Abstract
The goal of this study was to identify whether impaired cortical preparation may relate to impaired scaling of postural responses of people with Parkinson’s disease (PD). We hypothesized that impaired scaling of postural responses in participants with PD would be associated with impaired set-dependent cortical activity in preparation for perturbations of predictable magnitudes. Participants performed postural responses to backward surface translations. We examined the effects of perturbation magnitude (predictable small vs. predictable large) and predictability of magnitude (predictable vs. unpredictable-in-magnitude) on postural responses (center-of-pressure (CoP) displacements) and on preparatory electroencephalographic (EEG) measures of contingent negative variation (CNV) and alpha and beta event-related desynchronization (ERD). Our results showed that unpredictability of perturbation magnitude, but not the magnitude of the perturbation itself, was associated with increased CNV amplitude at the CZ electrode in both groups. While control participants scaled their postural responses to the predicted magnitude of the perturbation, their condition-related changes in CoP displacements were not correlated with condition-related changes in EEG preparatory activity (CNV or ERD). In contrast, participants with PD did not scale their postural responses to the predicted magnitude of the perturbation, but they did demonstrate greater beta ERD in the condition of predictably small-magnitude perturbations and greater beta ERD than the control participants at the CZ electrode. In addition, increased beta ERD in PD was associated with decreased adaptability of postural responses, suggesting that preparatory cortical activity may have a more direct influence on postural response scaling for people with PD than for control participants.
Keywords: Parkinson’s disease, electroencephalography, contingent negative variation, event related desynchronization, posture, preparation
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease that leads to postural instability and falls, with an impaired ability to recover from an induced loss of balance. One such impairment includes a decline in modifiability of postural responses in people with PD, which likely involves brain structures beyond the basal ganglia, such as the cortex (Horak et al. 1996; Bloem et al. 2001; Jacobs and Horak 2006). We have previously demonstrated that the magnitudes of postural responses are based not only on sensory drive from the perturbations, but also on central set – a neural state of readiness to receive a stimulus and generate a movement based on existing contextual factors, such as the predictability of impending postural perturbation characteristics (Horak et al. 1989; Prochazka 1989). We have also shown that PD impairs scaling of postural responses based on central set (Horak et al. 1996; Chong et al. 1999; Chong et al. 2000). This study sought to identify how PD affects preparatory cortical activity prior to postural perturbations induced by translations of the support surface.
The cortex is known to contribute to balance control (Dietz et al. 1985; Duckrow et al. 1999; Slobounov et al. 2005; Adkin et al. 2006; Jacobs and Horak 2007), and PD may adversely affect the ability of the cortex to prepare for postural perturbations. Recent evidence from our laboratory suggests a role for the cortex in preparatory adjustment of postural responses based on knowledge about the upcoming perturbation. Using electroencephalography (EEG), we demonstrated that contingent negative variation (CNV), a slow negative shift in EEG amplitude, is evident prior to pre-cued postural perturbations in healthy young adults, and the CNV amplitudes were associated with cue-related improvements in response displacements (Jacobs et al. 2008). Other researchers have also observed the presence of CNV prior to predictably timed perturbations (Adkin et al. 2008; Mochizuki et al. 2008; Mochizuki et al. 2010). In addition, we observed that healthy young adults scale the magnitudes of their postural responses to the magnitude of surface translations for predictable perturbations, but not for unpredictable-in-magnitude perturbations (Horak and Diener 1994). Other researchers have also demonstrated similar effects of previous experience on postural responses (Badke et al. 1987; Maki and Whitelaw 1993). Persons with PD, however, show smaller than normal postural responses and an impaired ability to adjust the magnitude of their postural responses based on previous experience (Beckley et al. 1993; Horak et al. 1996). We hypothesized that impaired preparatory cortical activity associated with PD may contribute to impaired postural responses.
We utilized two approaches to analyze our EEG data of cortical preparatory activity. We measured the amplitude of CNV and quantified event-related spectral changes in the upper alpha and beta bands. CNV was first identified as a slow, negative shift in EEG amplitude that occurs prior to voluntary movements performed in response to a warning cue that is followed by an imperative stimulus (Walter et al. 1964). The CNV has maximum negative amplitude over the vertex of the scalp and its amplitude peaks around movement onset. It represents both non-motor processes related to the anticipation of the second stimulus and sensorimotor processes related to preparation of the movement (van Boxtel and Brunia 1994a; Fischer et al. 2010). Known generators of the CNV motor components include the supplementary motor, lateral premotor and primary sensorimotor cortex (Lamarche et al. 1995; Hamano et al. 1997; Bares et al. 2007). Smaller amplitude CNVs have been observed in persons with PD on medication compared to control participants in tasks that require participants to respond by pushing a specific button (Aotsuka et al. 1996; Praamstra et al. 1996; Pulvermuller et al. 1996) or with a wrist extension movement (Ikeda et al. 1997). Another response-button task demonstrated smaller CNV amplitudes in persons with PD off medication compared to control participants (Oishi et al. 1995). It is not known whether abnormal CNV amplitudes would also be evident in people with PD when preparing for a response to an external postural perturbation.
Because the CNV potential represents a complex and poorly localized potential related to both cognitive and motor processes of anticipation for the response to the imperative stimulus, we also evaluated time-varying spectral changes preceding the postural response in the upper alpha and beta bands. Because the CNV precedes both voluntary movement and postural responses, we predicted that event-related spectral changes preceding postural responses would be similar to event-related spectral changes that are evident prior to voluntary movement: event-related desynchronization (ERD). ERD quantifies an event-related suppression of the EEG signal within a frequency band, representing a decrease in synchrony of oscillations of neural population firings (Pfurtscheller 1977; Pfurtscheller and Aranibar 1977; Pfurtscheller and Lopes da Silva 1999). Based on literature evaluating spectral changes in EEG signals prior to voluntary movements, decreases in synchronicity within the alpha band (8–13 Hz) represent increased activity in cortical areas involved in processing of sensory or cognitive information or production of motor behavior (Pfurtscheller 1992). Unlike the CNV, ERD within the upper alpha frequencies represents a topographically more focused change in activation state within the sensory-motor cortex. Specifically, when preceding voluntary foot or hand movements, ERD within the upper alpha frequencies isolates to the central-parietal electrodes somatotopically relevant to the movement (i.e., medial versus lateral electrodes), and is thought to represent enhanced cortical excitability or readiness for an impending movement (Pfurtscheller 2000). The upper beta ERD, however, localizes at mesial central electrodes, thought to represent changes in synchronization of circuits involving the supplementary motor area (Fogelson et al. 2006; Wheaton et al. 2008). Further, the upper alpha and beta bands appear to represent synchrony of distinct circuits among the cortex, basal ganglia, and thalamus (Fogelson et al. 2006; Klostermann et al. 2007). Thus, the measures of cortical activity (CNV, as well as the upper alpha and beta ERD) provide unique measures of cerebrocortical activity prior to movement (Babiloni et al. 1999; Filipovic et al. 2001; Bender et al. 2004).
The evaluation of cortical activity prior to an externally-induced loss of balance is extremely novel. Evidence of CNV prior to postural perturbations has only recently been described for young, healthy adults (Jacobs et al. 2008). Although alpha ERD was evident at right-hemisphere central-parietal electrodes when comparing eyes-open to eyes-closed conditions of standing sway (Del Percio et al. 2007) as well as when maintaining standing balance amidst audio biofeedback versus sham feedback (Pirini et al. 2011), we are unaware of any reports that evaluate ERD prior to postural perturbations. Thus, this study provides novel indices of cortical preparation prior to an anticipated, induced loss of balance in people with and without PD.
We predict that impaired response scaling in PD is associated with impaired preparatory cortical activity (CNVs and ERD) compared to healthy control participants. We propose, as have others (Mochizuki et al. 2010), that one of the many roles of anticipatory cortical activity is to scale the magnitude of postural responses for the anticipated magnitude of postural perturbations. We thus expected to see smaller CNV and differences in ERD before predictable small compared to predictable large perturbations. It is also possible, however, that knowing (vs. not knowing) the magnitude of the perturbation affects cortical preparation regardless of perturbation size, so we also compared predictable perturbations to unpredictable-in-magnitude perturbations. We collected EEG data of cortical preparation and kinetic center of pressure (CoP) data of postural responses in order to study both cortical preparation and subsequent postural responses.
Materials and Methods
Participants
Participants were 12 adults with moderate PD (Hoehn and Yahr Stage II or III) and 12 healthy control participants. There were no significant group differences in body mass index, height or age (see Table 1). Participants with PD were recruited from the Parkinson’s Center of Oregon and community support groups. They were sensitive to dopamine replacement medication and did not have history or evidence of physical or mental disability not associated with PD. Control participants had neurological and musculoskeletal function within normal limits. All participants were without pain or other conditions that would prevent standing independently for 30 minutes and had vision corrected to 20/40 or better. Participants were not taking medications known to affect balance or attention (other than medications for PD) and were without cognitive impairment (score >= 26 on the Montreal Cognitive Assessment (Nasreddine et al. 2005)).
Table 1.
Descriptive Characteristics: Mean (95% Confidence Interval of Mean) of Participants With Parkinson’s Disease (PD) and Control Participants
| PD | Control | Results for group differences (analysis of variance) | |
|---|---|---|---|
| Weight (kg) | 79.84 (74.57–85.10) | 68.41 (60.35–76.46) | F[1,22]= 6.84 p = 0.02 |
| Height (m) | 1.79 (1.74–1.84) | 1.72 (1.66–1.78) | F[1,22]= 3.61, p = 0.07 |
| Body Mass Index | 24.95 (23.32–26.59) | 23.09 (20.87–25.30) | F[1,22]= 2.23 p = 0.15 |
| Age (yrs) | 67.5 (63.1–72.9) | 62.9 (58.4–67.4) | F[1,22]= 2.60, p = 0.12 |
| Stance width (%)* | 9.5% (8.4%–10.6%) | 9.4% (8.6%–10.2%) | F[1,22]=0.01, p = 0.93 |
| Gender | 11m, 1f | 6m, 6f | |
| Duration of PD (mos) | 77 (47–107) | ||
| UPDRS Motor Score | 23 (18–29) |
stance width measured from calcaneuos to calcaneous and normalized to height as percent of height
Procedures
The Oregon Health and Science University Institutional Review Board approved all procedures. All participants came into the laboratory for one 2–2.5-hour visit. We explained all procedures to them and answered any questions before they signed an informed consent document. We administered a health history survey, the Montreal Cognitive Assessment and, for participants with PD, the Motor, Part IV of the Unified Parkinson’s Disease Rating Scale (original version). Participants with PD were tested in the ‘On’ medication state.
