Abstract
Background
Dysfunctional immune responses characterize sarcoidosis, but the status of cathelicidin, a potent immunoregulatory and antimicrobial molecule, has not been established in clinical disease activity.
Methods
Alveolar macrophage cathelicidin expression was determined in biopsy-proven sarcoidosis patients classified clinically as ‘severe’ (requiring systemic treatment) or ‘non-severe’ (never requiring treatment). Bronchoalveolar lavage (BAL) cells from sarcoidosis patients and healthy controls were analyzed for mRNA expression of cathelicidin, vitamin D receptor (VDR) and the VDR coactivator steroid receptor coactivator-3 (SRC3) by quantitative PCR. Cathelicidin-derived peptide LL-37 was determined by immunocytochemistry. Serum calcidiol (25-hydroxyvitamin D2; vitD2) and calcitriol (1,25-dihydroxyvitamin D3; vitD3) were quantified.
Results
The results indicated reduced BAL cell expression of cathelicidin and SRC3 in severe but not non-severe sarcoidosis compared to controls. Serum levels of biologically active vitD3 in both severe and non-severe patients were within the control range even though vitD2 levels in both groups were below the recommended level (30 ng/ml). Sarcoidosis and control alveolar macrophages were studied in vitro to determine cathelicidin responses to vitD3 and tumor necrosis factor-α (TNFα), a vitD3 antagonist elevated in active sarcoidosis. Alveolar macrophage cathelicidin was stimulated by vitD3 but repressed by TNFα, which also repressed SRC3.
Conclusions
These findings suggest that TNFα-mediated repression of SRC3 contributes to alveolar macrophage cathelicidin deficiency in severe sarcoidosis despite healthy vitD3 levels. Deficiency of cathelicidin, a multifunctional regulator of immune cells and proinflammatory cytokines, may impede resolution of inflammation in the lungs of patients with severe sarcoidosis.
Key Words: Cathelicidin, Vitamin D, Cytokines, Steroid receptor coactivators, Alveolar macrophage, Sarcoidosis
Introduction
Sarcoidosis is a relatively common systemic granulomatous disease producing significant morbidity. However, its etiology remains elusive and is speculated to be the result of complex interactions between environmental, genetic and epigenetic factors [1,2]. The pathophysiology of sarcoidosis is characterized by chronic T helper 1-type inflammatory responses leading to granulomas and variable degrees of fibrosis in several organs, especially the lung. The cytokine milieu in sarcoidosis lung is extremely complex and includes overexpression of both interferon-γ (IFN-γ) and tumor necrosis factor-α (TNFα) in bronchoalveolar lavage (BAL)-derived cells and fluids from patients with sarcoidosis. The clinical course is highly variable, ranging from acute self-limited disease to indolent progression terminating in significant morbidity and mortality. The factors responsible for progressive disease or, conversely, restoration of pulmonary homeostasis are unknown.
Cathelicidin represents a component of the innate immune system triggered by pattern recognition receptors [3]. A cathelicidin family of cationic host defense peptides is found in mammals, but the only human cathelicidin is the 37-amino acid peptide LL-37 [4,5]. LL-37 is stored within the cell as a propeptide, hCAP18, which is processed to LL-37 by cleavage with proteinase 3 [5]. Human cathelicidin is directly induced by 1,25-dihydroxyvitamin D3 (vitD3) [6] and is indirectly induced by Toll-like receptor-2/1 activation of macrophages in the presence of 25-hydroxyvitamin D2 (vitD2) [7]. Cathelicidin is present in neutrophils, keratinocytes, mucosal epithelium and macrophages and has a broad spectrum of antimicrobial activity, including binding to endotoxin [4,8]. Human alveolar macrophages infected in vitro with Mycobacterium tuberculosis display enhanced cathelicidin [9], and cathelicidin has been shown to be required for vitD3-induced antimicrobial activity against M. tuberculosis[7].
In addition to a role in innate immunity, the cathelicidin peptide LL-37 functions as a complex immunomodulator of inflammatory responses [4]. LL-37 enhances production of some immune mediators such as chemokines (e.g. monocyte chemoattractant protein-1) but downregulates proinflammatory cytokines such as TNFα and interleukin-12 [10]. In addition, LL-37 antagonizes many responses of immune cells to IFN-γ, a cytokine characteristic of sarcoidosis [4]. Thus, cathelicidin plays a versatile role in coordinating cellular responses to both microbial and endogenous immune stimuli.
Both vitD and mycobacteria, factors that elevate cathelicidin, have been implicated in the etiology/pathophysiology of sarcoidosis (reviewed by Brownell et al. [11]). Mycobacterial DNA or proteins have been detected in tissues of some sarcoidosis patients [12,13], and sarcoidosis lymphocytes display reactivity against mycobacteria-associated antigens including mKATg, SOD and ESAT-6 [12,13,14,15]. Abnormalities in vitD metabolism have long been recognized in sarcoidosis. Reports of endogenous overproduction of vitD in granulomatous diseases [16] led to the recognition that IFN-γ can augment macrophage synthesis of vitD [17]. Cathelicidin LL-37 has been detected in sarcoidosis tissue and BAL fluids, but comparative studies assessing the relationship to clinical status or healthy controls have not been performed [18].
