Abstract
Polysaccharides play a key role in enhancing immune function and facilitating cellular communication. Here, we purified Nymphaea rubra Roxb. polysaccharides (NR-PS) by treating them with pullulanase. They were then cultured with immature dendritic cells (DCs) derived from rat bone marrow hematopoietic cells (BMHCs). After treatment with bioactive NR-PS with a degree of polymerization (DP) value of 359.8, we found that the DCs underwent morphological changes indicative of activation. CD80/86 (87.16% ± 8.49%) and MHC class II (52.01% ± 10.11%) expression levels were significantly up-regulated by this treatment compared to the controls (65.45% ± 0.97% and 34.87% ± 1.96%). In parallel, endocytosis was also reduced (167.94% ± 60.59%) after treatment with 25 μg/mL of NR-PS as measured by the medium fluorescence intensity compared to the control (261.67% ± 47.26%). Furthermore, the DCs after treatment with 25 μg/mL NR-PS showed increased IL-12 (102.09 ± 10.16 to 258.78 ± 25.26 pg/mL) and IFN-γ (11.76 ± 0.11 to 15.51 ± 1.66 pg/mL) secretion together with reduced IL-10 secretion (30.75 ± 3.35 to 15.37 ± 2.35 pg/mL), which indicates a TH1 immune response. In conclusion, NR-PS exhibits stimulatory effects on rat DCs and promotes the secretion of TH1 cytokines. Taken together, our studies are the first to show that NR-PS is an immunomodulator affecting the maturation and functioning of DCs.
Keywords: Nymphaea rubra Roxb., pullulanase, polysaccharides, dendritic cells, TH1 immune response, functional foods
1. Introduction
The Nymphaeaceae include a wide range of flowering plants. They are also called water lilies and are distributed in tropical areas around the world, living on the banks of lakes and rivers [1,2]. The plants have a broad range of flower colors, including white, yellow, red and blue [3]. They are a perennial rhizomatous herb made up of six genera, namely Nymphaea, Nuphar, Barclaya, Victoria, Euryale and Ondinea, which include seventy species. Nymphaea is the major and most widely distributed genus and consists of 35 species of water lilies [2]. Many species of Nymphaea in Nepal, India and China are thought to acts as functional drug plants. Previous studies have reported that extracts of various organs from Nymphaea can be used as medicinal plant material. These include extracts of the rhizomes and flowers, which have anti-diabetic and anti-inflammatory effects [4–7], extracts of the rhizomes and seeds, which have the immunomodulatory activity [8], extracts of the stalks, which have an anti-pyretic effect [9], extracts of the leaves, flowers and stamens, which have been shown to have anti-oxidant effects [2,10,11], and extracts of seeds, which have been reported to have hepatoprotective and free radical scavenging effects [2,7,12,13].
Polysaccharides are polymers that consist of long carbohydrate molecules and are composed of monomer units linked together through glycosidic bonds [14]. There are a number of different types of polysaccharides in nature, such as storage polysaccharides (starches and glycogen) [15,16], structural polysaccharides (arabinoxylans, cellulose, chitin and pectins) [15,16], acidic polysaccharides [17] and bacterial polysaccharides (bacterial capsular polysaccharides) [1,14]. The polysaccharides extracted from higher plants, yeasts, fungi, bacteria and algae have been studied and are known to have a significant effect on the immune system. Polysaccharides have long been believed to have many different biological properties and certain polymers have recently been revealed to act as effective immunomodulating agents. However, while quite a few polysaccharide immunomodulators have been identified, most of these studies seem to be relatively unreliable and only a few polysaccharides have been examined in detail. Specifically, one example that has been studied in detail is the β-glucans and this has included the structure, functions and mechanisms of action of this type of polysaccharide.
It is thought that immunomodulating agents show antitumor activity by activating macrophages and natural killer cells via surface receptor binding [18,19]. Treatment with β-glucan polymers has been shown to result in an increased numbers of neutrophils and eosinophils as well as to cause an alteration in macrophage morphology. A previous report has show that mouse spleen cell proliferation is improved and that Sarcoma 180 tumor cell line growth is suppressed after treatment with polysaccharide from Flammulina velutipes, namely β-(1–3)-d-glucan [20]. Polysaccharides have also been shown to promote the innate immune system by interacting with dendritic cells (DCs), which are antigen-presenting cells that provide an important connection between innate and adaptive immunity [21]. Polysaccharides stimulate the maturation of DCs, allowing them to express MHC class II molecules as well as co-stimulatory molecules such as CD80/86 and CD40, all of which are essential for effective antigen presentation [22,23]. It is also known that several cytokines can be released by DCs, including IFN-γ, IL-12, and IL-4, and that these guide the development of T-cells down the path of cell-mediated or humoral immunity depending on the type of antigen captured and the maturation process [24–26].
Nymphaea rubra Roxb., a member of the Nymphaea genus, is grown mainly in India, where it is known as red water lily. The present study is the first to report polysaccharides from the carpel of the flowers of this plant as having immunomodulating activity. In addition, the immune cell-mediated functions of these polysaccharides are also explored. The present findings should help with the development and formulation of beneficial supplementary foods that ought to improve health via an improved innate immune system.