Force plate data were collected at 480 Hz with a custom QNX system (QNX Software Systems, Ottowa, Ontario, CA). We placed an Advanced Neuro Technology (ANT; Enschede, the Netherlands) Waveguard 32-channel EEG cap (sintered silver/silver-chloride electrodes; standard 10/20 system placement) on the participants and used gentle abrasion of the scalp and a conductive electrode gel (Electro-gel; Electro-Cap International; Eaton, OH, USA) to obtain impedances below 5 kΩ. To identify eye movement artifacts, electrooculographic (EOG) recordings were measured by silver/silver-chloride EMG electrodes placed above and below the right orbit and lateral to the right and left orbits. EEG and EOG data were collected at 512 Hz using an ANT high-density ASA-Lab amplifier and software. Electrodes were referenced to linked mastoid electrodes.
Participants stood with one foot on each force plate with their feet at a comfortable, self-selected, approximately hip-distance width (see Table 1). We used tape outlines to provide consistent foot placement from trial to trial. Participants wore a harness attached without tension to the ceiling and had a spotter standing on their left side. They were instructed to stand quietly, with their arms resting at their sides just in front of their hips and their vision focused on a target area 1.25 m high on a board 3.5 m in front of them.
Participants knew that they would see a visual warning stimulus 2 s prior to a posterior translation of the force plates (perturbation). There were three sets of trials, with 30 perturbations per set. Set-order was randomized by participant. Each set consisted of predictable small perturbations, predictable large perturbations or unpredictable-in-magnitude perturbations. For the predictable small or predictable large perturbations, the participants knew they would receive the same magnitude of perturbation for the entire set. For the unpredictable-in-magnitude perturbations, the participants did not know whether they would receive a small or large magnitude perturbation. We allowed them to experience the small and large perturbations once each as practice before we began data collection, so that they were familiar with what to expect in each set.
The small postural perturbation consisted of a 6-cm backward translation and the large postural perturbation consisted of a 12-cm backward translation. Velocity and acceleration were the same for the first 100 ms of force plate movement for the small and large perturbations so that the participant could not distinguish small from large during the first part of the unpredictable-in-magnitude perturbations trials. Trials were performed in blocks of 10, followed by a rest period. The experimenters did not speak to the participant during the block of 10 trials, other than to confirm that the trial was complete and the force plates were resetting to their original position. After the force plates reset, the experimenter waited to observe the participant standing quietly with a stable baseline EEG signal before sending the visual warning cue and subsequent platform perturbation.
Data Analysis
EEG Data: Cortical Preparation
Raw EEG data were imported into MATLAB (The MathWorks, Natick, MA) EEGLAB (Delorme and Makeig 2004) for all analysis. We analyzed 90 trials (30 for each of 3 conditions) for most participants. For 7 out of the 24 participants (4 control and 3 PD), the number of trials available was, on average, 25 instead of 30, with a range of 22–29 trials, due to missing data or technical difficulties.
EEG data were resampled to 480 Hz to match the other data sources and then detrended to remove the direct current (DC) mean from our DC amplifier. Specifically, we used the MATLAB function “detrend” to remove the mean value from each column of the matrix. The detrend procedure was performed to remove slow drift from our recording, however it is important to acknowledge that this procedure may remove some task-related CNV activity in addition to removing noise. Data were low-pass filtered at 40 Hz. We did not high-pass filter the data due to the low frequency of the CNV. We extracted epochs from 3 s before to 0.5 s after the perturbation. Epochs were baseline subtracted using the mean from −3 to −2 s. We used Independent Components Analysis to remove eye blinks and other obvious noise from the EEG epochs (Delorme and Makeig 2004). Components were rejected if they represented eye blinks or muscle activity or had a highly unstable baseline. Generally, 4–7 components were rejected per participant.
EEG signals were averaged by participant and condition. The CNV amplitude was then determined as the mean amplitude of the average EEG signal during the final 100 ms prior to perturbation onset. To examine the effects of magnitude and predictability on the amplitude of CNV, we chose to analyze data from the electrode in each group that demonstrated the most CNV activity. Out of the electrodes demonstrating negative voltage during the 100 ms preceding the perturbation, as shown in the topographies in Figure 1, we found the largest negative amplitude at CZ in both groups (highlighted in bold, see Table 2).
Fig 1.
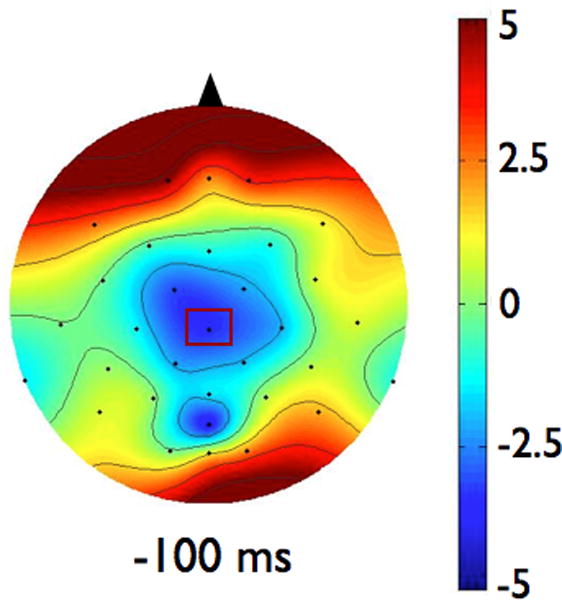
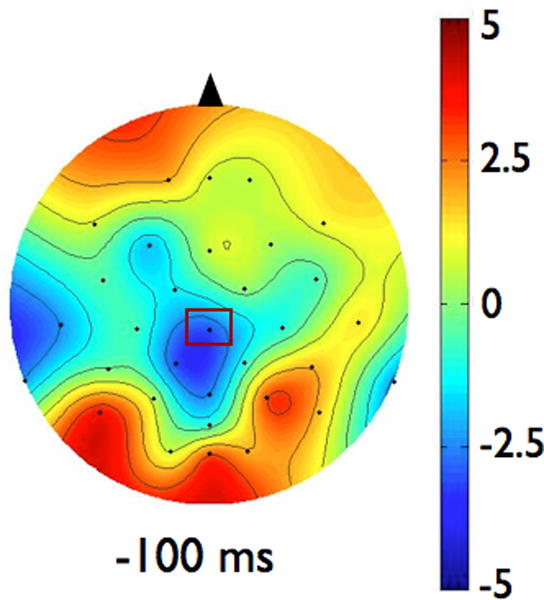
Scalp topographies represent the average voltage of the signal for each group across all electrodes at 100 ms before the imperative signal (postural perturbation). Scalp topographies were observed to be consistent across the last 500 ms so a representative time point is shown here. The view is of the head, from above, with the nose toward the top of the figure. Figure 1a shows data from the Parkinson’s disease (PD) group. Figure 1b presents data from the control group. To examine the effects of magnitude and predictability on the amplitude of CNV, we chose to analyze data from the electrode in each group that demonstrated the largest amplitude CNV. In both groups, largest CNV was at electrode CZ, which is marked in rectangles on the scalp topographies
Table 2.
Mean Contingent Negative Variation (CNV) and Standard Error of the Mean (SEM) by Electrode
| Electrode | PD Group Mean CNV (uV) |
PD Group SEM CNV (uV) |
Control Group Mean CNV (uV) |
Control Group SEM CNV (uV) |
|---|---|---|---|---|
| C3 | −1.02 | 1.10 | 0.54 | 1.38 |
| C4 | −2.05 | 0.64 | −1.79 | 1.37 |
| CP1 | −2.59 | 1.21 | −3.36 | 0.92 |
| CP2 | −2.32 | 1.03 | −1.62 | 1.15 |
| CZ | −3.92 | 1.21 | −4.00 | 1.31 |
| F3 | −0.67 | 0.55 | 0.14 | 1.32 |
| F4 | −1.09 | 0.60 | 0.10 | 0.78 |
| FC1 | −3.81 | 1.11 | −1.86 | 1.05 |
| FC2 | −2.81 | 0.70 | −1.90 | 1.02 |
| FZ | −0.84 | 0.85 | −1.95 | 1.53 |
| P3 | −0.17 | 0.86 | 0.98 | 1.23 |
| POZ | −3.91 | 2.66 | −0.12 | 1.54 |
| PZ | −2.06 | 1.13 | −2.88 | 1.70 |
For quantifying event-related spectral changes in expectation of ERD (Pfurtscheller and Lopes da Silva 1999), we analyzed all electrodes. The EEG signals from each trial were digitally re-referenced to a common average reference that included every cephalic electrode. This was done to obtain a reference-free analysis in order to identify a more focal spatial pattern of ERD (Pfurtscheller and Lopes da Silva 1999); due to a 32-electrode set, the average reference was chosen over the laplacian option. Continuous morlet wavelet transforms were performed within the upper alpha frequencies of 10–12 Hz and upper beta frequencies of 20–29 Hz for each of the artifact-free trials. The ERD of the upper bands was selected because they elicit a more focal spatial topography that is specific to the somatotopic representation of cortex relevant to the movement task (Pfurtscheller et al. 2000). These morlet coefficients were then rectified and averaged by condition and participant, followed by averaging across the upper alpha or beta bands. The time-varying alpha or beta coefficients were then normalized to a percentage change in alpha or beta activity from a baseline average calculated from the 500 ms before the warning cue.
