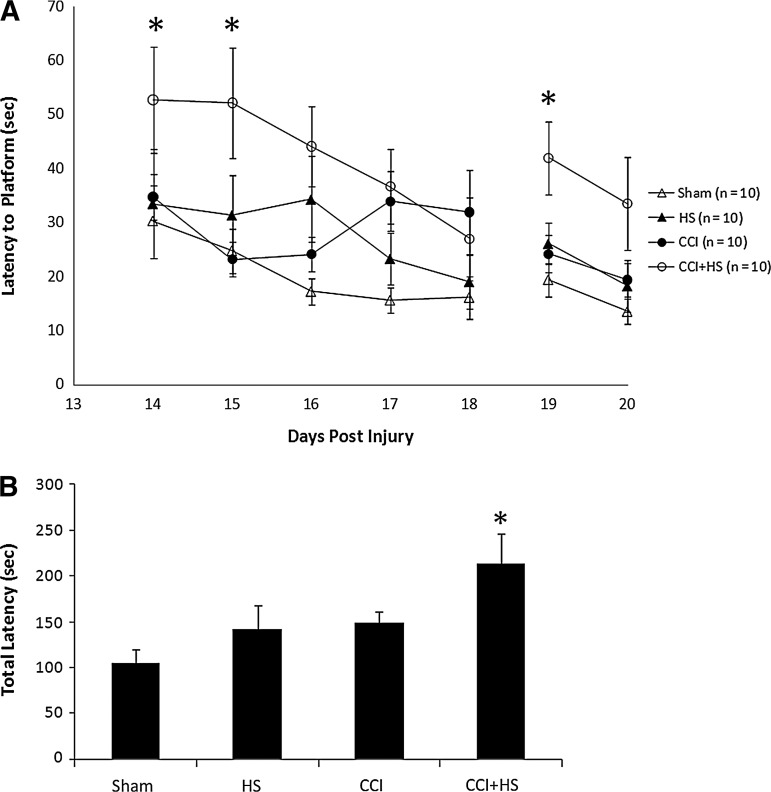
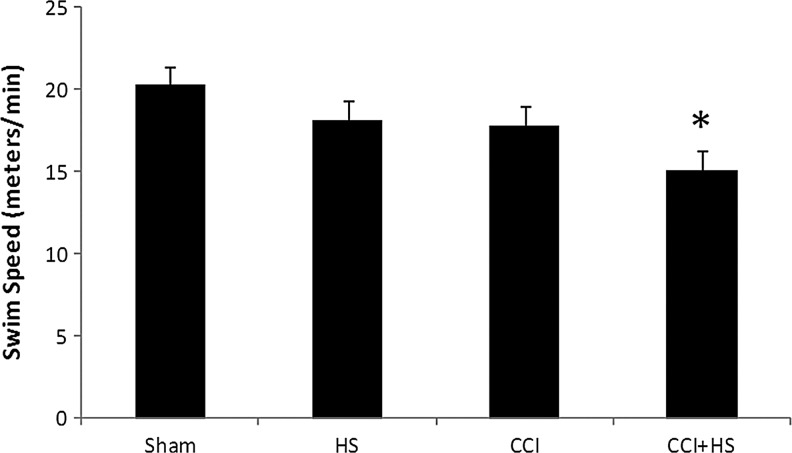
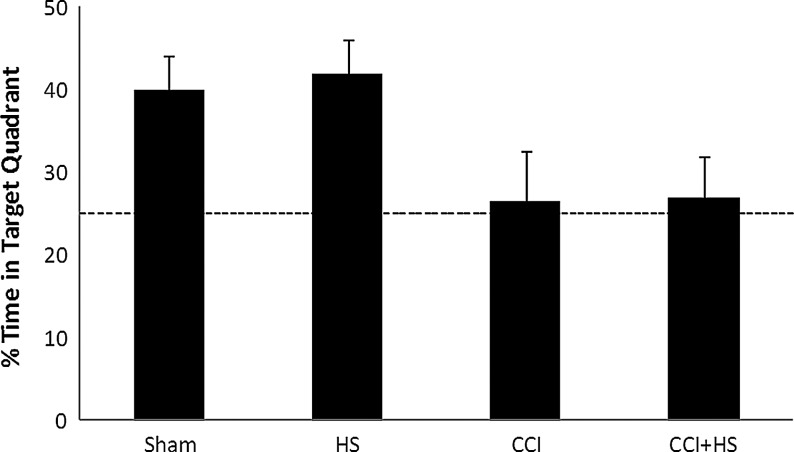
There was no difference in MWM latency between groups at the end of the training period (20.4±1.91, 26.5±7.32, 21.2±2.42, 20.6±1.85 sec in the sham, HS, CCI, and CCI+HS groups, respectively; p=0.6 by ANOVA). In contrast, after injury MWM latency to find the hidden platform was increased on days 14 and 15 of testing only in the TBI+HS group (p<0.05 versus all other groups; Fig. 5A). In addition, total latency to find the hidden platform was significantly increased in the CCI+HS group (p<0.05 versus all other groups; Fig. 5B). Surprisingly, both visible platform latency (Fig. 5A) and swim speed (Fig. 6) were also significantly impaired in the TBI+HS group (p<0.05 versus all other groups for both parameters). Mean latency to find the hidden platform was also significantly correlated with swim speed (Pearson's correlation coefficient=0.52, p=0.0005); however, the overall magnitude of the reduction in swim speed (∼20% versus sham animals) was much less than the effect on either MWM latency on days 14–15 (∼50%), or on total latency in the hidden platform trial (over 100%). In the probe trial, the percent of time spent in the target quadrant for both the CCI and CCI+HS groups was ∼25%, similar to random chance, in contrast to the sham and HS-only groups (Fig. 7). The overall ANOVA was significant at p=0.049; however, post-hoc comparisons did not isolate a specific individual difference.
FIG. 5.
(A) Graph depicting latency to find the platform on each day of testing in the Morris water maze (MWM) in the sham, hemorrhagic shock (HS) only, controlled cortical impact (CCI) only, and CCI+HS groups. The CCI-only insult was at a mild to moderate injury level that did not produce a significant MWM deficit during hidden platform testing. Also, HS alone also did not produce a MWM deficit versus sham animals. In contrast, the CCI+HS group showed an increased latency on days 14–18 (*p<0.05 versus all other groups), indicating that the secondary HS insult worsened functional outcome after CCI. Latency to find the platform was also increased on the first day of visible platform testing after CCI+HS (*p<0.05 versus all other groups), indicating that the MWM deficit in the acquisition phase of testing in the CCI+HS group cannot necessarily be attributed to spatial memory deficits. (B) Graph of total latency in the hidden platform paradigm (days 14–18) confirmed the deleterious effect of CCI+HS on MWM performance (p<0.05 versus all other groups).
FIG. 6.
Swim speed in the Morris water maze (MWM), assessed on days 19–20 for mice subjected to sham, hemorrhagic shock (HS) only, controlled cortical impact (CCI) only, and CCI+HS. Swim speed was significantly reduced only in the CCI+HS group (*p<0.05 versus sham animals by one-way analysis of variance and Student-Newman-Keuls testing).
FIG. 7.
Percent of time spent in the target quadrant during a probe trial for mice subjected to sham, hemorrhagic shock (HS) only, controlled cortical impact (CCI) only, and CCI+HS. The percent of time spent in the target quadrant was lower in mice in the CCI and CCI+HS groups than either sham or HS alone. Mice in both the CCI and CCI+HS groups exhibited maximally impaired performance on this task, given that their observed percent of time spent in the target quadrant was similar to random chance (p=0.049 for overall one-way analysis of variance effect).