2. Results and Discussion
2.1. Extraction and Analysis of Nymphaea rubra Roxb. Composition
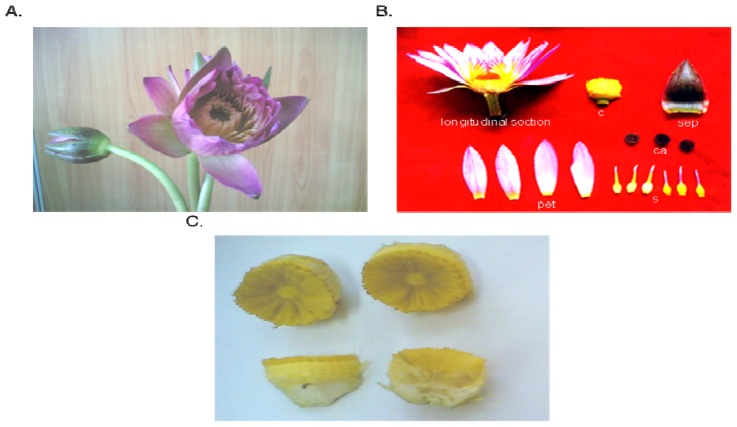
The carpels of Nymphaea rubra Roxb. were collected (Figure 1C) and ground to give a crude extract. We then prepared both crude and hydrolyzed Nymphaea rubra Roxb. extracts and analyzed their composition. As shown in Table 1, the yield of crude extraction was 1.43% (wet weight). As previously reported [27,28], β-1,3-d-glucan has important biological functions and therefore pullulanase was added to the crude extract to hydrolyze any α-1,6 linkages present and to increase the solubility of the crude extract without breaking the β-1,3-glycosidic linkages. The yield of the hydrolyzed sample was 42.00% (dry weight) relative to the crude extract. The total sugar content and reducing sugar content of the hydrolyzed extract were 51.3% and 2.45%, respectively. The total protein content of the crude and hydrolyzed extracts were 7.84% and 5.28% respectively. The DP value of the hydrolyzed extract was found to be at 359.8 kDa by Glass Capillary Viscometer with a degree of branching (DB) value of 0.36. Bohn and BeMiller reported that the most active polysaccharide polymers have a high molecular weight (up to 100 kDa) and a DB between 0.2 and 0.5 [29]. Ohno et al. reported that the polysaccharide fractions prepared from cultured A. blazei containing a highly branched 1,3-β-glucan segment are the most active and have the highest antitumor activity and immunomodulary effects [30]. The Nymphaea rubra Roxb. polysaccharides (NR-PS) preparation used in this study had a DB of 0.36 and was within the bioactive range of polysaccharide polymers.
Figure 1.
(A) The flower of Nymphaea rubra Roxb.; (B) Schematic of a longitudinal section through a quartered portion of the flower, (sep) sepals, (pet) petals, (s) stamen, (ca) carpellary appendage, (c) carpel; (C) The carpel of Nymphaea rubra Roxb.
Table 1.
Composition of the crude extracts and hydrolysate from Nymphaea rubra Roxb.
| Extract | Yield | Total sugar | Reducing sugar | Protein |
|---|---|---|---|---|
| Crude | 1.43 | 55.86 ± 0.80 a | 2.64± 0.34 a | 7.84 ± 0.63 a |
| Hydrolyzed | 42.00 | 51.30 ± 0.98 a | 2.45 ± 0.92 a | 5.28 ± 0.28 b |
Data in the same row with different superscripts are significantly different at p ≤ 0.05.
2.2. Morphological Changes and Activation of BMHC-Derived Immature Dendritic Cells (BHMC-imDCs) after NR-PS Treatment
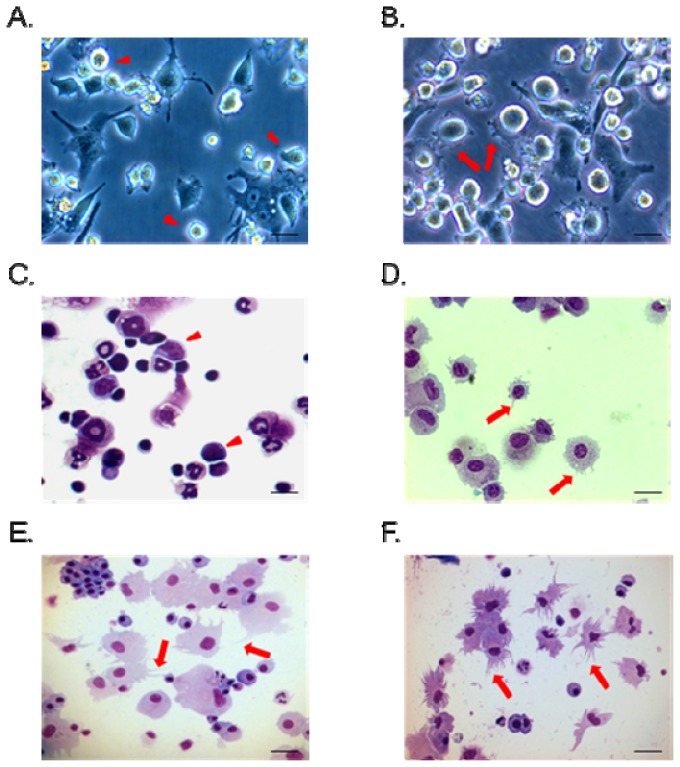
Bone marrow-derived DC (BMDC) maturation is attended by alteration of the cells’ morphological, phenotypic and functional properties [31,32]. According to Inaba et al., it was found that treatment with GM-CSF and IL-4 was able to stimulate rat BMHCs to form dendritic cells and that similar results were obtained in mice [33]. So, we used GM-CSF and IL-4 to stimulate bone marrow hematopoietic cells (BMHCs) to differentiation and become BMHC-imDCs as Talmor et al. reported [34]. In order to measure the bioactivity of NR-PS using BMHC-imDCs, the morphological changes in BMHC-imDCs were monitored after NR-PS treatment. First, using live cell observation, BMHC-imDCs were obtained by treating BMHCs with rGM-CSF and rIL-4 for nine days without NS-PS and these cells were compared with others that had been treated with a test concentration of 50 μg/mL NS-PS at the immature dendritic cell stage on the seventh day and then cultured until the ninth day. The BMHC-imDCs were activated by NR-PS, and this was shown by the presence of dendritic protrusions on the cell surface (Figure 2A,B). Figure 2 shows the cells after staining with Liu’s-stain in order to observe any morphological changes. The BHMC-imDCs can be seen to be round with polymorphic nuclei and small protrusions on the cell surface in the absence of stimulation (Figure 2C,D). However, after treated with 50 μg/mL NR-PS (Figure 2F) or 1 μg/mL LPS (positive control) for 48 h (Figure 2E), the cells are now larger, and the nuclei have become even more polymorphic. Furthermore, the dendritic protrusions on the cell surfaces have become more pronounced and elongated [34]. These findings strongly suggest that NR-PS treatment promotes dendritic cell maturation.
