Abstract
This randomized clinical trial retrospectively examined the effect of Post Traumatic Stress Disorder (PTSD) and contingency management (CM) on cocaine use in opioid and cocaine dependent individuals maintained on high or low-dose LAAM randomly assigned to CM or a yoked-control condition. Cocaine-positive urines decreased more rapidly over time in those without PTSD versus those with PTSD in the non-contingency condition. In participants with PTSD, CM resulted in fewer cocaine positive urines compared to the non-contingent condition. This suggests that CM may help improve the potentially worse outcomes in opioid-and cocaine dependent individuals with PTSD compared to those without PTSD.
Keywords: PTSD, cocaine dependent, opioid dependent, contingency management
Introduction
Although psychiatric co-morbidities, including Post Traumatic Stress Disorder (PTSD), are prevalent in persons with substance use disorders (1–4), their impact on treatment outcome is unclear. For instance, one of the earliest studies to examine the interaction between psychiatric severity, as measured by the Addiction Severity Index, and treatment modality on treatment outcomes found that alcohol- and other drug-dependent males with low psychiatric severity improved regardless of the treatment modality, while those with high psychiatric severity showed virtually no improvement in any treatment setting (5). Other studies utilizing different instruments to assess psychiatric comorbidity as well as different measures of treatment outcome had similar findings in heterogeneous substance abusing populations (6–8).
On the other hand, results of studies limited to opiate dependent populations are mixed in terms of the impact of psychiatric co-morbidity on treatment outcomes (9–12). For instance, comorbid psychiatric diagnosis has been shown to have a negative impact on functioning up to 2.5 years after seeking treatment (10, 11). However, although Axis I or II diagnosis has been shown to predict poorer psychosocial functioning and medical status at the time of admission and follow-up (10), comorbid psychiatric diagnosis had no impact on the number of positive urines for opiates or cocaine at 7 months follow-up (9,10). In contrast, patients with an antisocial personality disorder diagnosis reportedly have higher rates of cocaine and heroin use (12).
Whether comorbid psychiatric illness impacts retention in treatment is also somewhat unclear. Although the results of numerous studies demonstrated no impact of comorbid psychiatric illness on treatment outcome as determined by retention in treatment (10, 12–16), others found that patients with a personality disorder diagnosis were less likely to stay in treatment continuously for 7 months (9). In contrast, Gelkopf and colleagues (16) found that patients with a comorbid Axis I diagnosis stayed in treatment longer than those with no Axis I diagnosis.
Likewise, whether PTSD impacts treatment response is unclear. Two previous studies specifically examining the impact of PTSD on substance abuse treatment outcomes suggest that higher levels of PTSD are associated with negative substance use disorder treatment outcomes (17,18), whereas two other recent studies indicate no significant difference in outcome (19,20). Ouimette et al. (17) demonstrated that male heterogeneous substance abusing veterans with PTSD have more rapid relapse, greater substance use, and more problems from substance use following an intensive treatment or residential substance use disorder treatment program than veterans without PTSD. Moreover, Hien et al. (18) reported that new methadone patients with current PTSD had significantly more ongoing drug use, including cocaine use, at 3 months than those without PTSD. A more recent study examining 6-month treatment outcomes among cocaine-dependent outpatients with and without PTSD found that substance use disorder-PTSD (SUD-PTSD) patients were more impaired, and showed significantly less improvement in alcohol use and most addiction-related psychosocial problems compared with SUD-only patients (21). However, the two groups did not differ significantly in improvement over time on drug use or global psychological severity.
In contrast, Mills et al. (19) reported that, although PTSD was associated with continued physical and mental disability and reduced occupational functioning in heroin-dependent patients across a variety of settings, no significant differences in treatment retention and no significant differences in rates of other substance use were found between those with and without current PTSD across the two years of the study. Those with current PTSD were also less likely to use heroin than those without current PTSD (19). In addition, no significant differences in opiate or cocaine use were found between predominately male veterans in opiate agonist treatment with and without a PTSD diagnosis (20). Trafton et al. (20) suggest several possible reasons for the differences in outcomes across these studies, including differences in dropout-rate between the Hien et al. and Trafton et al. studies and the lack of detail regarding the treatment intervention such as methadone maintenance doses (20). In addition, methodological differences, such as the measures employed to assess outcome, methods to determine PTSD diagnosis or symptom severity, and comorbid drug dependence may be at least partial determinants of these seemingly conflicting data.
