Abstract
Melanin concentrating hormone (MCH) stimulates feeding driven by energy needs and reward and modifies anxiety behavior. Orexigenic peptides of similar characteristics, including nociceptin/orphanin FQ, Agouti-related protein and opioids, increase consumption also by reducing avoidance of potentially tainted food in animals displaying a conditioned taste aversion (CTA). Herein, using real-time PCR, we assessed whether expression levels of genes encoding MCH and its receptor, MCHR1, were affected in CTA in the rat. We also investigated whether injecting MCH intracerebroventricularly (ICV) during the acquisition and retrieval of LiCl-induced CTA, would alleviate aversive responses. MCHR1 gene was upregulated in the hypothalamus and brain stem of aversive animals, MCH mRNA was significantly higher in the hypothalamus, whereas a strong trend suggesting upregulation of MCH and MCHR1 genes was detected in the amygdala. Despite these expression changes associated with aversion, MCH injected prior to the induction of CTA with LiCl as well as later, during the CTA retrieval upon subsequent presentations of the aversive tastant, did not reduce the magnitude of CTA. We conclude that MCH and its receptor form an orexigenic system whose expression is affected in CTA. This altered MCH expression may contribute to tastant-targeted hypophagia in CTA. However, changing the MCH tone in the brain by exogenous peptide was insufficient to prevent the onset or facilitate extinction of LiCl-induced CTA. This designates MCH as one of many accessory molecules associated with shaping an aversive response, but not a critical one for LiCl-dependent CTA to occur.
Keywords: feeding, preference, avoidance, anorexia
INTRODUCTION
Melanin concentrating hormone (MCH) is a 19-amino acid cyclic peptide primarily expressed in the lateral hypothalamic (LH) and zona incerta (ZI) neurons (Risold et al., 2009). It binds two receptors: the MCHR1 and MCHR2; however rodents only express the MCHR1 (Tan et al., 2002). MCH has been implicated in a variety of processes including sleep (Peyron et al., 2009), mood disorders (Antal-Zimanyi & Khawaja, 2009), learning and memory (Adamantidis & de Lecea, 2009), and most pertinent to this project, feeding and energy homeostasis (for review see (Antal-Zimanyi & Khawaja, 2009) and (Griffond & Risold, 2009)).
Central administration of MCH increases feeding (Qu et al., 1996); MCH over-expression induces obesity and hyperphagia in transgenic mice (Ludwig et al., 2001), while MCH knockout (KO) mice are lean and hypophagic (Shimada et al., 1998). It has been suggested that MCH impacts energy balance either by altering feeding (Della-Zuana et al., 2002; Gomori et al., 2003; Ito et al., 2003) or also, as observed in KO models, by altering energy expenditure (Kokkotou et al., 2005). It is noteworthy that MCH may be involved in the regulation of reward-related processing of ingestive behavior. For example, central administration of MCHR1 agonists increases intake of palatable sucrose and ethanol and impacts the reinforcing value of these rewarding ingestants (Duncan et al., 2005; Sakamaki et al., 2005). Conversely, Morens et al. (2005) showed that antagonism of the MCHR1 leads to a more prominent decrease in consumption of preferred foods than of a regular bland diet. In addition, some of the orexigenic properties of MCH may stem from its anxiolytic properties (Monzon & De Barioglio, 1999; Kela et al., 2003; Morens et al., 2005).
The combination of orexigenic (through energy- and reward-related mechanisms) and anxiolytic properties of MCH led us to hypothesize that, similarly to other peptides sharing these characteristics, MCH signaling maybe crucial in the regulation of behaviors that underlie food avoidance. One such behavior is a conditioned taste aversion (CTA) which develops when exposure to a novel tastant is paired with an injection of a sickness/malaise inducing agent. The animal associates the unpleasant gastrointestinal sensation with the tastant and avoids this ingestant upon subsequent presentations. We have previously shown that opioids, whose main role is to mediate feeding reward and only to some extent feeding for calories, alleviate aversive responsiveness when co-administered with the CTA inducing toxin, lithium chloride (LiCl). Orexigenic opioid-like peptide, nociceptin/orphanin FQ (N/OFQ), reduces aversion; relative expression of genes encoding the components of the N/OFQ system in the brain is also altered in CTA (Olszewski et al., 2010a). In fact, our preliminary analysis of the tissues from that study (unpublished data) strongly suggested that MCH mRNA levels differ in the aversive state.