Volumetric assessments
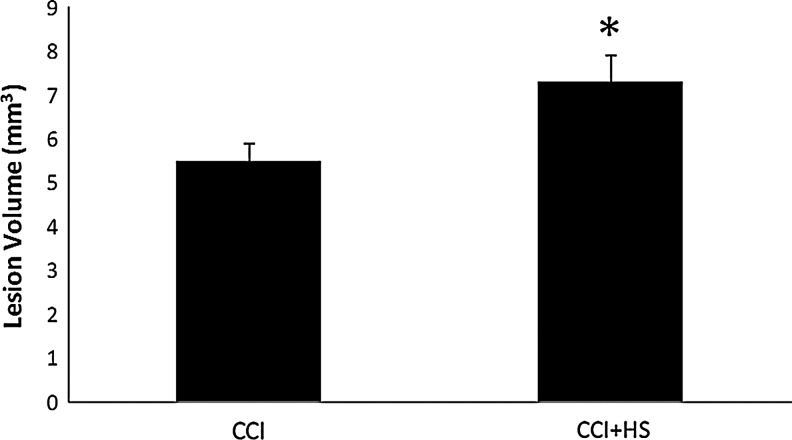
The CCI+HS group had a 33.0% increase in lesion volume versus the CCI-only group (7.33±0.64 versus 5.51±0.36 mm3, respectively, p<0.05; Fig. 8). Hemispheric volume loss, as assessed by the right-left hemispheric difference, was increased 33.3% in the CCI+HS versus the CCI group (17.32±1.14 versus 12.99±1.14 mm3, p<0.05; Fig. 9). Right (contralateral to impact) hemispheric volumes did not differ between groups (data not shown).
FIG. 8.
Graph depicting contusion volume in mice at 21 days after either controlled cortical impact (CCI), or CCI plus hemorrhagic shock (HS; CCI+HS). Mice in the sham and HS-only groups did not have visible lesions to quantify. Secondary HS after CCI significantly increased contusion volume, by over 33% (*p<0.05 for the CCI+HS group versus the CCI-only group).
FIG. 9.
Hemispheric tissue loss represented as right minus left (R-L) hemispheric volume in mice at 21 days after sham, hemorrhagic shock (HS) only, controlled cortical impact (CCI) only, and CCI+HS. In the CCI and CCI+HS groups, the injury was delivered to the left hemisphere. HS alone did not lead to a R-L hemispheric volume difference versus sham animals. CCI alone led to a R-L difference of 12.99 mm3, which represents ∼10% loss of volume (*p<0.05 versus sham or HS only) in the left hemisphere. Combined CCI+HS further expanded tissue loss, to over 15% of the left hemisphere (**p<0.05 versus all other groups), again indicating deleterious neuropathological consequences of a secondary HS insult on CCI.
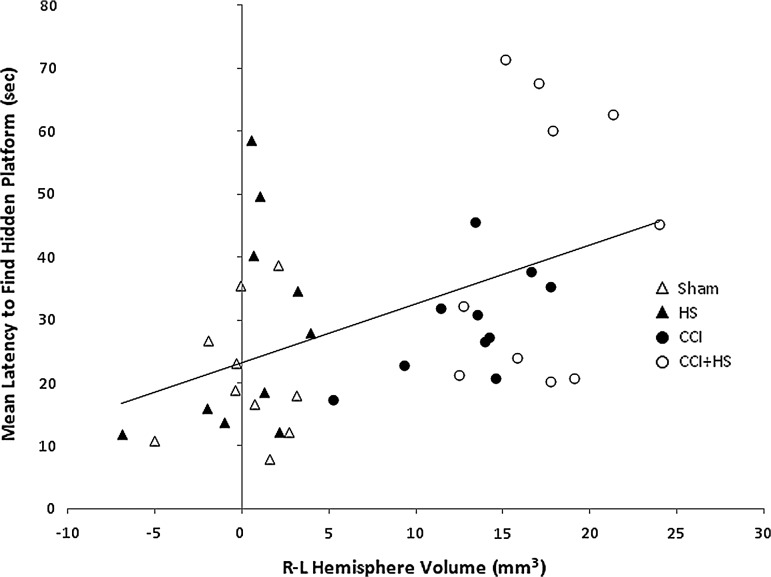
Relationship between functional outcome and hemispheric volume
Individual mean MWM latencies correlated with hemispheric volume loss (Pearson's correlation coefficient=0.434, p<0.005; Fig. 10), supporting a relationship between tissue loss and impairment in MWM performance in the CCI+HS group. However, there was no significant correlation between contusion volume and mean MWM latencies (Pearson's correlation coefficient r=0.18, p=0.79).
FIG. 10.
Correlation between tissue loss in the damaged left hemisphere, as quantified by the right-left (R-L) difference in hemispheric volume at 21 days after injury, and mean latency to find the hidden platform in the Morris water maze on days 14–18 after injury, in mice subjected to sham, hemorrhagic shock (HS) only, controlled cortical impact (CCI) only, and combined CCI+HS. A significant correlation between tissue loss and behavioral outcome was observed (Pearson's correlation coefficient n=0.434, p=0.005).
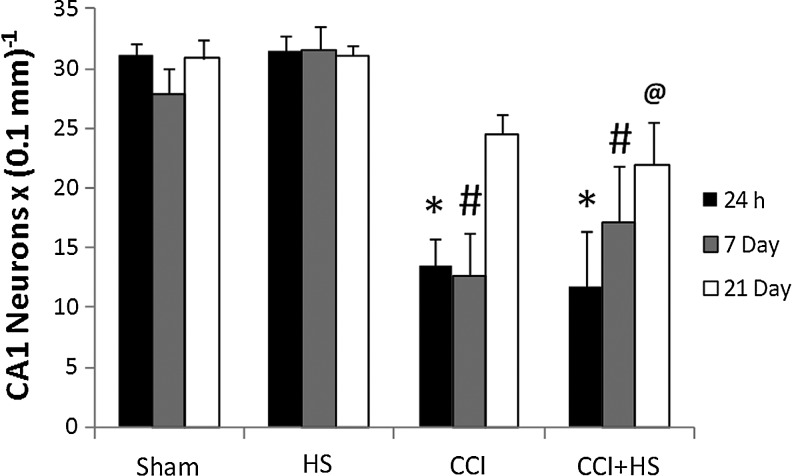
Time course of hippocampal neuron counts
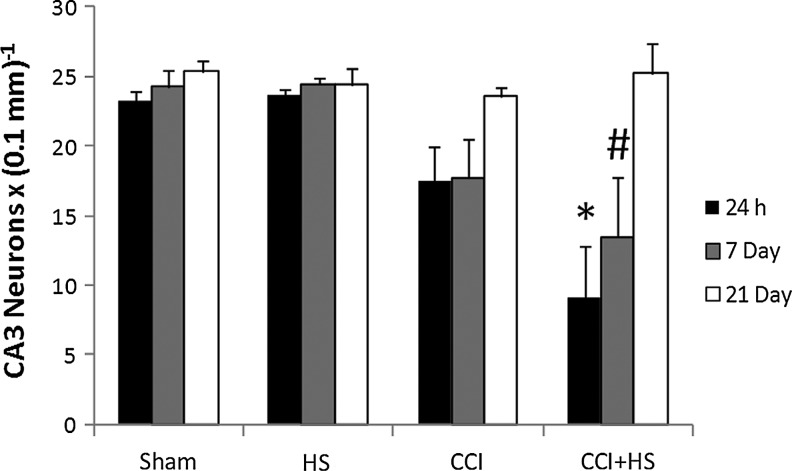
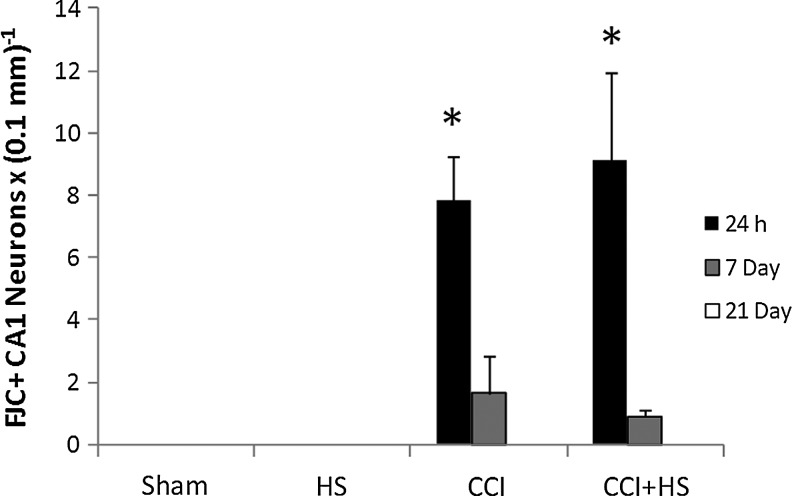
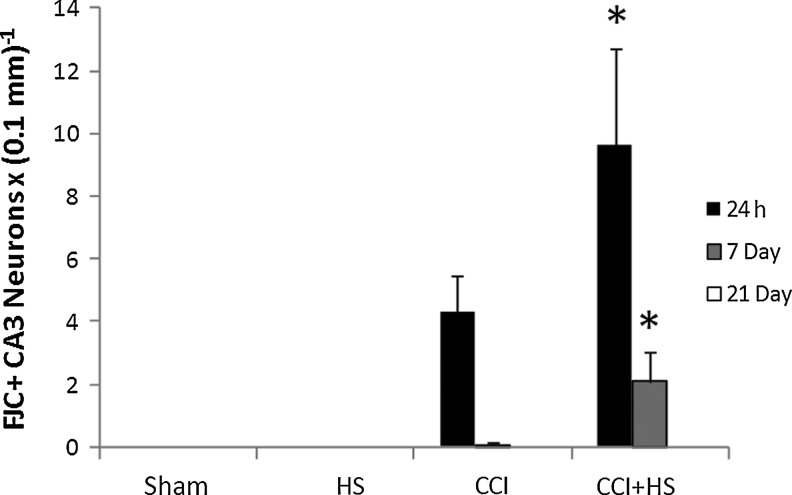
Hippocampal neuron counts as quantified in H&E-stained sections revealed obvious neuronal loss in CA1 at both 24 h and 7 days after both CCI and CCI+HS (both p<0.05 versus sham and HS animals; Fig. 11). There was no difference between the CCI and CCI+HS groups. However, by 21 days CA1 cell loss was only significantly reduced in the CCI+HS group (p<0.05 versus sham and HS animals). Neuron counts in CA3 early after injury were significantly reduced only in CCI+HS animals. At 24 h, numbers in the CCI+HS group were significantly reduced versus all other groups, while at 7 days the CCI+HS group was reduced versus the sham and HS groups (Fig. 12). However, there was a trend toward reduced cell counts after CCI in CA3 (∼25% reduction), at both 24 h and 7 days that did not reach significance. By 21 days, there were no differences between groups for CA3 neuron counts. Surprisingly, for both CA1 and CA3 neuron counts, the values were higher in both the CCI and CCI+HS groups at 21 days versus those observed in those groups at the 24-h and 7-day time points (Figs. 11 and 12). This was true for cell counts assessed by either cell density or total cell number (data not shown). We also quantified neuronal death in coronal brain sections stained with FJC. In general, these results corroborated the findings seen with H&E staining. In the CA1, FJC staining identified acute neuronal death at 24 h after injury in both the CCI and CCI+HS groups (p<0.05 versus the respective sham and HS groups at 24 h after the insult; Fig. 13). FJC positivity was not seen in the sham or HS groups in the CA1 at any time point, or in the CCI and CCI+HS groups at 21 days, consistent with the early use of this marker by others in experimental TBI (Hall et al., 2008). Again, corroborating the findings seen on H&E staining, CA3 neuronal death was found at 24 h and 7 days after the insult only in the CCI+HS group (p<0.05 versus the respective sham, HS, and CCI groups, at 24 h and 7 days after the insult; Fig. 14). Some FJC positivity was seen in the CA3 at 24 h after CCI alone; however, this did not reach significance, similar to the findings seen for H&E staining. Again, FJC positivity was not seen in the sham or HS groups in CA3 at any time point, or in the CCI and CCI+HS groups at 21 days.
FIG. 11.
