Abstract
Objective
Nonmotor symptoms are common in Parkinson’s disease (PD). Health-related quality of life (HRQoL) is negatively affected by different factors, of which pain and sleep disturbances are important contributors. This study was performed to evaluate and describe subjective experiences of pain, sleeping patterns, and HRQoL in a cohort of PD patients with chronic pain.
Methods
A total of 45 participants with established PD for more than 2 years, and PD-related pain for the preceding three months, were recruited from three sites in Sweden. Data regarding time point for onset, duration and degree of pain parameters, body localization of pain, external influences, and treatments were obtained. HRQoL was evaluated with the Short Form-36® Health Survey, and sleeping patterns were registered with the Parkinson’s disease Sleep Scale, both completed along with a questionnaire.
Results
In one-third of participants, pain preceded the PD diagnosis. Median pain score measured with a visual analog scale was 6.6 and 5.9 (for females and males, respectively) the week before the study. In almost half of the participants, pain was present during all their waking hours. Significantly more females described their pain as troublesome, while more males described their pain as irritating. Feelings of numbness and creeping sensations at night were strongly associated with the maximal visual analog scale scores. Polypharmacy was common; 89% used medication for anxiety/insomnia, and 18% used antidepressants. Only one-third of patients who reported pain relief with analgesics had these prescribed on their drug lists. Sleep was characterized by frequent awakenings. Urinary urgency and restless legs were frequently reported as troublesome. Patients rated HRQoL as significantly worse in all items compared with a healthy reference population matched for age and sex.
Conclusions
Experiences of chronic PD-related pain are complex; there is substantial sleep fragmentation and negative impact on HRQoL.
Keywords: data reporting, pain, Parkinson disease, sleep fragmentation
Introduction
Pain in Parkinson’s disease (PD) was described in 1817 by James Parkinson in “An essay on the shaking palsy.”1 Since that time, the symptom has occasionally been discussed in the literature. Ford2 categorized five modalities of pain in PD: musculoskeletal, radicular-neuropathic, dystonic, central pain and akathisia. Beiske et al found musculoskeletal and dystonic pain to be most common type of pain, and they estimated that the overall prevalence of chronic pain was over 80%.3
Muscular stiffness is common, and fluctuations due to “on” or “off ” states in chronic PD-related pain are well described in the literature.4,5 Pain is an early nonmotor symptom in PD,6 and often precedes the start of anti-PD medication.7
The reported prevalence of pain in PD varies in different studies. In 2008, Negre-Pages et al8 estimated the prevalence of chronic pain in PD to be 62%. Ten years earlier The Swedish Parkinson Association reported on a survey of nonmotor symptoms comprising almost 1000 PD respondents.9 They found that chronic pain was more common among females than males (54% and 45%, respectively). However, pain is also common in the general population, and there is a lack of studies on chronic noncancer pain. In a recent review article, the prevalence of moderate to severe, noncancer chronic pain was estimated to be 19% among adults.10
The origin of pain in PD is still obscure. Pathways other than those secondary to rigidity, tremor, or any other motor manifestations of the disease, are probable. The basal ganglia process somatosensory information in different ways. Increased subjective pain sensitivity with lower electrical- and heat-pain thresholds have been reported in PD patients.11 PD-related disorders such as multiple system atrophy show almost the same prevalence of pain as PD.12
Nocturnal symptoms with sleep disturbance are well known in PD.13,14 Sleep is disrupted in the majority of patients,15 and more than two-thirds of patients with PD are affected.16 As with PD-related pain, the origin of sleep disturbance is partially caused by the neurodegenerative process but also most probably due to the interaction with chronic pain. Sleep fragmentation and pain are common in PD, and negatively affect health-related quality of life (HRQoL).17,18
Previous descriptions of the diurnal recurrence and the migrational nature of pain are scarce, as are patients’ self-reported experiences, restrictions in their movements, experienced interference with sleep, and their views regarding causal factors and consequences in daily life.
An increased understanding of PD-related pain and its impact on everyday life, as well as the interactions with sleep disturbances, is essential for all caregivers caring for patients with this disease. Thus we decided to describe these variables in a group of outpatients with established PD and chronic PD-related pain.
Methods
Patient population
Patients with stable and well-defined PD for more than 2 years, who fulfilled the clinical criteria for diagnosis according to the United Kingdom Parkinson’s Disease Society brain bank criteria,19 and with chronic PD-related pain, were included. They were recruited during routine care visits at the outpatient departments of three medium-sized city hospitals in southern Sweden. Anamnestic reports of chronic pain from patients resulted in a more detailed analysis by the clinician. If inclusion and exclusion criteria were met, the patients were surveyed about their interest in participating in the study. Patients were asked to sign an informed consent. The study followed the tenets of the Declaration of Helsinki.