The present studies were undertaken to assess whether central mRNA levels of genes coding for MCH and its receptor are affected in the aversive state in the rat. We also sought to investigate whether supplying exogenous MCH during the acquisition and retrieval of a LiCl-induced CTA could alleviate aversive responses.
MATERIALS AND METHODS
Experiment 1: Expression of genes encoding MCH and MCHR1 in response to CTA
Expression levels of MCH and MCH receptor genes were established in the hypothalamus, amygdala and brainstem tissue collected from animals aversive to a novel solid diet. The CTA profile and initial mRNA data (housekeeping genes, nociceptin/orphanin peptide and receptor mRNA) from this experiment have been described in detail and published elsewhere (Olszewski et al., 2010a). In brief, 32 weight-matched (270-290 g) male Sprague-Dawley rats (Scanbur, Sweden) were housed individually in standard macrolon type IV cages with LD 12:12 (lights on at 07:00) in a temperature-controlled room (21-22 °C). The procedures were approved by the Uppsala Animal Ethics Committee. The animals were schedule-fed between 0900 and 1100 each day; standard chow (R36, Lactamin) was available. Food intake was measured daily and body weights were recorded every other day. After 3 days of acclimatizing to this schedule, the animals also received one i.p. saline injection daily for the next 3 days to allow animals to become accustomed to drug administration. On the 7th day, they were given a novel R6-38 (Lantmännen) diet instead of regular chow. Exposure to this novel diet was followed by an i.p. injection with saline or 3 meq LiCl. To ensure CTA acquisition, the protocol was repeated on the 9th and 11th day. The animals were randomly assigned to four different groups: (i) schedule-fed controls, receiving saline-injections immediately after exposure to the novel diet, (ii) schedule-fed CTA rats, injected with LiCl after exposure to the novel diet, (iii) schedule-fed rats, pair-fed to the CTA rats and injected only with saline after food exposure, and (iv) schedule-fed rats, pair-fed to the CTA rats, injected with LiCl 4 hours after exposure to the novel diet. The restricted group of animals was always pair-fed to the CTA group.
On the 13th day, the animals were exposed to the novel diet again, but no injections were given. Ninety minutes after food exposure, the animals were decapitated. A gross excision of the hypothalamus, amygdala and brain stem was performed according to the boundaries defined in the brain atlas of Paxinos and Watson (Paxinos & Watson, 1986). The tissue was kept at room temperature in the RNAlater solution (Ambion) for 3 h and then stored at −80°C.
RNA extraction and cDNA synthesis
RNA extraction and cDNA synthesis were performed according to the protocol described previously. In brief, homogenization of samples was carried out in the TRIzol reagent and chloroform was used to extract RNA followed by overnight incubation in isopropanol for RNA precipitation. After centrifugation, the pellets were washed, air-dried and dissolved in 1X DNase buffer, followed by 1.5 h incubation in RNase-free DNase I (Roche). RT-PCR was used to confirm the absence of genomic DNA. RNA concentration was measured using a nanodrop (ND-1000, Nanodrop). 5μg RNA samples were diluted in MilliQ waterto the final volume of 12μl. In order to reverse-transcribe RNA, a total volume of 20μl of the mix containing 1X mastermix and 1μl murine leukemia virus reverse transcriptase was used.
RT-PCR
The mastermix for each RT-PCR contained 2 μl MgCl2 free 10x Buffer, 1.6 μl 50 mM MgCl2, 0.2 μl 20 mM dNTP, 0.05 μl of both forward and reverse primer (100 pmol/μl), 1 μl dimethyl sulfoxide, 0.5 μl Sybr Green (1:50,000), 0.08 μl Taq polymerase (5U/μl) and 9.52 μl MilliQ water. Each plate included all samples in duplicates as well as negative controls. The following protocol was used for amplification: 3 min of denaturation at 95°C followed by 50 cycles of 15 sec denaturing at 95°C, 15 sec annealing at an appropriate temperature established for the primers (Table), and 30 sec extension at 72°C.