Meanwhile, behavioral interventions, notably contingency management procedures (CM), have been increasingly examined in drug dependent individuals, demonstrating some efficacy in facilitating illicit drug abstinence in general, as well as in dually cocaine and opioid dependent populations (22–30). Contingency management has also been shown to improve treatment retention in cocaine abusers (31). Yet, to our knowledge, only a few studies have examined the relative impact of psychiatric comorbidity and severity on outcome under contingency management conditions. For instance, in alcohol, cocaine or opioid-dependent individuals who received standard treatment or standard treatment plus contingency management, which rewarded negative urine screens and completion of goal-related activities, a significant interaction was found between psychiatric severity and treatment modality in relation to treatment retention, such that participants with greater psychiatric severity were more likely to dropout of treatment earlier in the standard treatment condition, while retention was similar across the psychiatric severity groups in the contingency management condition (22). In contrast, Saxon et al. (32) examined predictors of outcome at 18 months post-treatment entry for 353 admissions to methadone maintenance who received random assignment to one of three counseling conditions: (1) medication only, (2) standard counseling and (3) enhanced services with either no contingencies or contingency contracting. Subjects in contingency contracting were placed on contingency contracts that punished positive urine toxicology results and ultimately discharged patients for unremitting drug use. Lower ASI psychiatric composite scores consistent with lower psychiatric symptom severity predicted higher rates of total, opiate and cocaine positive urines. Higher rates of opiate positive urines were also predicted by assignment to medication only condition. Lower rates of cocaine positive urines were predicted by assignment to the enhanced contingency contracting condition. Overall, these findings suggest that contingency management and psychiatric severity impact treatment outcomes in a complex manner and deserve further study.
To our knowledge, only one study has examined the prognostic relevance of trauma history and PTSD symptoms on outcome in a randomized trial of contingency management (CM) compared to standard treatment in cocaine- or heroin-dependent outpatients (33). Results were that the severity of complex PTSD symptoms predicted poorer outcomes in terms of retention in treatment and objectively verified continuous abstinence from cocaine and heroin use in treatment. The absence of an interaction effect for complex PTSD symptoms and treatment condition suggested that the predictive relationship occurred both in standard treatment and CM. However, closer inspection of the results for each treatment condition showed that complex PTSD symptoms were predictive of outcome only in the CM condition. However, in this study a third of participants met criteria for opiate dependence, and only slightly more than one-fifth were receiving opiate agonist treatment at baseline. Taken together, these findings suggest that the impact of PTSD diagnosis and contingency management on treatment outcomes in cocaine abusing, opiate dependent patients needs further exploration.
A previous clinical trial examined the effects of levoalpha-acetyl methadyl (LAAM) maintenance dose and contingency management procedures aimed at facilitating illicit drug abstinence in dually cocaine- and opioid-dependent individuals (30). Results showed that contingency management increased cocaine abstinence over time to a greater degree in those maintained on an adequate dose of LAAM relative to a yoked control. Given that opioid maintenance dose was fixed and contingency management procedures were employed, we conducted a retrospective analysis to examine the prognostic significance of PTSD diagnosis on outcome in this sample. Our hypothesis was that those with PTSD would have poorer outcomes than those without PTSD and contingency management would facilitate better outcomes than the yoked control in those with PTSD.
Methods
Subjects
Data were obtained from a previously-reported study (30). One hundred forty male and female dually cocaine and opioid-dependent individuals (ages 21–55, including 45 females, 39 African-Americans, 10 Hispanics, and 91 Caucasians) seeking opioid maintenance treatment were recruited from the greater New Haven area after giving informed consent to participate in this randomized clinical trial approved by the Yale Human Investigations Committee and the VA Connecticut Human Studies Subcommittee. Subjects were entered into this study from 1997 through 2000. Thus data collected in this previous study were obtained prior to safety requirements for LAAM regarding ECG testing to monitor QTc interval or a review of other medications that might extend LAAM half-life or produce independent QTc interval changes. Individuals who could not tolerate LAAM were tapered off LAAM and referred to a local opioid maintenance program. Only two subjects were unable to tolerate LAAM at the dose outlined in the protocol. All participants met the Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) criteria for opioid and cocaine dependence, as determined from the Structured Clinical Interview for DSM-IV (SCID), and had urine toxicologies positive for both drugs. Exclusions included medical reasons contraindicating LAAM maintenance (e.g., pregnancy, respiratory condition such as asthma, abnormal liver enzyme levels, or use of medications that might interact with LAAM), a current diagnosis of other drug or alcohol physical dependence (other than tobacco), a history of major psychiatric disorder (psychosis, schizophrenia, bipolar), current suicidality, and an inability to read and understand the consent form. Alcohol physical dependence was defined as those having experienced tolerance or withdrawal during the preceding 12 months. Women of childbearing age were included provided they had a negative urine pregnancy test, agreed to use adequate contraception to prevent pregnancy during the study, and agreed to monthly pregnancy tests.
Research design and procedures
In this 12-week, randomized, double blind, outpatient clinical trial, 140 patients were randomized by sex to one of the following treatment groups: low-dose LAAM (30, 30, 39 mg/MWF) with contingency management procedures (LC); low-dose LAAM (30, 30, 39 mg/MWF) without contingency management procedures (LY); high-dose LAAM (100,100,130 mg/MWF) with contingency management procedures (HC); high-dose LAAM (100, 100, 130 mg/MWF) without contingency management procedures (HY). During LAAM induction, patients received an initial dose of 20 mg of LAAM on Monday of week 1. This dose was increased by 10 mg at next dosing such that patients received 30 mg on Wednesday of week 1. The patients in the low-dose group then received their maintenance doses of LAAM (30 mg on Mondays and Wednesdays; 39 mg on Fridays), while subjects in the high-dose group continued to be inducted onto LAAM in 10 mg dose increments until they received their maintenance dose of 100 mg by Friday of week 4. All patients were then maintained on their respective maintenance dose of LAAM through week 12. Further details regarding LAAM induction procedures are described elsewhere (30). At the end of the study, subjects were detoxified from LAAM over a 4-wk period unless they opted to transfer to a regular opioid maintenance program.