Chronic pain was defined as the occurrence of pain related to PD for three days or more per week during at least three months immediately prior to inclusion in the study. The pain was considered to be related to the disease when associated with fluctuations of the motor symptoms, cramps, or other pain sensation not explained by coexisting physical or mental problems. Exclusion criteria were musculoskeletal pain, such as the coexistence of arthrosis, tension headache, or neck ache, not associated with PD. Severe fluctuations in PD, concurrent existence of epilepsy, active malignancy, polyneuropathy, or other serious disease of somatic or psychiatric origin that could interfere with the study were exclusion criteria as well.
To avoid possible other coexistent chronic or acute diseases that might have concurrent influence on sensory systems, neuropathies, etcetera, patients with severe abnormalities in blood parameters, electrolytes, liver or renal parameters, such as bilirubin > 20 mmol/L, serum creatinine > 130 mmol/L, sedimentation rate > 30 mm, glucose 1-phosphate > 6.7 mmol/L (fasting), were also excluded. Participation in other studies was not allowed.
Patients’ evaluations of their pain, sleep and HRQoL
Before the study was started all patients received careful instructions about how to complete the self-reporting pain scales, and were assessed as being competent to complete their own forms. The week prior to their clinical visit, patients completed a 0–10 cm visual analog scale (VAS),20 marking maximal pain and duration of pain for each day, for five consecutive days. At the assessment visit, patients filled out a four-page “pain evaluation analysis” questionnaire, comprised of multiple-choice questions, body contours, and short free-text questions about different aspects of pain. Pain was also evaluated using the Pain-O-Meter (POM),21 which comprised a 10 cm VAS (POMvas), and a list of 15 sensory and 11 affective word descriptors with an assigned intensity value ranging from 1–5. A pain intensity score was calculated for the sensory, and for the affective components of pain. Experiences of sleep were evaluated using the Parkinson’s Disease Sleep Scale (PDSS).22 HRQoL was evaluated with the Short Form (36) Health Survey (SF-36®) (Swe.ver.1; Quality Metric Inc, Lincoln, RI).23
All medications were carefully registered and supervised by the study staff. At each site, a specialist trained in movement disorders completed the Unified Parkinson’s Disease Rating Scale (UPDRS) version 3.0, parts 1–4,24 and the modified Hoehn and Yahr scale.25
Statistical analyses
Comparisons between categorical variables with respect to proportions (presence of different symptoms or characteristics) were done by means of chi-square test or Fisher’s test.
When comparing variables of ordinal data type between different categories, the Mann–Whitney U test was used, and median with percentiles were presented.
Maximal pain above 7 (VAS ≥ 7) was used as the outcome in a logistic regression model, to explore factors with possible influence on pain.
The different domains of HRQoL levels in the study group were compared to mean levels in the Swedish norm population by means of a one sample t-test. As means of HRQoL in the norm population were presented as age- and sex-specific, and means within the study group were calculated as age-specific but not sex-specific, before comparisons we calculated weighted means based on norm data, and weighted by sex distribution in the study group, in order to exclude the sex effect.
Data were analyzed with STATISTICA© versions 8.0 and 10.0 (Statsoft Inc, Tulsa, OK) and SPSS© version 18.0 (IBM, Armonk, NJ).
Ethics
The study was approved by the Ethics Committees at the University of Gothenburg (Ö 762-03), and the University of Linkoping (D 03-673), Sweden.
Results
Our study included a convenience sample of 45 participants, 16 men and 29 women, who were 50–77 years of age. There were nine patients from Linkoping University Hospital, one of whom chose not to complete the study for personal reasons; 16 patients from Ryhov County Hospital; and 20 patients from Skaraborg County Hospital. Clinical characteristics of the PD population, severity of disease and pharmacological treatments are seen in Table 1.
Table 1.