Time course of neuronal survival as assessed in hematoxylin and eosin-stained coronal brain sections in the CA1 hippocampus in the sham, hemorrhagic shock (HS), controlled cortical impact (CCI), and CCI plus hemorrhagic shock (CCI+HS) groups. CA1 survival was reduced at 24 h and 7 days after CCI and CCI+HS versus their respective sham and HS groups. In contrast, CA1 neuron counts were reduced at 21 days after injury only in the CCI+HS group (*p<0.05 versus the respective sham and HS groups at 24 h after the insult; #p<0.05 versus the respective sham and HS groups at 7 days after the insult; @p<0.05 versus the respective sham and HS groups at 21 days after the insult).
FIG. 12.
Time course of neuronal survival as assessed in hematoxylin and eosin-stained coronal brain sections in the CA3 hippocampus in the sham, hemorrhagic shock (HS), controlled cortical impact (CCI), and CCI plus hemorrhagic shock (CCI+HS) groups. CA3 survival was significantly reduced at 24 h and 7 days only in the CCI+HS group (*p<0.05 versus the respective sham, HS, and CCI groups at 24 h after the insult; #p<0.05 versus the respective sham and HS groups at 7 days after the insult).
FIG. 13.
Time course of neuronal death as assessed in Fluoro-Jade C (FJC)-stained coronal brain sections in the CA1 hippocampus in the sham, hemorrhagic shock (HS), controlled cortical impact (CCI), and CCI plus hemorrhagic shock (CCI+HS) groups. CA1 cell death was significantly increased at 24 h after injury in the CCI and CCI+HS groups versus the sham and HS groups. FJC positivity was not seen in the sham or HS groups at any time point, or in the CCI and CCI+HS groups at 21 days (*p<0.05 versus the respective sham and HS groups at 24 h after the insult).
FIG. 14.
Time course of neuronal death as assessed in Fluoro-Jade C (FJC)-stained coronal brain sections in the CA3 hippocampus in the sham, hemorrhagic shock (HS), controlled cortical impact (CCI), and CCI plus hemorrhagic shock (CCI+HS) groups. CA3 cell death was significantly increased at 24 h and 7 days only in the CCI+HS group. FJC positivity was not seen in the sham or HS groups at any time point, or in the CCI and CCI+HS groups at 21 days (*p<0.05 versus the respective sham, HS, and CCI groups at 24 h or 7 days after the insult).
Neuropathologic observations: H&E, NeuN, GFAP, and FJC-stained sections
Paraffin sections stained with H&E, NeuN, FJC, and GFAP, addressed the coronal brain level containing the dorsal hippocampus (subjacent to contusion), and underlying thalamus and hypothalamus (at bregma −1.94 mm, based on Paxinos and Franklin [2001], with variations of ≤0.2 mm between mice). H&E-stained sections from the CCI and CCI+HS groups at 24 h revealed large numbers of degenerating neurons in the pyramidal layers (particularly CA1 and CA3) of the hippocampus on the side of the contusion. These neurons exhibited brightly-stained eosinophilic cytoplasm and pyknotic to fragmented (karyorrhectic) nuclei (Fig. 15A). Degenerating neurons were also seen in the dentate gyrus of the hippocampus and the underlying dorsal thalamus (and sometimes in the ventral thalamic nuclei). Similar neuronal degeneration was noted in FJC-stained sections of the CCI and CCI+HS groups at 24 h, and to a lesser extent at 7 days. No evidence of neuronal degeneration was seen in the H&E- or FJC-stained sections in the HS-only group. For the CCI and CCI+HS groups, differences were seen between H&E- and NeuN-stained sections at 24 h and 7 days. At 24 h, many eosinophilic (i.e., degenerative) neurons still showed staining for NeuN. However, the staining pattern had shifted from the nucleus (seen for normal neurons), to the cytoplasm (representing an abnormal staining pattern; Fig. 15B). At 7 days, on the other hand, many hippocampal pyramidal neurons that were normal in appearance in H&E-stained sections were NeuN-negative (Fig. 15C and D). Although small foci of neuron depletion were evident in the CA1 and CA3 of some of the CCI and CCI+HS group mice at 21 days, there was evidence of apparent recovery of the neuronal population in these sectors, and the pattern of NeuN staining had also returned to that of normal nuclear staining. Damage to dentate granule cells was more persistent, however, with prominent gaps in the dorsal blade, along with associated astrogliosis seen for 21 days after CCI and CCI+HS. While the NeuN stain was less useful at 24 h and 7 days, the GFAP stain showed subacute injury at 7 days and 21 days (GFAP stain was performed to ensure that none of the cells resembling newly-formed hippocampal pyramidal neurons within the H&E-stained sections actually represented astrocytes). Even at low-power magnification, GFAP readily showed elevated staining of astrocytes and their processes in the hippocampus, dentate, and thalamus (Fig. 16A–D). In hippocampus, the most intense GFAP staining was seen in the strata oriens and radiatum, and the molecular layer of the dentate gyrus (but not in the pyramidal neuron layer). Large numbers of reactive astrocytes with thickened cellular processes were seen in these same regions.