The following housekeeping genes were analyzed: glyceraldehyde-3-phosphate-dehydrogenase, β-tubulin, ribosomalprotein 19, histoneH3, cyclophilin, β-actin and succinate dehydrogenasecomplex subunit B). The RT-PCR experiments were performed using a MyiQthermal cycler (Bio-Rad Laboratories, Sweden).
Data analysis
Data analysis was carried out as described in Lindblom et al (2006) (Lindblom et al., 2006) using MyIQ version 1.04 (Bio-Rad). LinRegPCR (Ramakers et al., 2003) was used to calculate primer efficiencies to correct for efficiency differences. Normalization factors were calculated based on housekeeping gene expression using the GeNorm protocol (Vandesompele et al., 2002). Outliers were identified with Grubb’s test, and ANOVA followed by Fischer’s protected least significant difference test were used to analyze differences in gene expression between groups. Values of P<0.05 were considered different.
Experiment 2: Effect of MCH on acquisition and extinction of a CTA
Animals and surgeries
Male Sprague-Dawley rats (n=43; Charles River Laboratories, Wilmington, MA) weighing 260-370 g (Means: 320 ± 30 g) were individually housed in conventional hanging cages under the same conditions as described above. The study was approved by the University of Minnesota Institutional Animal Care and Use Committee. Rats were fitted with an indwelling stainless steel guide cannula (22 gauge, Plastics One, Roanoke, VA) descending into the third ventricle. They were anesthetized with an intraperitoneal (i.p.) injection of pentobarbital (Nembutal®, 60 mg/kg b.wt.). The surgical site was cleaned with povidone-iodine and ethanol. The rat was placed in the stereotaxic apparatus and the cannula was secured with dental cement and two screws inserted into the skull. Stereotaxic coordinates were as follows: 0 mm lateral to midline, 2.5 mm posterior to bregma, and 8.1 mm below the skull surface (injector extends 0.5 mm beyond the tip of the guide cannula). Immediately after surgery the rats were given subcutaneous injections of the analgesic carprofen (5 mg/kg) and an antibiotic ointment was applied to the wound site. The analgesic was administered daily for the next 2 days. The rats were given 7-10 days of postoperative recovery. To assess patency of the cannula, rats were tested for their drinking response to a central injection of 10 ng Ang II. Only rats that drank at least 5 ml of water within 30 min were used in the studies.
Drugs and Drug Administration
Angiotensin II (Ang II; Calbiochem, La Jolla, CA) was administered i.c.v. at a dose of 10 ng / 3μl. Melanin concentrating hormone (MCH; Sigma-Aldrich, St. Louis, MO), dissolved in artificial cerebrospinal fluid (aCSF), was administered i.c.v. at a dose of 5 μg / 3 μl. Lithium chloride (LiCl) (Sigma-Aldrich, St. Louis, MO) was dissolved in distilled water to obtain a 0.15M concentration and administered i.p. (2% of body weight; b.wt.). Central injections were administered over 30 s, followed by a 1 min delay before removing the injector from the cannula.
Feeding and Drinking Regimen
Food (Standard chow; Teklad Global Diet 2018) was allowed ad libitum until the start of each experimental trial, at which point food was removed for ca. 30 min. The rats’ water restriction regimen began 4 days prior to the start of the first experimental trial and continued throughout the duration of the study. The animals had 1 h access to a pre-weighed bottle of water every day. The rats’ body weights were monitored to ensure they did not drop below 85% of the pre-restriction values. On experimental days, instead of water, rats were given 1 h access to either a single burette containing a 0.1% solution of saccharin (CTA induction day) or 2 separate burettes containing the saccharin solution and water (tests for extinction of CTA).