Contingency Management Procedures
The contingency management procedures used in this study were adapted from those developed by Higgins et al (31, 34–35). Supervised urines were collected thrice-weekly (MWF) prior to being medicated in order to implement the contingency management procedures, which began during week 1 along with weekly group and weekly individual therapy sessions. During weeks 1–12, subjects in the contingency management group had the opportunity to obtain vouchers for illicit drug abstinence. Subjects received a monetary voucher for every drug-free urine sample. During weeks 1–12, the first drug-free urine sample was rewarded with a $3 monetary voucher. The value of the voucher increased by $1 for every consecutive drug-free urine sample thereafter. A drug-positive urine sample reset the voucher value back to $3. Those subjects not placed on contingency management procedures were each yoked to a subject in the contingency group, such that he/she received monetary vouchers whenever the subject in the contingency group received them. Subjects were blind to this schedule, but were informed that they may receive vouchers on a random basis. Subjects exchanged their vouchers for mutually agreed-upon goods or services at any time during the course of the study. Subjects in the contingency management group who remained abstinent during the first 12 weeks earned goods and services worth a maximum of $738.00. Additional details regarding contingency management procedures and weekly/individual therapy sessions can be found elsewhere (30).
Laboratory tests
Urine samples were analyzed for the presence of opioids and cocaine metabolites upon submission of a urine sample on a thrice-weekly basis. This frequency of urine testing was employed in order to detect most opioid and cocaine use. Breath analysis for alcohol was performed on a random basis or when recent alcohol use had been suspected. If the breath alcohol level was > 0.00 and < 0.05, one-half the LAAM dose was administered. Breath alcohol levels ≥ 0.05 resulted in the LAAM being withheld on that day. Urines were also screened for barbiturates and benzodiazepines on a weekly basis. A urine sample was rated positive if the quantity of opioids was greater than 200 ng/ml for opioids and quantity of other drugs and their metabolites was 300 ng/ml. Urine toxicology results were available the next day from the VA Clinical Laboratory. Blood chemistries (SMA 20, CBC) and electrocardiogram (ECG) as well as a general physical examination were performed during screening.
Psychosocial assessments
Self-reported opioid and cocaine use, as well as opiate withdrawal symptoms, were assessed at baseline and weekly using instruments we have developed in previous studies (36–37). Since medication hours were between 7 and 10 a.m., the time of day for these ratings was relatively standardized. Ratings were completed before LAAM dosing.
At intake, the SCID interview was completed for DSM-IV psychiatric diagnoses. Subjects identified to have been exposed to a traumatic event were first identified using the traumatic events booklet (38) and impact of events scale (39). Those events were then used as reference points during the PTSD portion of the SCID. Substance-related problems and psychosocial functioning were assessed at intake using the Addiction Severity Index (ASI), a 140-item structured clinical interview using both subjective and objective information to make severity ratings on 10-point scales in seven areas: alcohol and drug use, medical status, legal status, psychiatric symptoms, occupational functioning, and family/social functioning (40). Information on training of raters is published elsewhere (30).
Data analyses
Information regarding current PTSD diagnosis was available on 137 of 140 participants. The intent to treat sample of 137 participants was used for the statistical analyses. Subjects were grouped within each treatment cell by the presence or absence of current PTSD. Information about the lifetime rate of PTSD in this sample was unavailable. Baseline and retention data were analyzed between those participants with PTSD and those without PTSD with t-tests on the continuous variables and chi-square for categorical variables. For data sampled at several time points, participants were assigned to eight groups (HC with PTSD, HC without PTSD, HY with PTSD, etc.) Hierarchical Linear Modeling (HLM) was used to determine whether the number of positive urines decreased at a different rate across conditions over time (41, 42).
The primary outcome in the intent to treat sample was urine toxicology screening result. We compared the mean proportion of the three weekly urine samples that were opiate-positive, cocaine-positive, and negative from both each week ranging between 0 and 1 in a manner similar to that described previously (38, 39). Urinalyses were analyzed using hierarchical linear models (HLMs), which examine the linear trend of categorical and continuous data over time and the interaction of temporal trend with treatment factors and subject characteristics (42). The HLMs allow for inclusion of data from patients who did not complete treatment, varying assessment times, and different numbers of assessments per subject. Urinalysis data were first calculated as weekly proportion of urines positive for the target drug; zero reflected “no drug screens positive,” one of two, one of three, or two of three drug screens positive were recoded 1 for “some use”, and all drug screens positive was recoded 2, reflecting “all drug screens for that week positive”. These data were entered into 2 × 2 × 2 (high dose vs. low dose, contingency vs. non-contingency, and PTSD vs. no-PTSD) design over 12 time points to give an 8 × 12 ordinal response hierarchical linear model with random intercept (41,42) Slopes were compared over the 8 groups to compare rate of positive urine decrease over time. We used the MIXOR program of Hedeker (43) for ordinal HLM analyses and SAS for baseline analysis. These analyses yielded z-scores for pair wise comparison of slopes that assessed the linear increase or decrease in data values over the course of the study as a function of LAAM dose, contingency management condition, and PTSD diagnosis. This approach was selected to maintain the power of the analyses given smaller sample sizes in each of the 8 groups. For all analyses, statistical significance was inferred by a p value less than 0.05.