Basal characteristics of the study population, duration of pain, severity of PD and pharmacological treatment
| Sex | Agea (years) | Duration of paina (years) | UPDRS (3) scorea | UPDRS (1–4) scorea | H and Yb score | Levodopaa,c treatment (mg) | Antidepressants (n) | Anxiolytics/sedatives (n) |
|---|---|---|---|---|---|---|---|---|
| Females (n = 28) | 66.7/66.5 (60/73) | 6.5/4 (1/13) | 23.3/23 (10/37) | 36.3/36 (19/60) | 2 (0/3) | 634/562 (300/1140) | 6 | 23 |
| Males (n = 16) | 62.8/64.5 (54/69) | 4.8/4 (2/10) | 20.4/16.5 (12/36) | 34.5/32.5 (24/49) | 1.5 (0/3) | 768/758 (350/1205) | 2 | 16 |
| Total | 65.3/66 (59/73) | 5.8/4.0 (1.4/12) | 22.2/20 (10/36) | 36.3/35.5 (21/60) | 2 (0/3) | 682/650 (300/1205) | 8 | 39 |
Notes:
Values are given as mean/median (10/90th percentiles);
median;
LED levodopa equivalent doses, according to Tomlinson et al.37
Abbreviations: PD, Parkinson’s disease, UPDRS, Unified Parkinson’s Disease Rating Scale; H and Y, Hoehn and Yahr scale.
Results of basal analyses of pain parameters, the degree and duration of pain compared to time point for PD diagnosis, and the patients’ descriptions of pain are shown in Table 2.
Table 2.
Basal characteristics, onset, duration, and expression of pain
| Gender | Duration* of disease ≤5/>5 | Pain before/after PD diagnosis | Duration** of pain/day ≤10 h/10 h | VAS# ≤5/>5 | Pain expressions by participants | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Migrating | Irritating | Worrying | Trouble-some | Tiring | Suffocating | RLS† | |||||
| Females (28) | 11/16‡ | 10/17‡ | 20/8 | 13/14‡ | 13a | 5a | 3 | 20a | 20 | 1 | 5a |
| Males (16) | 9/7 | 6/10 | 9/7 | 9/7 | 4a | 9a | 5 | 5a | 9 | 0 | 10a |
Notes:
Less or more than 5 years;
maximal duration (less or more than 10 hours) of pain days 1–5;
visual analog scale maximal pain (less or more than 5 cm) days 1–5;
restless legs syndrome (yes/no);
1 missing data; a = statistical significant differences between sexes, P-value ≤0.05.
Abbreviations: PD, Parkinson’s disease; VAS, visual analog scale; RLS, restless legs syndrome.
Pain onset and characteristics of pain
Thirty-five percent of the patients experienced chronic pain before the time point of PD diagnosis, and in 45%, the onset of pain occurred within 5 years from diagnosis.
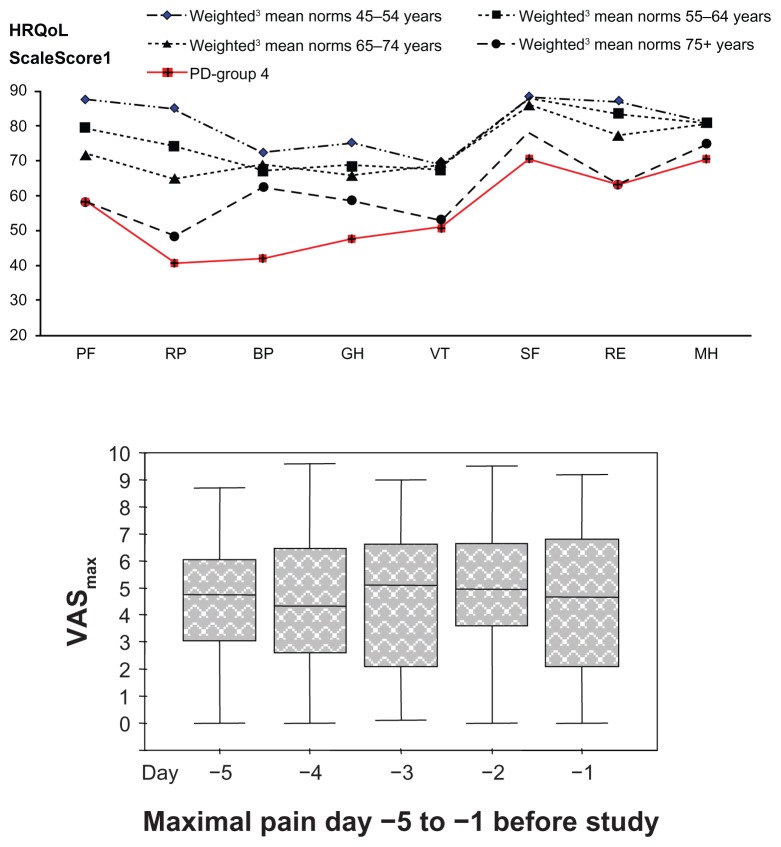
Maximal pain on the five consecutive days (VASmax) before assessment of the entire group is presented in Figure 1. The median score of the VASmax during the 5 days for all patients, was 5.0 (5.2 and 4.8 for females and males, respectively), in comparison with a median score of 3.3 (2.8 and 3.9 for females and males, respectively) at baseline, estimated by the POMVAS.