Based upon such evidence, we hypothesized that cathelicidin might be elevated in sarcoidosis alveolar macrophages. Surprisingly, the results presented here indicate that in freshly obtained BAL cells from patients with severe (treatment-requiring) pulmonary sarcoidosis, cathelicidin is deficient, as is expression of the vitamin D receptor (VDR) coactivator steroid receptor coactivator-3 (SRC3). These data demonstrate for the first time that cathelicidin, a molecule critical to innate immunity, is deficient in severe sarcoidosis in parallel with decreased SRC3. Results from in vitro studies suggest that TNFα antagonism of vitD transcription pathways may be involved in cathelicidin deficiency in sarcoidosis alveolar macrophages.
Methods
Study Population
Sarcoidosis subjects (n = 37) were recruited from patients undergoing routine clinical evaluation for initial diagnosis (n = 21) or confirmation (n = 16) of sarcoidosis (table 1). None had Löfgren's syndrome [19]. All diagnoses were confirmed by pulmonary histology demonstrating nonnecrotizing granulomas in the absence of infection or other etiologies. Prior to analyses, patient disease status was characterized as ‘severe’ or ‘non-severe’ based on whether there was an indication for systemic treatment of pulmonary disease at the time of bronchoscopy, as reported previously [20]. Nine sarcoidosis patients were receiving corticosteroids at the time of bronchoscopy; 2 patients were taking non-steroidal anti-inflammatory drugs, and the remaining patients were free of immunosuppressive drugs.
Table 1.
Demographics of patients and control subjects
| Sarcoidosis patients |
Healthy controls (n = 32) | ||
|---|---|---|---|
| non-severe (n = 14) | severe (n = 23) | ||
| Age, years | 50±14.6 | 44.7±11.4 | 31±8.3 |
| Female/male | 9/5 | 16/7 | 19/13 |
| Self-reported race | |||
| African-American | 11 | 18 | 13 |
| Caucasian | 3 | 5 | 16 |
| American Indian | 1 | ||
| Asian | 2 | ||
| Smokers | 5 (5 ex) | 6 (3 ex) | 7 (1 ex) |
| FVC, % predicted | 82.9±16.2 | 70.8±20.2 | |
| CXR stage | |||
| 0-1 | 5 | 3 | |
| 2-4 | 8 | 15 | |
| BAL macrophages, % | 84.9±11.6 | 84.7±10.3 | 95.0±3.9 |
| BAL lymphocytes, % | 14.0±9.6 | 11.3±7.7 | 4.8±3.7 |
| BAL PMNs, % | 1.3±2.5 | 4.0±7.3 | 0.5±1.1 |
| Main treatment indication(s) at time of bronchoscopy | not treated (13) | not treated (13) | |
| skin (1) | pulmonary (7) | ||
| ocular (1) | |||
| other1 (2) | |||
| Organ involvement | lung (14) | lung (23) | |
| ocular (1) | ocular (1) | ||
| skin (1) | central nervous system (1) | ||
| multiorgan (3) | multiorgan (3) | ||
Values represent means ± SD or number of subjects, as appropriate. FVC = Forced vital capacity; CXR = chest X-ray; PMNs = polymorphonuclear leukocytes.
Central nervous system (1), hypercalcemia (1).
The healthy control group (n = 32) was composed of individuals with no history of lung disease and no medication usage at the time of bronchoscopy. These healthy individuals volunteered to undergo bronchoscopy as part of an institutional review board-approved research program. The protocol was approved by the East Carolina University Institutional Review Board, and written informed consent was obtained from all patients and control subjects. The mean ± SD BAL cell counts for healthy controls were as follows: macrophages 95.0 ± 3.9%, lymphocytes 4.8 ± 3.7% and neutrophils 0.5 ± 1.1% (table 1).
Cell Collection and Culture
BAL cells were collected by fiberoptic bronchoscopy as previously described [21]. Differential cell counts were obtained from cytospins stained with a modified Wright's stain. Mean viability of lavage cells was greater than 95% as determined by trypan blue dye exclusion. For culture, BAL cells were plated into 24-well plates (300,000 alveolar macrophages per well) or chamber slides (60,000 cells/well) in RPMI 1640 medium supplemented with 5% human AB serum (Gemini, Calabasas, Calif., USA), L-glutamine and antibiotics, as described previously [20]. Adherence-purified alveolar macrophages (60 min) were cultured for 24 h in medium alone or medium supplemented with lipopolysaccharide (Salmonella typhimurium, Sigma; 500 ng/ml), calcitriol (vitamin D3, EMD-Calbiochem; 10–25 nM) or TNFα (250–500 units/ml; R & D Systems, Minneapolis, Minn., USA). Yields of patient or control BAL cells were not sufficient to allow performance of all experiments on each individual specimen. Bronchial epithelial cells were obtained as described previously [22].
