Abstract
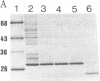
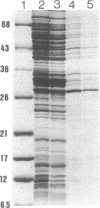
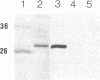
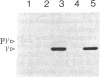
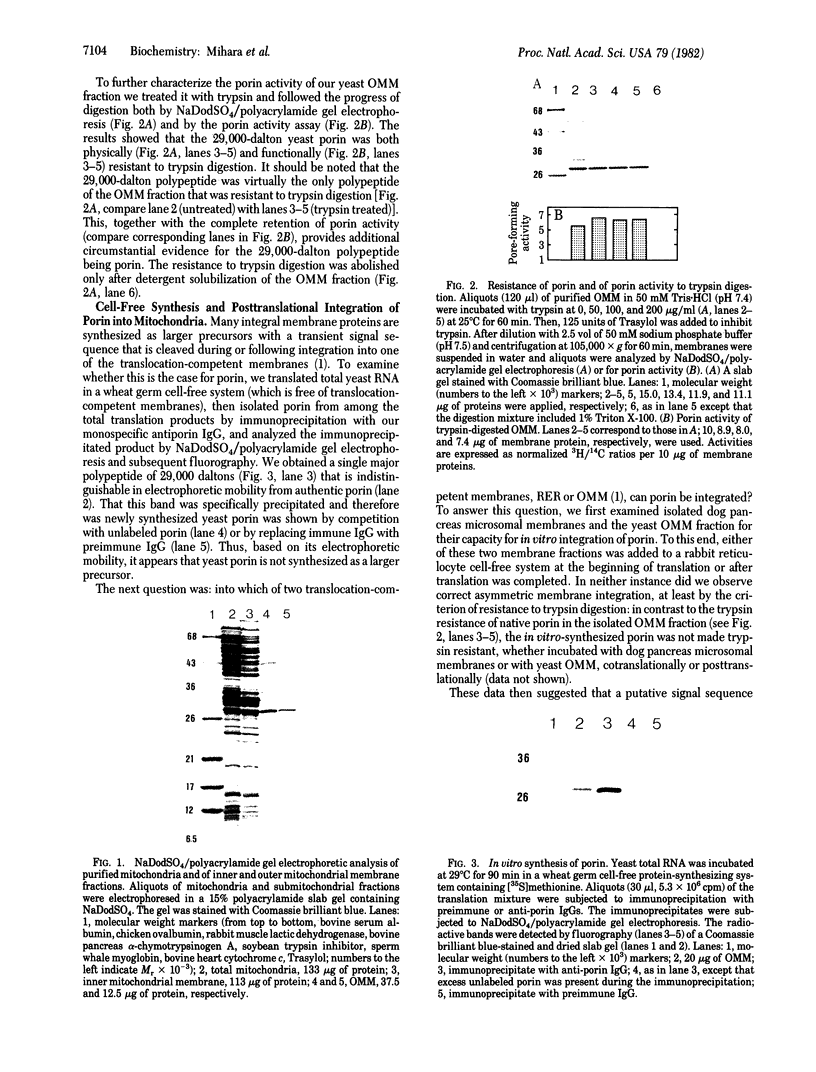
We have isolated an outer mitochondrial membrane (OMM) fraction from baker's yeast. Saccharomyces cerevisiae, that possesses porin activity and contains a major polypeptide of 29,000 daltons. By analogy to similar data for an OMM fraction from rat liver and mung bean [Zalman, L. S., Nikaido, N. & Kagawa, Y. (1980) J. Biol. Chem. 255, 1771-1774], the 29,000-dalton polypeptide of the isolated yeast OMM fraction has been tentatively identified as porin. Evidence to substantiate this identification was provided by the finding that both the porin activity and the 29,000-dalton polypeptide were entirely resistant when the OMM fraction was exposed to trypsin digestion, with the 29,000-dalton polypeptide being virtually the only polypeptide in the OMM fraction to be unaffected by trypsin digestion. There was no protection when trypsin digestion was carried out in the presence of detergent. Using monospecific antibodies, we have shown that yeast porin is apparently not synthesized as a larger precursor in a cell-free translation system. In vitro-synthesized porin could not be integrated into dog pancreas microsomal vesicles or into an isolated OMM fraction from yeast, either co- or posttranslationally. In vitro-synthesized porin, however, could be integrated posttranslationally into whole isolated mitochondria. This membrane specificity suggests that integration does not proceed by unassisted partitioning. The integration of porin into whole mitochondria occurred with fidelity by the criterion of its resistance to trypsin. Moreover, integration was not inhibited in the presence of the protonophore carbonyl cyanide m-chlorophenyl-hydrazone whereas translocation into the mitochondrial matrix of the in vitro-synthesized gamma subunit of F1-ATPase was inhibited.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borgese N., Meldolesi J. Localization and biosynthesis of NADH-cytochrome b5 reductase, an integral membrane protein, in rat liver cells. I. Distribution of the enzyme activity in microsomes, mitochondria, and golgi complex. J Cell Biol. 1980 Jun;85(3):501–515. doi: 10.1083/jcb.85.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Ito A. Cytochrome b5-like hemoprotein of outer mitochondrial membrane: OM cytochrome b. II. Contribution of OM cytochrome b to rotenone-insensitive NADH-cytochrome c reductase activity. J Biochem. 1980 Jan;87(1):73–80. doi: 10.1093/oxfordjournals.jbchem.a132754. [DOI] [PubMed] [Google Scholar]