CTA to a novel saccharin solution
Animals were trained to drink water for 1 h (10:00-11:00) each day on a restricted drinking schedule for 5 days to circumvent their natural neophobia and ensure that they would drink the novel test solution when it was presented to them. The rats were randomly divided into 4 groups that received (i) aCSF (3 μl) centrally and NaCl (0.9%, 2% of body weight) i.p., (ii) aCSF centrally and LiCl (0.15M, 2% of body weight) i.p., (iii) MCH (5 μg/3 μl) centrally and NaCl i.p. and (iv) MCH i.c.v. and LiCl i.p. The central injections were followed by exposure to a novel 0.1% saccharin solution. Thirty minutes later, the saccharin solution was removed and the peripheral injections were administered. Water restriction (1 h/day) continued for the next 72 h after which the two-bottle preference test was administered.
The strength of the aversion was determined 72 h post-injection with a two-bottle test. The rats were offered two burettes places in cages side-by-side. One bottle contained water and the other, the 0.1% saccharin. Liquid intake was measured after 30 min. In order to assess whether MCH treatment facilitated CTA extinction, the rats were again centrally administered the same injection they received on the day when the CTA was induced. These central injections were administered 3 days and 6 days after the first two-bottle test. On both these days immediately following the central injections, the rats were given access to 2 burettes with water in one, and 0.1% saccharin solution in the other. Liquid intake was measured 30 min after the central injections.
Data Analysis
Results are presented as means ± SEM. Liquid intake data were analyzed using two-factor ANOVAs. Values were considered significantly different when p<0.05.
RESULTS
Experiment 1
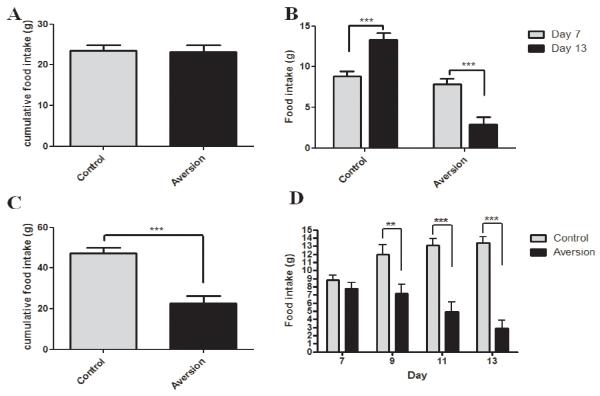
Over the first 3 days of the study the animals were becoming accustomed to the feeding schedule and, therefore, we disregarded food intake measurements obtained during this period. Cumulative chow intake in the animals over day 4-6, just prior to the first LiCl injection is displayed in Figure 1A (no difference between groups).
Fig 1.
A Cumulative chow intake in the control vs. aversion group over 3 days prior to the first LiCl injection. B Intake of the novel diet on day 7 (just prior to the first injection) compared to day 13 of the study. LiCl-injected animals decreased their food intake significantly (P<0.0001) whereas intake in the control group increased (P<0.0001). C Cumulative food intake over injection days 7,9,11 and 13 between the control and aversion group. Aversive animals ingested significantly less food compared to control animals (P<0.0001). D Overview over food intake on injection days for aversive animals compared to controls.
Figure 1B demonstrates the aversive effect of i.p. LiCl injections paired with the novel diet. Upon the initial exposure to the diet (preceding the first LiCl injection; day 7) animals consumed significantly (P<0.0001) more food than upon the last exposure (day 13). The saline-treated group on the other hand gradually increased food intake, likely as a result of greater acceptance of the novel diet.
Figure 1C shows the cumulative intake of the novel diet over injection days 7, 9, 11 and 13 between control and CTA rats. Intake of the novel diet was significantly lower in CTA rats compared to the control group (P<0.0001). Figure 1D displays all four exposure days and shows that the difference in cumulative food intake of the novel diet presented in figure 1C did not originate from one outlier day.