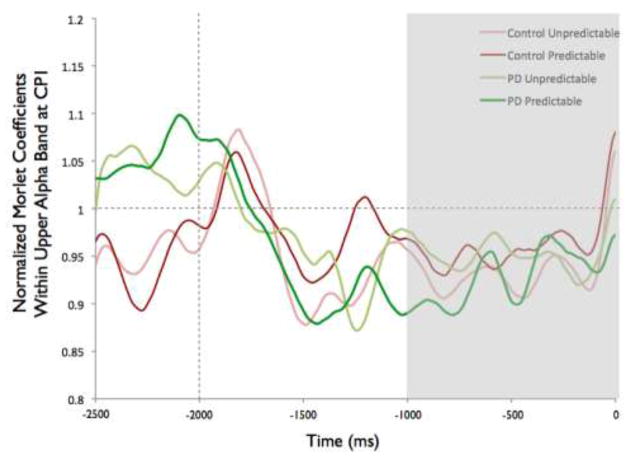
To examine the effects of magnitude and predictability on the amplitude of ERD, we chose to analyze data from the electrode in each group that demonstrated the most alpha or beta activity averaged over the final 1000 ms preceding the perturbation. For alpha ERD, as shown in the topographies in Figure 2, the largest amount of desynchronization occurred at the CP1 electrode in both groups (highlighted in bold, see Table 3). Figure 3 shows the topographies of the beta ERD response. The largest amount of beta ERD occurred at the CZ electrode in the PD group and at the CP2 electrode in the control group (highlighted in bold, see Table 3).
Fig 2.
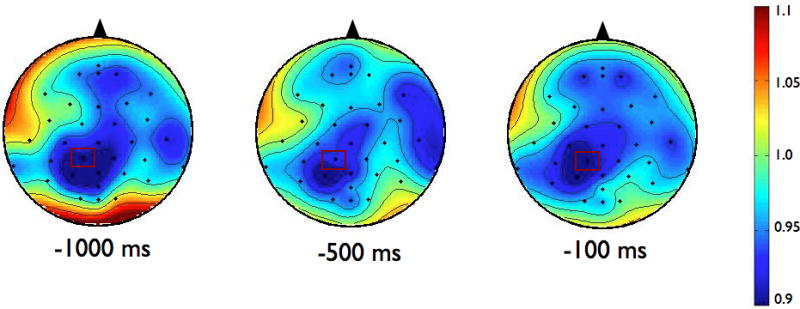
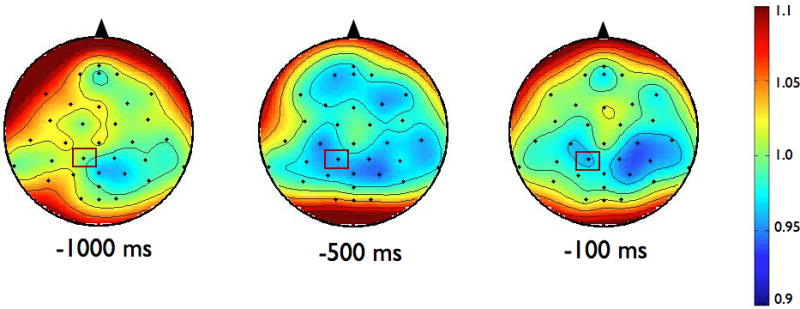
Scalp topographies of the upper alpha-band (10–12 Hz) event-related desynchronization (ERD). Figure 2a presents the Parkinson’s disease (PD) group while 2b shows the control group. To examine the effects of magnitude and predictability on alpha ERD, we chose to analyze data across the 1000 ms preceding the perturbation from the electrode in each group that demonstrated the most desynchronization. In both groups, this occurred at CP1, which is marked in rectangles on the scalp topographies
Table 3.
Mean Event-Related Desynchronization (ERD) and Standard Error of the Mean (SEM) by Electrode: Morlet Coeffiicents, Normalized to Baseline
| Electrode | PD Group Mean Alpha ERD |
PD Group SEM Alpha ERD |
Control Group Mean Alpha ERD |
Control Group SEM Alpha ERD |
PD Group Mean Beta ERD |
PD Group SEM Beta ERD |
Control Group Mean Beta ERD |
Control Group SEM Beta ERD |
|---|---|---|---|---|---|---|---|---|
| C3 | 0.96 | 0.02 | 0.99 | 0.02 | 0.95 | 0.01 | 0.97 | 0.01 |
| C4 | 0.97 | 0.02 | 0.95 | 0.02 | 0.99 | 0.01 | 0.96 | 0.01 |
| CP1 | 0.94 | 0.02 | 0.94 | 0.03 | 0.97 | 0.01 | 0.97 | 0.01 |
| CP2 | 0.95 | 0.02 | 0.95 | 0.03 | 0.97 | 0.01 | 0.95 | 0.01 |
| CP5 | 0.98 | 0.02 | 0.99 | 0.02 | 0.98 | 0.01 | 0.99 | 0.01 |
| CP6 | 1.02 | 0.02 | 0.99 | 0.02 | 1.00 | 0.01 | 0.98 | 0.01 |
| CZ | 0.96 | 0.02 | 0.97 | 0.02 | 0.92 | 0.01 | 0.98 | 0.01 |
| F3 | 1.01 | 0.01 | 0.99 | 0.02 | 0.97 | 0.01 | 0.97 | 0.01 |
| F4 | 0.99 | 0.02 | 0.99 | 0.02 | 0.97 | 0.01 | 0.96 | 0.01 |
| F7 | 1.00 | 0.01 | 1.00 | 0.02 | 0.98 | 0.01 | 0.98 | 0.01 |
| F8 | 0.97 | 0.02 | 0.96 | 0.02 | 0.97 | 0.01 | 0.97 | 0.01 |
| FC1 | 0.99 | 0.02 | 0.98 | 0.02 | 0.95 | 0.01 | 0.97 | 0.01 |
| FC2 | 0.96 | 0.02 | 0.98 | 0.02 | 0.95 | 0.01 | 0.96 | 0.01 |
| FC5 | 1.01 | 0.01 | 1.02 | 0.02 | 0.98 | 0.01 | 0.97 | 0.01 |
| FC6 | 0.99 | 0.02 | 0.98 | 0.02 | 0.98 | 0.01 | 0.98 | 0.01 |
| FP1 | 0.99 | 0.02 | 0.98 | 0.02 | 0.99 | 0.01 | 0.97 | 0.01 |
| FP2 | 0.99 | 0.02 | 1.02 | 0.02 | 0.98 | 0.01 | 0.97 | 0.01 |
| FPZ | 0.99 | 0.02 | 0.98 | 0.02 | 0.98 | 0.01 | 0.97 | 0.01 |
| FZ | 0.99 | 0.02 | 1.00 | 0.02 | 0.97 | 0.01 | 0.98 | 0.01 |
| O1 | 1.01 | 0.02 | 1.02 | 0.03 | 1.00 | 0.01 | 1.01 | 0.01 |
| O2 | 1.03 | 0.03 | 0.98 | 0.03 | 1.00 | 0.01 | 0.99 | 0.01 |
| OZ | 1.01 | 0.03 | 0.99 | 0.02 | 1.00 | 0.01 | 0.99 | 0.01 |
| P3 | 0.99 | 0.02 | 1.00 | 0.02 | 0.99 | 0.01 | 0.99 | 0.01 |
| P4 | 1.02 | 0.02 | 0.97 | 0.02 | 0.97 | 0.01 | 0.98 | 0.01 |
| P7 | 0.96 | 0.02 | 0.99 | 0.02 | 0.95 | 0.01 | 0.97 | 0.01 |
| P8 | 0.97 | 0.02 | 0.95 | 0.02 | 0.99 | 0.01 | 0.96 | 0.01 |
| POZ | 0.94 | 0.02 | 0.94 | 0.03 | 0.97 | 0.01 | 0.97 | 0.01 |
| PZ | 0.95 | 0.02 | 0.95 | 0.03 | 0.97 | 0.01 | 0.95 | 0.01 |
| T7 | 0.98 | 0.02 | 0.99 | 0.02 | 0.98 | 0.01 | 0.99 | 0.01 |
| T8 | 1.02 | 0.02 | 0.99 | 0.02 | 1.00 | 0.01 | 0.98 | 0.01 |
Fig 3.
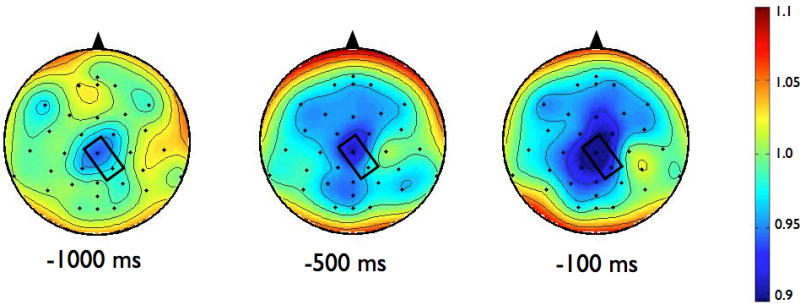
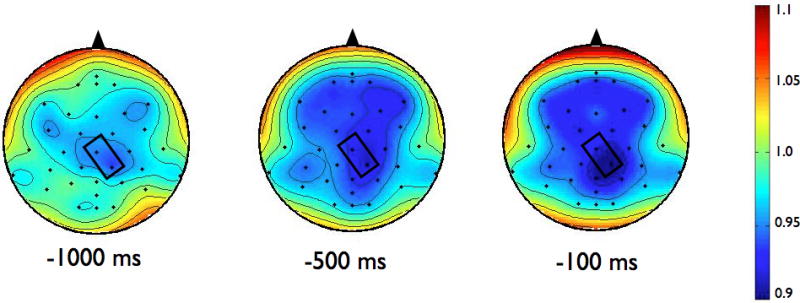
Scalp topographies of the upper beta-band (20–29 Hz) event-related desynchronization (ERD). Figure 3a presents the Parkinson’s disease (PD) group while 3b shows the control group. To examine the effects of magnitude and predictability, we chose to analyze data across the 1000 ms preceding the perturbation from the electrode in each group that demonstrated the most desynchronization. For beta ERD, the largest amount of desynchronization occurred at the CZ electrode in the PD group and at the CP2 electrode in the control group. Both electrodes are marked in rectangles on the scalp topographies
Kinetic Data: Postural Responses
Center of pressure (CoP) was calculated from the force plate data (Henry et al. 1998) and low-pass filtered at 10 Hz. Initial rate of change of the CoP was calculated from the slope of the anterior-posterior CoP between 100 and 200 ms after the perturbation. This time period was chosen to represent the initial active postural response, after the passive response and before long loop feedback correction (Horak et al. 1989; Horak et al. 1996; Horak and MacPherson 1996). Studies suggest that the cortex can influence this initial, active postural response based on knowledge of the upcoming perturbation (e.g., central set) (Jacobs and Horak 2007; Jacobs et al. 2008). This measure thus reflects preparation of the postural response, before any feedback adjustments have been made.