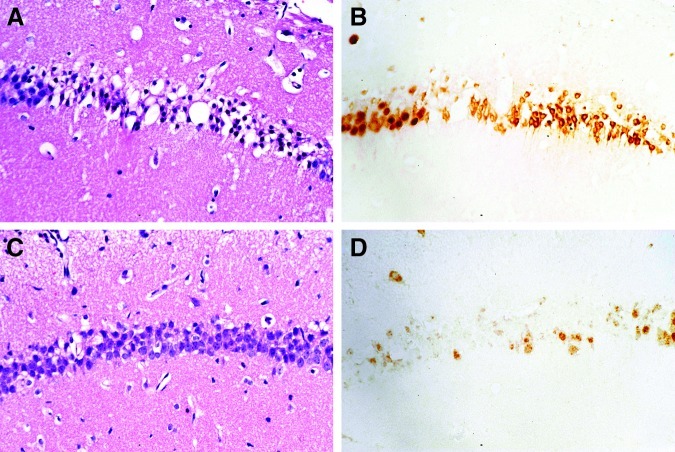
FIG. 15.
These panels show hematoxylin and eosin (H&E)- and NeuN-stained sections from mice necropsied at 24 h (A and B), and at 7 days (C and D). A and B are from the same 24-h controlled cortical impact (CCI)-group mouse, are of the same magnification, and represent the exact same region of the CA1 sector of the hippocampus. The H&E-stained section (A) is characterized by extensive eosinophilic neuron degeneration. Although most of the degenerating pyramidal neurons still stain with NeuN, the staining pattern is marked by a transition from nuclear staining, as seen in the left-hand margin of panel B (which is normal), to that of cytoplasmic staining. In contrast, at 7 days (C), the H&E-stained section shows many normal-appearing neurons in the CA1 sector of this CCI-group mouse. However, the bulk of these neurons do not stain (or show only weak nuclear staining) with NeuN (D).
FIG. 16.
Hematoxylin and eosin (H&E)- and glial fibrillary acidic protein (GFAP)-stained sections of hippocampi from mice necropsied at 7 days and 21 days post-trauma. All four images are at the same magnification. Panel A is an H&E-stained section from a controlled cortical impact (CCI) mouse necropsied at 7 days. At this magnification, little evidence of degeneration is evident. Panel B is of a GFAP-stained section of hippocampus from a sham mouse, whereas panels C and D show GFAP-stained hippocampal sections from a CCI-group mouse necropsied at 21 days (C), and of a CCI+HS-group mouse necropsied at 7 days. These panels demonstrate the consistent astrocyte response seen at 7 days and 21 days after CCI. At higher magnifications, many reactive astrocytes were also evident within the hippocampus and underlying thalamus of the 7-day and 21-day CCI and CCI+HS rats.
Amino cupric silver-stained sections
At 24 h, some sham animals as well as mice in all three treatment groups had foci of neuronal and axonal degeneration that involved the rostral portion of the left frontal cortex, and extended into the underlying striatum (Fig. 17A). This was likely secondary to the placement of temperature probes in this region and the high level of sensitivity of CuAg to detect injury. There was no difference in the degrees of this pattern of degeneration between groups, but one HS-group mouse necropsied at 24 h had foci of neuron degeneration in the cerebral cortex (left side only), that extended caudally from the frontal cortex to involve the parietal cortex, and eventually the occipital cortex (Fig. 17B). All CCI and CCI+HS group mice at 24 h had extensive trauma-induced degeneration in the cerebral cortex, and the subjacent hippocampus and thalamus (Fig. 17C and D). Such degeneration was not present in any of the six HS-group mice.
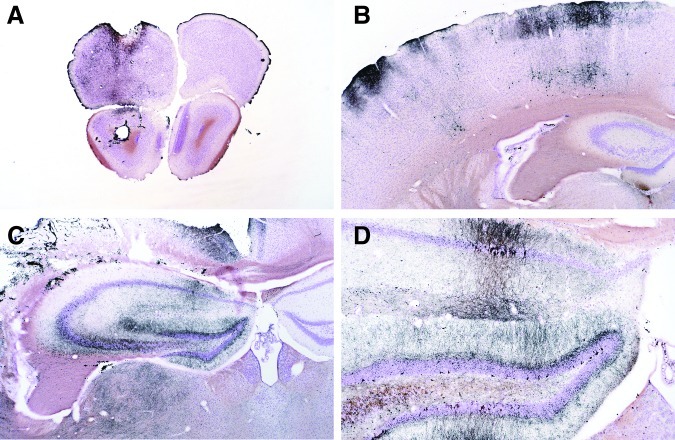
FIG. 17.
Representative micrographs of brain sections from mice 24 h after injury. In all micrographs, the left side of the brain (the injured side) is on the left. All sections were stained with cupric silver, so cells and processes staining black are degenerative. Reflecting the sensitivity of the cupric silver stain, panel A shows subtle degeneration in the frontal lobe after temperature probe placement in striatum in a controlled cortical impact plus hemorrhagic shock (CCI+HS) mouse. This injury pattern was seen in all groups. Panel B shows neuron degeneration in the parietal cortex of one of two HS-only mice at 24 h. This pattern of cortical damage, which extended from the frontal to the occipital cortex in this mouse, was limited to the left side of the brain, suggesting that HS can exacerbate brain injury resulting from even a subtle insult such as temperature probe placement. Panels C and D show low- and medium-power magnifications of the same hippocampal section of a 24-h CCI mouse. Many neurons are stained in both the pyramidal sectors (particularly CA1 and CA3), and the dentate gyrus and the underlying dorsal thalamus. Staining is not evident in the contralateral hippocampus at 24 h (partially shown in C).
At 72 h, prominent CuAg staining of neuronal processes in the strata oriens, radiatum, and molecular layer of the dentate gyrus, was more prominent in the left hippocampus, and was also seen in the right hippocampus. However, no neuron degeneration was seen in the right hippocampus (Fig. 18A–D). CCI and CCI+HS mice necropsied at 72 h also had moderate degrees of neuronal degeneration in the left superior colliculus. By 14 days, neuronal process staining was reduced in the hippocampus, but there was increased staining of degenerating neurons in the left superior colliculus. At 14 days, CuAg staining also indicated prominent degrees of axonal injury in selected fiber pathways, including (but not restricted to) the anterior commissure, corpus callosum, cerebral peduncle, nigrostriatal tract, medial lemniscus, and pyramids (Fig. 19A–F). Except for crossing tracts (i.e., the corpus callosum and anterior commissure), degenerative axonal staining was primarily restricted to the left side of the brain. This pattern of axonal injury was most prominent in mice from the CCI and CCI+HS groups.
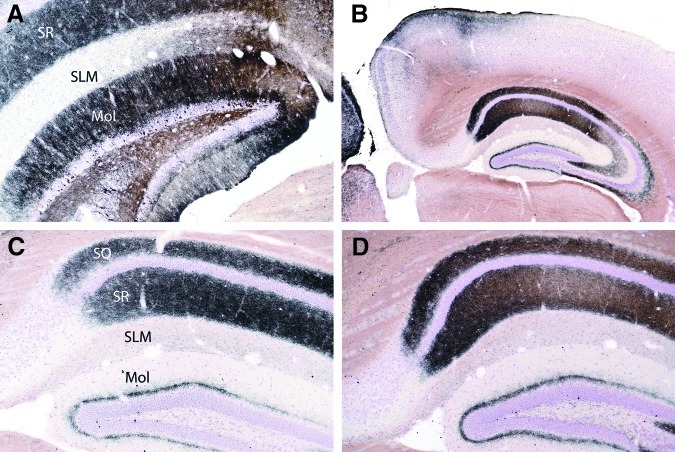
FIG. 18.