Figure 1.
Pain (VASmax) and HRQoL (SF-36®, Swe.ver.1) in the study group.
Notes: 10 (worst) −100 (best); 2Visual analog scale: 0 cm (no pain) −10 cm (worst pain); 3presented within age groups of reference population; (mean) weighted according to sex distribution within PD group; 64%/36%, (females/males); 4PD-group; (mean [SD]): PF: 58.4 (22.0), RP: 40.5 (41.0), BP: 41.9 (16.4), GH: 47.6 (17.5), VT: 50.6 (20.2), SF: 70.5 (26.7), RE: 62.9 (44.8), MH: 70.4 (21.2).
Abbreviations: VASmax, visual analog scale maximum pain; HRQoL, health-related quality of life; SF-36, Short-Form Health Survey; PD, Parkinson’s disease; PF, Physical Functioning; RP, Physical Roles; BP, Bodily Pain; GH, General Health; VT, Vitality; SF, Social Functioning; RE, Emotional Role; MH, Mental Health.
The individual descriptions of pain characteristics differed between sexes but this was not influenced by age or disease duration (Table 2).
Dystonic cramps were seen in twelve females and eight males, all of whom experienced benefits regarding pain from anti Parkinson therapy. Three females and two males suffered from dystonic cramps for more than half of the day.
Significantly more patients with a long duration of PD (≥5 years) described their pain symptoms as troublesome compared with those with a short (<5 years) duration (74% versus 26%, respectively; P = 0.006).
Descriptive terms such as “tiring” and “worrying” increased with disease duration, but not significantly. We did not find any association between the descriptive terms and disease severity as expressed by the UPDRS parts 1–4 scores.
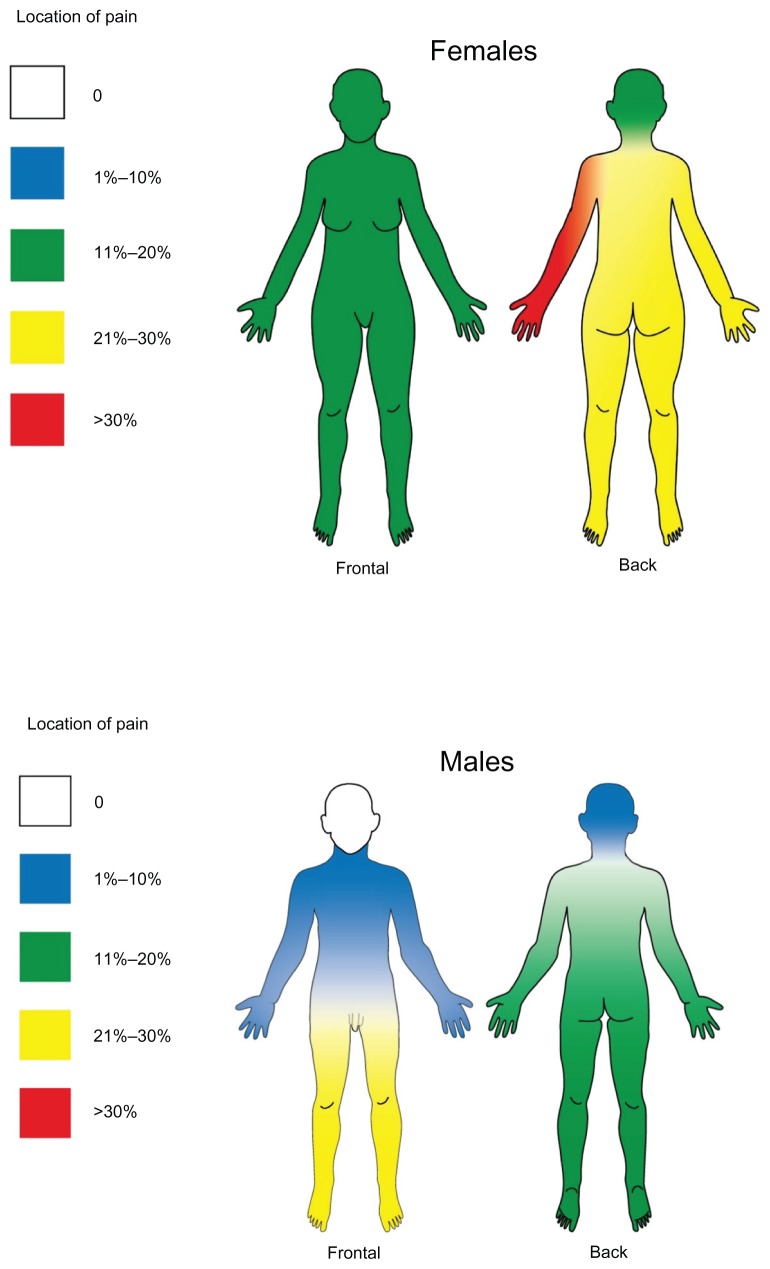
Thirty-four percent of the patients suffered from restless legs, which was significantly more common in males than in females (62.5% versus 17.9%, [P = 0.003]). Figure 2 shows, in percentage of participants, a summary of localizations of chronic pain and the differences between sexes. More males than females experienced pain from the front of the lower extremities (P-values = 0.014 and 0.019, for left and right, respectively).