Statistical Analysis
To test for effects of magnitude, we compared the predictable small and predictable large conditions, using an average value created from 30 trials per condition. To test for the effects of predictability, we compared the average of the unpredictable-in-magnitude condition (30 trials) to an average created from the odd numbered trials of 30 predictable small and 30 predictable large (thus creating an average of 30 trials, 15 predictable small and 15 predictable large, evenly distributed across testing time).
In order to examine whether each group significantly modified their CoP initial rate of change based on central set, we used paired t-tests in each group to test for significant differences between predictable small vs. predictable large and predictable vs. unpredictable-in-magnitude conditions.
We used linear mixed models to test for differences in cortical preparation between groups and conditions. Dependent variables were CNV, alpha ERD and beta ERD. For CNV and alpha ERD we used 2-group (PD or control) by 2-condition (predictable small vs. predictable large or predictable vs. unpredictable-in-magnitude) linear mixed models to test for differences in the final segment: 100 ms preceding perturbation (CNV at CZ) or 1000 ms preceding perturbation (alpha ERD at CP1). We tested for group and condition main effects and a group-by-condition interaction. We specified condition as a repeated measure, used an unstructured covariance structure, and adjusted for multiple comparisons with the Bonferroni correction. For beta ERD we used a 2-group (PD or control) by 2-condition (predictable small vs. predictable large or predictable vs. unpredictable-in-magnitude) by 2-electrode (CP2 vs. CZ) linear mixed model to test for differences in the average ERD of the final 1000 ms preceding the perturbation. We tested for group, condition and electrode main effects and group-by-condition and group-by-electrode interactions. We specified condition and electrode as repeated measures, used an unstructured covariance structure, and adjusted for multiple comparisons with the Bonferroni correction.
We used Predictive Analytics Software (SPSS: An IBM Company, Chicago, IL) version 18 for statistical analysis and set our alpha level of significance at 0.05.
Results
Postural Response: Effects of Perturbation Magnitude
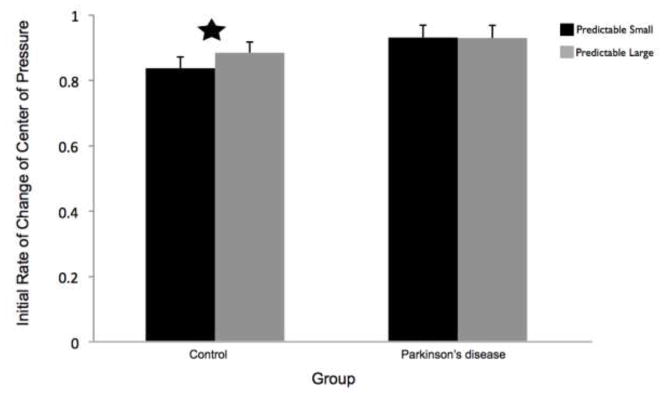
CoP initial rate of change was significantly slower in the predictable small compared to predictable large conditions for the control group (t11 = −2.89, p = 0.02) but not the PD group (t11 = 0.32, p = 0.98). Group means by condition are shown in Figure 4.
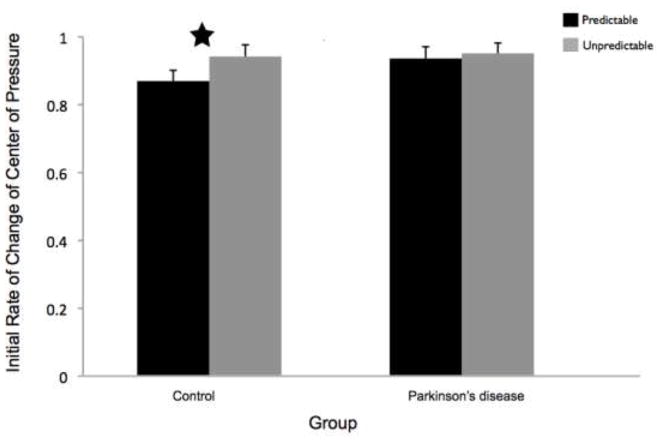
Fig 4.
The initial rate of change of the center of pressure (CoP) was calculated as the slope of the anterior-posterior direction CoP between 100 and 200 ms following the perturbation. Data are group means and 95% confidence intervals by condition. Figure 4a presents the effects of magnitude (predictable small vs. predictable large). Figure 4b presents the effects of predictability (predictable vs. unpredictable-in-magnitude). The star indicates conditions that were significantly different from each other. PD = Parkinson’s disease
Postural Response: Effects of Perturbation Predictability
CoP initial rate of change was significantly slower in the predictable, compared to unpredictable, trials in the control group (t11 = −3.18, p = 0.01) but not in the PD group (t11 = −0.54, p = 0.60). Group means by condition are shown in Figure 4.
CNV Cortical Preparation: Effects of Perturbation Magnitude
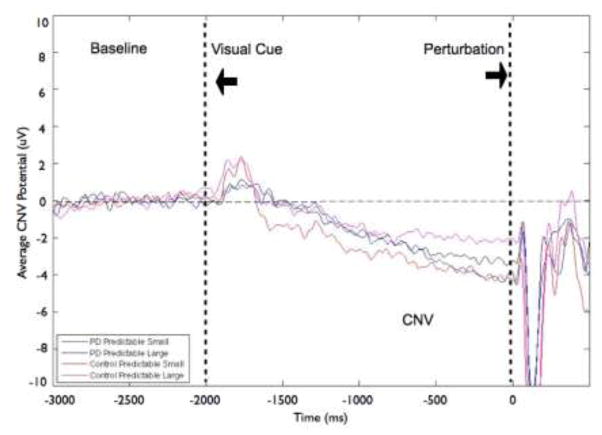
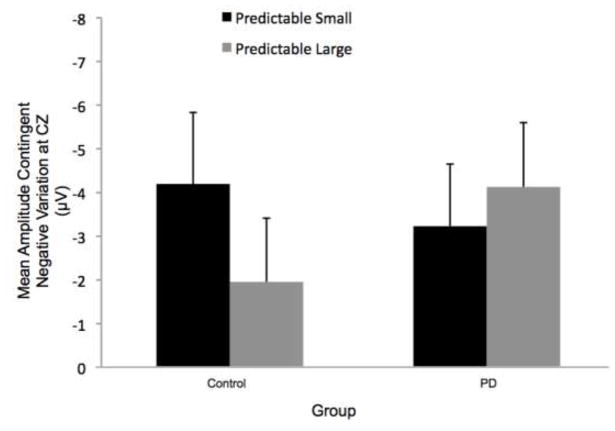
The magnitude of the perturbation (predictable small vs. predictable large) did not significantly affect CNV amplitude. Figure 5 displays the effects of magnitude on the grand average CNV at the CZ electrode by group (PD or control) and condition. Figure 6 shows the corresponding group means. There was no significant group effect (F[1,22]= 0.11, p = 0.75), condition effect (F[1,22]= 0.41, p = 0.53) or group-by-condition interaction (F[1,22]= 2.25, p = 0.15). However, 9 of the 12 control participants demonstrated larger amplitude CNV in the predictable small compared to predictable large condition, whereas the PD group’s CNV amplitudes were evenly distributed. Six of 12 PD participants showed larger amplitude CNV in the predictable small compared to the predictable large condition.
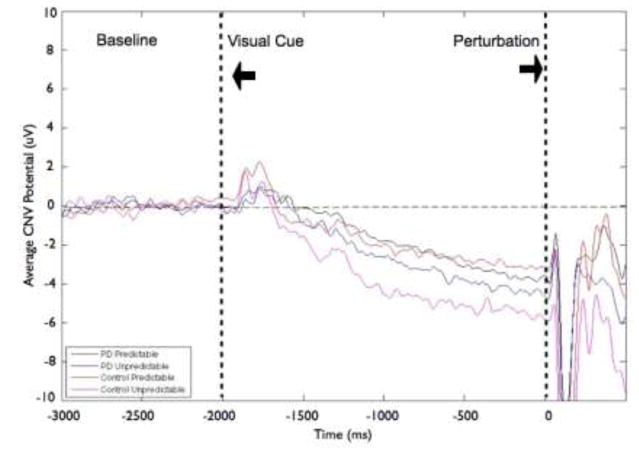
Fig 5.
Grand averages of contingent negative variation (CNV) amplitudes at the CZ electrode by group and condition. Figure 5a shows data from the Parkinson’s disease (PD) and control groups for the effects of magnitude (predictable small vs. predictable large). Figure 5b presents data from the PD and control groups for the effects of predictability (predictable vs. unpredictable-in-magnitude). Data were low-pass filtered at 15 Hz for display purposes only (not during analysis). Baseline is −3000 to −2000 ms, followed by the visual warning cue at −2000 ms and perturbation onset at 0 ms
Fig 6.