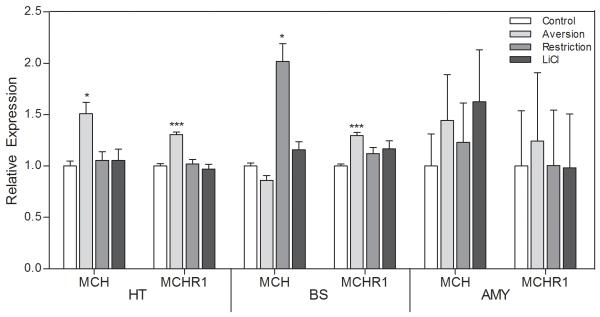
Aversion was associated with increased levels of both MCH and MCHR1 mRNA. Upregulation of MCHR1 gene expression was found in both the hypothalamus and brain stem of the CTA rats, whereas MCH mRNA was significantly higher in the hypothalamus. Although a trend suggesting upregulation of both MCH and MCHR1 genes was detected also in the amygdala of these animals, it did not reach the significance level (MCH: p=0.094; MCHR1: p=0.097).
When a decrease in food intake was due to deprivation rather than the malaise/aversion-dependent avoidance of the diet, only the brainstem pool of the MCH gene was affected. In these animals, pair-fed to match food intake in the CTA group, no other changes in expression of genes encoding MCH peptide or receptor occurred (Figure 2).
Fig 2.
Relative expression of the MCH and MCH receptor 1 genes in the hypothalamus (HT), brain stem (BS), and amygdala (Amy) of rats displaying CTA toward the presented diet (aversion), non-aversive animals pair-fed to the CTA rats (restriction), nonaversive animals pair-fed to the CTA rats, injected with LiCl 4 hours after exposure to the diet (LiCl), and unrestricted non-CTA controls (control). Animals had access to food during a scheduled 2-h period. *P < 0.05.
In order to rule out any effect of LiCl on the expression of these genes, one group of animals was injected with LiCl 4 hours after exposure to the novel diet. Analysis of MCH and MCHR1 gene expression revealed no significant changes compared to the control group.
Experiment 2
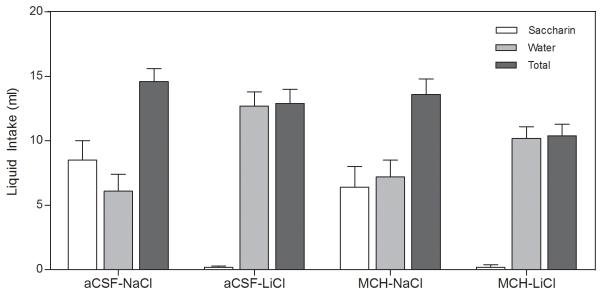
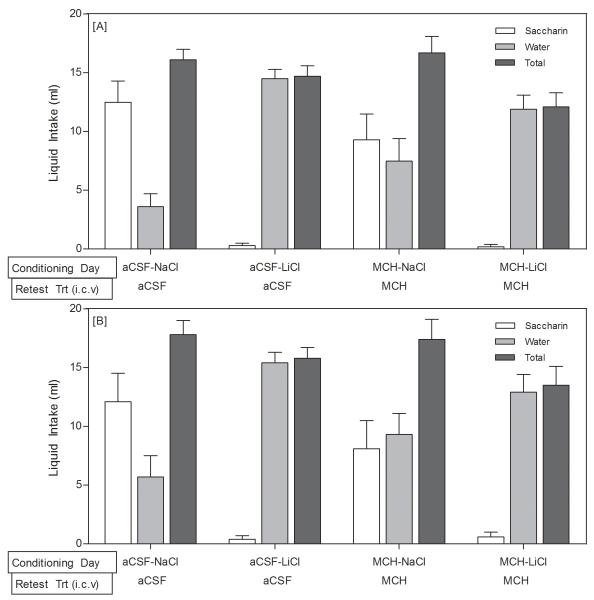
On the day of the CTA induction (upon the first presentation of saccharin, immediately preceding LiCl injections) the rats in all 4 groups drank a statistically similar amount of saccharin (p>0.05). The strength of the aversion was assessed by the two-bottle choice test 72 hours after the induction of the CTA. There was no effect of the central (i.c.v.) injections (F1,39=0.329, p>0.05, Figure 3); there was only an effect of the peripheral injection (F1,39=17.496, p<0.001, Figure 3) such that rats that had been treated with LiCl drank water almost exclusively, regardless of whether they had received a central injection of MCH or aCSF on the CTA induction day. In order to examine whether MCH treatment facilitated extinction of the aversion, the central injections were repeated 3 days and 6 days after the two-bottle choice test and the rats were again given access to both water and saccharin. Again there was no effect of the central injections (F1,39=0.237, p>0.05, Figure 4A; F1,39=0.127, p>0.05, Figure 4B), but there was a robust effect of the peripheral injection, such that the rats that had been treated with LiCl continued to avoid the saccharin and almost exclusively consumed water (F1,39=32.345, p<0.001, Figure 4A; F1,39=19.690, p<0.001; Figure 4B).