Figure 2.
Visualization of the localization of chronic pain and differences between sexes.
Duration and fluctuation of chronic pain
More males than females experienced pain from the front of the lower extremities (P-values = 0.014 and 0.019, for the left and right, respectively). Duration of pain was 6.7/5.5; (2/14) years for females, and 5.7/4.5; (2/12) years for males. Almost the whole study population (90%) suffered from daily pain attacks, with no differences between sexes. One third (36%) of the patients registered pain all their waking hours, and only one out of six (16%) reported less than 1 hour of pain per day.
Pharmacological treatment
We could not find any significant correlations between the amount of levodopa and the intensity of pain that was reported (not shown). About half of the patients confirmed pain relief from anti-PD medication. Although 67% of participants indicated relief of pain from analgesics, these drugs were documented in the drug lists of only 27% of those patients. Paracetamol and nonsteroidal anti-inflammatory drugs were the most commonly used analgesics (10/44 and 6/44, respectively). Anxiolytics and medication for insomnia were very commonly prescribed (Table 1). The majority (4/6) of participants who were prescribed antidepressants had a long duration of PD.
Patients’ experiences of nonpharmacological treatments and their thoughts concerning pain relief and pain origin
There was a tendency for more females than males to have tried treatments other than pharmacotherapy, for pain. Taking a bath was most common (n = 18), and four females and four males worked out to alleviate pain. Acupuncture (4), rest (4), active movements (3), and cooling the aching area (3), were other reported examples of self-treatments. Massage, transcutaneous nerve stimulation, and sonography were practiced by one patient each. Approximately three out of four patients had an opinion about which nonpharmacological therapy could be useful for relief of pain. No associations were seen with regard to age or sex.
Almost all patients had an opinion about the origin of pain; most patients (71%) thought that the pain originated from the musculoskeletal system, and 28% said it came from the nervous system. Individual patients indicated that the pain originated from the kidneys, the skin, or that the origin was mental. One fourth of the patients (26%) indicated more than one origin, and three (6%) had no idea of the origin.
There were some restrictions due to chronic pain, where the most common conditions that had a negative impact on pain were “physical strain” (50%), “cold” (29%), and “ walking” (29%). A “sitting position” increased the sensation of pain (48%), more so in patients with severe PD.
Sleep and nightly pain characteristics
The length of unfractioned sleep during the night was 3–4 hours (median) for both sexes. Females woke up two times per night, and males two to three times per night. These values corresponded well to the responses of the item “ difficulty staying asleep,” plotted to in median three to four on the VAS scale in the PDSS questionnaire (worst = 0, best = 10). Females arose a median of twice nightly to urinate, while males arose once. Three females reported distressing pain at night. Nightly muscle cramps, numbness, and burning pain were reported by ten, five, and two patients, respectively. The sex distributions were equal.
Impact on health-related quality of life (HRQoL)
HRQoL was significantly lower in the study group compared with mean levels in a reference population, matched for age and sex (P-value <0.001) for all items except Emotional Role (P-value = 0.021).
Even when compared with a reference group over 75 years of age (ie, older than the study group), there was a statistically significant lower HRQoL within the study group with respect to bodily pain, general health, and social functioning (P-value = <0.001, <0.001, and 0.037, respectively).
Correlations between maximal pain (VASmax ≥ 7) and factors with possible influence on pain were analyzed by logistic regression. There was significant positive correlations between VASmax and POM-VASemon (P-value = 0.03, odds-ratio (OR) = 2.33), the PDSS item “numbness and creeping sensation” (P-value = 0.03, OR 1.33). Age, sex, PD duration, POM-VAS, physUPDRS I-IV, UPDRS depression, PDSS total and PDSS unexpected day sleep were also analyzed, but there were no significant correlations.