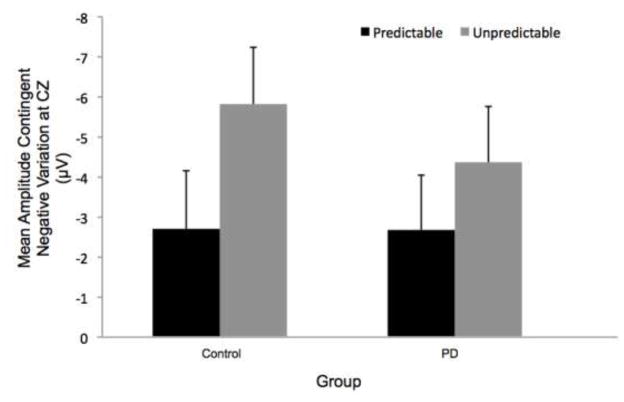
Group means for contingent negative variation (CNV) amplitudes by condition. Figure 6a presents the effects of magnitude (predictable small vs. predictable large). Figure 6b presents the effects of predictability (predictable vs. unpredictable-in-magnitude). There was a statistically significant condition main effect due to larger CNV amplitude in the unpredictable-in-magnitude compared to predictable trials. Error bars represent the standard error of the mean. PD = Parkinson’s disease
CNV Cortical Preparation: Effects of Perturbation Predictability
We found larger amplitude of CNV at the CZ electrode for the unpredictable-in-magnitude condition compared to the predictable trials (significant condition main effect: F[1,22]= 10.45, p < 0.01). There was no significant group effect (F[1,22]= 0.17, p = 0.68) or group-by-condition interaction (F[1,22]= 0.84, p = 0.37). Figure 5 displays the effects of predictability on the grand average CNV at the CZ electrode by group (PD or control) and condition. Figure 6 indicates that the larger CNV amplitude in the unpredictable-in-magnitude condition (condition effect) was driven more by the control group, however there was no significant group main effect or group-by-condition interaction. Eight of the 12 control participants demonstrated their largest CNV in the unpredictable-in-magnitude condition, whereas the largest CNV amplitudes for participants with PD were distributed across conditions; four participants with PD exhibited their largest CNV amplitudes in each of 3 conditions (predictable small, predictable large, unpredictable-in-magnitude).
Alpha ERD Cortical Preparation: Effects of Perturbation Magnitude
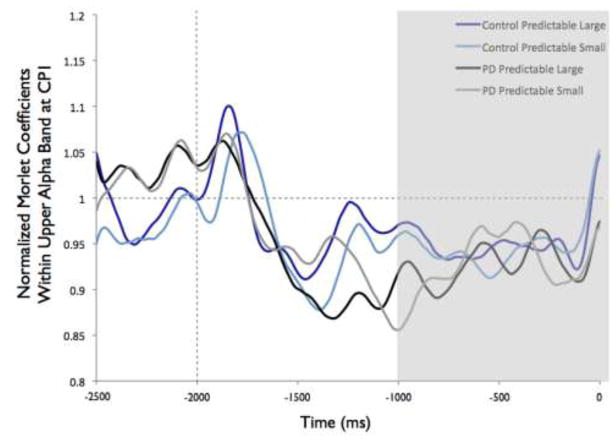
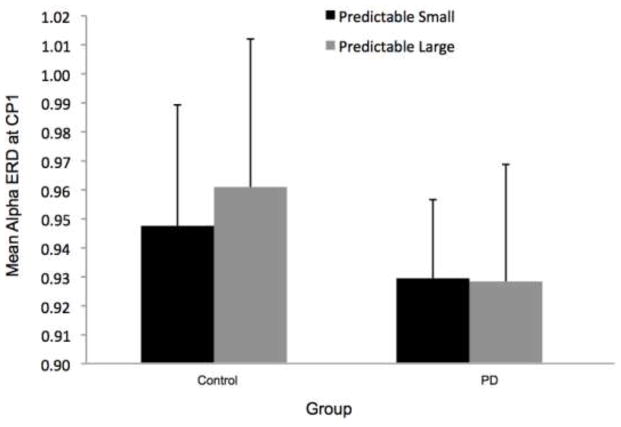
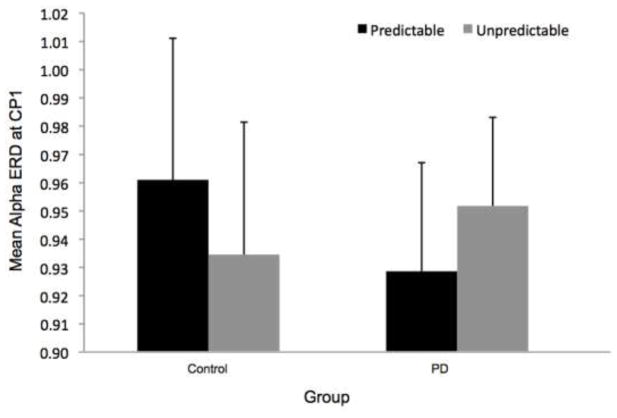
Overall, the PD group appeared to demonstrate more widespread alpha ERD across electrodes, as shown in Figure 2. Despite the different overall patterns, the CP1 electrode was the site of the most alpha ERD in both groups. Figure 7 shows the time-varying morlet coefficients at CP1. There were no significant effects of magnitude on alpha ERD at the CP1 electrode. There was not a significant group effect (F[1,22]= 0.16, p = 0.69), condition effect (F[1,22] < 0.01, p = 0.98) or group-by-condition interaction (F[1,22] < 0.01, p = 0.95), as shown in Figure 7.
Fig 7.
Group means for alpha event-related desynchronization at electrode CP1 by condition. Figure 7a presents time-varying morlet coefficients, normalized to baseline activity, of upper alpha-band (10–12 Hz) activity, showing the effects of magnitude (predictable small vs. predictable large). The vertical gray line at −2000 ms represents the onset of the warning cue and 0 s represents perturbation onset. The gray background highlights the final 1000-ms epoch prior to the perturbation used to calculate an average ERD for statistical analysis, which is further summarized in Figure 7b. Smaller values represent greater ERD, referenced to a baseline value of 1. Error bars represent the standard error of the mean. PD = Parkinson’s disease. Figures 7c and 7d similarly present the effects of predictability (predictable vs. unpredictable-in-magnitude)
Alpha ERD Cortical Preparation: Effects of Perturbation Predictability
Figure 7 presents the time-varying morlet coefficients for the predictable and unpredictable-in-magnitude trials. There were no effects of predictability on alpha ERD at the CP1 electrode. There was not a significant group effect (F[1,22] = 0.02, p = 0.90), condition effect (F[1,22] < 0.01, p = 0.93) or group-by-condition interaction (F[1,22] = 1.90, p = 0.18), as demonstrated in Figure 7.
Beta ERD Cortical Preparation: Effects of Perturbation Magnitude
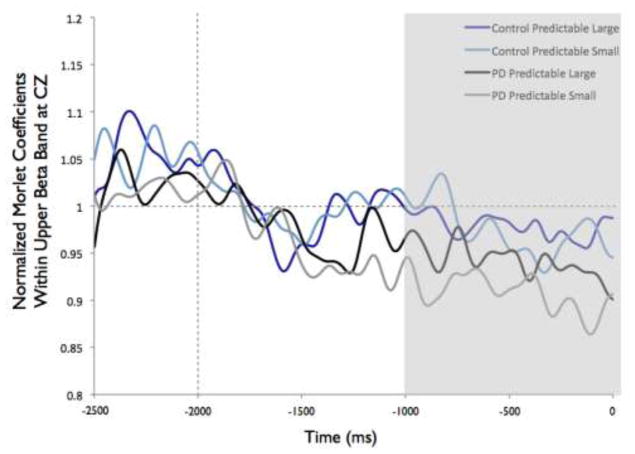
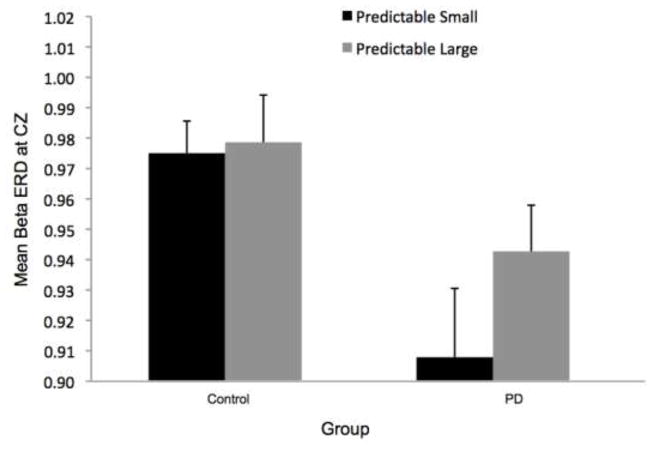
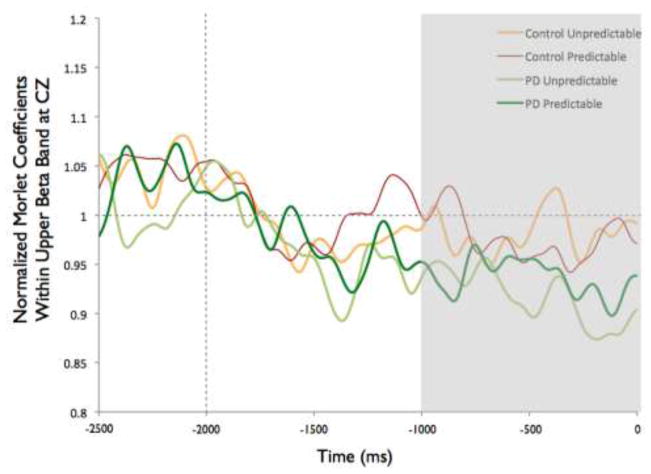
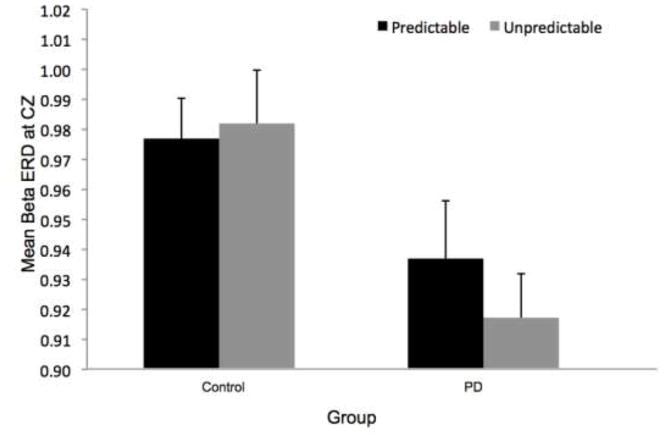
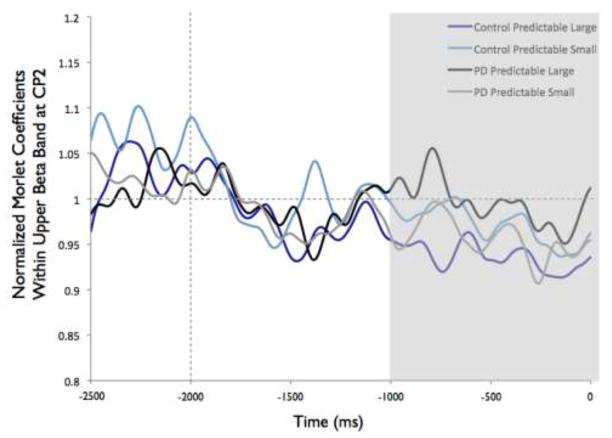
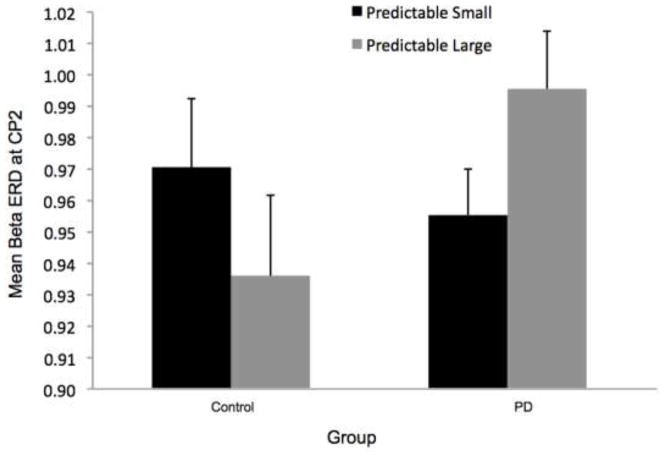
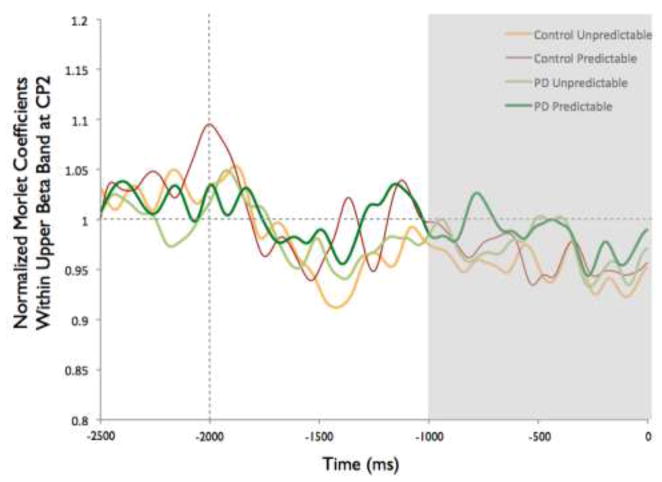
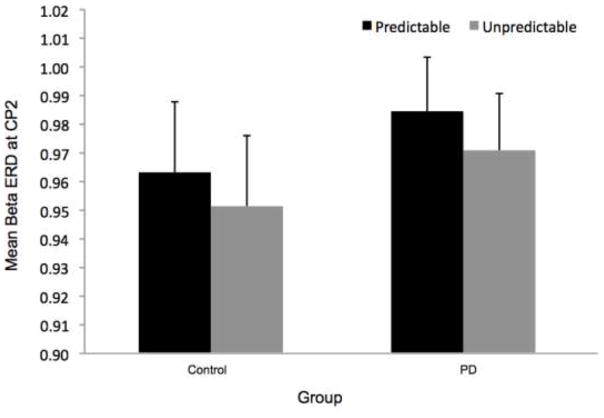
Figure 8 shows the time-varying morlet coefficients for beta ERD at the CZ and CP2 electrodes. Overall, the PD and control groups demonstrated different patterns of beta ERD responses, as shown in Figure 3. There was a significant group-by-condition interaction (F[1,22]= 10.16, p < 0.01) and a significant group-by-electrode interaction (F[1,22]= 9.58, p < 0.01) (see Figure 8). There was not a significant group (F[1,22]= 1.73, p = 0.20), condition (F[1,22]= 0.38, p = 0.55) or electrode (F[1,22]= 2.34, p = 0.14) effect. Follow-up analysis of the group-by-condition interaction revealed that the PD group demonstrated greater beta ERD in the predictable small condition than in the predictable large condition (p = 0.01), while the control group did not significantly differ between conditions (p = 0.08). The PD group demonstrated significantly greater beta ERD than the control group in the predictable small condition (p = 0.01) but not in the predictable large condition (p = 0.74). Follow up analysis of the group-by-electrode interaction revealed that the PD group demonstrated greater beta ERD at the CZ electrode compared to the CP2 electrode (p < 0.01), while the control group did not differ between electrodes (p = 0.28). The PD group demonstrated significantly greater beta ERD than the control group at the CZ electrode (p < 0.01) but the groups were not different at the CP2 electrode (p = 0.76).