Results
Baseline characteristics and treatment retention
Baseline and demographic characteristics of 137 subjects based on the presence or absence of a PTSD diagnosis are shown in Table 1. Thirty-one of 137 (22.6 %) of participants met DSM IV criteria for PTSD. Participants with PTSD were more likely to be female, have a diagnosis of alcohol dependence, and have a diagnosis of major depression. Baseline cocaine-positive urine screens did not differ across groups.
Table 1.
BASELINE SUBJECT CHARACTERISTICS AND STUDY RETENTION
| Measure | No PTSD (SD) | PTSD (SD) | T | Chi2 | P |
|---|---|---|---|---|---|
| N | 106 | 31 | |||
| Age (yrs) | 35.3 (7.3) | 34.2 (7.8) | 0.14 | 0.90 | |
| Race (C/AA/H) | 69/32/5 | 21/6/4 | 3.5 | 0.17 | |
| Gender (F/M) | 28/78 | 14/17 | 8.8 | 0.003 | |
| Alcohol dependence | 13 | 9 | 5.0 | 0.03 | |
| Education (#≥HS grad) | 81 | 23 | 0.07 | 0.80 | |
| Employment (#≥p/t) | 46 | 12 | 0.22 | 0.64 | |
| SCID Dx depression | 10 | 18 | 34.9 | <.0001 | |
| Wks in study | 14.7 (8.7) | 12.0 (9.5) | 1.5 | 0.15 | |
| SCID Dx opiate depend | 106 | 30 (1-missing) | |||
| SCID Dx cocaine depend | 99 | 30 |
Race: C, Caucasian; AA, African American; H, Hispanic
Employment: #≥p/t = number of subjects with part time or full time employment
No PTSD = No current PTSD diagnosis
PTSD = Current PTSD diagnosis
There was a trend toward a longer duration of participation in those without PTSD than those with PTSD. Participants with PTSD were more likely to leave the study early compared to those without PTSD in the absence of contingency management (t=2.46, p=0.02), but not in the contingency management condition (t=0.45, p=0.65).
Cocaine use during the trial
Illicit opioid use and the combination of opioids and cocaine use are not reported here as there were no significant differences in urine toxicology results when examining participants with and without a diagnosis of PTSD. Complete results without PTSD as a factor have already been published elsewhere (30).
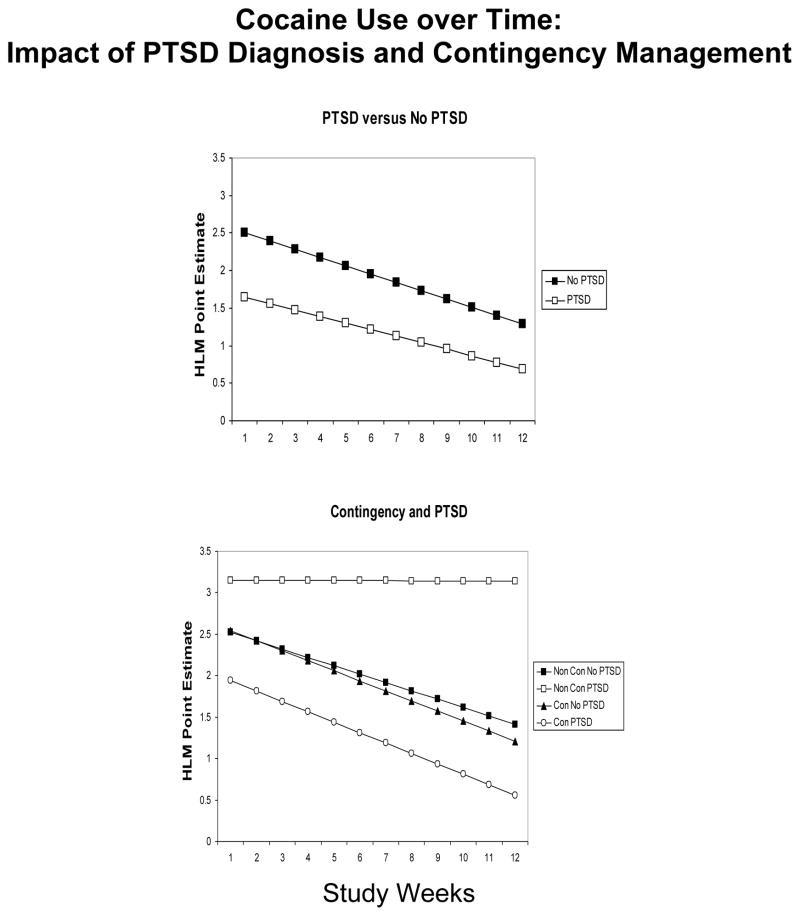
The overall impact of PTSD and contingency management on cocaine use is shown in Figure 1. Regardless of LAAM dose and contingency management condition, changes in cocaine-positive urines over time did not differ by the presence or absence of PTSD (z = 0.8, p = 0.43). Furthermore, the presence or absence of PTSD did not impact cocaine positive urines in the contingency management condition (z = −0.1, p = 0.92). However, for participants without contingency management, cocaine-positive urines decreased more rapidly over time in those without a PTSD diagnosis relative to those with a PTSD diagnosis (z = 2.3, p = 0.02). In participants with PTSD contingency management was associated with fewer positive urines for cocaine over time compared to the yoked control (z = 2.5, p = 0.014).