Representative micrographs of hippocampal sections from mice 72 h after injury (all sections stained with cupric silver). Panel A shows degenerating neurons within the dentate gyrus, and extensive staining of neuronal processes in the dentate molecular layer (Mol), and within the stratum radiatum (SR) of the pyramidal layer. The stratum lacunosum molecular (SLM) is not affected. Panels B, C, and D are micrographs of the contralateral (non-injured) side of the brain (B and D are low- and medium-power micrographs from the same controlled cortical impact plus hemorrhagic shock [CCI+HS] mouse; C is from a CCI-only mouse). At 72 h the contralateral hippocampi were characterized by extensive neuronal process degeneration within the stratum oriens (SO) and stratum radiatum (SR) of the pyramidal layer, as well as granular staining within the molecular layer (Mol) of the dentate.
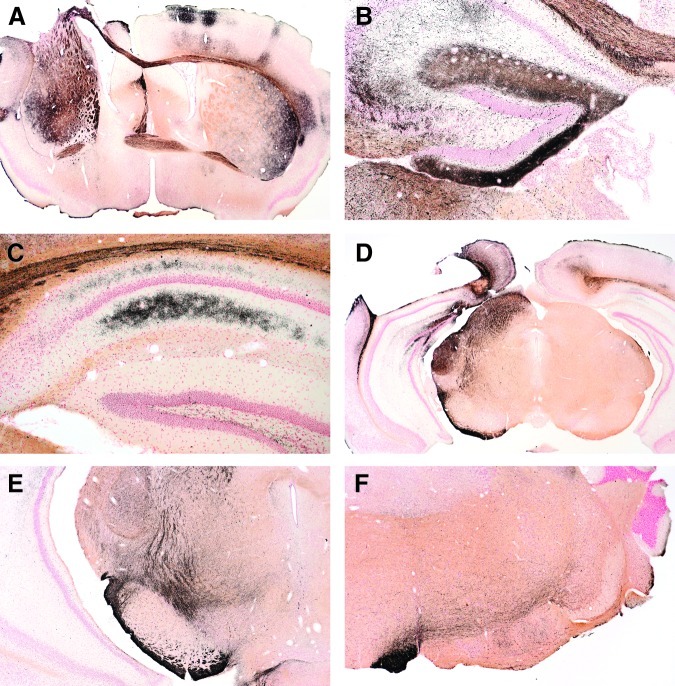
FIG. 19.
Cupric silver-stained sections from controlled cortical impact (CCI) and CCI plus hemorrhagic shock (CCI+HS) group mice at 14 days. In contrast to the 72-h time point, brains from 14-day mice showed staining (i.e., degeneration) in a variety of nerve fiber tracts. Panel A (CCI+HS) shows staining of the anterior commissure, corpus callosum, and striatum bilaterally. Panel B (CCI) shows persistence of staining in the molecular layer of the dentate and the corpus callosum, the Schaffer collaterals of the hippocampus, and the underlying thalamus (on the injured side). Panel C (CCI+HS) shows staining of processes in the strata oriens and radiatum of the contralateral hippocampus and overlying corpus callosum. Panel D (CCI) shows sub-contusion staining of the superior colliculus, medial geniculate, and cerebral peduncle, whereas E (CCI+HS) shows staining of the cerebral peduncle and fiber tracts associated with the pars compacta of the substantia nigra (nigrostriatal pathway). Panel F, from a more caudal midbrain level of a CCI+HS mouse, shows staining of the pyramidal tract on the left side (rostral to its decussation within the medulla), and staining of associated crossing fibers.
Discussion
We characterized a clinically-relevant pressure-controlled model of TBI+HS in mice. Brief, severe, pressure-controlled HS after CCI, followed by a MAP-targeted resuscitation, results in robust functional deficits on multiple elements of the MWM task, and exacerbates neuropathological damage versus CCI alone.
Secondary insults are common after TBI (Chesnut, 1995). Hypotension was highly detrimental in patients with severe TBI, occurring in 34.6% of cases, and increasing mortality by 150% (Chesnut et al., 1993). Impaired CBF autoregulation after TBI likely increases this vulnerability (Chaiwat et al., 2009). In combat casualty care, secondary insults after TBI are common and are associated with increased mortality (Ling et al., 2009), including blast TBI (Nelson et al., 2006). Experimental blast models corroborate the clinical condition, since even moderate hemorrhagic hypotension is poorly tolerated (Garner et al., 2010). Despite this important epidemiological target, studies of neuroprotective strategies in CCI+HS are rare (Robertson et al., 2011).
Work in large-animal models of TBI+HS has furthered our knowledge of strategies to enhance MAP, while limiting intracranial hypertension. Optimizing CPP is important, but the best way to accomplish this is controversial, as is the optimal MAP. Various therapies have reduced the volume of fluid given, such as vasopressors (Feinstein et al., 2005), colloids (Crookes et al., 2004), hypertonic saline (Sell et al., 2008), or blood substitutes (Gibson et al., 2002; Stern et al., 2009; Teranishi et al., 2010). However, clinical trials have failed in TBI resuscitation (Bulger et al., 2010; SAFE Study Investigators, 2007).
In contrast to TBI, studies of TBI+HS in rodent models are uncommon, and studies in murine models are rare. DeWitt and Prough (2009) identified 10 studies in rat models of TBI that superimposed hemorrhage or ischemia (Cherian et al., 1996; Giri et al., 2000; Hellmich et al., 2005; Ishige et al., 1988; Jenkins et al., 1989; Kroppenstedt et al., 1999; Lammie et al., 1999; Matsushita et al., 2001; Stiefel et al., 2005; Trabold et al., 2006). Five of these 10 focused on biochemical or molecular outcomes, while five examined neuropathology and revealed increased lesion volume and/or CA1 neuronal death after the insult. One study showed increased CA3 neuronal death. In general, in these studies a volume-controlled hemorrhage was used with a MAP of 40–60 mm Hg for ≥30 min. Also, Schütz and associates (2006) studied the effect of hemorrhage (MAP 50–60 mm Hg) for 30 min after FPI in rats, and reported increased impairment of MWM performance by hemorrhage. However, the shed blood was not re-infused, resulting in sustained anemia.
We published three reports on a new model of CCI+HS in mice (Dennis et al., 2009; Exo et al., 2009; Shellington et al., 2011). We noted that 90 min, but not 60 min, of a moderate volume-controlled HS (MAP 35–45 mm Hg) after CCI increased hippocampal CA1 cell death at 7 days. HS at 90 min also exacerbated the CBF reduction produced versus CCI alone (Dennis et al., 2006). We used the model to screen resuscitation fluids focused on mitigating CA1 cell death (Exo et al., 2009; Shellington et al., 2011). In that model, volume-controlled HS of 0.2 mL/10 g produced MAP values of ∼35–45 mm Hg. After 90 min of HS, a brief pre-hospital resuscitation period of 30 min was used, resulting in modest resuscitation volumes of ∼30–60 mL/kg. Exacerbation of CA1 cell death was seen versus CCI alone; studies of long-term function or neuropathology were not carried out. Since CPP is a key determinant of CBF, the use of pressure-controlled rather than volume-controlled HS after TBI would be expected to produce a more consistent insult, and help define the impact of various target MAPs on outcome. In pressure-controlled HS models, MAP is rigorously maintained at a fixed target, unlike volume-controlled HS, for which a set volume of blood is removed and MAP is simply observed.
As expected, we were able to maintain a tight MAP reduction during HS; however, after TBI, despite aggressive fluid administration we were unable to maintain MAP ≥70 mm Hg during resuscitation. This may also have contributed to the detrimental effects of our combined insult on outcomes.