Fig 8.
Group means for beta event-related desynchronization by condition at electrodes CP2 and CZ. Figure 8a presents time-varying morlet coefficients, normalized to baseline activity, of upper beta-band (20–29 Hz) activity at CZ, showing the effects of magnitude (predictable small vs. predictable large). The vertical gray line at −2000 ms represents the onset of the warning cue and 0 s represents perturbation onset. The gray background highlights the final 1000-ms epoch prior to the perturbation used to calculate an average ERD for statistical analysis, which is further summarized in Figure 8b. Smaller values represent greater ERD, referenced to a baseline value of 1. Error bars represent the standard error of the mean. PD = Parkinson’s disease. Figures 8c and 8d similarly present the effects of predictability (predictable vs. unpredictable-in-magnitude). Figures 8e–8h present electrode CP2
Beta ERD Cortical Preparation: Effects of Perturbation Predictability
There was a significant group-by-electrode interaction for beta ERD at the CZ and CP2 electrodes (F[1,22]= 13.32, p < 0.01). There was not a significant group (F[1,22]= 0.75, p = 0.40), condition (F[1,22]= 1.23, p = 0.27) or electrode (F[1,22]= 2.50, p = 0.13) effect, nor was there a group-by-condition interaction (F[1,22]= 0.23, p = 0.64). Follow up analysis of the group-by-electrode interaction, as shown in Figure 8, revealed that the PD group demonstrated greater beta ERD at the CZ electrode compared to the CP2 electrode (p < 0.01), whereas the control group did not differ significantly between electrodes (p = 0.16). The PD group demonstrated significantly greater beta ERD than the control group at the CZ electrode (p = 0.01) but the groups were not different at the CP2 electrode (p = 0.59).
Follow-Up Correlation Analyses
We wanted to test whether modification of postural responses by condition was related to measures of preceding cortical activity. We tested for Pearson correlations (r) between CoP initial rate of change and 1) CNV amplitude at CZ, 2) alpha ERD at CP1 and 3) beta ERD at CZ and CP2. We ran correlation analyses separately for each group, by condition, because of their different patterns of responses.
In the control group, there were no significant correlations between CoP initial rate of change and the EEG measures of cortical preparation in any of the conditions (Predictable Small: CNV amplitude at CZ r = −0.03, p = 0.93, alpha ERD at CP1 r = −0.27, p = 0.39, beta ERD at CZ r = −0.19, p = 0.56, beta ERD at CP2 r = −0.24, p = 0.44; Predictable Large: CNV amplitude at CZ r = 0.04, p = 0.90, alpha ERD at CP1 r = −0.08, p = 0.81, beta ERD at CZ r = −0.14, p = 0.65, beta ERD at CP2 r = −0.24, p = 0.46; Unpredictable-in-Magntitude: CNV amplitude at CZ r = 0.26, p = 0.41, alpha ERD at CP1 r = −0.04, p = 0.91, beta ERD at CZ r = 0.45, p = 0.14, beta ERD at CP2 r = −0.41, p = 0.18). In contrast, the CoP initial rate of change of the PD group was significantly negatively correlated with the beta ERD at CZ (r = −0.58, p = 0.047) in the predictable small condition. Faster CoP initial rate of change responses were correlated with greater ERD, as lower values for ERD represent greater desynchronization. There were no significant correlations in the other predictable small variables or any of the variables for the predictable large or unpredictable conditions in the PD group (Predictable Small: CNV amplitude at CZ r = −0.41, p = 0.19, alpha ERD at CP1 r = 0.15, p = 0.65, beta ERD at CP2 r = −0.09, p = 0.78; Predictable Large: CNV amplitude at CZ r = −0.16, p = 0.61, alpha ERD at CP1 r = −0.44, p = 0.16, beta ERD at CZ r = −0.46, p = 0.13, beta ERD at CP2 r = −0.11, p = 0.75; Unpredictable-in-Magntitude: CNV amplitude at CZ r = 0.04, p = 0.89, alpha ERD at CP1 r = −0.38, p = 0.23, beta ERD at CZ r = −0.48, p = 0.11, beta ERD at CP2 r = 0.46, p = 0.13).
Because modifying postural responses could be related to the ability to modify cortical activity among conditions, as opposed to the overall amount of CNV or ERD, we also compared the difference in initial rate of change of CoP to changes in CNV and ERD from the predictable small to the predictable large conditions. We ran the correlation analyses separately for each group due to their different patterns of responses. In the control group, there were no significant correlations between the difference in CoP initial rate of change from the predictable small to predictable large conditions and the difference from the predictable small to predictable large conditions in CNV amplitude at CZ (r = −0.22, p = 0.49), alpha ERD at CP1 (r = 0.15, p = 0.64), beta ERD at CZ (r = 0.34, p = 0.28) and beta ERD at CP2 (r = −0.08, p = 0.81). In contrast, the PD group’s difference in CoP initial rate of change from the predictable small to predictable large condition was significantly negatively correlated with the difference in beta ERD at CZ (r = −0.64, p = 0.02) and positively correlated with the difference in beta ERD at CP2 (r = 0.61, p = 0.04). Thus, a smaller adjustment in CoP initial rate of change correlated with greater change in beta ERD at CZ and smaller change in beta ERD at CP2 from the predictable small to large condition. It was not correlated with the difference in CNV amplitude at CZ (r = 0.28, p = 0.38) or the difference in alpha ERD at CP1 (r = −0.12, p = 0.70).