Figure 1.
Cocaine use as a function of time over a 12 week period. Left panel: HLM Point Estimate to show lack of any change over time in cocaine positive urines in participants with PTSD (open squares) versus those without PTSD (closed squares). Right panel: HLM Point Estimate to show direction of change in cocaine positive urines in participants with PTSD receiving contingency (open circles), those with PTSD and no contingency (open squares), those without PTSD receiving contingency (closed triangles) and those without PTSD and no contingency (closed squares).
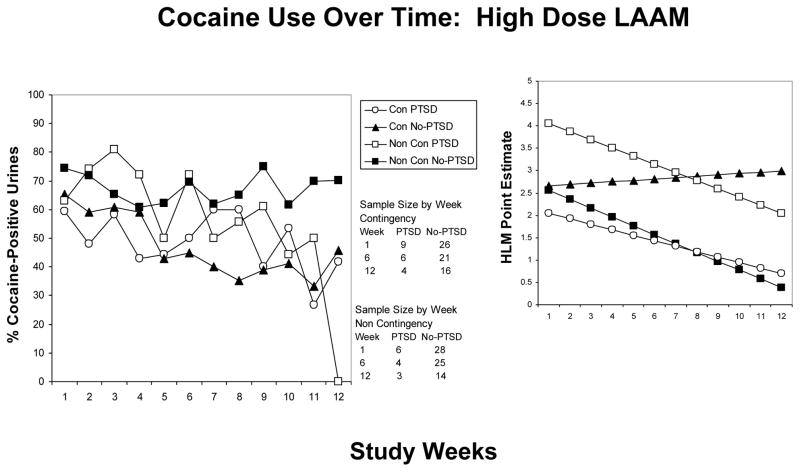
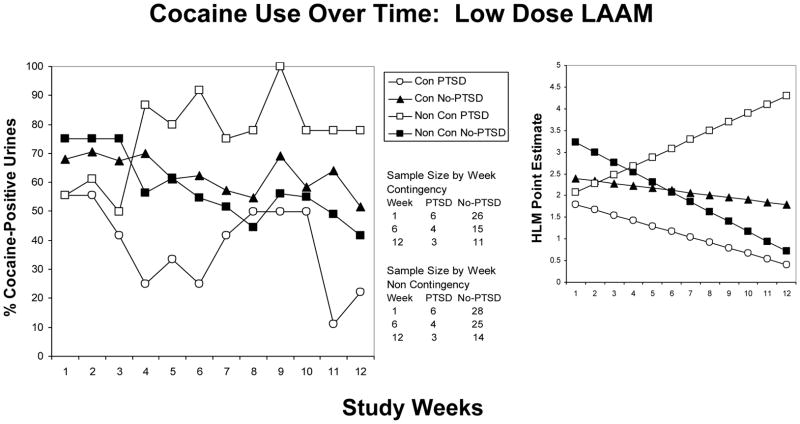
When controlling for both LAAM dose and contingency management condition, cocaine positive urines decreased more rapidly over time in the HY group without PTSD relative to the HY group with PTSD (z = −2.4, p = 0.015) (see figure 2). Cocaine use decreased over time in the LY group without PTSD while increasing over time in the LY group with PTSD (z = 4.1, p < 0.001). The change in the proportion of cocaine positive urines over time did not differ in the HC group with PTSD relative to HC group w/o PTSD (z = −0.65, p =0.51). Similarly, changes in the proportion of cocaine positive urines over time did not differ between LC group with PTSD and without PTSD (z = −1.0, p = 0.32) (see figure 3).
Figure 2.
Cocaine use as a function of time over 12 weeks in participants receiving high dose LAAM. Left panel: Mean percentage of cocaine positive urines in participants with PTSD receiving contingency (HC-open circles), those with PTSD and no contingency (HY-open squares), those without PTSD receiving contingency (HC-closed triangles) and those without PTSD and no contingency (HY-closed squares). Right panel: HLM generated estimates to show the direction of changes, if any.
Figure 3.
Cocaine use as a function of time over 12 weeks in participants receiving low dose LAAM. Left panel: Mean percentage of cocaine positive urines in participants with PTSD receiving contingency (LC-open circles), those with PTSD and no contingency (LY-open squares), those without PTSD receiving contingency (LC-closed triangles) and those without PTSD and no contingency (LY-closed squares). Right panel: HLM generated estimates to show the direction of changes, if any.