Immunocytochemistry
Expression of cathelicidin-derived proteins and SRC3 was analyzed in cytospins of freshly obtained BAL-derived cells. Primary antibodies used were as follows: (1) murine monoclonal anti-CAP-18 (sc-130552; 1:50), (2) goat polyclonal antibody directed against the LL-37 peptide (sc-21578; 1:50) and (3) goat polyclonal anti-SRC3 (sc-14605; 1:100; all from Santa Cruz Biotechnology, Santa Cruz, Calif., USA). Alexa 488-conjugated antibodies antimurine (1) and antigoat (2) and (3) (Invitrogen, Grand Island, N.Y., USA) were used as secondary antibodies (1:1,000). Slides were examined by fluorescent microscopy. Chambers receiving only secondary antibody were used to verify the specificity of primary antibody staining. Epithelial cells were characterized by the presence of cytokeratin (Abcam, Cambridge, Mass., USA; 1:50).
Gene Array
Analysis was carried out by the Gene Expression Array Core Facility at the Comprehensive Cancer Center at Case Western Reserve University. Total RNA was extracted from whole BAL cells from 5 subjects with non-severe sarcoidosis and 4 subjects with severe sarcoidosis; RNA integrity was confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, Calif., USA). Amplified labeled cRNA was generated from 5–8 µg of total RNA by a SuperScript kit (Invitrogen, Carlsbad, Calif., USA) and processed using high-density oligonucleotide HG-U133A arrays (Affymetrix, Santa Clara, Calif., USA). The probe intensities were analyzed using Microarray suite 5.0 software (Affymetrix). For analysis, the samples were matched for the percentage of BAL lymphocytes to account for variances in cell populations and compared by paired t test, with a corrected p value of <0.05.
Quantitative mRNA Expression
Total RNA was extracted from BAL cells or cultured alveolar macrophages by an RNeasy protocol (Qiagen, Valencia, Calif., USA). Expression of mRNA was determined by real-time RT-PCR using the ABI Prism 7300 Detection System (TaqMan, Applied Biosystems, Foster City, Calif., USA) described previously [22,23]. RNA specimens were analyzed in duplicate using primer-probe sets for a housekeeping gene (GAPDH, Cat. No. 4333764T), cathelicidin (Cat. No. Hs00189038), VDR (Cat. No. Hs01045840; ABI) or SRC-3 (Cat. No. Hs00180722). Raw changes in threshold cycle values for genes of interest were used in statistical analyses after normalization to GAPDH. These data were also utilized to calculate the relative quantity (expressed as fold change) of mRNA expression in sarcoidosis patients compared to healthy controls or for in vitro studies in which vitD-treated or TNFα-treated cells were compared to untreated cells (medium alone).
Quantitation of LL-37
Levels of LL-37 peptide were determined in BAL fluids and conditioned media from alveolar macrophages cultured with or without calcitriol (10 nM) by ELISA according to the manufacturer's instructions (Cell Sciences, Canton, Mass., USA). Results were expressed as nanograms of LL-37 per milliliter.
Measurement of VitD
Serum samples from sarcoidosis patients were precipitated with acetonitrile and analyzed by a liquid chromatography-tandem mass spectrometry assay as described elsewhere [24]. Results were expressed as nanograms of vitD2 per milliliter or picograms of vitD3 per milliliter.
Statistical Analyses
Intrinsic data from control and patient cells were analyzed for significance by analysis of variance and Tukey's test using Prism software (Graph Pad, San Diego, Calif., USA). Data from in vitro studies were evaluated by Student's t test.