Figure 2.
Morphology of dendritic cells after various treatments. (A) Bone marrow hematopoietic cells-imdendritic cells (BMHC-imDC) were grown from rat bone marrow in completed RPMI media 1640 containing 20 ng/mL recombinant rat GM-CSF and 10 ng/mL IL-4; (B) BMHC-imDC treated with 50 μg/mL NR-PS on day seven (200×); (C) A photomicrogaph of rat bone marrow hematopoietic cells (BMHC) isolated from four to seeks week old SD rats after staining with Liu’s-stain (200×); (D) BMHC-imDCs were generated from BMHC by treatment with 20 ng/mL GM-CSF and 10 ng/mL IL-4 for seven days. Note the presence of the short dendritic processes (arrow) associated with the plasma membrane (Liu’s staining, 200×); (E) Lipopolysaccharide(1 μg/mL) was used to stimulate the maturation of BMHC-imDC into BMHC-mDC. Note the presence of the long dendritic processes (arrow) associated with the plasma membrane (Liu’s staining, 200×); (F) Bone marrow hematopoietic cell-derived immature dendritic cells (BMHC-imDCs) treated with 50 μg/mL NR-PS for 48hr (Liu’s stain, 200×).
2.3. CD11c, MHC Class II and CD80/86 Expression by Dendritic Cells after NR-PS Activation
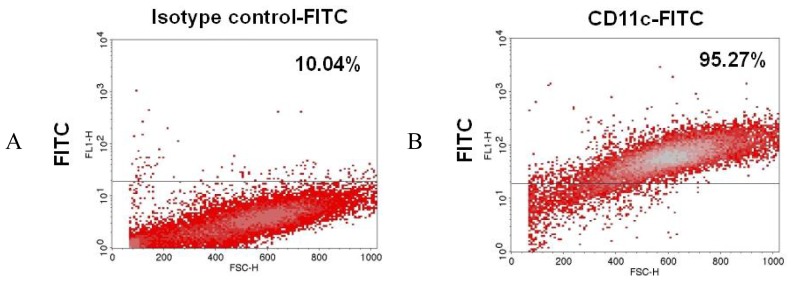
Maturation of DCs is distinguished by a reduction in antigen-processing capacity together with an increase in cell surface expression of MHC class II molecules and the cell markers CD80, CD86, CD11c and CD40 [34,35]. The cell marker CD11c (LFA-1) was used to monitor the rat DCs [36,37]. After cytokine treatment, the BMHCs differentiated into BMHC-imDCs and these cells were shown to be 95.27% CD11c+ by fluorescence activated cell sorting (FACS) analysis (Figure 3). This process was used to set up a BMHC-imDCs assay system that measured the maturation of DCs after stimulation by NR-PS. In order to examine the activation of dendritic cells by NR-PS, the presence of the indicator molecules CD80 and CD86 as well as MHC class II expression were measured. In terms of T cell activation, MHC class II is functional during antigen presentation, while CD80 and CD86 (B7-1 and B7-2, respectively) are important co-stimulatory molecules. We used various concentrations of NR-PS (3.125, 6.25, 12.5, 25, 50 and 100 μg/mL) to treat the BMHC-imDCs for 48 h. The proportion of positive cells and the mean fluorescence intensity of the cells for CD80, CD/86 and MHCclass II were measured (Tables 2 and 3). When compared with the untreated control (65.45% ± 0.97% for positive cell ratio and 24.60% ± 3.19% for mean fluorescence intensity) and positive control treated with 1 μg/mL LPS (85.76% ± 3.06% for positive cell ratio and 49.00% ± 9.18% for mean fluorescence intensity), all NR-PS treatments showed an increase in the positive cell ratio (84.49% ± 10.86%, 84.80% ± 7.94%, 85.68% ± 18.66%, 87.16% ± 8.49%, 84.44% ± 6.35% and 84.56% ± 7.62%) (Table 2). There was a similar increase in mean fluorescence intensity to 47.23% ± 3.72%, 42.73% ± 4.20%, 45.69% ± 3.58%, 48.48% ± 4.26%, 38.03% ± 6.27% and 43.70% ± 7.61% (Table 2). Furthermore, similar results were also obtained for MHC class II expression. The untreated control was 34.87% ± 1.96% for the positive cell ratio and 46.55% ± 4.97% for mean fluorescence intensity, while the LPS positive control gave 73.20% ± 6.16% for positive cell ratio and 57.62% ± 1.87% for mean fluorescence intensity. On treatment with various concentrations of NR-PS, the positive cell ratio was increased to 45.06% ± 8.11%, 44.88% ± 5.11%, 49.18% ± 10.41%, 52.01% ± 10.11%, 49.27% ± 5.03% and 50.27% ± 5.24%, respectively (Table 3), while the mean fluorescence intensity was increased to 51.09% ± 4.73%, 51.94% ± 2.89%, 55.62% ± 8.21%, 60.74% ± 10.06%, 57.30% ± 4.76% and 57.70% ± 2.68%, respectively (Table 3). Thus treating DCs with NR-PS increases the positive cell ratio and the mean fluorescence intensity for all markers compared to the untreated control with the highest activation of the DCs cells being at 25 μg/mL NR-PS treatment (Tables 2 and 3). This confirms clearly that NR-PS is able to enhance the maturation of DCs.