We noted a spectrum of functional deficits rarely seen when CCI is studied alone (Abrahamson et al., 2009; Smith et al., 1995; Tehranian et al., 2006,2008; Whalen et al., 1999a,1999b; You et al., 2008). We chose a mild to moderate CCI to limit functional deficits and neuropathology due to CCI alone, so that the full impact of secondary HS could be revealed. We also used a level of HS that although severe, was not itself associated with MWM deficits. CCI+HS produced a >50% increase in latency to find the hidden platform in the MWM. The impairment after CCI+HS was manifest in the first 2 days of MWM testing. This may result from the fact that the mice were trained in the MWM before injury, and although we used a new platform location in post-injury testing, habituation of the task could have been selectively lost after CCI+HS. We also noted impaired swim speed after CCI+HS, despite using a fairly mild CCI. This is uncommon after CCI alone in numerous studies (Ahmad et al.,2008; Tehranian et al., 2006,2008; Whalen et al., 1999a,1999b). It suggests marked detrimental effects of a second insult on function. We recognize that the fact that swim speed was impaired and correlated with MWM latencies indicates that the MWM dysfunction seen after CCI+HS could be influenced by a persistent impairment of motor function. However, the magnitude of the motor deficit, although statistically significant, was fairly modest (∼20% reduction) versus the marked increase in latency to find the hidden platform, which ranged from 50% on days 14–15 to over 100% overall. We also noted that visible platform testing was only impaired after CCI+HS; thus the MWM deficit cannot be attributed solely to spatial memory deficits. MWM performance can be influenced by various functional domains (motor, attention, acquisition, retention, and visuospatial), and we did not define the specific components that are disrupted by TBI+HS. Nevertheless, these MWM deficits and additional tasks, such as rotarod testing, may also aid in assessing functional impairments after CCI+HS. In any case, our findings suggest the possibility that severe HS—even for a brief interval—can have global consequences for functional outcome.
Although the CCI alone used in this report did not produce deficits in the acquisition phase of testing versus sham or HS animals, performance after CCI in the probe trial was at a level that was similar to random chance. The probe trial is often highly sensitive to injury (Abrahamson et al., 2009; Lok et al., 2007), and in our case shows some level of functional impairment after CCI alone. It is also a task that is largely independent of swim speed. Probe trial performance after CCI+HS was also commensurate with random chance, unlike the sham or HS-only groups.
Although the HS alone used in this report did not produce significant deficits in either the acquisition phase of testing or on the probe trial, we used a severe level of HS, with MAP well below the reported lower limit of CBF autoregulation, even in normal C57BL/6 mice, which may be as high as 60 mm Hg (Lacombe et al., 2005). Examination of the individual latencies in each mouse in the HS-only group suggests that three mice had a deficit. Thus the HS-only insult we used was at the threshold of producing injury. Kroppenstedt and colleagues (1999) showed that MAP levels <70 mm Hg for 30 min in rats after CCI can expand lesion volume in a MAP-dependent manner, down to 40 mm Hg. Further study is needed to determine the effect of various levels and durations of HS on pathology and function after CCI.
CCI+HS also exacerbated tissue loss in the contusion and the hemisphere ipsilateral to impact. These targets each showed a ∼33% increase in loss, indicating that HS after CCI exacerbates injury. There was a significant correlation between latency to find the hidden platform and hemispheric loss, suggesting that the detrimental effects of HS on function after CCI may have a histological substrate outside of the contusion.
Hippocampal neuron counts as assessed by both H&E and FJC revealed that the CA1 and CA3 were injured after CCI or CCI+HS, but that the extent of damage was greater in the CCI+HS group. Early CA1 cell death was readily seen in the CCI and CCI+HS groups, but by 21 days CA1 cell loss was only seen in the CCI+HS group. Early CA3 cell death was seen by FJC staining in both models, but was greater in the CCI+HS group at 24 h and 7 days. However, by 21 days, significant CA3 cell death was not evident in either insult. HS alone did not produce appreciable CA1 or CA3 death as assessed by FJC or H&E cell counts, consistent with our prior work (Carrillo et al., 1998; Dennis et al., 2009). Adding an HS insult with a MAP of ∼35–45 mm Hg for 90 min after CCI exacerbated only CA1 cell death (Dennis et al., 2009). In contrast, in the current report, more severe, brief HS (MAP 25–27 mm Hg) exacerbated neuronal death in the CA1 at 21 days, and in the CA3 at 24 h and 7 days. Thus the exact pattern of neuronal death in the hippocampus that is exacerbated by HS depends on the magnitude and duration of the HS insult, along with the duration of outcome. It was also curious that hippocampal neuron counts, quantified per 0.1-mm length of the respective hippocampal subfield, appeared to normalize between 7 and 21 days after the various insults, except in CA1 of the CCI+HS group. This could result from progressive volume loss in the hippocampus over time, giving the illusion of recovery, or possibly some recovery of CA3 and CA1 related to neurogenesis (Dong et al., 2003; Luo et al., 2007). A similar temporal pattern of delayed recovery in the CA1 has been reported after incomplete forebrain ischemia in rats (Elsersy et al., 2004). We performed neuron counts on H&E-stained sections, but NeuN-stained sections of 21-day post-injury mice also suggested a potential for recovery. NeuN (which stands for neuronal nuclei) is primarily present in the nuclei and perinuclear cytoplasm of post-mitotic neurons, and would not be detected in neuroblasts. NeuN staining may also be lost in neurons experiencing sublethal injury (Liu et al., 2009; Ünal-Çevik et al., 2004). Whether the apparent recovery in the numbers of hippocampal pyramidal neurons seen at 21 days represents re-population of the pyramidal cell layer by neuroblasts, or recovery of shrunken (uncounted) neurons, merits additional study. Brain edema during the initial days after the insult would resolve by 21 days, and the impact of this on tissue volume could also differentially influence the reported cell counts. Stereological cell counts at multiple time points are needed, along with Brd-U labeling and/or the use of other cellular markers of neurogenesis such as nestin or doublecortin.
Several conclusions arose out of the neuropathologic evaluations. First, the temporal aspects of injury should be considered when using NeuN for neuron enumeration, because neurons in the early stages of degeneration may not be negative for this stain, whereas neurons observed during the recovery phase may be NeuN-negative. The GFAP stain revealed a consistent astrocyte response at 7 and 21 days. Second, the CuAg stain, as expected, was more sensitive for revealing not only neuronal injury, but associated degeneration in neuronal processes and fiber tracts. Third, placement of a temperature probe is not innocuous, especially in mice further stressed by HS. Although the neuropathologic evaluations did not reveal definitive differences in severities or patterns of injury between the CCI and CCI+HS groups, this does not negate the validity of the intergroup differences demonstrated by the neuronal counts performed in this study for several reasons. First, for the CuAg-stained sections (the most sensitive indicator of microscopic injury), there were only two mice per group at each time point; thus intergroup differences may not have been detected. For the paraffin sections, subjective grades of neuropathologic injury were based not just on numbers of dead/dying neurons, but also on other histologic features, such as vacuolation, overall cellularity (including microglial cell infiltrates and astrogliosis), and for the hippocampus and thalamus, approximations of the percentage of each neuroanatomic region affected.
We conclude that, brief, severe, pressure-controlled HS after CCI with a clinically-relevant MAP-targeted resuscitation produces robust and unique functional deficits on multiple outcomes of the MWM task, and exacerbates neuropathology versus CCI or HS alone. These behavioral and neuropathological deficits should serve as targets to test novel therapies and study mutant mouse strains in the deleterious setting of TBI+HS.
Acknowledgments
We thank the Defense Advanced Research Projects Agency (DARPA) Prevent Program (N66001-10-C2124 to P.M.K.), the United States Army (W81XWH-06-1-0247 and W81XWH-09-2-0187 to P.M.K.; T32 HD040686 to J.L.E.; NS 30218 and NS30318 to C.E.D.) for support. The views, opinions, and findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of DARPA or the U.S. Department of Defense.
Author Disclosure Statement
No competing financial interests exist.