Results
Alveolar Macrophage Cathelicidin Deficiency Parallels Disease Severity
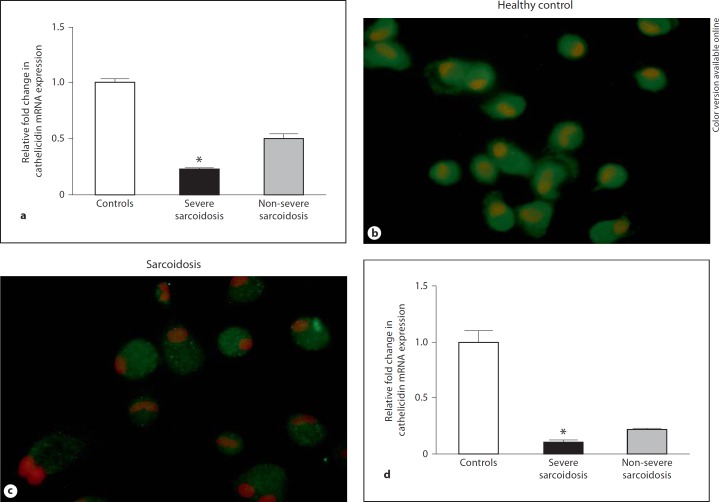
Initial studies were carried out to determine whether global gene expression would reveal significant differences between groups of severe (n = 4) and non-severe (n = 5) sarcoidosis patients. Data indicated that compared to non-severe patients, BAL cells from severe sarcoidosis patients exhibited an 8-fold decrease in cathelicidin gene expression. To validate microarray results, freshly obtained BAL cells from sarcoidosis patients and healthy controls were analyzed by quantitative PCR for intrinsic expression of cathelicidin mRNA. Compared to healthy controls (n = 21), intrinsic cathelicidin mRNA expression was significantly reduced (p < 0.05) by 4-fold in BAL cells of sarcoidosis patients (n = 17) characterized as having severe, treatment-requiring disease (fig. 1a). Cathelicidin was also decreased in patients with non-severe sarcoidosis (n = 12), but to a lesser extent (2-fold) and did not differ significantly from controls (fig. 1a). Prednisone treatment did not appear to affect cathelicidin expression in severe sarcoidosis. Cathelicidin expression was comparably reduced by 4-fold in either prednisone-treated (n = 7) or non-prednisone-treated severe sarcoidosis patients (n = 12), with no statistically significant difference between these groups. Immunocytochemistry of alveolar macrophages from severe sarcoidosis patients (n = 2) also indicated reduced levels of the cathelicidin peptide LL-37 compared to healthy controls (n = 2; fig. 1b, c).
Fig. 1.
Deficiency in cathelicidin mRNA expression correlates with clinical disease severity of sarcoidosis. a Samples of cDNA derived from BAL cells of sarcoidosis patients with severe (n = 16) or non-severe (n = 12) disease and healthy controls (n = 21) were evaluated for cathelicidin expression by quantitative PCR. Data represent the relative fold change in cathelicidin expression after subtraction of raw GAPDH cycle values and normalization to healthy control threshold cycle values. Means and SEMs are shown. * p < 0.05. b, c Immunocytochemistry of alveolar macrophage cytospins illustrates cathelicidin peptide LL-37 levels in a healthy control (b) and reduced cathelicidin peptide LL-37 in a severe sarcoidosis patient (c) (n = 2). d Cathelicidin mRNA expression is also significantly decreased in sarcoidosis pulmonary epithelial cells. Means and SEMs are shown. Controls: n = 6; severe sarcoidosis patients: n = 4; non-severe sarcoidosis patients: n = 2. * p < 0.05.
We also examined cathelicidin expression in epithelial cells from sarcoidosis lung because pulmonary epithelial cells have been shown to process hCAP-18 protein to active LL-37 peptide [25,26]. Interestingly, quantitative analysis of cathelicidin mRNA in epithelial cell brushings from severe sarcoidosis patients (n = 4) indicated significantly (p < 0.05) reduced cathelicidin mRNA expression compared to brushings from healthy controls (n = 6; fig. 1d). Epithelial cells were characterized by cytokeratin expression as reported previously [22]. Cathelicidin proteins were not detectable in BAL fluids (below 0.6 ng/ml).
Circulating VitD3 Levels Are within the Healthy Range in Sarcoidosis Patients
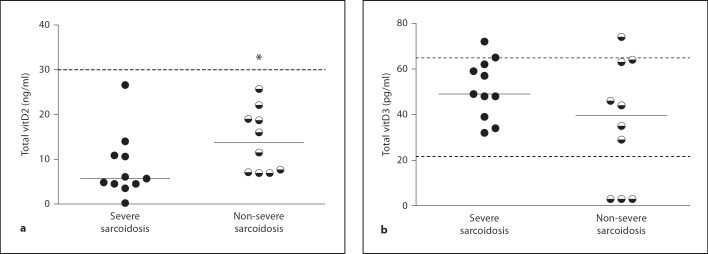
Levels of vitD metabolites were examined because vitD is known to be essential to cathelicidin expression. As a first step, intrinsic expression of the VDR was examined, and results indicated no differences between sarcoidosis and healthy control BAL cells (data not shown). Next, both vitD2 and vitD3 were quantified in sera and BAL fluid specimens from severe and non-severe sarcoidosis patients. Neither vitD2 nor vitD3 were detectable in BAL fluids (data not shown). Median levels of serum vitD2 in severe patients (5.7 ng/ml; n = 11) were significantly (p < 0.05) lower than levels in non-severe patients (13.8 ng/ml; n = 10; fig. 2a). VitD2 results from both groups of patients were well below the recommended level for healthy individuals (30 ng/ml; fig. 2a). However, analyses of vitD3 levels revealed no significant difference between groups (severe: median 49.0 pg/ml; non-severe: 39.5 pg/ml), and the majority of sarcoidosis values were within the healthy range (fig. 2b). Thus, the presence of healthy levels of vitD3 could not account for the deficiency of cathelicidin expression in alveolar macrophages.
Fig. 2.