Discussion
In this study we have analyzed different aspects of chronic PD-related pain and sleeping patterns, focusing on the patients’ own experiences, as well as on the impact on quality of life. In contrast, previous studies in this area have categorized PD-related pain into classical subgroups (musculoskeletal, radicular-neuropathic, dystonic, central pain and akathisia).2
Pain
Characteristics of onset of pain
As chronic pain in the general population without PD is common and increases with age, we attempted to exclude chronic pain due to concomitant diseases unrelated to PD.3,26 The oldest PD patients were excluded from the study. The time point for onset of pain in PD differs with respect to age at onset of the disease.8 In recent studies focusing on early premotor symptoms of PD, pain has been identified as one of ten risk indicators for disease.27 In agreement with other studies, approximately one-third of the participants experienced pain as an early phenomenon.5
Location of pain and type of pain
We found sex differences in the relative locations of pain as described in Figure 2. Results from earlier studies in this field have been contradictory; Marinkovic et al28 found the most affected parts of the body to be upper and lower limbs in about 70% of the studied population. In another study neck and paraspinal cramps were predominant.29 Our findings showed topographical dominance for lower extremities in males compared with females.
Dystonic cramps in the extremities are a common phenomenon in PD.3 Prominent dystonia affecting the feet and toes is not uncommon, and in special cases paroxysmal exercise-induced dystonia of the feet has been described as having preceded the onset of more classical PD symptoms.30 These observations are in agreement with our results.
Pharmacological aspects
One-third of our patients reported pain relief in connection with intake of anti-PD medication. High concentrations of striatal dopamine (hyperdopaminergic state) are associated with the “on” phase, and can contribute to the development of some sensory symptoms, such as peak-dose akathisia, although this symptom is usually associated with a hypodopaminergic state.4 A temporal relation between onset of pain symptoms and medication intake may reveal an association of pain with wearing “off ” periods, such as in early morning dystonia.29 Adjusting anti-PD medication can be more effective than the administration of analgesics in these cases.
Intraventricular or striatal microinjections of the dopamine agonist apomorphine have been shown to result in a dose-dependent decrease in nociceptive responses, supporting the theory of dopamine as a pain modulator. Mood and anxiety disorders have also been associated with an increased likelihood of developing chronic pain symptoms. This reciprocal relationship may be partially due to shared dysfunctions in central dopamine signaling, which plays a role in these disorders, as well as in pain processing.31
Patients’ subjective opinions regarding pain treatment
Our study reveals a variety of measures are employed to attain pain relief. About 30% of the patients were prescribed analgesics, most frequently paracetamol and nonsteroidal anti-inflammatory drugs. Surprisingly, many patients took anxiolytics, sedatives and/or antidepressants on a daily basis. The outcome of using analgesics in PD and related disorders has not been reported in any controlled trial, and systematic reports are also lacking.32 Hence, pain is probably underreported and many neurologists might consider it difficult to treat pain associated with PD and PD-related disorders.
Sleep
Undisturbed sleep was rare in our study. This is consistent with the findings by van Hilten et al,33 who found more severely disturbed sleep maintenance in the PD group than healthy controls, and that nocturia, pain, stiffness, and problems with turning over in bed were the main causes of frequent awakening in PD patients. Difficulties in remaining asleep were revealed by both the PDSS and the pain evaluation analysis scales in our study. Numbness and “creeping” sensations at night were strongly associated with the maximal VAS scores, which strengthens our assumption that pain is an important cause of sleep disruptions in PD. Frequent awakenings to urinate, and early awakenings with painful posturing of arms and/or legs, were common.
Sleep disturbances can occur at any stage of PD, but they gradually worsen as the disease progresses.16 Excessive daytime sleepiness and daytime sleep attacks are common features in PD, and are only partially an effect of the type and dosage of anti-PD drug treatment. Sleepwalking and sleep talking, nightmares, sleep terrors, and panic attacks are common,13 and most certainly contributes to a low HRQoL in this patient group. Painful leg cramps, back pain, limb/facial dystonia, and difficulty in turning over in bed are also common symptoms verified in our study.