Discussion
To our knowledge, this is the first study to examine the impact of PTSD diagnosis on the efficacy of contingency management procedures in a dually cocaine- and opioid-dependent population maintained at different doses of an opioid agonist. The prevalence of PTSD in our study population (i.e., 22.6 %) is consistent with other clinical studies that report high rates of PTSD among people in treatment for opioid dependence (life-time: 14–41%; current: 20–31%) (15, 18, 44, 45) relative to those observed among the general population (life-time: 1–8%; current: 1–4%) (46–49). That those with PTSD were more likely to be female, be alcohol dependent or depressed is also consistent with previous studies. For instance, PTSD diagnosis is reportedly more common in females (18–20). In addition, patients diagnosed with PTSD have significantly longer histories of drinking alcohol to intoxication (20) and are also more likely to be diagnosed with depression (19, 20).
Our study did not show any significant differences in outcome with regard to opiate positive urines in participants with PTSD compared to those without PTSD. It has been postulated that the need for an adequate dose of opiate medication is perhaps the most important factor in treatment outcome, especially as it pertains to opiate use (13, 20, 32). That opioid use differed between the high and low LAAM maintenance dose conditions (30), but not between those with and without PTSD within each dose condition supports this view.
The finding that cocaine positive urines did not differ between those with and without a PTSD diagnosis regardless of treatment condition during the study would seem to be in agreement with recent studies (20, 21). However, one of these studies reported that patients with PTSD received higher doses of opiate medication, attended more psychosocial treatment sessions for substance-use disorder, and had better treatment retention (20). These additional contacts may have contributed to outcomes that were similar between those with and without PTSD.
In contrast, a trend toward earlier dropout for participants with PTSD compared to those without PTSD was observed in the present study. Furthermore, the addition of the contingency management condition was associated with an elimination of the differences seen in dropout between those with and without PTSD. That participants with PTSD had worse outcomes than participants without PTSD in terms of retention in treatment and cocaine positive urines in the absence of contingency management is in keeping with the findings of others demonstrating worse outcomes in patients with higher levels of PTSD (18,19).
The fact that participants with PTSD in the contingency management conditions had greater reductions in cocaine use over time relative to participants with PTSD in the non-contingency condition suggests that negative outcomes due to the presence of PTSD may be diminished by incorporating contingency management procedures that reward positive behavior change into the treatment program. To our knowledge, this is the first report that contingency management procedures using positive reinforcement for drug abstinence may improve upon treatment outcomes for those with PTSD. As such these findings extend findings from earlier studies that demonstrate some efficacy in facilitating illicit drug abstinence (22–30) to facilitating abstinence in the context of a comorbid psychiatric disorder. Thus, contingency management procedures may be especially beneficial in substance abusers with PTSD. Even within the context of a fixed opioid maintenance dose, those with PTSD had a more rapid decline in cocaine positive urines over time in the contingency management group relative to the yoked control. Moreover, within the yoked control low LAAM dose condition cocaine positive urines increased over time in those with PTSD, but decreased over time in those without PTSD. This is consistent with previous findings (32) that lower methadone dose predicts higher rates of cocaine positive urines. However, this earlier study did not specifically examine the impact of PTSD on treatment outcomes. The Hien et al. (18) study also reported that new methadone patients with current PTSD had significantly more ongoing cocaine use, at 3 months than those without PTSD. This study did not specifically examine the interaction between dose and treatment outcome so it is difficult to draw comparisons between these previous studies and the current study which examines both the impact of dose and PTSD as well as contingency management on outcomes.
It could be argued that one of the main limitations of this study relates to baseline characteristics associated with the PTSD diagnosis. Indeed in our study population PTSD diagnosis was associated with being female, having a lifetime alcohol diagnosis and having a depression diagnosis. However, similar analyses including each of these as a co-factor did not show similar patterns of effect observed with PTSD in the contingent versus non-contingent conditions. (These data are not shown due to being beyond the scope of the current paper.) Thus, the co-occurring presence of female sex, lifetime alcohol dependence diagnosis or depression diagnosis does not appear to account for the more rapid decrease in cocaine positive urines over time in those with PTSD under contingency management relative to yoked control.
Nevertheless, there were several limitations of this study. For instance, these data were analyzed retrospectively. Thus, treatment groups had not been stratified on PTSD diagnosis. Be that as it may, there were not significant differences in the proportion of those with and without PTSD across treatment groups. Moreover, retention was relatively low, with only 53% of participants completing the 12-week trial. However, the analytic strategy employed (i.e., HLM) does allow inclusion of data from participants who have missing data, including those who drop out prior to study completion, and uses existing data to estimate a linear trend of changes over time, if any. Nevertheless, we cannot account for potential sample bias due to the relatively small sample size for those with PTSD relative to those without, although the association of PTSD with other subject characteristics did not appear to impact outcome. In addition, given that LAAM maintenance is no longer a common opioid agonist treatment because of the black box warnings related to adverse cardiac events, whether these findings extend to those on methadone or buprenorphine maintenance is unclear. Additional work is necessary in cocaine-dependent methadone and buprenorphine maintained individuals to clarify better both the impact of PTSD diagnosis on outcome and the potential of contingency management to improve outcomes.
Conclusion
These results suggest that dually cocaine and opioid-dependent individuals with PTSD may have worse treatment outcomes than those without PTSD. However, in the presence of contingency management procedures, dually cocaine and opioid dependent patients with and without PTSD have similar treatment outcomes during opioid maintenance.