Circulating vitD2 levels parallel disease severity in sarcoidosis. VitD2 (a) and vitD3 (b) levels were quantified in sera from patients with severe (n = 11) and non-severe (n = 10) sarcoidosis. Medians are shown, and dotted lines indicate the recommended normal levels of vitD2 (a) and the normal range of vitD3 (b) are provided. * p < 0.05.
VitD Responsiveness Is Not Deficient in Sarcoidosis
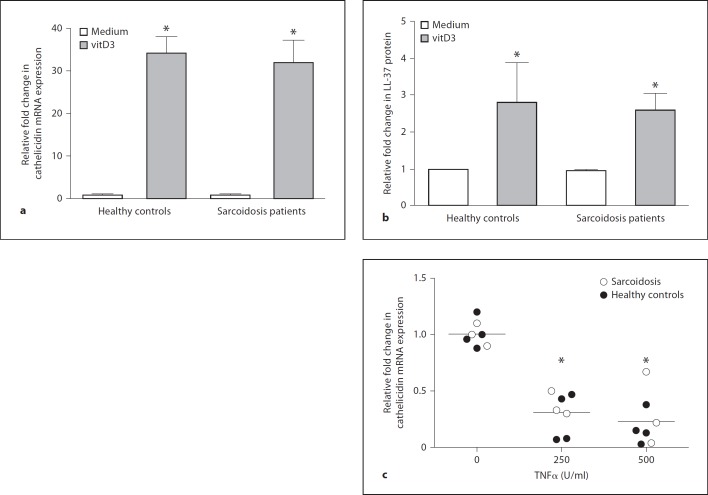
To ascertain whether sarcoidosis alveolar macrophages were capable of responding to VitD, we examined cathelicidin expression in cultures of alveolar macrophages exposed to vitD3. In vitro culture of alveolar macrophages with vitD3 (10–25 nM) yielded comparable upregulation of cathelicidin mRNA (fig. 3a) and LL-37 peptide (fig. 3b) in sarcoidosis patients and healthy controls. Results suggested that sarcoidosis alveolar macrophages were not refractory to vitD stimulation.
Fig. 3.
Cathelicidin expression is upregulated by vitD3 and downregulated by TNFα in sarcoidosis and healthy control alveolar macrophages. a Quantitative PCR analysis ofcDNA samples from sarcoidosis and healthy control alveolar macrophages cultured for 24 h in medium with or without vitD3 (10 nM). Values for cathelicidin mRNA were determined after normalization to GAPDH. Data are expressed as the fold change in cathelicidin mRNA expression relative to untreated cells (medium alone). n = 5 per group. * p < 0.05. b Cathelicidin-derived LL-37 peptide synthesis in supernatant fluids from sarcoidosis and healthy control alveolar macrophages cultured in vitro with or without vitD3. Data are normalized to untreated (medium) cultured cells. Controls: n = 4; sarcoidosis patients: n = 3. * p < 0.05. c A dot plot illustrates repression of alveolar macrophage cathelicidin mRNA expression by TNFα (250–500 units/ml) in sarcoidosis patients (n = 3) and healthy controls (n = 5). * p < 0.05.
TNFα Is a Repressor of Cathelicidin Expression
Because results indicated that intrinsic expression of cathelicidin in sarcoidosis alveolar macrophages cells was deficient despite healthy VDR levels and vitD responsiveness, we hypothesized that repressive factors might be responsible. Previously, we reported that the transcription factor nuclear factor (NF)-ĸB is activated in sarcoidosis BAL cells, indicating ongoing inflammatory transcriptional activity [21]. NF-ĸB activation has been shown to inhibit vitD-stimulated transcription [27]. Further, elevated TNFα production has been reported in alveolar macrophages from patients with severe sarcoidosis [28], and TNFα has been shown to decrease vitD-mediated transcription via NF-ĸB [29]. To determine whether TNFα antagonized cathelicidin expression, alveolar macrophages from healthy controls and sarcoidosis patients were cultured in vitro with or without TNFα (250–500 U/ml) for 24 h (fig. 3c). Results indicated significant (p < 0.05) repression of cathelicidin mRNA by TNFα compared to culture in medium alone (fig. 3c), suggesting a potential role for TNFα in sarcoidosis-associated cathelicidin deficiency.