Nonpharmacological aspects of sleep treatment
A majority of the study population used sedatives and/or anxiolytics (Table 1). There is a lack of scientific studies of complementary therapies addressing treatment of sleep disturbances. Tactile massage has shown positive effects in relation to pain, sleep, relaxation, energy, and mood in healthy adults in one Swedish study from 2009.34 Further studies are warranted in this field, as many PD patients currently are using different complementary and alternative medical therapies.35
HRQoL
HRQoL decreases with increased age in healthy people.23 Even when compared to a reference population of the oldest ages, the study patients’ HRQoL was significantly lower in several items, indicating the powerful negative impact of PD symptoms.
Most obvious regarding HRQoL was the importance of sleep interruption, fidgeting in bed, nightmares, painful muscle cramps whilst sleeping, and urinating during the night.
We compared the results of HRQoL in our PD patients with the results of a group of patients (N = 104) with the same country of origin and the same age, who were suffering from sequelae of ischemic stroke.36 The same instrument, SF-36, was used for both groups. Our study group had lower scores in six out of nine HRQoL items. Major negative differences in the PD group were in the items relating to general health and bodily pain. Both items closely associated to our research questions.
Limitations of the study
Relatively few patients were included. Diff iculties in separating PD-related pain from pain of non-PD origin was harder among the oldest PD patients, who therefore were underrepresented.
Conclusion
Our study has confirmed that chronic PD-related pain is complex in nature. A considerable proportion of the patients had onset of pain many years before diagnosis of PD. Patients with PD-related pain had high scores on the VAS scales. Individual descriptions of pain and experiences with nonpharmacological therapies varied between sexes. A severe impact on sleep with disrupted sleep patterns was seen. Concomitant pharmacotherapy with anxiolytics, analgesics, and antidepressant was common, with significant differences in prescriptions between the sexes. HRQoL in PD patients with chronic pain was significantly lower than in an age-matched healthy reference population. Future studies should address pain-reducing therapies, both pharmacological and nonpharmacological.
Acknowledgments
Financial support for this study was provided by the Research Fund at Skaraborg Hospital, the Research and Development Council of Skaraborg County, the Palle Ferb Foundation, FUTURUM, the Academy for Healthcare, Jonkoping County Hospital, the Medical Research Council of Southeast Sweden, and the Skaraborg Institute of Research and Development and Else Torgards Fund. We acknowledge Salmir Nasic for excellent statistical support. And last but not least, we acknowledge and are grateful to the study participants.
Authors’ contributions
CJT, OS, GH, PAF, MC, HS, UL, BB were responsible for the study conception, data collection, and design. OS performed the data analysis. OS was responsible for drafting the manuscript, with support from CJT and JL.
Disclosure
The authors declare no conflicts of interests in this work.
References
- 1.Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14(2):223–236. doi: 10.1176/jnp.14.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Ford B. Pain in Parkinson’s disease. Clin Neurosci. 1998;5(2):63–72. [PubMed] [Google Scholar]
- 3.Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson’s disease: Prevalence and characteristics. Pain. 2009;141(1–2):173–177. doi: 10.1016/j.pain.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Bayulkem K, Lopez G. Clinical approach to nonmotor sensory fluctuations in Parkinson’s disease. J Neurol Sci. 2011;310(1–2):82–85. doi: 10.1016/j.jns.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Giuffrida R, Vingerhoets FJ, Bogousslavsky J, Ghika J. Pain in Parkinson’s disease. Rev Neurol (Paris) 2005;161(4):407–418. doi: 10.1016/s0035-3787(05)85070-2. French. [DOI] [PubMed] [Google Scholar]
- 6.Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology. 1976;26(5):423–439. doi: 10.1212/wnl.26.5.423. [DOI] [PubMed] [Google Scholar]
- 7.Defazio G, Berardelli A, Fabbrini G, et al. Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol. 2008;65(9):1191–1194. doi: 10.1001/archneurol.2008.2. [DOI] [PubMed] [Google Scholar]
- 8.Nègre-Pagès L, Regragui W, Bouhassira D, Grandiean H, Rascol O for DoPaMiP Study Group. Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord. 2008;23(10):1361–1369. doi: 10.1002/mds.22142. [DOI] [PubMed] [Google Scholar]
- 9.Borgman A. Parkinsonjournalen. Swedish: 2002. Parkinsonenkät-98. [study-overview] [Google Scholar]
