Abstract
Antipsychotic drug therapy, e.g., risperidone, can be associated with endocrine abnormalities, including an increase in serum prolactin level (sPrl) due to a drug-induced benign pituitary tumor (prolactinoma). A few case reports have noted a resolution of hyperprolactinemia and prolactinoma after cessation of risperidone treatment. We report a similar finding for a woman with schizoaffective disorder, manic type.
Due to a neurological disorder involving the tongue (tardive dyskinesia), a woman with schizoaffective disorder switched from 50mg thioridazine after 21 years to 2mg of risperidone at bedtime for 10 years. Elevated sPrl was noted in June and August 2005 (83.8 and 100.1µg/L; normal: 3.4–24.1µg/L) and a cranial magnetic resonance imaging scan showed evidence of a small area of decreased signal in the pituitary gland consistent with a microadenoma. The subject transitioned slowly to ziprasidone and off risperidone in October and November of 2005. The prolactinoma completely resolved with the switch to ziprasidone. It is recommended that sPrl be measured annually in patients taking antipsychotic drugs to test for any indication of pituitary prolactinoma that could suggest the need to switch the primary treatment to another drug.
Keywords: Prolactinoma, schizoaffective disorder, mania, risperidone, ziprasidone, pituitary tumor, prolactin
Introduction
It has been established that antipsychotic drug therapy with either first-generation or second-generation agents can be associated with side effects such as weight gain and endocrine abnormalities noted in metabolic profiles for cholesterol, triglycerides, glucose, hemoglobin and/or prolactin.1,2 An observed increase in serum prolactin level is often due to a drug-induced benign pituitary tumor (prolactinoma),3–8 with risperidone utilization by far outnumbering other antipsychotic medications in causing pituitary tumors,9 especially in women.10 A few case reports have noted a resolution of hyperprolactinemia and prolactinoma after cessation of risperidone treatment.11,12 We report a similar finding for a woman with schizoaffective disorder, manic type.
Case Report
The subject, a Caucasian woman, was 36 years old when she had her first hospitalization for a psychotic break. The treatment included electroconvulsive therapy and initiation of medication management with thioridazine. The onset of this affective psychosis, involving cognitive as well as behavior problems, occurred acutely in June of 1974, at which time she began taking 50mg thioridazine hydrochloride a day. There was good premorbid adjustment and no prior psychiatric history.
Through two structured interviews, a diagnosis of schizoaffective disorder (Research Diagnostic Criteria schizoaffective disorder, manic type) was made and later confirmed (see Diagnostic and Statistical Manual of Mental Disorders, Third Edition). The subject manifested false sensory impressions, a distortion of the outside world, elation, and religious experiences, but not passivity or withdrawal. In the intermediate psychotic areas of schizoaffective disorder, symptoms and characteristics of both schizophrenia and affective illness were present. There were enough affective symptoms to make the diagnosis of schizophrenia unlikely and sufficient thought and behavior disorder to make the diagnosis of affective disorder unlikely. A relapse of severe symptomatology instigated treatment with lithium at 300mg 3 to 4 times per day between June 6 and August 31, 1980, which was effective in ameliorating the schizophrenic-manic symptoms.
Subsequent spontaneous manic episodes were milder (hypomania) and were successfully treated at home with thioridazine. During intervals between manic episodes, the subject was euthymic, not experiencing clinical depression, but did retain certain residual symptoms, including delusions and preoccupation unaccompanied by strong affect. At no time was psychosis associated with alcohol, drug abuse, or known organic disease. Thioridazine was tapered significantly the year before a second hospitalization at age 54 for two weeks in June of 1992.
The subject, an educator with an interest in the science of biological rhythms (chronobiology) in regard to health, participated in several biomedical experiments throughout her illness. Circannual (about yearly) and menstrual cycle rhythms were found in the onset of eleven manic episodes when self-measured basal body temperatures collected daily over 11 years were analyzed for periodicity. The timing of the manic episodes was not random, but occurred during distinct portions of the menstrual cycle and the year: the onset of nearly all manic days occurred near menses and during the follicular phase of the menstrual cycle (up to ovulation) and between December and May (winter to spring).13 A second study involving self-measurement of several physiologic and psychologic variables every three hours during waking over a 7.5-month span from 1979 to 1980, including around-the-clock urine collections during four 1.5 to 4-week subspans, found an increase in the 24-hour mean levels of urinary epinephrine and norepinephrine and a delay in the circadian acrophase (i.e., peak of a 24-hour cosine) for these two catecholamines just prior to the onset of a manic episode, indicating that the peak values of the 24-hour rhythm occurred later in the day than normal.14 A third study, a four-year case report based upon daily recording of 125 variables by the subject upon awakening and at bedtime, corroborated that mania occurred in the spring.15
A neurological disorder characterized by involuntary fasciculation (contractions or twitching) of various muscles of the tongue developed after several years using thioridazine, possibly indicating the onset of tardive dyskinesia (TD). As newer drugs were developed that had less chance of TD, she switched off of thioridazine in early 1995 near age 57 and began taking 2mg of risperidone (Risperdal®, Janssen Pharma) at bedtime for 10 years. The risperidone was efficacious and created great emotional stability in the subject with no manic episodes. On June 17, 2005, a healthcare provider brought the Wall St. Journal’s article “J&J’s Risperdal Raises Tumor Issue”16 to the subject’s attention. As a result, her serum prolactin level was tested on June 23, 2005, and was found to be very elevated at 83.8µg/L (normal range=3.4–24.1µg/L for nonpregnant women). Since this was not a fasting test, subsequent prolactin levels were always measured 1.5 to 2 hours after awakening with fasting, due to a significant diurnal rhythm in prolactin and its increase by eating.6
Subsequently, a cranial magnetic resonance imaging (MRI) scan with and without contrast showed evidence of a small area of decreased signal in the pituitary gland of 2mm consistent with a microadenoma. There was no impression on the optic chiasm, and the cavernous sinuses were normal. An August 31, 2005, prolactin level was 100.1µg/L. The subject subsequently agreed to transition slowly to ziprasidone (Geodon®, Pfizer) by an increase of 40mg and taper off from risperidone by 0.5mg less every other week, beginning on October 10, 2005. The final switch was November 28, 2005, to 80mg of ziprasidone at breakfast and 80mg at 8PM.