The VitD Coactivator SRC3 Is Repressed by TNFα
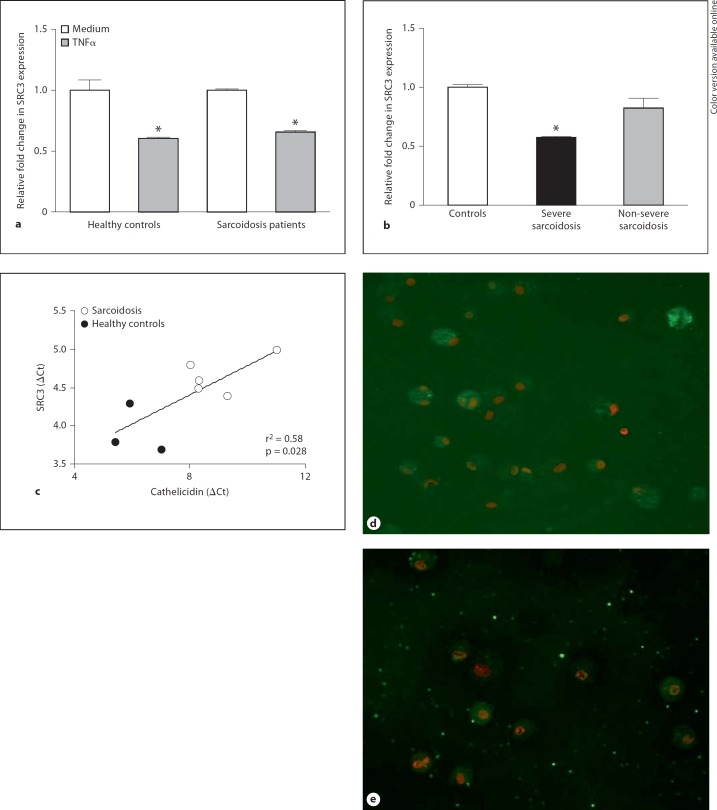
VitD-mediated stimulation of cathelicidin requires VDR recruitment of SRC3 in order to initiate transcriptional activity in human keratinocytes [30,31]. We hypothesized that this mechanism might also be active in human alveolar macrophages and susceptible to TNFα repression. Data from in vitro culture of alveolar macrophages with and without TNFα (500 units/ml) supported this concept. TNFα significantly (p < 0.05) repressed alveolar macrophage expression of SRC3 mRNA in both healthy controls and sarcoidosis patients (fig. 4a). To determine if intrinsic expression of SRC3 was impaired in sarcoidosis, SRC3 mRNA was evaluated in freshly derived, noncultured sarcoidosis and control BAL cells. Compared to healthy control values, SRC3 mRNA expression was significantly (p < 0.05) reduced in BAL cells from sarcoidosis patients with severe disease, but values in BAL cells from patients with non-severe disease were comparable to controls (fig. 4b). Regression analysis of intrinsic mRNA expression in healthy controls and sarcoidosis patients indicated a significant correlation (r2 = 0.58; p = 0.028) between SRC3 and cathelicidin values (fig. 4c). SRC3 protein expression was also diminished in alveolar macrophages of patients with severe sarcoidosis compared to healthy controls (n = 2; fig. 4d, e). Investigation of SRC1, a coactivator not implicated in vitD regulation of cathelicidin [32,33], did not yield any differences between control and sarcoidosis groups (data not shown).
Fig. 4.
Expression of SRC3 is repressed by TNFα and decreased in severe sarcoidosis. a Quantitative PCR analysis of SRC3 expression was performed in cDNA samples from healthy control and sarcoidosis alveolar macrophages cultured for 24 h in medium with or without TNFα (500 units/ml). Means and SEMs are given. Controls: n = 4; sarcoidosis patients: n = 2. * p < 0.05. b SRC3 mRNA expression was quantitated in BAL cells derived from healthy controls (n = 3) and patients with severe (n = 4) or non-severe (n = 3) sarcoidosis. Data were normalized to healthy controls. * p < 0.05. c Intrinsic alveolar macrophage SRC3 and cathelicidin mRNA values show significant correlation by regression analysis. ΔCt = Change in threshold cycle values. d, e Immunocytochemistry illustrates marked SRC3 protein expression in alveolar macrophages from a healthy control (d) and reduced SRC3 in a patient with severe sarcoidosis (e) (n = 2).
Discussion
VitD and Sarcoidosis
Data presented here are the first to show an association between alveolar macrophage cathelicidin deficiency and clinical disease status in sarcoidosis. Reduced cathelicidin mRNA expression was apparent in patients with severe sarcoidosis by global gene analysis. These results were verified by quantitative TaqMan assays indicating significantly depressed cathelicidin expression in patients with severe sarcoidosis. Systemic levels of vitD2 but not vitD3 were decreased in sarcoidosis patients, with severe patients showing marked reduction. These results suggest that circulating vitD2 is rapidly metabolized in severe sarcoidosis for extrarenal synthesis of vitD3 by macrophages and other cells. The systemic vitD levels reported here for sarcoidosis patients are consistent with those reported previously [34,35], although comparative studies of vitD levels and clinical disease status were not carried out in those earlier studies.
VitD exerts a potent regulatory effect on the immune system [summarized in [36]. Dysregulation of vitD synthesis is well recognized in sarcoidosis, a disease characterized by hyperproduction of proinflammatory immune mediators. Alveolar macrophages from sarcoidosis patients with active disease are known to constitutively convert vitD2 to vitD3, unlike alveolar macrophages from healthy donors [37,38]. These findings support the concept of extrarenal sources of vitD3 in sarcoidosis as contributors to disordered calcium metabolism [17]. In vitro studies demonstrating IFN-γ promotion of vitD3 synthesis in differentiated THP-1 cells [17,38,39] led to the recognition that proinflammatory cytokine-mediated activation of macrophages contributed to vitD3 synthesis. Our data suggest that proinflammatory cytokines (e.g. TNFα) also antagonize vitD3 pathways in sarcoidosis, thus generating a deficiency of cathelicidin despite ongoing metabolism of vitD in activated alveolar macrophages.