Fig 3.
Acquisition of CTA: LiCl-treated rats avoided consuming saccharin regardless of whether they had received aCSF or MCH centrally. Data are presented as means ± SEM (N = 10-11/group).
Fig 4.
Magnitude and Retrieval of the CTA: [A] Three days after the 2-bottle choice test, the LiCl-treated rats continued to avoid saccharin. [B] Six days after the 2-bottle choice test, the LiCl-treated rats continued to avoid saccharin despite the MCH treatment. On both of these test days, no peripheral injections were administered. Data are presented as means ± SEM (N = 10-11/group).
DISCUSSION
The current study was conducted to investigate the role of MCH in CTA by examining expression of genes coding for MCH and its receptor in the aversive state and by testing the ability of MCH to reduce CTA. We found that central MCH and MCHR1 mRNA levels were elevated in aversive rats. Despite the changes in gene expression of the MCH system during aversion, intracranial administration of MCH at an orexigenic dose did not diminish LiCl CTA-driven reduction in consumption.
Acquisition and maintenance of aversive responses engage a variety of central systems, such as those involved in stress, learning and memory. Importantly, as food avoidance is a crucial component of the aversive state, feeding-related systems are also affected (Thiele et al., 1997; Renner et al., 2010). In line with that, CTA is associated with changes in activity of neurons and expression of genes involved in controlling a meal size and perceived palatability (thus a rewarding value) of presented food (Lee et al., 2009; Olszewski et al., 2010b). In turn, these neural and molecular adaptations appear to support short- and long-term hypophagic responsiveness upon presentation of potentially tainted tastants. For example, increase in activation of anorexigenic OT and VP neurons occurs upon LiCl injections. Elevated c-Fos immunoreactivity has been found in undefined cells in the nucleus of the solitary tract in the brain stem as well as in the hypothalamus of rats treated with LiCl (Olszewski et al., 2000; Olszewski et al., 2010a; Renner et al., 2010; Schwarz et al., 2010). Rinaman (2000) reported that neurons synthesizing glucagon-like peptide-1 (GLP1), including those that project to the PVN, are activated by LiCl (Rinaman, 1999). Hypophagia during aversion retrieval also depends on multiple factors, such as OT and cholecystokinin (CCK) (Onaka & Yagi, 1998). We have recently reported that expression of genes encoding the components of the N/OFQ system in the brain is altered in CTA (Olszewski et al., 2010a). The present project delineates MCH/MCHR1 as another orexigenic system whose activity is affected during aversion, which reflects the decrease in the animal’s drive to consume food. It is noteworthy that upregulation in MCH and MCHR1 mRNA levels was not observed only in the hypothalamus, but also a clear trend persisted in the amygdala and significant changes in MCHR1 mRNA were detected in the brainstem. Hence, the MCH peptide/receptor gene expression response in CTA was common for several brain regions.
Although Qu et al. (1996) have shown that hypothalamic MCH expression in starved mice increases roughly fourfold compared to ad libitum-fed controls (Qu et al., 1996), we did not observe upregulation in the restricted group. This may be due to differences in feeding paradigms, since all our animals were schedule-fed, which by itself constitutes mild food restriction. That MCH and MCHR1 mRNA levels were not affected by energy deprivation itself, but only in association with CTA, serves as evidence linking this system with aversion.