Figure 3.
Analysis of the surface phenotypes of BMHC-imDC using fluorescence activated cell sorting (FACS) scan. On day nine, non-adherent cells were stained with (A) fluorescein isothiocyanate (FITC) conjugated isotype control; (B) conjugated anti-rat CD11c antibody.
Table 2.
Effects of various concentrations of NR-PS on the expression of cell surface antigens on BMHC-imDCs.
| Groups | Percentages of cells with a positive cell surface antigen reaction * | |
|---|---|---|
|
| ||
| CD 80/86 ** | MHC II ** | |
| Control | 65.45 ± 0.97 | 34.87 ± 1.96 |
| LPS (μg/mL) | ||
| 1 | 85.76 ± 3.06 | 73.20 ± 6.16 |
| NR-PS (μg/mL) | ||
| 3.125 | 84.49 ± 10.86 | 45.06 ± 8.11 |
| 6.25 | 84.80 ± 7.94 | 44.88 ± 5.11 |
| 12.5 | 85.68 ± 18.66 | 49.18 ± 10.41 |
| 25 | 87.16 ± 8.49 | 52.01 ± 10.11 |
| 50 | 84.44 ± 6.35 | 49.27 ± 5.03 |
| 100 | 84.56 ± 7.62 | 50.27 ± 5.24 |
Cell surface antigen expression was analyzed by flow cytometry and data in the same column with different superscripts are significantly different with p ≤ 0.05;
Data are expressed as mean ± SD: n = 3.
Table 3.
Comparison of the mean fluorescence intensity of dendritic cells across various concentrations of NR-PS.
| Groups | Mean fluorescence intensity of dendritic cells * | |
|---|---|---|
|
| ||
| CD 80/86 ** | MHC II ** | |
| Control | 24.60 ± 3.19 | 46.55 ± 4.97 |
| LPS (μg/mL) | ||
| 1 | 49.00 ± 9.18 | 57.62 ± 1.87 |
| NR-PS (μg/mL) | ||
| 3.125 | 47.23 ± 3.72 | 51.09 ± 4.73 |
| 6.25 | 42.73 ± 4.20 | 51.94 ± 2.89 |
| 12.5 | 45.69 ± 3.58 | 55.62 ± 8.21 |
| 25 | 48.48 ± 4.26 | 60.74 ± 10.06 |
| 50 | 38.03 ± 6.27 | 57.30 ± 4.76 |
| 100 | 43.70 ± 7.61 | 57.70 ± 2.68 |
Cell surface antigen expression was analyzed by flow cytometry and data in the same column with different superscripts are significantly different with p ≤ 0.05;
Data are expressed as mean ± SD: n = 3.
2.4. The Endocytosis of Dendritic Cells Was Reduced after NR-PS Treatment
The expression of the mannose receptor is the determinant for the receptor-mediated endocytosis of dextran, and in mammals this receptor is expressed at high levels on immature DCs and is low or absent on mature DCs [38,39]. In the mammalian immune system, dendritic cells act as antigen presentation cells and function by endocytosing, processing and presenting antigens in order to activate the adaptive immune system [40,41]. As previously reported, immature DCs show higher levels of endocytosis and processing as mature DCs. In contrast, mature DCs show lower endocytosis, but have higher antigen-presenting activity [42]. Various concentrations of NR-PS were added in order to observe their effect on the level of endocytosis of BMHC-imDCs; this was done over 48 h using a FITC-dextran uptake assay (Table 4). The results showed that the mean fluorescence intensity was reduced to 167.94% after treatment with 25 μg/mL NR-PS compared to the control level of 261.67%. This reduction in endocytotic activity, as shown by the reduced dextran uptake, strongly suggests that NS-PS treatment leads to the activation of DCs.
Table 4.