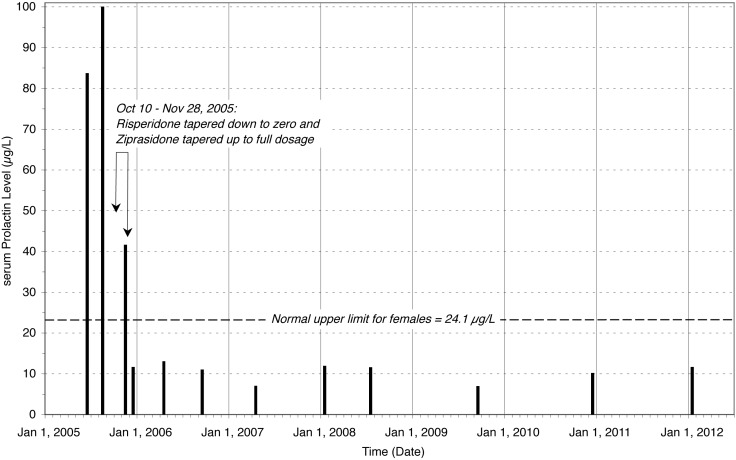
On Nov 10, 2005, a month after beginning to switch to ziprasidone only, the prolactin level had significantly dropped to 41.7µg/L, was in the middle of the normal range at 11.7µg/L on Dec 15, 2005, and on April 18, 2006 it remained normal at 13.1µg/L. A follow-up MRI on May 26, 2006, showed normal enhancement of the pituitary gland and stalk and no further signal attenuation corresponding to the area previously considered suspicious for adenoma. Subsequent prolactin levels (in µg/L) over the following 5.5 years all remained within the normal range: 11.1 on Sept 5, 2006; 7.1 on April 5, 2007; 12.0 on Jan 8, 2008; 11.6 on July 15, 2008; 7.0 on Sept 17, 2009; 10.2 on Dec 30, 2010, and 11.7 on Jan 12, 2012 (Figure 1).
Figure 1.
Risperidone-induced prolactinoma resolved in a woman with schizoaffective disorder by switching to ziprasidone. Risperidone was taken at bedtime for 10 years beginning in 1995, and elevated prolactin levels were found on June 23 and Aug 31, 2005. The subject transitioned slowly to ziprasidone and off risperidone from October 10 to Nov 28, 2005, after which all follow-up prolactin levels over the next 5.5 years through Jan 12, 2012 remained within the normal range.
Risperidone was reportedly more efficacious than ziprasidone, since the subject had no exacerbation of symptoms when experiencing significant Axis IV stressors, whereas ziprasidone led to more subjective experience of instability. After switching to ziprasidone and the subsequent decrease in prolactin, the subject experienced increased libido and improved weight loss.
Discussion
Because the release of prolactin from the pituitary gland is inhibited by dopamine, any process resulting in a reduction in dopamine increases prolactin levels.17 Some antipsychotic drugs block dopamine in the tuberoinfundibular pathway, which can cause an increase in blood prolactin levels (hyperprolactinemia). The tuberoinfundibular tract refers to a population of short axons in the arcuate nucleus of the mediobasal hypothalamus (the “tuberal region”) that project to the median eminence (the “infundibular region”) that release dopamine into the portal veins of the pituitary gland. It is one of the four major dopamine pathways in the brain. Risperidone has been found to persistently elevate prolactin in human studies, despite its impact on other receptor sites, while ziprasidone has been found to have little effect or produce decreases in prolactin.18 Thus, even though ziprasidone is a dopamine antagonist, it is less likely than risperidone to inhibit dopamine receptors enough to induce certain types of pituitary hyperplasia.19
Conclusion
It is recommended that a serum prolactin level be measured at least annually in patients taking risperidone or similar antipsychotic drugs20 to see if there is any indication of a medication-induced pituitary prolactinoma, which could suggest the need to stop primary drug treatment and/or switch to an alternative drug.10,21
Footnotes
FUNDING: No funding was received for the development of this article.