References
- Abrahamson E.E. Ikonomovic M.D. Dixon C.E. DeKosky S.T. Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels. Ann. Neurol. 2009;66:407–414. doi: 10.1002/ana.21731. [DOI] [PubMed] [Google Scholar]
- Ahmad M. Rose M.E. Vagni V. Griffith R.P. Dixon C.E. Kochanek P.M. Hickey R.W. Graham S.H. Genetic disruption of cyclooxygenase-2 does not improve histological or behavioral outcome after traumatic brain injury in mice. J. Neurosci. Res. 2008;86:3605–3612. doi: 10.1002/jnr.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger E.M. May S. Brasel K.J. Schreiber M. Kerby J.D. Tisherman S.A. Newgard C. Slutsky A. Coimbra R. Emerson S. Minei J.P. Bardarson B. Kudenchuk P. Baker A. Christenson J. Idris A. Davis D. Fabian T.C. Aufderheide T.P. Callaway C. Williams C. Banek J. Vaillancourt C. van Heest R. Sopko G. Hata J.S. Hoyt D.B. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2010;304:1455–1464. doi: 10.1001/jama.2010.1405. ROC Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo P. Takasu A. Safar P. Tisherman S. Stezoski S.W. Stolz G. Dixon C.E. Radovsky A. Prolonged severe hemorrhagic shock and resuscitation in rats does not cause subtle brain damage. J. Trauma. 1998;45:239–248. doi: 10.1097/00005373-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Chaiwat O. Sharma D. Udomphorn Y. Armstead W.M. Vavilala M.S. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J. Neurotrauma. 2009;26:657–663. doi: 10.1089/neu.2008.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L. Robertson C.S. Goodman J.C. Secondary insults increase injury after controlled cortical impact in rats. J. Neurotrauma. 1996;13:371–383. doi: 10.1089/neu.1996.13.371. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Secondary brain insults after head injury: clinical perspectives. New Horiz. 1995;3:366–375. [PubMed] [Google Scholar]
- Crookes B.A. Cohn S.M. Bonet H. Burton E.A. Nelson J. Majetschak M. Varon A.J. Linden J.M. Proctor K.G. Building a better fluid for emergency resuscitation of traumatic brain injury. J. Trauma. 2004;57:547–554. doi: 10.1097/01.ta.0000135162.85859.4c. [DOI] [PubMed] [Google Scholar]
- Dennis A.M. Foley L.M. Melick J.A. Hitchens T.K. Jenkins L.W. Clark R.S. Ho C. Kochanek P.M. Effect of hemorrhagic shock on cerebral blood flow in experimental traumatic brain injury: Magnetic resonance imaging assessment. Crit. Care Med. 2006;34:A5. [Google Scholar]
- Dennis A.M. Haselkorn M.L. Vagni V.A. Garman R.H. Janesko-Feldman K. Bayır H. Clark R.S. Jenkins L.W. Dixon C.E. Kochanek P.M. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J. Neurotrauma. 2009;26:889–899. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J. Neurotrauma. 2009;26:877–887. doi: 10.1089/neu.2007.0439. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Taylor C.L. Whitley J.M. Deal D.D. Vines S.M. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am. J. Physiol. 1992;263:H1276–H1284. doi: 10.1152/ajpheart.1992.263.4.H1276. [DOI] [PubMed] [Google Scholar]
- Dong H. Csernansky C.A. Goico B. Csernansky J.G. Hippocampal neurogenesis follows kainic acid-induced apoptosis in neonatal rats. J. Neurosci. 2003;23:1742–1749. doi: 10.1523/JNEUROSCI.23-05-01742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsersy H. Sheng H. Lynch J.R. Moldovan M. Pearlstein R.D. Warner D.S. Effects of isoflurane versus fentanyl-nitrous oxide anesthesia on long-term outcome from severe forebrain ischemia in the rat. Anesthesiology. 2004;100:1160–1166. doi: 10.1097/00000542-200405000-00018. [DOI] [PubMed] [Google Scholar]
- Exo J.L. Shellington D.K. Bayir H. Vagni V.A. Janesko-Feldman K. Ma L. Hsia C.J. Clark R.S. Jenkins L.W. Dixon C.E. Kochanek P.M. Resuscitation of traumatic brain injury and hemorrhagic shock with polynitroxylated albumin, hextend, hypertonic saline, and lactated Ringer's: Effects on acute hemodynamics, survival, and neuronal death in mice. J. Neurotrauma. 2009;26:2403–2408. doi: 10.1089/neu.2009.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein A.J. Patel M.B. Sanui M. Cohn S.M. Majetschak M. Proctor K.G. Resuscitation with pressors after traumatic brain injury. J. Am. Coll. Surg. 2005;201:536–545. doi: 10.1016/j.jamcollsurg.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Garner J. Watts S. Parry C. Bird J. Cooper G. Kirkman E. Prolonged permissive hypotensive resuscitation is associated with poor outcome in primary blast injury with controlled hemorrhage. Ann. Surg. 2010;251:1131–1139. doi: 10.1097/SLA.0b013e3181e00fcb. [DOI] [PubMed] [Google Scholar]
- Gibson J.B. Maxwell R.A. Schweitzer J.B. Fabian T.C. Proctor K.G. Resuscitation from severe hemorrhagic shock after traumatic brain injury using saline, shed blood, or a blood substitute. Shock. 2002;17:234–244. doi: 10.1097/00024382-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Giri B.K. Krishnappa I.K. Bryan R.M., Jr. Robertson C. Watson J. Regional cerebral blood flow after cortical impact injury complicated by a secondary insult in rats. Stroke. 2000;31:961–967. doi: 10.1161/01.str.31.4.961. [DOI] [PubMed] [Google Scholar]
- Glass T.F. Fabian M.J. Schweitzer J.B. Weinberg J.A. Proctor K.G. Secondary neurologic injury resulting from nonhypotensive hemorrhage combined with mild traumatic brain injury. J. Neurotrauma. 1999;16:771–782. doi: 10.1089/neu.1999.16.771. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Bryant Y.D. Cho W. Sullivan P.G. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Hellmich H.L. Garcia J.M. Shimamura M. Shah S.A. Avila M.A. Uchida T. Parsley M.A. Capra B.A. Eidson K.A. Kennedy D.R. Winston J.H. DeWitt D.S. Prough D.S. Traumatic brain injury and hemorrhagic hypotension suppress neuroprotective gene expression in injured hippocampal neurons. Anesthesiology. 2005;102:806–814. doi: 10.1097/00000542-200504000-00017. [DOI] [PubMed] [Google Scholar]
- Ishige N. Pitts L.H. Berry I. Nishimura M.C. James T.L. The effects of hypovolemic hypotension on high-energy phosphate metabolism of traumatized brain in rats. Neurosurgery. 1988;68:129–136. doi: 10.3171/jns.1988.68.1.0129. [DOI] [PubMed] [Google Scholar]
- Jenkins L.W. Moszynski K. Lyeth B.G. Lewelt W. DeWitt D.S. Allen A. Dixon C.E. Povlishock J.T. Majewski T.J. Clifton G.L. Young H.F. Becker D.P. Hayes R.L. Increased vulnerability of the mildly traumatized rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;477:211–224. doi: 10.1016/0006-8993(89)91409-1. [DOI] [PubMed] [Google Scholar]
- Kroppenstedt S.N. Kern M. Thomale U.W. Schneider G.H. Lanksch W.R. Unterberg A.W. Effect of cerebral perfusion pressure on contusion volume following impact injury. J. Neurosurg. 1999;90:520–526. doi: 10.3171/jns.1999.90.3.0520. [DOI] [PubMed] [Google Scholar]
- Lacombe P. Oligo C. Domenga V. Tournier-Lasserve E. Joutel A. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke. 2005;36:1053–1058. doi: 10.1161/01.STR.0000163080.82766.eb. [DOI] [PubMed] [Google Scholar]
- Lammie G.A. Piper I.R. Thomson D. Brannan F. Neuropathologic characterization of a rodent model of closed head injury—addition of clinically relevant secondary insults does not significantly potentiate brain damage. J. Neurotrauma. 1999;16:603–615. doi: 10.1089/neu.1999.16.603. [DOI] [PubMed] [Google Scholar]