- Kellems R. E., Butow R. A. Cytoplasmic-type 80 S ribosomes associated with yeast mitochondria. I. Evidence for ribosome binding sites on yeast mitochondria. J Biol Chem. 1972 Dec 25;247(24):8043–8050. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Corte G., Pietrini G., Borgese N. Localization and biosynthesis of NADH-cytochrome b5 reductase, an integral membrane protein, in rat liver cells. II. Evidence that a single enzyme accounts for the activity in its various subcellular locations. J Cell Biol. 1980 Jun;85(3):516–526. doi: 10.1083/jcb.85.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Blobel G. The four cytoplasmically made subunits of yeast mitochondrial cytochrome c oxidase are synthesized individually and not as a polyprotein. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4160–4164. doi: 10.1073/pnas.77.7.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Müller H. Synthesis and processing of in vitro and in vivo precursors of the vacuolar yeast enzyme carboxypeptidase Y. J Biol Chem. 1981 Dec 10;256(23):11962–11965. [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. Distinct repressible mRNAs for cytoplasmic and secreted yeast invertase are encoded by a single gene. Cell. 1981 Aug;25(2):525–536. doi: 10.1016/0092-8674(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., McKemmie E. Post-translational processing and transport of the polyprotein precursor to subunits IV to VII of yeast cytochrome c oxidase. J Biol Chem. 1979 Jul 25;254(14):6772–6780. [PubMed] [Google Scholar]
- Shore G. C., Power F., Bendayan M., Carignan P. Biogenesis of a 35-kilodalton protein associated with outer mitochondrial membrane in rat liver. J Biol Chem. 1981 Aug 25;256(16):8761–8766. [PubMed] [Google Scholar]
- Thomas P. E., Korzeniowski D., Ryan D., Levin W. Preparation of monospecific antibodies against two forms of rat liver cytochrome P-450 and quantitation of these antigens in microsomes. Arch Biochem Biophys. 1979 Feb;192(2):524–532. doi: 10.1016/0003-9861(79)90122-x. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Nikaido H., Kagawa Y. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J Biol Chem. 1980 Mar 10;255(5):1771–1774. [PubMed] [Google Scholar]
- Zimmermann R., Hennig B., Neupert W. Different transport pathways of individual precursor proteins in mitochondria. Eur J Biochem. 1981 Jun 1;116(3):455–460. doi: 10.1111/j.1432-1033.1981.tb05357.x. [DOI] [PubMed] [Google Scholar]