Acknowledgments
The authors wish to thank Nichole Sanders who assisted in the creation of the figures included in the manuscript.
Footnotes
The authors report no conflicts of interest.
The authors alone are responsible for the content and writing of the paper.
References
- 1.Rounsaville BJ, Weissman MM, Kleber H, Wilber C. Heterogeneity of psychiatric diagnosis in treated opiate addicts. Arch Gen Psychiatry. 1982;39:161–166. doi: 10.1001/archpsyc.1982.04290020027006. [DOI] [PubMed] [Google Scholar]
- 2.Dansky B, Saladin M, Brady K, Kilpatrick D, Resnick H. Prevalence of victimization and posttraumatic stress disorder among women with substance use disorders. International Journal of Addiction. 1995;30:1079–1099. doi: 10.3109/10826089509055829. [DOI] [PubMed] [Google Scholar]
- 3.Fullilove MT, Fullilove RE, Smith M, Winkler K, et al. Violence, trauma, and post-traumatic stress disorder among women drug users. J Trauma Stress. 1993;6:533–543. [Google Scholar]
- 4.Najavits L, Gastfriend D, Barber J, et al. Cocaine dependence with and without PTSD among subjects in the national institute on drug abuse collaborative cocaine treatment study. Am J Psychiatry. 1998;155:214–219. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- 5.McLellan AT, Luborsky L, Woody GE, O’Brien CP, Druley KA. Predicting Response to alcohol and drug abuse treatments: role of psychiatric severity. Arch Gen Psychiatry. 1983;40:620–625. doi: 10.1001/archpsyc.1983.04390010030004. [DOI] [PubMed] [Google Scholar]
- 6.Mertens JR, Weisner CM. Predictors of substance abuse treatment retention among women and men in an HMO. Alcohol Clin Exp Res. 2000;24:1525–1533. [PubMed] [Google Scholar]
- 7.Compton WM, Cottler LB, Jacobs JL, Ben-Abdallah A, Spitznagel EL. The role of psychiatric disorders in predicting drug dependence treatment outcomes. Am J Psychiatry. 2003;160:890–895. doi: 10.1176/appi.ajp.160.5.890. [DOI] [PubMed] [Google Scholar]
- 8.Charney DA, Palacios-Boix J, Negrete JC, Dobkin PL, Gill KJ. Association between concurrent depression and anxiety and six-month outcome of addiction treatment. Psych Serv. 2005;56:927–933. doi: 10.1176/appi.ps.56.8.927. [DOI] [PubMed] [Google Scholar]
- 9.Cacciola JS, Rutherford MJ, Alterman AI, McKay JR, Snider EC. Personality disorders and treatment outcome in methadone maintenance patients. J Nerv Ment Dis. 1996;184(4):234–239. doi: 10.1097/00005053-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cacciola JS, Alterman AI, Rutherford MJ, McKay JR, Mulvaney FD. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 2001;61:271–280. doi: 10.1016/s0376-8716(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 11.Rounsaville BJ, Kosten TR, Weissman MM, Kleber HD. Prognostic significance of psychopathology in treated opiate addicts. Arch. Gen Psychiatry. 1986;43:739–745. doi: 10.1001/archpsyc.1986.01800080025004. [DOI] [PubMed] [Google Scholar]
- 12.King VL, Kidorf MS, Stoller KB, Carter JA, Brooner RK. Influence of antisocial personality subtypes on drug abuse treatment response. J Nerv Ment Dis. 2001;189(9):593–601. doi: 10.1097/00005053-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Magura S, Nwakeze PC, Demsky S. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93(1):51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- 14.Maremmani I, Zolesi O, Aglietti M, et al. Methadone dose and retention during treatment of heroin addicts with Axis I psychiatric comorbidity. J Addict Dis. 2000;19(2):29–41. doi: 10.1300/J069v19n02_03. [DOI] [PubMed] [Google Scholar]
- 15.Milby JB, Sims MK, Khuder S, Schumacher JE, Huggins N. Psychiatric comorbidity: prevalence in methadone maintenance treatment. Am J Drug Alcohol Abuse. 1996;22:95–107. doi: 10.3109/00952999609001647. [DOI] [PubMed] [Google Scholar]
- 16.Gelkopf M, Weizman T, Melamed Y, Adelson M, Bleich A. Does psychiatric comorbidity affect drug abuse treatment outcome? A prospective assessment of drugabuse, treatment tenure and infectious diseases in an Israeli methadone maintenance clinic. Isr J Psychiatry Relat Sci. 2006;43(2):126–136. [PubMed] [Google Scholar]
- 17.Ouimette PC, Brown PJ, Najavits LM. Course and treatment of patients with both substance use and posttraumatic stress disorders. Addict Behav. 1998;23:785–795. doi: 10.1016/s0306-4603(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 18.Hien DA, Nunes E, Levin FR, Fraser D. Posttraumatic stress disorder and short-term outcome in early methadone treatment. J Subst Abuse Treat. 2000;19:31–37. doi: 10.1016/s0740-5472(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 19.Mills KL, Teesson M, Ross J, Darke S. The impact of post-traumatic stress disorder on treatment outcomes for heroin dependence. Addiction. 2007;102:447–454. doi: 10.1111/j.1360-0443.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- 20.Trafton JA, Minkel J, Humphreys K. Opioid substitution treatment reduces substance use equivalently in patients with and without posttraumatic stress disorder. Journal of Studies on Alcohol. 2006;67:228–235. doi: 10.15288/jsa.2006.67.228. [DOI] [PubMed] [Google Scholar]