Effect of various concentrations of NR-PS on the pinocytosis activity of BMHC-imDC.
| Groups | Uptake of FITC-dextran * |
|---|---|
|
| |
| Mean fluorescence intensity ** | |
| Control 37 °C | 261.67 ± 47.26 |
| LPS (μg/mL) | |
| 1 | 121.60 ± 31.05 |
| NR-PS (μg/mL) | |
| 3.125 | 227.87 ± 35.88 |
| 6.25 | 192.47 ± 52.47 |
| 12.5 | 176.50 ± 28.52 |
| 25 | 167.94 ± 60.59 |
| 50 | 199.41 ± 24.52 |
| 100 | 211.49 ± 33.65 |
Pinocytosis activity was measured by the uptake of FITC-dextran (40,000 dalton; Sigma) and analyzed by FACScan flow cytometry. The data in the same column with different superscripts are significantly different at p ≤ 0.05;
Data are expressed as mean ± SD: n = 3.
2.5. NR-PS Treatment of Dendritic Cells Results in Increased IL-12/IFN-γ Secretion and Reduced IL-10 Secretion
Cytokines are secreted by DCs when they activate native CD4 T-cells, and these determine the developmental path of the CD4 T-cells. IL-12 and IFN-γ promote the development of CD4 T-cells into TH1 cells, while IL-10 favors development into TH2 cells. As shown in Table 5, both IL-12 and IFN-γ levels were elevated after treatment with various concentrations of NR-PS. When compared to the untreated group, the secretion of IL-12 and IFN-γ was significantly increased after treatment with 25 μg/mL NR-PS. IL-12 is an important cytokine involved in production of IFN-γ by T cells and NK cells and plays an important role in the differentiation of the TH1 cell population [18]. Moreover, the level of IL-10 secreted by the NP-PS treated cells decreased as the dose increased. When compared to the untreated control (30.75 ± 3.35 pg/mL), treatment with 25 μg/mL and 50 μg/mL NR-PS resulted in a reduction in IL-10 production to 15.37 ± 2.35 pg/mL and 14.83 ± 3.8 pg/mL (Table 5). These results were better than those reported previously by us for the functional food okra [27]. In the human immunosystem, DCs can modulate the TH1–TH2 balance according to their microbial interactions [43]. DCs accomplish this, at least in part, through differences in the production of IL-10 and IL-12, since IL-10 is concerned with initiating TH2 responses while IL-12 potently induces IFN-γ producing Th1 cells [44,45]. Taken together, the combined findings for DCs showing increased IL-12/IFN-γ and reduced IL-10 production indicates that NR-PS leads to a TH1 response.
Table 5.
Effect of various concentrations of NR-PS on IL-12, IL-10 and IFN-γ production by BMHC-im DCs.
| Groups | Level of cytokine * (pg/mL) | ||
|---|---|---|---|
|
| |||
| IL-12 ** | IL-10 ** | IFN-γ ** | |
| Control | 102.09 ± 10.16 | 30.75 ± 3.35 | 11.76 ± 0.11 |
| NR-PS (μg/mL) | |||
| 3.125 | 158.59 ± 37.85 | 23.20 ± 8.47 | 15.40 ± 1.52 |
| 6.25 | 221.40 ± 11.90 | 22.48 ± 5.44 | 15.33 ± 2.62 |
| 12.5 | 220.65 ± 17.09 | 18.16 ± 4.17 | 15.96 ± 2.66 |
| 25 | 258.78 ± 25.26 | 15.37 ± 2.35 | 15.51 ± 1.66 |
| 50 | 254.62 ± 36.22 | 14.83 ± 3.80 | 15.01 ± 1.47 |
| 100 | 194.48 ± 27.61 | 21.22 ± 2.11 | 15.67 ± 1.24 |
IL-12, IL10 and IFN-γ levels in the supernatants by ELISA. Data in the same row with different superscripts are significantly different at p ≤ 0.05;
Data are expressed as mean ± SD: n = 3.
3. Experimental Section
3.1. Rats
Four-week-old male CD (SD) IGS rats with initial weights between 75 g and 125 g were purchased from BioLasco (Taipei, Taiwan). In the experiments, the rats were fed a basal diet for more than a week with free access to water and feed to allow adjustment to their environment. The animal rooms were kept at 25 °C and 60% humidity with a 12 h light and dark cycle (8 am to 8 pm). All animal treatments were approved by the Animal Ethics Committee at the National Pingtung University of Science and Technology, Taiwan.
3.2. Preparation of Crude and Hydrolyzed Nymphaea rubra Roxb. Extract
Nymphaea rubra Roxb. (Figure 1) was purchased from the market. To produce the crude extract, the carpels of the flowers were cleaned, diced and immersed in 95 °C ddH2O for 4 h before centrifugation at 10,000× g for 10 min at 4 °C. Then 95% alcohol was used to precipitate the polysaccharides in the crude extract, and these were collected by centrifugation at 10,000× g for 10 min at 4 °C. An equal volume of acetone was added for decolorization before a second centrifugation at 10,000× g for 10 min at 4 °C. The residual acetone was allowed to evaporate in a hood, and the resulting crude extract was then lyophilized, ground and stored at −20 °C until use.
In order to prepare hydrolyzed Nymphaea rubra Roxb. extract, 5 mg of the crude extract was dissolved in 25 mL of 0.1 M sodium acetate buffer (pH 5.2), then 5 U of pullulanase (Sigma, USA) was added and the mixture incubated for 6 h at 45 °C. The reaction mixture was then cooled for 5 min on ice before being terminated with 20% trichloroacetic acid (TCA). The supernatant was collected by centrifugation at 10,000× g for 10 min at 4 °C. Ethanol to a final concentration of 80% was then added to allow precipitation, and the mixture was then incubated at 4 °C for 24 h. The precipitated polysaccharides were centrifuged at 10,000× g for 10 min at 4 °C, and then washed three times with 95% alcohol before being lyophilized, ground and stored at −20 °C until use.