Chronic Inflammation and Cathelicidin Deficiency
The specific mechanisms by which chronic inflammation alters alveolar macrophage cathelicidin expression levels in sarcoidosis remain to be elucidated. Sarcoidosis is associated with persistent hyperproduction of IFN-γ and other cytokines related to T helper 1 cell activity in BAL fluids, lymph nodes, granulomas and blood [reviewed in [1]. IFN-γ has been shown to antagonize vitD-mediated transcription [40] and to repress cathelicidin promoter activity in A549 cells [41]. Interestingly, the cathelicidin peptide LL-37 blocks macrophage and dendritic cell responses to IFN-γ [42]; thus, the deficiency of cathelicidin in sarcoidosis may impede resolution of inflammatory activity.
Our data also suggest that TNFα, the production of which is reported to be elevated in severe sarcoidosis [28], may be another candidate antagonist for cathelicidin. TNFα is known to antagonize VDR transcriptional activity via the NF-ĸB p65 subunit [27,29]. After association with proteins in the VDR complex, p65 impedes binding of the VDR to coactivators such as SRC3, thus preventing VDR binding to vitD response elements in cathelicidin and other target genes [27,29]. SRC3 is known to be required for VDR-mediated transcription of cathelicidin [31]. Interestingly, alveolar macrophage cathelicidin deficiency in severe sarcoidosis did not appear to be affected by steroid therapy since expression levels did not differ among patients on or off prednisone treatment. More recently, anti-TNFα therapy such as infliximab has shown promise in sarcoidosis [43]. In light of our current findings suggesting TNFα involvement in cathelicidin deficiency, investigation of alveolar macrophages from sarcoidosis patients before and after anti-TNFα therapy may provide helpful data.
Conclusion
Data indicate that both intrinsic cathelicidin and SRC3 levels are decreased in alveolar macrophages from patients with severe sarcoidosis. Of relevance here are studies indicating that mice deficient in SRC3 display reduced expression of VDR target genes [32]. Thus, our studies suggest that overproduction of TNFα in severe sarcoidosis blocks vitD-mediated cathelicidin transcription via downregulatory effects on SRC3 despite the presence of localized vitD3 synthesis. Although the precise mechanisms of action of TNFα on cathelicidin and SRC3 remain to be determined, findings suggest that TNFα-mediated repression of SRC3 may constitute one pathway by which cathelicidin expression remains deficient in severe sarcoidosis. Moreover, deficiency of cathelicidin, a source of immunoregulatory proteins, may retard resolution of sarcoidosis inflammatory pathways.
Acknowledgements
This study was supported by National Institutes of Health grant HL-077652 (to M.J.T.) and Department of Health and Human Services grant D52MP02105 (to M.S.K.).
References
- 1.Chen ES, Moller DR. Sarcoidosis - scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–467. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 3.Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 4.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009;16:41–47. doi: 10.1097/moh.0b013e32831ac517. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 6.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 8.Gombart A, Bhan I, Borregaard N, Tamez H, Camargo J, Koeffler H, Thadhani R. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48:418–424. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, Torres T, Sada E. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun. 2008;76:935–941. doi: 10.1128/IAI.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 11.Brownell I, Ramirez-Valle F, Sanchez M, Prystowsky S. Evidence for mycobacteria in sarcoidosis. Am J Respir Cell Mol Biol. 2011;45:899–905. doi: 10.1165/rcmb.2010-0433TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC, West EE, McDyer JF, Zhang Y, Eklund A, Grunewald J, Moller DR. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181:8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Bajoghli A, Kubba A, Bhawan J. Identification of mycobacterial DNA in cutaneous lesions of sarcoidosis. J Cutan Pathol. 1999;26:271–278. doi: 10.1111/j.1600-0560.1999.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 14.Drake WP, Dhason MS, Nadaf M, Shepherd BE, Vadivelu S, Hajizadeh R, Newman LS, Kalams SA. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75:527–530. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oswald-Richter KA, Culver DA, Hawkins C, Hajizadeh R, Abraham S, Shepherd BE, Jenkins CA, Judson MA, Drake WP. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect Immun. 2009;77:3740–3748. doi: 10.1128/IAI.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams JS, Gacad MA, Anders A, Endres DB, Sharma OP. Biochemical indicators of disordered vitamin D and calcium homeostasis in sarcoidosis. Sarcoidosis. 1986;3:1–6. [PubMed] [Google Scholar]
