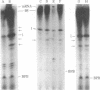
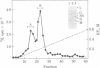
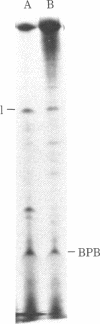
Abstract
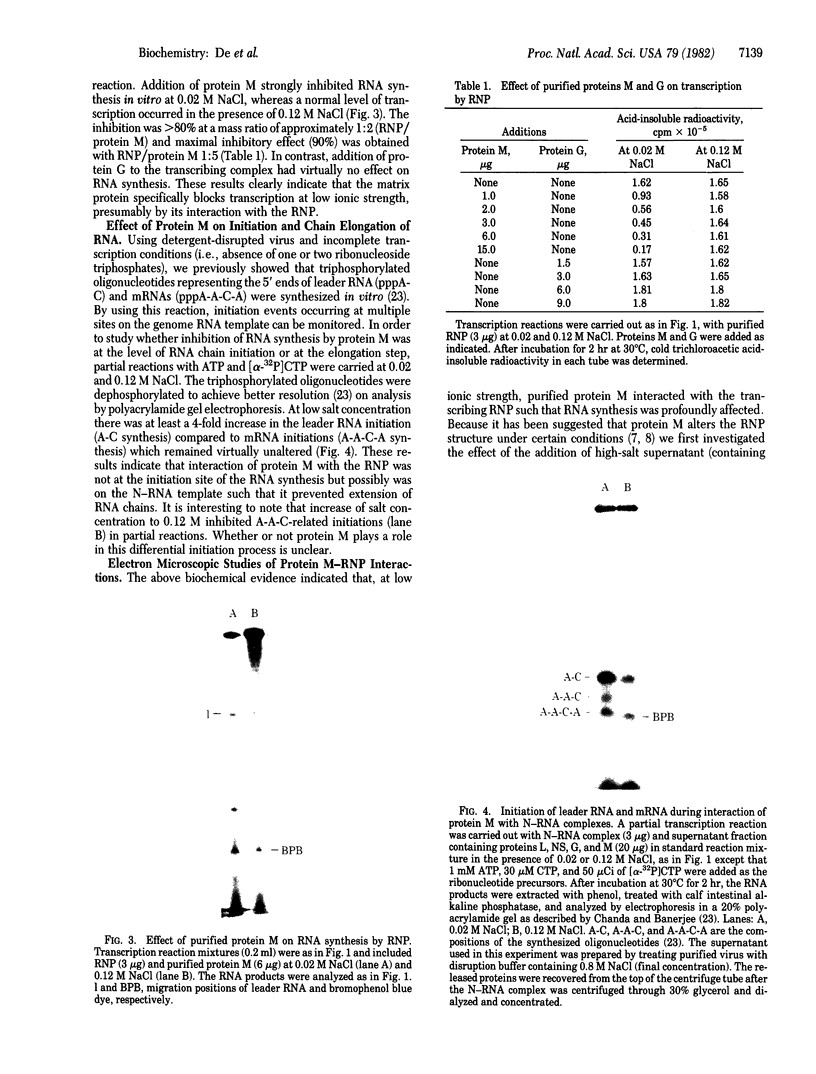
One of the major structural proteins of vesicular stomatitis virus is a small, nonglycosylated, matrix protein which associates with the nucleocapsid core during final stages of morphogenesis and budding. Biochemical and genetic studies suggested that the matrix protein regulates RNA synthesis both in vitro and in vivo. We have purified biologically active matrix protein from the virus and have directly shown that it significantly inhibits RNA synthesis in vitro mediated by the virion-associated RNA polymerase at low ionic strength (0.02 M). The inhibition was greater than 80% when the ratio of matrix protein to the major nucleocapsid protein in the transcribing complex was 2:1 (wt/wt). The inhibition was found to be at the level of RNA chain elongation and not at the initiation step. Electron microscopic studies revealed that inhibition of transcription by matrix protein was accompanied by a profound structural change of the transcribing nucleocapsid from an extended structure to a highly compact form. At higher ionic strength (0.12 M), the matrix protein failed to interact with the nucleocapsid. The matrix protein appears to be involved in condensing the nucleocapsid and blocking transcription during maturation of the virus particle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. The nature of the RNA products synthesized in vitro by subviral components of visicular stomatitis virus. Virology. 1976 May;71(1):230–241. doi: 10.1016/0042-6822(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Moyer S. A., Rhodes D. P. Studies on the in vitro adenylation of RNA by vesicular stomatitis virus. Virology. 1974 Oct;61(2):547–558. doi: 10.1016/0042-6822(74)90289-x. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Identification of promoter-proximal oligonucleotides and a unique dinucleotide, pppGpC, from in vitro transcription products of vesicular stomatitis virus. J Virol. 1981 Jul;39(1):93–103. doi: 10.1128/jvi.39.1.93-103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Scheid A., Choppin P. W. Conformation of the helical nucleocapsids of paramyxoviruses and vesicular stomatitis virus: reversible coiling and uncoiling induced by changes in salt concentration. Proc Natl Acad Sci U S A. 1980 May;77(5):2631–2635. doi: 10.1073/pnas.77.5.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin M. Intracellular potassium and macromolecular synthesis in mammalian cells. Nature. 1967 Feb 4;213(5075):451–453. doi: 10.1038/213451a0. [DOI] [PubMed] [Google Scholar]
- Martinet C., Combard A., Printz-Ané C., Printz P. Envelope proteins and replication of vesicular stomatitis virus: in vivo effects of RNA+ temperature-sensitive mutations on viral RNA synthesis. J Virol. 1979 Jan;29(1):123–133. doi: 10.1128/jvi.29.1.123-133.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Kolakofsky C. M., Summers D. F. Comparison o;f vesicular stomatitis virus intracellular and virion ribonucleoproteins. J Virol. 1980 Feb;33(2):856–865. doi: 10.1128/jvi.33.2.856-865.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. doi: 10.1128/jvi.39.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Kingsbury D. T. Inhibitor of vesicular stomatitis virus transcriptase in purified virions. Nature. 1974 Mar 1;248(5443):45–47. doi: 10.1038/248045a0. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J., Chanda P. K., Thornton G. B., Luk D., Banerjee A. K. Activation of the virion-associated RNA polymerase of vesicular stomatitis virus by melittin. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1265–1271. doi: 10.1016/0006-291x(82)90923-8. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Dickson C., Weiss R. A. Morphological and biochemical characterization of viral particles produced by the tsO45 mutant of vesicular stomatitis virus at restrictive temperature. J Virol. 1979 Jan;29(1):185–195. doi: 10.1128/jvi.29.1.185-195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Sogo J. M., Koller T. A routine method for protein-free spreading of double- and single-stranded nucleic acid molecules. Proc Natl Acad Sci U S A. 1975 Jan;72(1):83–87. doi: 10.1073/pnas.72.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Bennett P. L. Assembly of membrane glycoproteins studied by phenotypic mixing between mutants of vesicular stomatitis virus and retroviruses. Virology. 1980 Jan 30;100(2):252–274. doi: 10.1016/0042-6822(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]