Abstract
The energies of three- and five-chain antiparallel and parallel beta-sheets were minimized. Each chain consisted of six L-valine residues with CH3CO and NHCH3 end groups; the chains were considered to be equivalent, but all dihedral angles of a given chain were allowed to vary independently during energy minimization. The minimum-energy structures had a considerable right-handed twist, as observed in globular proteins. This right-handed twist is due primarily to intrachain nonbonded interactions. Such interactions between the C gamma 1H3 group of the ith residue and the C gamma 2H3 group of the (i + 2)th residue of the same chain favor a twist of either handedness over the flat structure. However, many small intrastrand pair-wise interatomic interactions involving the C gamma 1H3 and C gamma 2H3 groups, especially the interactions of these groups with the O and amide H atoms of the neighboring peptide groups, make the right-handed twisted structure energetically more favorable than the left-handed one. The intrastrand side-chain torsional energy plays a small additional role in favoring the right-twisted structure over both the flat and the left-twisted structures. The interstrand interactions favor flat structures, but they are not strong enough to overcome the intrastrand interactions that favor the twisted structure; they only decrease somewhat the extent of the right-handed twist of the beta-sheets.
Full text
PDF

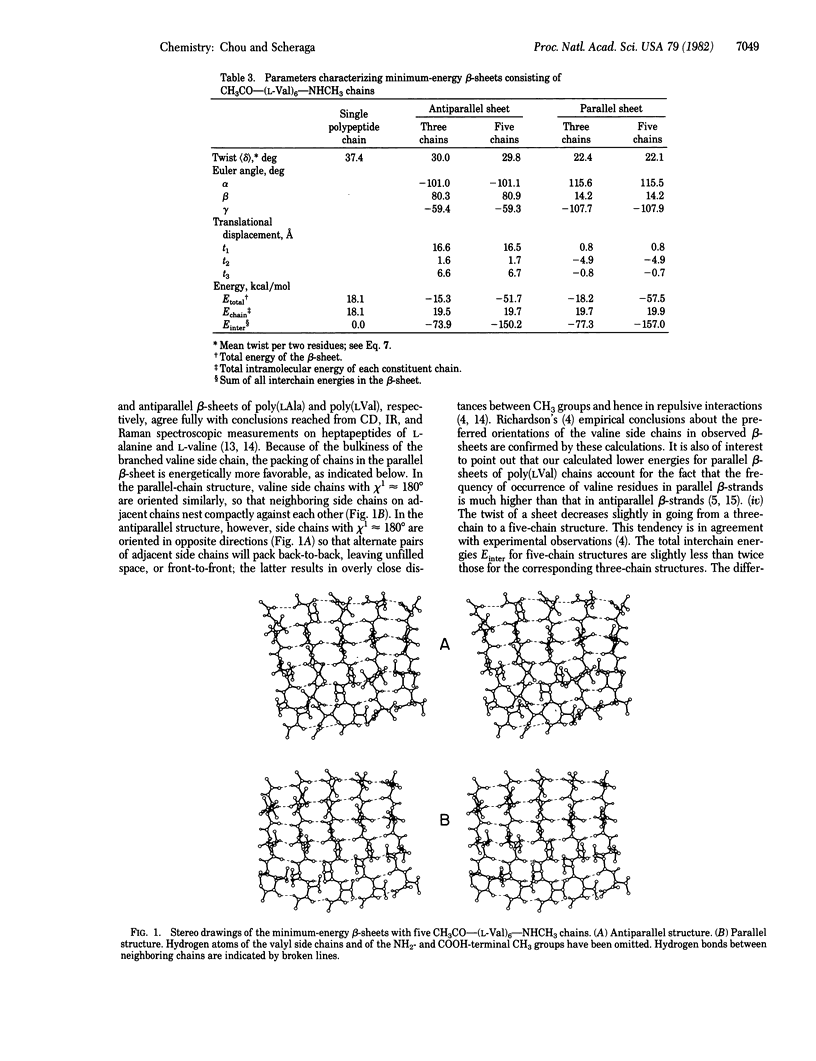

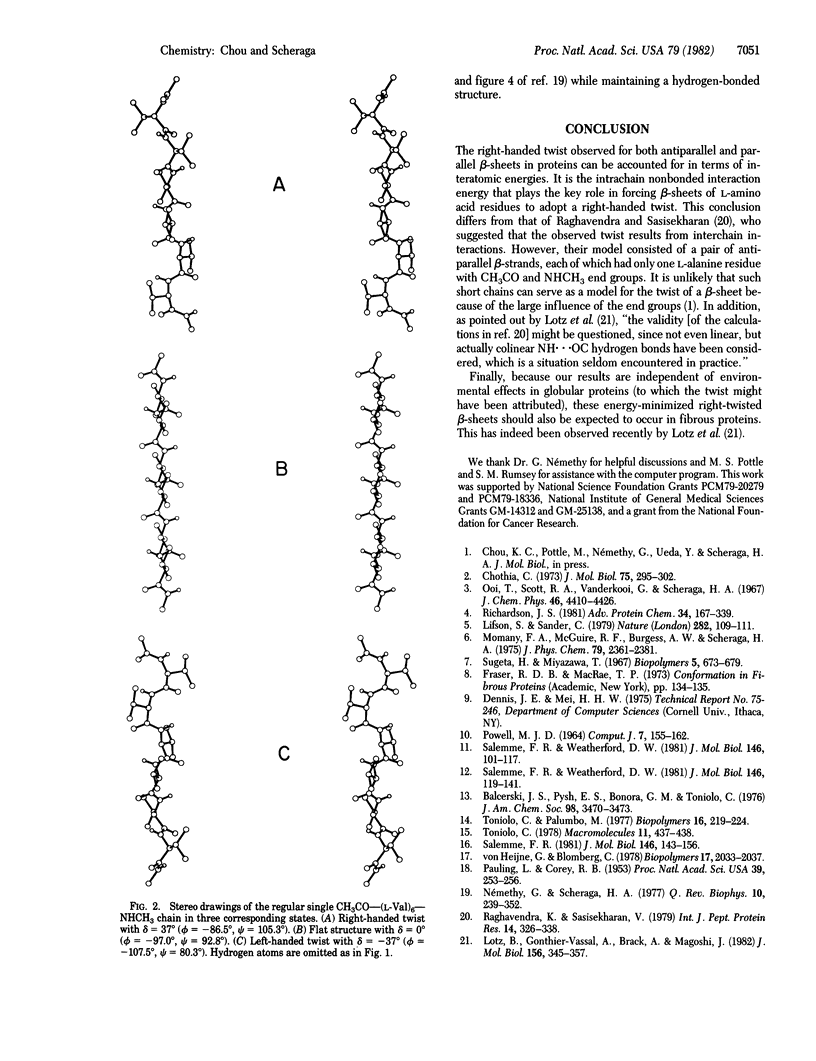
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcerski J. S., Pysh E. S., Bonora G. M., Toniolo C. Vacuum ultraviolet circular dichroism of beta-forming alkyl oligopeptides. J Am Chem Soc. 1976 Jun 9;98(12):3470–3473. doi: 10.1021/ja00428a013. [DOI] [PubMed] [Google Scholar]
- Chothia C. Conformation of twisted beta-pleated sheets in proteins. J Mol Biol. 1973 Apr 5;75(2):295–302. doi: 10.1016/0022-2836(73)90022-3. [DOI] [PubMed] [Google Scholar]
- Lifson S., Sander C. Antiparallel and parallel beta-strands differ in amino acid residue preferences. Nature. 1979 Nov 1;282(5734):109–111. doi: 10.1038/282109a0. [DOI] [PubMed] [Google Scholar]