3.3. Measurement of Sugar, Protein and Polymerization Levels of the Nymphaea rubra Roxb. Extract
Measurement of the total sugar content of the extract was carried out by a colorimetric method that has been previously described [46]. The total reducing sugar content was measured by the 3,5-dinitro-salicyclic acid method as previously described [47]. The absorbance of the sample solution was read at 540 nm. Glucose was used as a standard for both the total sugar content and reducing sugar content analyses. The protein concentration was determined by the Bradford method using a protein assay kit (Bio-Rad, USA) according the manufacturer’s instructions. Polymerization was measured by Glass Capillary Viscometer as previous reported [48].
3.4. Isolation of Rat BMHCs
We used the femur bones of sacrificed rats (between six and eight weeks old) to extract bone marrow using RPMI 1640 containing 5% FBS and 1% penicillin/streptomycin (P/S). The cells were collected and resuspended in RPMI 1640 complete medium (90% RPMI 1640 medium, 10% FBS, 1% penicillin-streptomycin, 2000 U GM-CSF, 10 U IL-4). Then 1 × 106 cells/mL were seeded into a 75 cm2 culture flask and incubated at 37 °C with 5% CO2, and the medium was changed on the third and sixth day of culture.
Stimulation of the BMHC-imDCs involved treatment with NR-PS. On the sixth day of culture, the cells were collected by centrifugation, resuspended in RPMI 1640 complete medium, and seeded into 24-well flat bottom culture plates (1 × 106 cells/well). The BMHC-imDCs were found to have differentiated after 24 h of incubation. On the seventh day the cells were treated with various concentrations of hydrolyzed NR-PS (0, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL) or 1 μg/mL lipopolysaccharide (LPS) (as a positive control) for 48 h at 37 °C with 5% CO2. Cell morphology, surface antigen presentation, cytokine secretion, and phagocytosis were then analyzed.
3.5. Observation of the Cell Morphology of the BMHC-imDCs
The morphology of the BMHCs was visualized using Liu’s stain, which comprises two staining solutions, namely Liu’s A stain (0.5 g/L methylene blue and 1.7 g/L eosin yellow in methyl alcohol) to visualize the cytoplasm, and Liu’s B stain (1.3 g/L azure and 1.4 g/L methylene blue) to visualize the nucleus. The cells were placed in a cytospin (Thermo Scientific, UK), centrifuged at 350× g for 10 min and stained with Liu’s reagent. The cell morphology was observed using a microscope.
3.6. Measurement of the BMHC-imDC Surface Markers by FACS Analysis
Cells (2 × 105 cells/mL) were collected, washed with FACS buffer (1% BSA and 0.1% sodium acid in PBS) and resuspended in 100 μL of FACS buffer. Fluorescent monoclonal antibodies (2 μL) against CD11c, MHC class II and CD 80/86 surface antigens (eBioscience, USA) were added to the cells, and the mixture incubated in the dark for 30 min. Cells were then washed with FACS buffer and resuspended in 0.5 mL of 3.8% paraformaldehyde (in PBS), which was followed by analysis on a FACScan flow cytometer (Becton Dickinson, USA).
3.7. Assessing the Endocytotic Activity of BMHC-imDCs
In order to measure the endocytotic activity of the BMHC-imDCs, 4 μL of 1 mg/mL FITC-dextran (Sigma) was added to 96 μL of 1 × 106 cells (in PBS buffer) that had been washed three times with PBS buffer. The cells were divided into two tubes. One tube was incubated at 4 °C for 1 h, and the other tube was incubated at 37 °C for 1 h. After the reaction, all cells were washed three times with cold PBS buffer (4 °C) and resuspended in 0.5 mL FACS buffer for analysis. The uptake of FITC-dextran was analyzed cytofluorimetrically using a FACScan flow cytometer (Becton Dickinson, USA).
3.8. Enzyme-Linked Immunosorbent Assay (ELISA) for Cytokine Detection
We used various concentrations of NR-PS (0, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL) to stimulate the BMHC-imDCs, and after nine days the supernatants were collected by centrifugation at 400× g for 8 min at 4 °C and were then stored at −80 °C. An IL-12 p40 ELISA kit was used to determine IL-12 expression according to the manufacturer’s instructions (Biosource, USA), while the amount of IFN-γ and IL-10 was determined using a cytokine assay kit (Bender, Austria) according to the manufacturers’ instructions.
3.9. Statistical Analysis
The data were analyzed using SAS software (SAS institute, USA) as previous described [27]. One-way analysis of variance (one-way ANOVA) and Duncan’s test were used to determine the statistical significance between groups. Differences were considered statistically significant at p ≤ 0.05.
4. Conclusion
This is the first study to examine the immunopotentiating effect of Nymphaea rubra Roxb. polysaccharides. This study demonstrates that NR-PS treatment promotes murine DC maturation as shown by the presence of morphological changes that are consistent with maturation, namely a reduction in endocytosis and an increase in the expression of surface molecules important for antigen presentation. Furthermore, NR-PS seems to induce a TH1 response. Our findings should help the development of new products that have health food applications, including functional foods that are able to immunomodulate. However, the production of Nymphaea rubra Roxb. extracts still needs to be improved in order to increase the production of β-glucan after pullulanase treatment. Further research is also needed to determine the best procedure for preparing functional foods containing Nymphaea rubra Roxb. So that they can be eaten directly and thus improve human immunity.
