Abstract
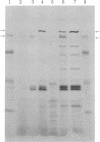
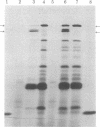
Multiplication of several amber mutants of bacteriophage T7 was decreased in two polyamine-deficient mutants of Escherichia coli K-12 carrying amber suppressors, relative to the multiplication of wild type bacteriophage T7 in the same hosts. In contrast the same T7 amber bacteriophages multiplied well in these strains when supplemented with polyamines. The requirement for polyamines for optimal translation of amber codons in vivo was confirmed by showing that infection of polyamine-depleted E. coli with bacteriophage T7 carrying an amber mutation in gene 1 resulted in an increased accumulation of the amber fragment of the gene 1 protein and a decreased accumulation of the full-length gene 1 protein compared with infection of an amine-supplemented culture. These results indicate that one important function of polyamines in vivo is concerned with protein translation and the protein-synthesizing ribosomal complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- ANDERSON T. F., RAPPAPORT C., MUSCATINE N. A. On the structure and osmotic properties of phage particles. Ann Inst Pasteur (Paris) 1953 Jan;84(1):5–14. [PubMed] [Google Scholar]
- Abraham A. K., Olsnes S., Pihl A. Fidelity of protein synthesis in vitro is increased in the presence of spermidine. FEBS Lett. 1979 May 1;101(1):93–96. [PubMed] [Google Scholar]
- Algranati I. D., Goldemberg S. H. Initiation, elongation and termination of polypeptide synthesis in cell-free systems from polyamine-deficient bacteria. Biochem Biophys Res Commun. 1981 Nov 16;103(1):8–15. doi: 10.1016/0006-291x(81)91653-3. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echandi G., Algranati I. D. Defective 30S ribosomal particles in a polyamine auxotroph of Escherichia coli. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1185–1191. doi: 10.1016/0006-291x(75)90798-6. [DOI] [PubMed] [Google Scholar]
- Goldemberg S. H., Algranati I. D. Polyamine requirement for streptomycin action on protein synthesis in bacteria. Eur J Biochem. 1981 Jul;117(2):251–255. doi: 10.1111/j.1432-1033.1981.tb06330.x. [DOI] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Hirashima A., Harigai H., Watanabe I. Enhancing effect of magnesium ion on cell-free synthesis of read-through protein of bacteriophage Qbeta. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1046–1051. doi: 10.1016/0006-291x(79)91514-6. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K., Kishida K., Kakegawa T., Hirose S. Decrease in the S1 protein of 30-S ribosomal subunits in polyamine-requiring mutants of Escherichia coli grown in the absence of polyamines. Eur J Biochem. 1981;114(1):127–131. doi: 10.1111/j.1432-1033.1981.tb06182.x. [DOI] [PubMed] [Google Scholar]
- Jorstad C. M., Morris D. R. Polyamine limitation of growth slows the rate of polypeptide chain elongation in Escherichia coli. J Bacteriol. 1974 Sep;119(3):857–860. doi: 10.1128/jb.119.3.857-860.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D., Kramer G., Pinphanichakarn P., Hardesty B. Polyamines are necessary for maximum in vitro synthesis of globin peptides and play a role in chain initiation. Arch Biochem Biophys. 1975 Jul;169(1):192–198. doi: 10.1016/0003-9861(75)90332-x. [DOI] [PubMed] [Google Scholar]
- Lövgren T. N., Petersson A., Loftfield R. B. The mechanism of aminoacylation of transfer ribonucleic acid. The role of magnesium and spermine in the synthesis of isoleucyl-tRNA. J Biol Chem. 1978 Oct 10;253(19):6702–6710. [PubMed] [Google Scholar]
- Maas W. K. Mapping of genes involved in the synthesis of spermidine in Escherichia coli. Mol Gen Genet. 1972;119(1):1–9. doi: 10.1007/BF00270439. [DOI] [PubMed] [Google Scholar]
- McGrogan M., Spector D. J., Goldenberg C. J., Halbert D., Raskas H. J. Purification of specific adenovirus 2 RNAs by preparative hybridization and selective thermal elution. Nucleic Acids Res. 1979 Feb;6(2):593–607. doi: 10.1093/nar/6.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch M. D., Benicourt C. Polyamines stimulate suppression of amber termination codons in vitro by normal tRNAs. Eur J Biochem. 1980 Apr;105(3):445–451. doi: 10.1111/j.1432-1033.1980.tb04519.x. [DOI] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöthig-Laslo V., Weygand-Durasević I., Zivković T., Kućan Z. Binding of spermine to tRNATyr stabilizes the conformation of the anticodon loop and creates strong binding sites for divalent cations. Eur J Biochem. 1981 Jul;117(2):263–267. doi: 10.1111/j.1432-1033.1981.tb06332.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Pochon F., Cohen S. S. 4-Thiouridine and the conformation of E. coli tRNA induced by spermidine. Biochem Biophys Res Commun. 1972 May 26;47(4):720–726. doi: 10.1016/0006-291x(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Polyamines as cellular regulators. Med Biol. 1981 Dec;59(5-6):269–461. [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier A. A., Schimmel P. R. Interaction of polyamines with fragments and whole molecules of yeast phenylalanine-specific transfer RNA. J Mol Biol. 1975 Apr 5;93(2):323–329. doi: 10.1016/0022-2836(75)90136-9. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J Bacteriol. 1980 Dec;144(3):952–956. doi: 10.1128/jb.144.3.952-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Cohn M. S., Hafner E. W. Streptomycin resistance (rpsL) produces an absolute requirement for polyamines for growth of an Escherichia coli strain unable to synthesize putrescine and spermidine [delta(speA-speB) delta specC]. J Bacteriol. 1981 Aug;147(2):702–704. doi: 10.1128/jb.147.2.702-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Dix D. B., Gerson R. B., Karim A. M. Effect of Mg2+ concentration, polyamines, streptomycin, and mutations in ribosomal proteins on the accuracy of the two-step selection of aminoacyl-tRNAs in protein biosynthesis. J Biol Chem. 1981 Jul 10;256(13):6676–6681. [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Regulation of macromolecular synthesis by putrescine in a conditional Escherichia coli putrescine auxotroph. J Bacteriol. 1972 Oct;112(1):30–39. doi: 10.1128/jb.112.1.30-39.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]