- 21.Najavits LM, Harned MS, Gallop RJ, et al. Six-month treatment outcomes of cocaine-dependent patients with and without PTSD in a multisite national trial. J Stud Alcohol Drugs. 2007;68(3):353–61. doi: 10.15288/jsad.2007.68.353. [DOI] [PubMed] [Google Scholar]
- 22.Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend. 2007;87:288–296. doi: 10.1016/j.drugalcdep.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend. 1994;34:87–97. doi: 10.1016/0376-8716(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 24.Silverman K, Higgins ST, Brooner RK, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996 May;53(5):409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- 25.Vocci FJ, Montoya ID. Psychological treatment for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263–268. doi: 10.1097/YCO.0b013e32832a3b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic and White methadone maintenance clients. Psychol Addict Behav. 2009;23(1):168–174. doi: 10.1037/a0014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preston KL, Ghitza UE, Scmittner JP, Schroeder JR, Epstein DH. Randomized trial comparing two treatment strategies using prize-based reinforcement in cocaine and opiate users. J Appl Behav Anal. 2008;41(4):551–563. doi: 10.1901/jaba.2008.41-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, Preston KL. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend. 2009;101(1–2):92–100. doi: 10.1016/j.drugalcdep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stitzer ML, Vandrey R. Contingency management: utility in the treatment of drug abuse disorders. Clin Pharmcol Ther. 2008;83(4):644–677. doi: 10.1038/sj.clpt.6100508. [DOI] [PubMed] [Google Scholar]
- 30.Oliveto A, Poling J, Sevarino KA, et al. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine dependent patients. Drug Alcohol Depend. 2005;79:157–165. doi: 10.1016/j.drugalcdep.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- 32.Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre-treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction. 1996;91(8):1197–1209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- 33.Ford JD, Hawke J, Alessi S, Ledgerwood D, Petry N. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther. 2007;45 (10):2417–31. doi: 10.1016/j.brat.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg FE, Badger GJ. Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry. 1993;150:763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- 35.Higgins ST, Delaney DD, Budney AJ, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 36.Kosten TR, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 37.Oliveto A, Feingold A, Shottenfeld R, Jatlow P, Kosten TR. Desipramine in opioid-dependent cocaine abusers maintained on buprenorphine vs. methadone. Arch Gen Psychiatry. 1999;56:812–820. 1999. doi: 10.1001/archpsyc.56.9.812. [DOI] [PubMed] [Google Scholar]
- 38.Davidson JRT, Smith RD. Traumatic experiences in a psychiatric outpatient population. J Trauma Stress. 1990;3:459–474. [Google Scholar]
- 39.Weiss D, Marmar C. The impact of event scale-revised. In: Wilson J, Keane T, editors. Assessing Psychological Trauma and PTSD. New York: Guildford; 1997. pp. 399–411. [Google Scholar]
- 40.McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. J Nerv Ment Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psych Bull. 1987;101:147–158. [Google Scholar]
- 42.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analyses of longitudinal psychiatric data. Arch Gen Psychiatry. 1983;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 43.Hedeker D, Gibbons RD. MIXOR: a computer program for mixed-effects ordinal regression analysis. Comput Methods Programs Biomed. 1996 Mar;49 (2):157–176. doi: 10.1016/0169-2607(96)01720-8. [DOI] [PubMed] [Google Scholar]
- 44.Clark HW, Masson CL, Delucchi KL, Hall SM, Sees KL. Violent traumatic events and drug abuse severity. J Subst Abuse Treat. 2001;20:121–7. doi: 10.1016/s0740-5472(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 45.Villagomez RE, Meyer TJ, Lin MM, Brown LS. Posttraumatic stress disorder among inner city methadone maintenance patients. J Subst Abuse Treat. 1995;12:253–7. doi: 10.1016/0740-5472(95)00025-z. [DOI] [PubMed] [Google Scholar]
- 46.Creamer M, Burgess P, McFarlene C. Post-traumatic stress disorder: findings from The Australian National Survey of Mental Health and Well-being. Psychol Med. 2001;31:1237–47. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- 47.Helzer JE, Robins LN, McEvoy L. Post-traumatic stress disorder in the general population: findings of the Epidemiologic Catchment Area survey. N Engl J Med. 1987;317:1630–1634. doi: 10.1056/NEJM198712243172604. [DOI] [PubMed] [Google Scholar]
- 48.Kessler RC, Sonnega A, Bromet E, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 49.Kessler RC, Zhao S, Katz SJ, et al. Past-year use of outpatient services for psychiatric problems in the National Comorbidity Survey. Am J Psychiatry. 1999;156:115–23. doi: 10.1176/ajp.156.1.115. [DOI] [PubMed] [Google Scholar]