Acknowledgement
The authors would like to thank all their colleagues and students who contributed to this study. This research was supported by grants from the National Science Council, Taiwan (Grant nos. NSC- 93-2745-B-020-001 and NSC101-2321-B-039-007).
Abbreviations
- NR-PS
Nymphaea rubra Roxb. Polysaccharides
- DCs
dendritic cells
- BMHCs
bone marrow hematopoietic cells
- BHMC-imDCs
BMHC-derived immature dendritic cells
- DP
degree of polymerization
- TCA
trichloroacetic acid
- LPS
lipopolysaccharides
- DB
degree of branching
References
- 1.Agnihotri V.K., Elsohly H.N., Khan S.I., Smillie T.J., Khan I.A., Walker L.A. Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry. 2008;69:2061–2066. doi: 10.1016/j.phytochem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J., Liu T., Ma L., Yan M., Gu Z., Huang Y., Xu F., Zhao Y. Antioxidant and Preventive Effects of Extract from Nymphaea candida Flower on In vitro Immunological Liver Injury of Rat Primary Hepatocyte Cultures. Evid. Based Complement. Alternat. Med. 2009 doi: 10.1093/ecam/nep003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckett K.A. The Concise Encyclopedia of Garden Plants. Orbis Publishing; London, UK: 1984. p. 268. [Google Scholar]
- 4.Rajagopal K., Sasikala K. Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats. Singapore Med. J. 2008;49:137–141. [PubMed] [Google Scholar]
- 5.Mukherjee P.K., Saha K., Das J., Pal M., Saha B.P. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med. 1997;63:367–369. doi: 10.1055/s-2006-957705. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee P.K., Saha K., Pal M., Saha B.P. Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. J. Ethnopharmacol. 1997;58:207–213. doi: 10.1016/s0378-8741(97)00107-4. [DOI] [PubMed] [Google Scholar]
- 7.Raja M.K., Sethiya N.K., Mishra S.H. A comprehensive review on Nymphaea stellata: A traditionally used bitter. J. Adv. Pharm. Technol. Res. 2010;1:311–319. doi: 10.4103/0110-5558.72424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee D., Khatua T.N., Venkatesh P., Saha B.P., Mukherjee P.K. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. J. Ethnopharmacol. 2010;128:490–494. doi: 10.1016/j.jep.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Sinha S., Mukherjee P.K., Mukherjee K., Pal M., Mandal S.C., Saha B.P. Evaluation of antipyretic potential of Nelumbo nucifera stalk extract. Phytother. Res. 2000;14:272–274. doi: 10.1002/1099-1573(200006)14:4<272::aid-ptr556>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Jung H.A., Kim J.E., Chung H.Y., Choi J.S. Antioxidant principles of Nelumbo nucifera stamens. Arch. Pharm. Res. 2003;26:279–285. doi: 10.1007/BF02976956. [DOI] [PubMed] [Google Scholar]
- 11.Wu M.J., Wang L., Weng C.Y., Yen J.H. Antioxidant activity of methanol extract of the lotus leaf (Nelumbo nucifera Gertn.) Am. J. Chin. Med. 2003;31:687–698. doi: 10.1142/S0192415X03001429. [DOI] [PubMed] [Google Scholar]
- 12.Sohn D.H., Kim Y.C., Oh S.H., Park E.J., Li X., Lee B.H. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine. 2003;10:165–169. doi: 10.1078/094471103321659889. [DOI] [PubMed] [Google Scholar]
- 13.Bhandarkar M.R., Khan A. Antihepatotoxic effect of Nymphaea stellata willd., against carbon tetrachloride-induced hepatic damage in albino rats. J. Ethnopharmacol. 2004;91:61–64. doi: 10.1016/j.jep.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Varki A. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: p. 2009. [PubMed] [Google Scholar]
- 15.Sheeler P., Bianchi D.E., Sheeler P.C.B. Cell and Molecular Biology. 3rd ed. Wiley; New York, NY, USA: p. 1987. [Google Scholar]
- 16.Taylor D., Soper R., Green N.P.O., Stout W. Biological Science. 3rd ed. Cambridge University Press; Cambridge, UK: p. 1997. [Google Scholar]
- 17.Fang X., Jiang B., Wang X. Purification and partial characterization of an acidic polysaccharide with complement fixing ability from the stems of Avicennia marina. J. Biochem. Mol. Biol. 2006;39:546–555. doi: 10.5483/bmbrep.2006.39.5.546. [DOI] [PubMed] [Google Scholar]
- 18.Tzianabos A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000;13:523–533. doi: 10.1128/cmr.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S.P., Kang M.Y., Kim J.H., Nam S.H., Friedman M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J. Agric. Food Chem. 2011;59:9861–9869. doi: 10.1021/jf201944n. [DOI] [PubMed] [Google Scholar]
- 20.Leung M.Y., Fung K.P., Choy Y.M. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology. 1997;35:255–263. doi: 10.1016/s0162-3109(96)00157-9. [DOI] [PubMed] [Google Scholar]