FINANCIAL DISCLOSURES: All authors report no financial interests or any other potential conflicts of interest.
References
- 1.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA, Stroup TS. The NIMH-CATIE Schizophrenia Study: what did we learn? Am J Psychiatry. 2011;168:770–775. doi: 10.1176/appi.ajp.2011.11010039. [DOI] [PubMed] [Google Scholar]
- 3.Popli A, Gupta S, Rangwani SR. Risperidone-induced galactorrhea associated with a prolactin elevation. Ann Clin Psychiatry. 1998;10:31–33. doi: 10.1023/a:1026150629199. [DOI] [PubMed] [Google Scholar]
- 4.Dickson R, Glazer W. Neuroleptic-induced hyperprolactinemia. Schizophr Res. 1999;35 doi: 10.1016/s0920-9964(98)00159-5. S75-86 Review. [DOI] [PubMed] [Google Scholar]
- 5.Hariharan J, Moshin J. Risperidone induced galactorrhea: a case analysis. WMJ. 2002;101:41–43. [PubMed] [Google Scholar]
- 6.Halbreich U, Kahn LS. Hyperprolactinemia and schizophrenia: mechanisms and clinical aspects. J Psychiatr Pract. 2003;9:344–353. doi: 10.1097/00131746-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinemia in adolescents. J Clin Psychopharmacol. 2006;26:167–171. doi: 10.1097/01.jcp.0000203194.58087.9a. [DOI] [PubMed] [Google Scholar]
- 8.Kopecek M, Bares M, Horacek J, Mohr P. Low dose risperidone augmentation of antidepressants or anxiolytics is associated with hyperprolactinemia. Neuro Endocrinol Lett. 2006;27:803–806. [PubMed] [Google Scholar]
- 9.Szarfman A, Tonning JM, Levine JG, Doraiswamy PM. Atypical antipsychotics and pituitary tumors: a pharmacovigilence study. Pharmacotherapy. 2006;26:748–758. doi: 10.1592/phco.26.6.748. [DOI] [PubMed] [Google Scholar]
- 10.Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Hyperprolactinemia in response to antipsychotic drugs: characterization across comparative clinical trials. Psychoneuroendocrinol Suppl. 2003;2:69–82. doi: 10.1016/s0306-4530(02)00128-2. [DOI] [PubMed] [Google Scholar]
- 11.Pal JK, Sarino WA. Effect of risperidone on prolactinoma growth in a psychotic woman. Psychosom Med. 2000;62:736–738. doi: 10.1097/00006842-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Mendhekar DN, Jiloha RC, Srivastava PK. Effect of risperidone on prolactinoma - a case report. Pharmacopsychiatry. 2004;37:41–42. doi: 10.1055/s-2004-815474. [DOI] [PubMed] [Google Scholar]
- 13.Sothern R, Slover G, Morris R. Circannual and menstrual rhythm characteristics in manic episodes and body temperature. Biol Psychiatry. 1993;33:194–203. doi: 10.1016/0006-3223(93)90139-5. [DOI] [PubMed] [Google Scholar]
- 14.Slover G, Sothern R, Scheving L, et al. Urinary and self-measured circadian and circatrigintan rhythms before and after a human manic episode. In: Halberg F, Reale L, Tarquini B, editors. Proc II Intl Symp on Chronobiologic Approach to Social Medicine. Florence, Oct. 2, 1984. Rome: Instituto Italiano di Medicina Sociale; 1984. pp. 427–441. [Google Scholar]
- 15.Slover G, Morris RW, Stroebel CF. Case study of psychophysiological diary: Infradian rhythms. Pauly JE, Scheving LE, editors. Advances in Chronobiology, Part B Proc XVIIth Intl Conf Intl Soc Chronobiology, Little Rock, Nov 1985. New York: Alan R Liss; 1987:439-444. [PubMed] [Google Scholar]
- 16.Abboud L. J&J’s Risperdal raises tumor issue. Wall Street J. 2005 Jun 17; pp A3. [Google Scholar]
- 17.Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res. 1999;35(Suppl):S67–S73. doi: 10.1016/s0920-9964(98)00158-3. [DOI] [PubMed] [Google Scholar]
- 18.Goodnick PJ, Rodriguez L, Santana O. Antipsychotics: impact on prolactin levels. Expert Opin Pharmacother. 2002;3:1381–1391. doi: 10.1517/14656566.3.10.1381. [DOI] [PubMed] [Google Scholar]
- 19.Molitch ME. Medication-induced hyperprolactinemia. Mayo Clin Proc. 2005;80:1050–1057. doi: 10.4065/80.8.1050. [DOI] [PubMed] [Google Scholar]
- 20.Gianfrancesco FD, Pandina G, Mahmoud R, et al. Potential bias in testing for hyperprolactinemia and pituitary tumors in risperidone-treated patients: a claims-based study. Ann Gen Psychiatry. 2009;8:5. doi: 10.1186/1744-859X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65:265–273. doi: 10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]