- 10.Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–462. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- 11.Mylius V, Engau I, Teepker M, et al. Pain sensitivity and descending inhibition of pain in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80(1):24–28. doi: 10.1136/jnnp.2008.145995. [DOI] [PubMed] [Google Scholar]
- 12.Tison F, Wenning GK, Volonte MA, Poewe WR, Henry P, Quinn NP. Pain in multiple system atrophy. J Neurol. 1996;243(2):153–156. doi: 10.1007/BF02444007. [DOI] [PubMed] [Google Scholar]
- 13.Thorpy MJ. Sleep disorders in Parkinson’s disease. Clin Cornerstone. 2004;6(Suppl 1A):S7–S15. doi: 10.1016/s1098-3597(04)90013-0. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson’s disease. Mov Disord. 2002;17(4):775–781. doi: 10.1002/mds.10167. [DOI] [PubMed] [Google Scholar]
- 15.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11(6):512–519. doi: 10.1097/00002826-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Partinen M. Sleep disorder related to Parkinson’s disease. J Neurol. 1997;244(4 Suppl 1):S3–S6. doi: 10.1007/BF03160564. [DOI] [PubMed] [Google Scholar]
- 17.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008;23(10):1428–1434. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- 18.Quittenbaum BH, Grahn B. Quality of life and pain in Parkinson’s disease: a controlled cross-sectional study. Parkinsonism Relat Disord. 2004;10(3):129–136. doi: 10.1016/j.parkreldis.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Gibb WR, Lees AJ. The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol Appl Neurobiol. 1989;15(1):27–44. doi: 10.1111/j.1365-2990.1989.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 20.Huskinson EC. Visual analogue scales. In: Melzack R, editor. Measurement and Assessment. New York, NY: Raven Press; 1983. pp. 33–37. [Google Scholar]
- 21.Gaston-Johansson F. Measurement of pain: the psychometric properties of the Pain-O-Meter, a simple, inexpensive pain assessment tool that could change health care practices. J Pain Symptom Manage. 1996;12(3):172–181. doi: 10.1016/0885-3924(96)00128-5. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73(6):629–635. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan M, Karlsson J, Ware JE., Jr The Swedish SF-36 Health Survey – I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41(10):1349–1358. doi: 10.1016/0277-9536(95)00125-q. [DOI] [PubMed] [Google Scholar]
- 24.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 25.Goetz CG, Poewe W, Rascol O, et al. for Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 26.Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18(3):299–314. doi: 10.1016/0304-3959(84)90824-8. [DOI] [PubMed] [Google Scholar]
- 27.Winkler J, Ehret R, Büttner T, et al. Parkinson’s disease risk score: moving to a premotor diagnosis. J Neurol. 2011;258(Suppl 2):S311–S315. doi: 10.1007/s00415-011-5952-x. [DOI] [PubMed] [Google Scholar]
- 28.Marinković Z, Kostić V, Coviković-Sternić N, Marinković S. Pain in patients with Parkinson disease. Srp Arh Celok Lek. 1990;118(11–12):463–466. Serbian. [PubMed] [Google Scholar]
- 29.Goetz CG, Tanner CM, Levy M, Wilson RS, Garron DC. Pain in Parkinson’s disease. Mov Disord. 1986;1(1):45–49. doi: 10.1002/mds.870010106. [DOI] [PubMed] [Google Scholar]
- 30.Bozi M, Bhatia KP. Paroxysmal exercise-induced dystonia as a presenting feature of young-onset Parkinson’s disease. Mov Disord. 2003;18(12):1545–1547. doi: 10.1002/mds.10597. [DOI] [PubMed] [Google Scholar]
- 31.Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153(4):744–754. doi: 10.1016/j.pain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boivie J. Pain in Parkinson’s disease (PD) Pain. 2009;141(1–2):2–3. doi: 10.1016/j.pain.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 33.van Hilten JJ, Weggeman M, van der Velde EA, Kerkhof GA, van Dijk JG, Roos RA. Sleep, excessive daytime sleepiness and fatigue in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1993;5(3):235–244. doi: 10.1007/BF02257678. [DOI] [PubMed] [Google Scholar]
- 34.Andersson K, Törnkvist L, Wändell P. Tactile massage within the primary health care setting. Complement Ther Clin Pract. 2009;15(3):158–160. doi: 10.1016/j.ctcp.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Lökk J, Nilsson M. Frequency, type and factors associated with the use of complementary and alternative medicine in patients with Parkinson’s disease at a neurological outpatient clinic. Parkinsonism Relat Disord. 2010;16(8):540–544. doi: 10.1016/j.parkreldis.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Sprigg N, Gray LJ, Bath PM, et al. for TAIST Investigators. Quality of life after ischemic stroke varies in western countries: data from the Tinzaparin in acute ischaemic stroke trial (TAIST) J Stroke Cerebrovasc Dis. 2011 Mar 10; doi: 10.1016/j.jstrokecerebrovasdis.2011.01.007. [Epub before print.] [DOI] [PubMed] [Google Scholar]
- 37.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]