- 17.Dusso AS, Kamimura S, Gallieni M, Zhong M, Negrea L, Shapiro S, Slatopolsky E. γ-Interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997;82:2222–2232. doi: 10.1210/jcem.82.7.4074. [DOI] [PubMed] [Google Scholar]
- 18.Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, Eklund A, Gudmundsson GH. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160:283–290. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- 19.Statement on sarcoidosis Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 20.Barna BP, Culver DA, Abraham S, Malur A, Bonfield TL, John N, Farver CF, Drazba JA, Raychaudhuri B, Kavuru MS, Thomassen MJ. Depressed peroxisome proliferator-activated receptor gamma (PPARgamma) is indicative of severe pulmonary sarcoidosis: possible involvement of interferon gamma (IFN-gamma) Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:93–100. [PubMed] [Google Scholar]
- 21.Culver DA, Barna BP, Raychaudhuri B, Bonfield TL, Abraham S, Malur A, Farver CF, Kavuru MS, Thomassen MJ. Peroxisome proliferator-activated receptor gamma activity is deficient in alveolar macrophages in pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 2004;30:1–5. doi: 10.1165/rcmb.2003-0304RC. [DOI] [PubMed] [Google Scholar]
- 22.Bonfield TL, Farver CF, Barna BP, Malur A, Abraham S, Raychaudhuri B, Kavuru MS, Thomassen MJ. Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am J Respir Cell Mol Biol. 2003;29:677–682. doi: 10.1165/rcmb.2003-0148OC. [DOI] [PubMed] [Google Scholar]
- 23.Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS, Thomassen MJ. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1132–L1136. doi: 10.1152/ajplung.00216.2003. [DOI] [PubMed] [Google Scholar]
- 24.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 25.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 27.Lu X, Farmer P, Rubin J, Nanes MS. Integration of the NFkappaB p65 subunit into the vitamin D receptor transcriptional complex: identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J Cell Biochem. 2004;92:833–848. doi: 10.1002/jcb.20143. [DOI] [PubMed] [Google Scholar]
- 28.Ziegenhagen MW, Rothe ME, Zissel G, Muller-Quernheim J. Exaggerated TNFalpha release of alveolar macrophages in corticosteroid resistant sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:185–190. [PubMed] [Google Scholar]
- 29.Farmer PK, He X, Schmitz ML, Rubin J, Nanes MS. Inhibitory effect of NF-kappaB on 1,25-dihydroxyvitamin D(3) and retinoid X receptor function. Am J Physiol Endocrinol Metab. 2000;279:E213–E220. doi: 10.1152/ajpendo.2000.279.1.E213. [DOI] [PubMed] [Google Scholar]
- 30.Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol. 2009;129:1367–1378. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, Bikle DD, Gallo RL. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 32.Liao L, Chen X, Wang S, Parlow AF, Xu J. Steroid receptor coactivator 3 maintains circulating insulin-like growth factor I (IGF-I) by controlling IGF-binding protein 3 expression. Mol Cell Biol. 2008;28:2460–2469. doi: 10.1128/MCB.01163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 34.Basile JN, Liel Y, Shary J, Bell NH. Increased calcium intake does not suppress circulating 1,25-dihydroxyvitamin D in normocalcemic patients with sarcoidosis. J Clin Invest. 1993;91:1396–1398. doi: 10.1172/JCI116342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamada K, Nagai S, Tsutsumi T, Izumi T. Ionized calcium and 1,25-dihydroxyvitamin D concentration in serum of patients with sarcoidosis. Eur Respir J. 1998;11:1015–1020. doi: 10.1183/09031936.98.11051015. [DOI] [PubMed] [Google Scholar]
- 36.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab. 1987;65:1201–1209. doi: 10.1210/jcem-65-6-1201. [DOI] [PubMed] [Google Scholar]
- 39.Overbergh L, Stoffels K, Waer M, Verstuyf A, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin D-1alpha-hydroxylase in human monocytic THP1 cells: mechanisms of interferon-gamma-mediated induction. J Clin Endocrinol Metab. 2006;91:3566–3574. doi: 10.1210/jc.2006-0678. [DOI] [PubMed] [Google Scholar]
- 40.Vidal M, Ramana CV, Dusso AS. Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance Stat1-mediated transcription. Mol Cell Biol. 2002;22:2777–2787. doi: 10.1128/MCB.22.8.2777-2787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elloumi HZ, Holland SM. Complex regulation of human cathelicidin gene expression: novel splice variants and 5′UTR negative regulatory element. Mol Immunol. 2008;45:204–217. doi: 10.1016/j.molimm.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nijnik A, Pistolic J, Wyatt A, Tam S, Hancock RE. Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs. J Immunol. 2009;183:5788–5798. doi: 10.4049/jimmunol.0901491. [DOI] [PubMed] [Google Scholar]
- 43.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, Albera C, Brutsche M, Davis G, Donohue JF, Muller-Quernheim J, Schlenker-Herceg R, Flavin S, Lo KH, Oemar B, Barnathan ES;, Sarcoidosis Investigators Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]