Importantly, simultaneous upregulation of the receptor and ligand encoding genes was somewhat puzzling and expression data per se do not provide the sufficient basis to speculate whether changes in the activity of these genes are indeed critical for the formation and/or maintenance of CTA. We therefore performed an injection experiment to examine whether centrally administered MCH at the dose known to induce consumption for energy and reward (Duncan et al., 2005; Sakamaki et al., 2005) can also increase the consummatory response in CTA animals. In fact, MCH has been shown to elevate palatable saccharin intake under some conditions (Furudono et al., 2006), however, in the current experiment involving daily scheduled drinking, no effect of MCH on saccharin solution intake was observed, likely due to very high volumes of consumed fluid. Several orexigens have been found to reduce aversive responsiveness. Those include peptides that induce hunger for calories or feeding for reward, such as Agouti-related protein (AgRP), opioids and N/OFQ (Olszewski et al., 2000; Wirth et al., 2002). The fact that N/OFQ reduces CTA, while the NOP receptor antagonist delays extinction of LiCl-induced CTA, seemed of particular importance, as changes in N/OFQ and NOP mRNA levels had been previously detected in the same tissue that was used in the current study. Unlike N/OFQ, intracerebroventricular MCH injected at the orexigenic dose just before the onset of aversion failed to hamper the development of CTA. We also attempted to alleviate aversive responses during the CTA retrieval process by injecting MCH just before saccharin presentation and this treatment was done on CTA post-induction days 3 and 6. The rats continued to avoid the saccharin solution, demonstrating that MCH did not promote extinction of aversion. These data suggest that MCH does not retain its orexigenic properties in the context of food avoidance resulting from aversion; and that MCH does not counteract the onset or maintenance of aversive responsiveness.
Overall, the current set of data offers an interesting insight into our understanding of the aversion process. It defines MCH and its receptor as an orexigenic system whose expression is affected in the brain of animals displaying CTA, and this effect is independent of energy status of the organism. This altered MCH expression profile may serve as one of the factors contributing to tastant-targeted hypophagia in CTA. However, changing the MCH tone in the brain by supplying exogenous peptide is insufficient to prevent the onset or facilitate extinction of food avoidance caused by LiCl-induced CTA. This designates MCH as one of many accessory molecules associated with shaping an aversive response, but not a critical one for LiCl-dependent CTA to occur. Despite negative data obtained in the injection experiment, future studies on utilizing MCH in pharmacological interventions aimed at reducing aversion are needed, for example in the context of CTAs caused by factors other than LiCl or in relation to usability of MCH in combination treatment with other anti-aversive agents.
Research Highlights for “Expression levels of genes encoding melanin concentrating hormone (MCH) and MCH receptor change in taste aversion, but MCH injections do not alleviate aversive responses”.
-
>
We examine using real-time PCR, whether the expression levels of genes encoding MCH and its receptor, MCHR1, were affected in conditioned taste aversion (CTA) in the rat.
-
>
We also examine whether injecting MCH intracerebroventricularly during the acquisition and retrieval of LiCl-induced CTA, would alleviate aversive responses.
-
>
MCHR1 mRNA was up-regulated in the hypothalamus and brain stem, MCH mRNA was significantly higher in the hypothalamus, and there was a strong trend suggesting an elevation of MCH and MCHR1 genes in the amygdala.
-
>
However central administration of MCH did not reduce the magnitude of Li-Cl dependant CTA.