- 21.Zou Y., Meng J., Chen W., Liu J., Li X., Li W., Lu C., Shan F. Modulation of phenotypic and functional maturation of murine dendritic cells (DCs) by purified Achyranthes bidentata polysaccharide (ABP) Int. Immunopharmacol. 2011;11:1103–1108. doi: 10.1016/j.intimp.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Chan W.K., Cheung C.C., Law H.K., Lau Y.L., Chan G.C. Ganoderma lucidum polysaccharides can induce human monocytic leukemia cells into dendritic cells with immuno-stimulatory function. J. Hematol. Oncol. 2008;1:9. doi: 10.1186/1756-8722-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bimczok D., Wrenger J., Schirrmann T., Rothkotter H.J., Wray V., Rau U. Short chain regioselectively hydrolyzed scleroglucans induce maturation of porcine dendritic cells. Appl. Microbiol. Biotechnol. 2009;82:321–331. doi: 10.1007/s00253-008-1813-7. [DOI] [PubMed] [Google Scholar]
- 24.Zanoni I., Ostuni R., Capuano G., Collini M., Caccia M., Ronchi A.E., Rocchetti M., Mingozzi F., Foti M., Chirico G., et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 25.Granucci F., Zanoni I. The dendritic cell life cycle. Cell Cycle. 2009;8:3816–3821. doi: 10.4161/cc.8.23.9998. [DOI] [PubMed] [Google Scholar]
- 26.Lanzavecchia A. Dendritic cell maturation and generation of immune responses. Haematologica. 1999;84:23–25. [PubMed] [Google Scholar]
- 27.Sheu S., Lai M. Composition analysis and immuno-modulatory effect of okra (Abelmoschus esculentus L.) extract. Food Chemistry. 2012;134:1906–1911. doi: 10.1016/j.foodchem.2012.03.110. [DOI] [PubMed] [Google Scholar]
- 28.Dong Q., Yao J., Yang X.T., Fang J.N. Structural characterization of a water-soluble β-d-glucan from fruiting bodies of Agaricus blazei Murr. Carbohydr. Res. 2002;337:1417–1421. doi: 10.1016/s0008-6215(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 29.Bohn J.A., BeMiller J.N. (1→3)-β-d-glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohyd. Polym. 1995;28:3–14. [Google Scholar]
- 30.Ohno N., Furukawa M., Miura N.N., Adachi Y., Moto M., Yadomae T. Antitumor β-Glucan from the Cultured Fruit Body of Agaricus blazei. Biol. Pharm. Bull. 2001;24:820–828. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 31.Fina D., Sarra M., Fantini M.C., Rizzo A., Caruso R., Caprioli F., Stolfi C., Cardolini I., Dottori M., Boirivant M., et al. Regulation of gut inflammation and Th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Istrate C., Douagi I., Charpilienne A., McInerney G.M., Hidmark A., Johansen K., Larsson M., Magnusson K.E., Poncet D., Svensson L., et al. Bone marrow dendritic cells internalize live RF-81 bovine rotavirus and rotavirus-like particles (RF 2/6-GFP-VLP and RF 8*2/6/7-VLP) but are only activated by live bovine rotavirus. Scand. J. Immunol. 2007;65:494–502. doi: 10.1111/j.1365-3083.2007.01907.x. [DOI] [PubMed] [Google Scholar]
- 33.Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talmor M., Mirza A., Turley S., Mellman I., Hoffman L.A., Steinman R.M. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur. J. Immunol. 1998;28:811–817. doi: 10.1002/(SICI)1521-4141(199803)28:03<811::AID-IMMU811>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Acosta C., Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J. Neurosci. Res. 2008;86:1077–1086. doi: 10.1002/jnr.21565. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L., Kaneko T., Okiji T., Takagi M., Suda H. Immunoelectron microscopic analysis of CD11c-positive dendritic cells in the periapical region of the periodontal ligament of rat molars. J. Endod. 2006;32:1164–1167. doi: 10.1016/j.joen.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi T., Ohno N., Ohno T. Maturation of dendritic cells induced by Candida β-d-glucan. Int. Immunopharmacol. 2002;2:1503–1508. doi: 10.1016/s1567-5769(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 38.Kato M., Neil T.K., Fearnley D.B., McLellan A.D., Vuckovic S., Hart D.N. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int. Immunol. 2000;12:1511–1519. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 39.Menges M., Baumeister T., Rossner S., Stoitzner P., Romani N., Gessner A., Lutz M.B. IL-4 supports the generation of a dendritic cell subset from murine bone marrow with altered endocytosis capacity. J. Leukoc. Biol. 2005;77:535–543. doi: 10.1189/jlb.0804473. [DOI] [PubMed] [Google Scholar]
- 40.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 41.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 42.Keller R. Dendritic cells: Their significance in health and disease. Immunol. Lett. 2001;78:113–122. doi: 10.1016/s0165-2478(01)00247-4. [DOI] [PubMed] [Google Scholar]
- 43.Moser M., Murphy K.M. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 44.Stumbles P.A., Thomas J.A., Pimm C.L., Lee P.T., Venaille T.J., Proksch S., Holt P.G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trinchieri G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 46.Dubois M., Gilles K., Hamilton J.K., Rebers P.A., Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 47.Miller G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- 48.Shlieout G., Arnold K., Muller G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS PharmSciTech. 2002;3:E11. doi: 10.1208/pt030211. [DOI] [PMC free article] [PubMed] [Google Scholar]