Discussion
Beta ERD, but not alpha ERD or CNV, exhibited differences between the groups with and without PD, particularly regarding the location of beta ERD and the relationship of beta ERD to the groups’ ability to scale their postural responses to predictable perturbation magnitudes based on central set. As predicted, the group with PD did not modify their initial rate of CoP displacement with knowledge of perturbation magnitude, while the control group did. The control group demonstrated faster CoP initial rate of change in the predictable large compared to predictable small condition, reflecting a condition-appropriate response. They also demonstrated faster CoP initial rate of change in the unpredictable-in-magnitude condition compared to predictable trials. This likely reflects a strategy to generate a large postural response in anticipation of a potentially large perturbation, even if such a response is less efficient or unnecessary when randomly presented with a small perturbation, in order to ensure a sufficient response when presented with a large perturbation (Beckley et al. 1991; Mochizuki et al. 2010). Given our predicted observation of impaired response modification based on central set with PD, we next interpret the functional significance of our findings for each measure of cortical preparation in relation to their modifiability based on knowledge of perturbation magnitude.
Effects of Magnitude Predictability on CNV Suggests Enhanced Anticipation With Unpredictable Perturbations In Participants With and Without PD
Consistent with previous findings on young healthy adults (Mochizuki et al. 2010), both groups with and without PD demonstrated larger CNV at CZ in the unpredictable-in-magnitude condition compared to the predictable trials. However, anticipating perturbation magnitude (predictable small versus predictable large) did not significantly affect CNV amplitude. The CNV reflects processes of both anticipation for the imperative stimulus (in this study, represented by perturbation onset) and motor preparation (van Boxtel and Brunia 1994a; van Boxtel and Brunia 1994b; Fischer et al. 2010). CNV amplitudes were not significantly affected by perturbation magnitude and did not correlate with initial CoP responses, suggesting the CNV was not sensitive to changes in motor preparation for the postural response. Previous studies, however, identified that the CNV is most sensitive to knowing the timing of perturbation onset (Adkin et al. 2008; Jacobs et al. 2008; Mochizuki et al. 2008), suggesting that enhanced CNV prior to postural perturbations reflect this measure’s underlying processes of anticipation for the perturbation’s onset. In addition, research in healthy young adults has shown that peak CNV amplitudes prior to postural perturbations are earlier and larger with increasing balance difficulty due to initial leaning positions that decreased the available initial distance between the center of mass and the limits of support (Fujiwara et al. 2011). In our protocol, it is possible that not knowing the amplitude of the perturbation increased task difficulty and/or heightened anticipation of perturbation timing, thereby eliciting a larger CNV in the unpredictable-in-magnitude condition.
Group differences were not significant with regard to the CNV, suggesting that participants with PD similarly exhibited enhanced anticipation of perturbation timing when the perturbation magnitude was unpredictable. Although statistically insignificant, we did observe that the largest CNV was more consistently observed in the unpredictable condition by more control participants than by the participants with PD, suggesting that the modifiability of the CNV potential based on the predictability of perturbation magnitude is dependent on individual participant characteristics. A larger study is thus needed to examine whether impaired CNV modifiability based on central set is related to the progress of disease-related factors, perhaps such as symptom severity, disease duration, or medication dose.
A Lack of Effect on Alpha ERD Suggests No Effects of Magnitude or of Magnitude Predictability on Sensory Processing Prior to the Perturbation
Alpha ERD was observed but was not significantly affected by predictability or magnitude in either group, suggesting this measure does not reflect changes in motor preparation for postural responses based on knowledge of perturbation magnitude. Alternatively, this measure may reflect sensory processing prior to the perturbation. The alpha ERD was most evident over central-parietal regions of sensory processing, and previous literature identified event-related spectral changes in alpha activity at central-parietal regions during stance between different sensory conditions (Del Percio et al. 2007; Pirini et al. 2011). These results thus suggest the alpha ERD may reflect processes of sensory integration or reweighting for maintaining standing balance. The lack of change in alpha ERD by either group in this study, therefore, suggests sensory processing prior to perturbation onset was similar regardless of advanced knowledge of the upcoming perturbation magnitude.
Changes in Beta ERD Associated With Perturbation Magnitude for the Group With PD Suggests a More Direct Cortical Influence on Central-Set Related Motor Preparation for People With PD
Patterns of beta ERD were different between groups: (1) the PD group showed a more anterior location of peak beta ERD with more beta ERD at CZ than the control group, (2) the PD group demonstrated significantly greater beta ERD than the control group in the predictable small condition, and (3) beta ERD amplitudes and condition-related changes in beta ERD amplitudes significantly associated with those of the initial rate of CoP displacement for only the group with PD. Although the control participants scaled their postural responses to the known magnitude of the perturbation, there was not a correlated change in preparatory EEG activity. The lack of correlation in the control group may reflect a minimized role for the cortex in favor of a more automated formation of central set among the basal ganglia and postural centers within the brainstem (Grillner et al. 2005; Jacobs and Horak 2007).
In contrast, the beta ERD for the group with PD was localized more anterior than that of the control participants to regions overlying primary and supplementary motor cortex, and the beta ERD significantly associated with their initial postural responses. Given that beta ERD has been suggested to represent synchrony of cortico-striatal circuits known to be impaired with PD (Klostermann et al. 2007), the results together suggest that the participants with PD attempted to compensate for impaired basal-ganglia function by enhancing the influence of motor preparation by the cerebral cortex to modify upcoming postural responses based on central set.
The association of increased beta ERD with less modified postural responses based on central set suggests attempts at a compensatory mechanism. Greater beta ERD at CZ in participants with PD appears to represent an unsuccessful attempt to influence postural responses based on the known small perturbation, because the participants with PD did not significantly alter their initial rate of CoP displacement with knowledge of perturbation magnitude. It could also be, however, that increased beta ERD causes the lack of postural response scaling. In either case, increased cortical beta ERD in PD is associated with decreased adaptability of postural responses. Our interpretation is limited, however, as the effect was pronounced in the predictable small condition but not observed in the predictable large condition. One possibility is that the more abrupt movement of the force plate during the small perturbation (6 cm translation as opposed to 12 cm for the large perturbation) was more difficult and thus elicited more cortical preparatory activity. An increase in beta ERD in the sensorimotor cortex during motor preparation has been associated with task-related uncertainty (Tzagarakis et al. 2010).
Methodological Considerations
The visualization of scalp topographies suggested that the participants with PD exhibited a more frontal CNV, a more widespread and perhaps more lateral alpha ERD, and a less widespread beta ERD than the control participants. Our intent here, however, was to study the effects of magnitude and predictability. While our results raise interesting questions about group differences in the overall scalp patterns of EEG activity preceding a postural response, these questions need to be answered with a prospectively-designed study with only one type of perturbation and perhaps a more dense array of electrodes. Because the design of our study included three different types of perturbations, our results are inherently affected by predictability and magnitude effects, thus we are limited in our ability to comment on the overall distribution of EEG activity preceding postural responses in general. Larger future studies that are prospectively designed with more electrodes are therefore warranted to explore differences due to PD in spatial patterns of EEG preparatory activity and the functional significance of these potentially altered EEG topographies.
Our observation of similar CNV amplitudes between persons with and without PD is in contrast to previous findings of smaller CNV amplitudes in persons with PD compared to healthy control participants during voluntary motor tasks (Oishi et al. 1995; Aotsuka et al. 1996; Praamstra et al. 1996; Pulvermuller et al. 1996; Ikeda et al. 1997). Although all CNV paradigms have inherent temporal predictability in the imperative stimulus, one possible explanation for the similar CNV amplitudes in our study is that PD participants may rely more on the timing of an upcoming perturbation in the case of an externally-induced postural cue than during a self-initiated movement, a characteristic which has been previously suggested for persons with PD (Jacobs and Horak 2006). In fact, many studies show that participants with PD compensate for poor centrally-initiated movement with externally-triggered movements (Morris et al. 1996; Hanakawa et al. 1999).
The ERD results in our study are also quite different from other studies. Our study, however, uses induced loss of balance, a very novel task compared to voluntarily generated limb movements. We believe that this study’s results reflect the uniqueness of the postural task. ERD has previously been reported to be of small amplitude (and perhaps only transiently evident) in voluntary tasks, even when performing lower-limb movements (Crone et al. 1998; Wheaton et al. 2008). In addition, using the same methods of analysis yielded a more sustained alpha ERD of larger amplitudes when testing a voluntary arm movement task that did not involve a balance perturbation (Jacobs et al. 2010). The less sustained alpha ERD between the preparatory and postural cues in our task may represent the transient role of the cortex for preparing anticipated postural responses. Unlike voluntary movements, for which it more directly generates the behavior, the cortex is thought to influence postural responses by priming the context-specific synergy of a postural response within sub-cortical centers of posture control that are subsequently triggered by the perturbation (Jacobs and Horak 2007).
Conclusions
Surprisingly, for the participants without PD, cortical preparatory activity overlying the primary and supplementary motor areas was not related to the modification of postural responses to predicted perturbation characteristics based on central set, suggesting that subcortical areas are responsible for this type of central set for postural control. However, increased beta ERD in PD was associated with decreased adaptability of postural responses, indicating the possibility that preparatory cortical activity may have more direct influence on postural response scaling for people with PD. Whether increased beta ERD activity is causing poor postural response performance or is an attempt to compensate for poor postural response performance remains to be determined. Next, we will ask if cortical preparatory activity in persons with PD can be positively affected by cuing and directing attention, a question that has important implications for understanding current rehabilitation strategies in persons with PD (Morris et al. 2009).