- Ling G. Bandak F. Armonda R. Grant G. Ecklund J. Explosive blast neurotrauma. J. Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Liu F. Schafer D.P. McCullough L.D. TTC, Fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J. Neurosci. Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J. Wang H. Murata Y. Zhu H.H. Qin T. Whalen M.J. Lo E.H. Effect of neuregulin-1 on histopathological and functional outcome after controlled cortical impact in mice. J. Neurotrauma. 2007;24:1817–1822. doi: 10.1089/neu.2007.0372. [DOI] [PubMed] [Google Scholar]
- Luo C.X. Zhu X.J. Zhou Q.G. Wang B. Wang W. Cai H.H. Sun Y.J. Hu M. Jiang J. Hua Y. Han X. Zhu D.Y. Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J. Neurochem. 2007;103:1872–1882. doi: 10.1111/j.1471-4159.2007.04915.x. [DOI] [PubMed] [Google Scholar]
- Matsushita Y. Bramlett H.M. Kuluz J.W. Alonso O. Dietrich W.D. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2001;21:847–856. doi: 10.1097/00004647-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Nelson T.J. Wall D.B. Stedje-Larsen E.T. Clark R.T. Chambers L.W. Bohman H.R. Predictors of mortality in close proximity blast injuries during Operation Iraqi Freedom. J. Am. Coll. Surg. 2006;202:418–422. doi: 10.1016/j.jamcollsurg.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Franklin K.B.J. The Mouse Brain in Stereotaxic Coordinates. 2nd. Academic Press; San Diego: 2001. [Google Scholar]
- Riches A.C. Sharp J.G. Thomas D.B. Smith S.V. Blood volume determination in the mouse. J. Physiol. 1973;228:279–284. doi: 10.1113/jphysiol.1973.sp010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C.S. Cherian L. Shah M. Garcia R. Navarro J.C. Grill R.J. Hand C.C. Tian T.S. Hannay H.J. Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J. Neurotrauma. 2011;29:1156–1166. doi: 10.1089/neu.2011.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFE Study Investigators; Australian New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health. Myburgh J. Cooper D.J. Finfer S. Bellomo R. Norton R. Bishop N. Kai Lo S. Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N. Engl. J. Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- Schmued L.C. Stowers C.C. Scallet A.C. Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Schütz C. Stover J.F. Thompson H.J. Hoover R.C. Morales D.M. Schouten J.W. McMillan A. Soltesz K. Motta M. Spangler Z. Neugebauer E. McIntosh T.K. Acute, transient hemorrhagic hypotension does not aggravate structural damage or neurologic motor deficits but delays the long-term cognitive recovery following mild to moderate traumatic brain injury. Crit. Care Med. 2006;34:492–501. doi: 10.1097/01.ccm.0000198326.32049.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S.L. Avila M.A. Yu G. Vergara L. Prough D.S. Grady J.J. DeWitt D.S. Hypertonic resuscitation improves neuronal and behavioral outcomes after traumatic brain injury plus hemorrhage. Anesthesiology. 2008;108:873–881. doi: 10.1097/ALN.0b013e31816c8a15. [DOI] [PubMed] [Google Scholar]
- Shellington D.K. Du L. Wu X. Exo J. Vagni V. Ma L. Janesko-Feldman K. Clark R.S. Bayir H. Dixon C.E. Jenkins L.W. Hsia C.J. Kochanek P.M. Polynitroxylated pegylated hemoglobin: a novel neuroprotective hemoglobin for acute volume-limited fluid resuscitation after combined traumatic brain injury and hemorrhagic hypotension in mice. Crit. Care Med. 2011;39:494–505. doi: 10.1097/CCM.0b013e318206b1fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinz E.H. Kochanek P.M. Dixon C.E. Clark R.S. Carcillo J.A. Schiding J.K. Chen M. Wisniewski S.R. Carlos T.M. Williams D. DeKosky S.T. Watkins S.C. Marion D.W. Billiar T.R. Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J. Clin. Invest. 1999;104:647–656. doi: 10.1172/JCI6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.H. Soares H.D. Pierce J.S. Perlman K.G. Saatman K.E. Meaney D.F. Dixon C.E. McIntosh T.K. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Statler K.D. Alexander H. Vagni V. Holubkov R. Dixon C.E. Clark R.S. Jenkins L. Kochanek P.M. Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res. 2006;1076:216–224. doi: 10.1016/j.brainres.2005.12.106. [DOI] [PubMed] [Google Scholar]
- Statler K.D. Kochanek P.M. Dixon C.E. Alexander H.L. Warner D.S. Clark R.S. Wisniewski S.R. Graham S.H. Jenkins L.W. Marion D.W. Safar P.J. Isoflurane improves long-term neurologic outcome versus fentanyl after traumatic brain injury in rats. J. Neurotrauma. 2000;17:1179–1189. doi: 10.1089/neu.2000.17.1179. [DOI] [PubMed] [Google Scholar]
- Stiefel M.F. Tomita Y. Marmarou A. Secondary ischemia impairing the restoration of ion homeostasis following traumatic brain injury. J. Neurosurg. 2005;103:707–714. doi: 10.3171/jns.2005.103.4.0707. [DOI] [PubMed] [Google Scholar]
- Stern S. Rice J. Philbin N. McGwin G. Arnaud F. Johnson T. Flournoy W.S. Ahlers S. Pearce L.B. McCarron R. Freilich D. Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock. 2009;31:64–79. doi: 10.1097/SHK.0b013e3181778dc3. [DOI] [PubMed] [Google Scholar]
- Strebel S. Lam A.M. Matta B.F. Newell D.W. Impaired cerebral autoregulation after mild brain injury. Surg. Neurol. 1997;47:128–131. doi: 10.1016/s0090-3019(96)00459-4. [DOI] [PubMed] [Google Scholar]
- Tehranian R. Rose M.E. Vagni V. Griffith R.P. Wu S. Maits S. Zhang X. Clark R.S. Dixon C.E. Kochanek P.M. Bernard O. Graham S.H. Transgenic mice that overexpress the anti-apoptotic Bcl-2 protein have improved histological outcome but unchanged behavioral outcome after traumatic brain injury. Brain Res. 2006;1101:126–135. doi: 10.1016/j.brainres.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Tehranian R. Rose M.E. Vagni V. Pickrell A.M. Griffith R.P. Liu H. Clark R.S. Dixon C.E. Kochanek P.M. Graham S.H. Disruption of Bax protein prevents neuronal cell death but produces cognitive impairment in mice following traumatic brain injury. J. Neurotrauma. 2008;25:755–767. doi: 10.1089/neu.2007.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi K. Scultetus A. Haque A. Stern S. Philbin N. Rice J. Johnson T. Auker C. McCarron R. Freilich D. Arnaud F. Traumatic brain injury and severe uncontrolled haemorrhage with short delay pre-hospital resuscitation in a swine model. Injury. 2010;43:585–593. doi: 10.1016/j.injury.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Trabold R. Schueler O.G. Eriskat J. Plesnila N. Baethmann A.J. Back T. Arterial hypotension triggers perifocal depolarizations and aggravates secondary damage in focal brain injury. Brain Res. 2006;1071:237–244. doi: 10.1016/j.brainres.2005.11.095. [DOI] [PubMed] [Google Scholar]
- Ünal-Çevik I. Kılınç M. Gürsoy-Özdemir Y. Gurer G. Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015:169–174. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Whalen M.J. Carlos T.M. Dixon C.E. Schiding J.K. Clark R.S. Baum E. Yan H.Q. Marion D.W. Kochanek P.M. Effect of traumatic brain injury in mice deficient in intercellular adhesion molecule-1: assessment of histopathologic and functional outcome. J. Neurotrauma. 1999a;16:299–309. doi: 10.1089/neu.1999.16.299. [DOI] [PubMed] [Google Scholar]
- Whalen M.J. Clark R.S. Dixon C.E. Robichaud P. Marion D.W. Vagni V. Graham S.H. Virag L. Hasko G. Stachlewitz R. Szabo C. Kochanek P.M. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 1999b;19:835–842. doi: 10.1097/00004647-199908000-00002. [DOI] [PubMed] [Google Scholar]
- You Z. Savitz S.I. Yang J. Degterev A. Yuan J. Cuny G.D. Moskowitz M.A. Whalen M.J. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]