Abstract
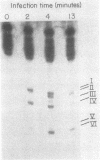
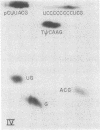
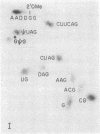
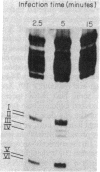
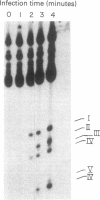
The fate of host tRNAs during T4 bacteriophage infection was investigated with Escherichia coli CTr5x, the only known host strain that is restrictive to RNA ligase and polynucleotide kinase mutants. Three CTr5x tRNA species were cleaved during infection. One was leucine tRNA1, which was cleaved in the extra arm, as reported elsewhere for E. coli B infected with bacteriophage T2 or T4. The other two were specific to E. coli CTr5x and were not cleaved in various other hosts. One of the cleaved CTr5x-specific tRNAs had an anticodon sequence of the E. coli B "major" isoleucine tRNA but otherwise little sequence homology. Both CTr5x-specific tRNAs were cleaved by a distinct T4-induced endonuclease, other than that of leucine tRNA1, because the CTr5x-specific cleavages (i) were induced later in infection, (ii) persisted with a T4 mutant deficient in leucine tRNA1 endonuclease, and (iii) occurred in the anticodon loop. The specific manifestation of the anticodon-directed endonuclease activity in T4-infected E. coli CTr5x suggests roles for RNA ligase and polynucleotide kinase in processing of host tRNA species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Collinsworth W. L., Mathews C. K. Biochemistry of DNA-defective amber mutants of bacteriophage T4. IV. DNA synthesis in plasmolyzed cells. J Virol. 1974 Apr;13(4):908–915. doi: 10.1128/jvi.13.4.908-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V., Sarid S., Littauer U. Z. Coding by T4 phage DNA of soluble RNA containing pseudouridylic acid. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1403–1409. doi: 10.1073/pnas.60.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Vekstein R., Kaufmann G. RNA ligase reaction products in plasmolyzed Escherichia coli cells infected by T4 bacteriophage. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5430–5434. doi: 10.1073/pnas.76.11.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Cozzarelli N. R. Genetics and physiology of bacteriophage T4 3'-phosphatase: evidence for involvement of the enzyme in T4 DNA metabolism. J Virol. 1974 Apr;13(4):888–897. doi: 10.1128/jvi.13.4.888-897.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C., McClain W. H. Conditionally lethal mutants of bacteriophage T4 defective in production of a transfer RNA. J Mol Biol. 1973 Dec 5;81(2):137–155. doi: 10.1016/0022-2836(73)90185-x. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Cozzarelli N. R. DNA-joining enzymes: a review. Methods Enzymol. 1979;68:50–71. doi: 10.1016/0076-6879(79)68006-0. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Kano-Sueoka T., Sueoka N. Characterization of a modified leucyl-tRNA of Escherichia coli after bacteriophage T2 infection. J Mol Biol. 1968 Nov 14;37(3):475–491. doi: 10.1016/0022-2836(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Kallenbach N. R. Determination of recognition sites of T4 RNA ligase on the 3'-OH and 5' -P termini of polyribonucleotide chains. Nature. 1975 Apr 3;254(5499):452–454. doi: 10.1038/254452a0. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Watanabe S., Harada F., Nishimura S. Primary structure of AUA-specific isoleucine transfer ribonucleic acid from Escherichia coli. Biochemistry. 1980 May 13;19(10):2085–2089. doi: 10.1021/bi00551a013. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton T. C., Paddock G., Abelson J. Nucleotide sequence determination of bacteriophage T4 leucine transfer ribonucleic acid. J Biol Chem. 1973 Sep 25;248(18):6348–6365. [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels J. M., Soltis D., Hey T., Snyder L. Genetic and physiological studies of the role of the RNA ligase of bacteriophage T4. J Mol Biol. 1982 Jan 15;154(2):273–286. doi: 10.1016/0022-2836(82)90064-x. [DOI] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Cooley W., Runnels J., Snyder L. R. A role in true-late gene expression for the T4 bacteriophage 5' polynucleotide kinase 3' phosphatase. J Mol Biol. 1978 Aug 5;123(2):221–233. doi: 10.1016/0022-2836(78)90322-4. [DOI] [PubMed] [Google Scholar]
- Snopek T. J., Wood W. B., Conley M. P., Chen P., Cozzarelli N. R. Bacteriophage T4 RNA ligase is gene 63 product, the protein that promotes tail fiber attachment to the baseplate. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3355–3359. doi: 10.1073/pnas.74.8.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r1–55. [PMC free article] [PubMed] [Google Scholar]
- Weiss S. B., Hsu W. T., Foft J. W., Scherberg N. H. Transfer RNA coded by the T4 bacteriophage genome. Proc Natl Acad Sci U S A. 1968 Sep;61(1):114–121. doi: 10.1073/pnas.61.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H. Function of the bacteriophage T4 transfer RNA's. J Mol Biol. 1973 Mar 15;74(4):753–757. doi: 10.1016/0022-2836(73)90065-x. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., Kim J. S., Abelson J. N. Bacteriophage T4 transfer RNA. 3. Clustering of the genes for the T4 transfer RNA's. J Mol Biol. 1972 Nov 28;71(3):547–556. doi: 10.1016/s0022-2836(72)80022-6. [DOI] [PubMed] [Google Scholar]
- Yarus M., Barrell B. G. The sequence of nucleotides in tRNA Ile from E. coli B. Biochem Biophys Res Commun. 1971 May 21;43(4):729–734. doi: 10.1016/0006-291x(71)90676-0. [DOI] [PubMed] [Google Scholar]
- Yudelevich A. Specific cleavage of an Escherichia coli leucine transfer RNA following bacteriophage T4 infection. J Mol Biol. 1971 Aug 28;60(1):21–29. doi: 10.1016/0022-2836(71)90444-x. [DOI] [PubMed] [Google Scholar]