- Lotz B., Gonthier-Vassal A., Brack A., Magoshi J. Twisted single crystals of Bombyx mori silk fibroin and related model polypeptides with beta structure. A correlation with the twist of the beta sheets in globular proteins. J Mol Biol. 1982 Apr 5;156(2):345–357. doi: 10.1016/0022-2836(82)90333-3. [DOI] [PubMed] [Google Scholar]
- Némethy G., Scheraga H. A. Protein folding. Q Rev Biophys. 1977 Aug;10(3):239–252. doi: 10.1017/s0033583500002936. [DOI] [PubMed] [Google Scholar]
- Ooi T., Scott R. A., Vanderkooi G., Scheraga H. A. Conformation of analysis of macromolecules. IV. Helical structures of poly-L-alanine, poly-L-valine, poly-beta-methyl-L-aspartate, poly-gamma-methyl-L-glutamate, and poly-L-tyrosine. J Chem Phys. 1967 Jun 1;46(11):4410–4426. doi: 10.1063/1.1840561. [DOI] [PubMed] [Google Scholar]
- Pauling L., Corey R. B. Two Rippled-Sheet Configurations of Polypeptide Chains, and a Note about the Pleated Sheets. Proc Natl Acad Sci U S A. 1953 Apr;39(4):253–256. doi: 10.1073/pnas.39.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra K., Sasisekharan V. Conformational analysis of the right-hand twisted antiparallel beta-structure. Int J Pept Protein Res. 1979 Oct;14(4):326–338. doi: 10.1111/j.1399-3011.1979.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. Conformational and geometrical properties of beta-sheets in proteins. III. Isotropically stressed configurations. J Mol Biol. 1981 Feb 15;146(1):143–156. doi: 10.1016/0022-2836(81)90370-3. [DOI] [PubMed] [Google Scholar]
- Salemme F. R., Weatherford D. W. Conformational and geometrical properties of beta-sheets in proteins. I. Parallel beta-sheets. J Mol Biol. 1981 Feb 15;146(1):101–117. doi: 10.1016/0022-2836(81)90368-5. [DOI] [PubMed] [Google Scholar]
- Salemme F. R., Weatherford D. W. Conformational and geometrical properties of beta-sheets in proteins. II. Antiparallel and mixed beta-sheets. J Mol Biol. 1981 Feb 15;146(1):119–141. doi: 10.1016/0022-2836(81)90369-7. [DOI] [PubMed] [Google Scholar]
- Toniolo C., Palumbo M. Solid-state infrared absorption spectra and chain arrangement in some synthetic homooligopeptides in the intermolecularly hydrogen-bonded pleated-sheet beta-conformation. Biopolymers. 1977 Jan;16(1):219–224. doi: 10.1002/bip.1977.360160116. [DOI] [PubMed] [Google Scholar]