Acknowledgments
We would like to thank our participants. We would like to thank Kristin Lucas, PT, DPT, for her invaluable assistance with data collections. We would like to acknowledge our funding sources: National Institutes of Health, National Institute of Aging R37 A60006457 (FBH), National Institutes of Health, National Institute of Child Health and Human Development F32 HD070796 (BAS), Medical Research Foundation of Oregon (BAS), the Foundation for Physical Therapy (BAS) and National Institutes of Health, National Institute of Aging T32AG023477. Funding sources provided funding only and did not influence the work. Abstracts of this work were presented at the Society for Neuroscience Annual Conferences in 2010 (San Diego, CA) and 2011 (Washington, DC), the Oregon Physical Therapy Association Annual Conference (Portland, OR, April 2011) and the Pacific Northwest Basal Ganglia Coterie (Hood River, OR, May 2011).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Adkin AL, Campbell AD, Chua R, Carpenter MG. The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci Lett. 2008;435:120–125. doi: 10.1016/j.neulet.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Adkin AL, Quant S, Maki BE, McIlroy WE. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp Brain Res. 2006;172:85–93. doi: 10.1007/s00221-005-0310-9. [DOI] [PubMed] [Google Scholar]
- Aotsuka A, Weate SJ, Drake ME, Jr, Paulson GW. Event-related potentials in Parkinson’s disease. Electromyogr Clin Neurophysiol. 1996;36:215–220. [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, Babiloni F. Human movement-related potentials vs desynchronization of EEG alpha rhythm: a high-resolution EEG study. NeuroImage. 1999;10:658–665. doi: 10.1006/nimg.1999.0504. [DOI] [PubMed] [Google Scholar]
- Badke MB, Duncan PW, Di Fabio RP. Influence of prior knowledge on automatic and voluntary postural adjustments in healthy and hemiplegic subjects. Phys Ther. 1987;67:1495–1500. doi: 10.1093/ptj/67.10.1495. [DOI] [PubMed] [Google Scholar]
- Bares M, Nestrasil I, Rektor I. The effect of response type (motor output versus mental counting) on the intracerebral distribution of the slow cortical potentials in an externally cued (CNV) paradigm. Brain Res Bull. 2007;71:428–435. doi: 10.1016/j.brainresbull.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Beckley DJ, Bloem BR, Remler MP. Impaired scaling of long latency postural reflexes in patients with Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1993;89:22–28. doi: 10.1016/0168-5597(93)90080-9. [DOI] [PubMed] [Google Scholar]
- Beckley DJ, Bloem BR, Remler MP, Roos RA, Van Dijk JG. Long latency postural responses are functionally modified by cognitive set. Electroencephalogr Clin Neurophysiol. 1991;81:353–358. doi: 10.1016/0168-5597(91)90024-r. [DOI] [PubMed] [Google Scholar]
- Bender S, Resch F, Weisbrod M, Oelkers-Ax R. Specific task anticipation versus unspecific orienting reaction during early contingent negative variation. Clin Neurophysiol. 2004;115:1836–1845. doi: 10.1016/j.clinph.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Bloem BR, van Vugt JP, Beckley DJ. Postural instability and falls in Parkinson’s disease. Adv Neurol. 2001;87:209–223. [PubMed] [Google Scholar]
- Chong RK, Horak FB, Woollacott MH. Parkinson’s disease impairs the ability to change set quickly. J Neurol Sci. 2000;175:57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Chong RK, Jones CL, Horak FB. Postural set for balance control is normal in Alzheimer’s but not in Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 1999;54:M129–135. doi: 10.1093/gerona/54.3.m129. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;12:2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Brancucci A, Bergami F, Marzano N, Fiore A, Di Ciolo E, Aschieri P, Lino A, Vecchio F, Iacoboni M, Gallamini M, Babiloni C, Eusebi F. Cortical alpha rhythms are correlated with body sway during quiet open-eyes standing in athletes: a high-resolution EEG study. NeuroImage. 2007;36:822–829. doi: 10.1016/j.neuroimage.2007.02.054. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W, Schenck E. Cerebral potentials and leg muscle e.m.g. responses associated with stance perturbation. Exp Brain Res. 1985;57:348–354. doi: 10.1007/BF00236540. [DOI] [PubMed] [Google Scholar]
- Duckrow RB, Abu-Hasaballah K, Whipple R, Wolfson L. Stance perturbation-evoked potentials in old people with poor gait and balance. Clin Neurophysiol. 1999;110:2026–2032. doi: 10.1016/s1388-2457(99)00195-9. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Jahanshahi M, Rothwell JC. Uncoupling of contingent negative variation and alpha band event-related desynchronization in a go/no-go task. Clin Neurophysiol. 2001;112:1307–1315. doi: 10.1016/s1388-2457(01)00558-2. [DOI] [PubMed] [Google Scholar]
- Fischer T, Langner R, Diers K, Brocke B, Birbaumer N. Temporo-Spatial Dynamics of Event-Related EEG Beta Activity during the Initial Contingent Negative Variation. PLoS One. 2010;5:e12514. doi: 10.1371/journal.pone.0012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson’s disease. Cereb Cortex. 2006;16:64–75. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Kiyota N, Maeda K. Contingent negative variation and activation of postural preparation before postural perturbation by backward floor translation at different initial standing positions. Neurosci Lett. 2011;490:135–139. doi: 10.1016/j.neulet.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hamano T, Luders HO, Ikeda A, Collura TF, Comair YG, Shibasaki H. The cortical generators of the contingent negative variation in humans: a study with subdural electrodes. Electroencephalogr Clin Neurophysiol. 1997;104:257–268. doi: 10.1016/s0168-5597(97)96107-4. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson’s disease. Ann Neurol. 1999;45:329–336. doi: 10.1002/1531-8249(199903)45:3<329::aid-ana8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. Control of stance during lateral and anterior/posterior surface translations. IEEE Trans Rehabil Eng. 1998;6:32–42. doi: 10.1109/86.662618. [DOI] [PubMed] [Google Scholar]
- Horak FB, Diener HC. Cerebellar control of postural scaling and central set in stance. J Neurophysiol. 1994;72:479–493. doi: 10.1152/jn.1994.72.2.479. [DOI] [PubMed] [Google Scholar]
- Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62:841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- Horak FB, MacPherson JM. Postural Orientation and Equilibrium. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology. Oxford University Press; Oxford: 1996. pp. 255–288. [Google Scholar]
- Ikeda A, Shibasaki H, Kaji R, Terada K, Nagamine T, Honda M, Kimura J. Dissociation between contingent negative variation (CNV) and Bereitschaftspotential (BP) in patients with parkinsonism. Electroencephalogr Clin Neurophysiol. 1997;102:142–151. doi: 10.1016/s0921-884x(96)95067-5. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Fujiwara K, Tomita H, Furune N, Kunita K, Horak FB. Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin Neurophysiol. 2008;119:1431–1442. doi: 10.1016/j.clinph.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Nagle KJ. Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clin Neurophysiol. 2010;121:431–440. doi: 10.1016/j.clinph.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience. 2006;141:999–1009. doi: 10.1016/j.neuroscience.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann F, Nikulin VV, Kuhn AA, Marzinzik F, Wahl M, Pogosyan A, Kupsch A, Schneider GH, Brown P, Curio G. Task-related differential dynamics of EEG alpha- and beta-band synchronization in cortico-basal motor structures. Eur J Neurosci. 2007;25:1604–1615. doi: 10.1111/j.1460-9568.2007.05417.x. [DOI] [PubMed] [Google Scholar]
- Lamarche M, Louvel J, Buser P, Rektor I. Intracerebral recordings of slow potentials in a contingent negative variation paradigm: an exploration in epileptic patients. Electroencephalogr Clin Neurophysiol. 1995;95:268–276. doi: 10.1016/0013-4694(95)00117-h. [DOI] [PubMed] [Google Scholar]
- Maki BE, Whitelaw RS. Influence of expectation and arousal on center-of-pressure responses to transient postural perturbations. J Vestib Res. 1993;3:25–39. [PubMed] [Google Scholar]
- Mochizuki G, Boe S, Marlin A, McIlRoy WE. Perturbation-evoked cortical activity reflects both the context and consequence of postural instability. Neuroscience. 2010;170:599–609. doi: 10.1016/j.neuroscience.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Mochizuki G, Sibley KM, Esposito JG, Camilleri JM, McIlroy WE. Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clinical Neurophys. 2008;119:1626–1637. doi: 10.1016/j.clinph.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- Morris ME, Martin CL, Schenkman ML. Striding out with Parkinson disease: evidence-based physical therapy for gait disorders. Phys Ther. 2009;90:280–288. doi: 10.2522/ptj.20090091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Du C, Takasu T. Contingent negative variation and movement-related cortical potentials in parkinsonism. Electroencephalogr Clin Neurophysiol. 1995;95:346–349. doi: 10.1016/0013-4694(95)00084-c. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Graphical display and statistical evaluation of event-related desynchronization (ERD) Electroencephalogr Clin Neurophysiol. 1977;43:757–760. doi: 10.1016/0013-4694(77)90092-x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 1992;83:62–69. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Spatiotemporal ERD/ERS patterns during voluntary movement and motor imagery. Suppl Clin Neurophysiol. 2000;53:196–198. doi: 10.1016/s1567-424x(09)70157-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol. 1977;42:817–826. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin Neurophysiol. 2000;111:1873–1879. doi: 10.1016/s1388-2457(00)00428-4. [DOI] [PubMed] [Google Scholar]
- Pirini M, Mancini M, Farella E, Chiari L. EEG correlates of postural audio-biofeedback. Hum Mov Sci. 2011;30:249–261. doi: 10.1016/j.humov.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Meyer AS, Cools AR, Horstink MW, Stegeman DF. Movement preparation in Parkinson’s disease. Time course and distribution of movement-related potentials in a movement precueing task. Brain. 1996;119:1689–1704. doi: 10.1093/brain/119.5.1689. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Lutzenberger W, Muller V, Mohr B, Dichgans J, Birbaumer N. P3 and contingent negative variation in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1996;98:456–467. doi: 10.1016/0013-4694(96)95537-6. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Hallett M, Stanhope S, Shibasaki H. Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol. 2005;116:315–323. doi: 10.1016/j.clinph.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 2010;30:11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel GJ, Brunia CH. Motor and non-motor aspects of slow brain potentials. Biol Psychol. 1994a;38:37–51. doi: 10.1016/0301-0511(94)90048-5. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, Brunia CH. Motor and non-motor components of the Contingent Negative Variation. Int J Psychophysiol. 1994b;17:269–279. doi: 10.1016/0167-8760(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent Negative Variation: An Electric Sign of Sensorimotor Association and Expectancy in the Human Brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Carpenter M, Mizelle JC, Forrester L. Preparatory band specific premotor cortical activity differentiates upper and lower extremity movement. Exp Brain Res. 2008;184:121–126. doi: 10.1007/s00221-007-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]