Acknowledgments
We thank Shahrzad Shirazi Fard for technical help with RNA preparation. The studies were supported by the Swedish Research Council, The Novo Nordisk Foundation and the Brain Research Foundation, National Institute on Drug Abuse Grants R01DA021280, National Institute of Diabetes and Digestive and Kidney Diseases P30DK50456, and the National Institute of Dental and Craniofacial Research Grant T32DE007288.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adamantidis A, de Lecea L. A role for Melanin-Concentrating Hormone in learning and memory. Peptides. 2009;30:2066–2070. doi: 10.1016/j.peptides.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal-Zimanyi I, Khawaja X. The role of melanin-concentrating hormone in energy homeostasis and mood disorders. J Mol Neurosci. 2009;39:86–98. doi: 10.1007/s12031-009-9207-6. [DOI] [PubMed] [Google Scholar]
- Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res. 2005;29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- Furudono Y, Ando C, Yamamoto C, Kobashi M, Yamamoto T. Involvement of specific orexigenic neuropeptides in sweetener-induced overconsumption in rats. Behav Brain Res. 2006;175:241–248. doi: 10.1016/j.bbr.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, Tanaka T, Tokita S, Moriya M, Iwaasa H, Kanatani A. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone. Am J Physiol Endocrinol Metab. 2003;284:E583–588. doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- Griffond B, Risold PY. MCH and feeding behavior-interaction with peptidic network. Peptides. 2009;30:2045–2051. doi: 10.1016/j.peptides.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Ito M, Gomori A, Ishihara A, Oda Z, Mashiko S, Matsushita H, Yumoto M, Sano H, Tokita S, Moriya M, Iwaasa H, Kanatani A. Characterization of MCH-mediated obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E940–945. doi: 10.1152/ajpendo.00529.2002. [DOI] [PubMed] [Google Scholar]
- Kela J, Salmi P, Rimondini-Giorgini R, Heilig M, Wahlestedt C. Behavioural analysis of melanin-concentrating hormone in rats: evidence for orexigenic and anxiolytic properties. Regul Pept. 2003;114:109–114. doi: 10.1016/s0167-0115(03)00114-9. [DOI] [PubMed] [Google Scholar]
- Kokkotou E, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R117–124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee JH, Moon YW, Chun BG, Jahng JW. Proteomic analysis of lithium-induced gene expression in the rat hypothalamus. Int J Neurosci. 2009;119:1267–1281. doi: 10.1080/00207450902889201. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Johansson A, Holmgren A, Grandin E, Nedergard C, Fredriksson R, Schioth HB. Increased mRNA levels of tyrosine hydroxylase and dopamine transporter in the VTA of male rats after chronic food restriction. Eur J Neurosci. 2006;23:180–186. doi: 10.1111/j.1460-9568.2005.04531.x. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzon ME, De Barioglio SR. Response to novelty after i.c.v. injection of melanin-concentrating hormone (MCH) in rats. Physiol Behav. 1999;67:813–817. doi: 10.1016/s0031-9384(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Morens C, Norregaard P, Receveur JM, van Dijk G, Scheurink AJ. Effects of MCH and a MCH1-receptor antagonist on (palatable) food and water intake. Brain Res. 2005;1062:32–38. doi: 10.1016/j.brainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Grace MK, Fard SS, Le Greves M, Klockars A, Massi M, Schioth HB, Levine AS. Central nociceptin/orphanin FQ system elevates food consumption by both increasing energy intake and reducing aversive responsiveness. Am J Physiol Regul Integr Comp Physiol. 2010a;299:R655–663. doi: 10.1152/ajpregu.00556.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schioth HB, Levine AS. Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology. 2010b;151:4736–4744. doi: 10.1210/en.2010-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Shi Q, Billington CJ, Levine AS. Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1504–1511. doi: 10.1152/ajpregu.2000.279.4.R1504. [DOI] [PubMed] [Google Scholar]
- Onaka T, Yagi K. Oxytocin release from the neurohypophysis after the taste stimuli previously paired with intravenous cholecystokinin in anaesthetized rats. J Neuroendocrinol. 1998;10:309–316. doi: 10.1046/j.1365-2826.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Academic Press; Sydney ; Orlando: 1986. [Google Scholar]
- Peyron C, Sapin E, Leger L, Luppi PH, Fort P. Role of the melanin-concentrating hormone neuropeptide in sleep regulation. Peptides. 2009;30:2052–2059. doi: 10.1016/j.peptides.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Renner E, Szabo-Meltzer KI, Puskas N, Toth ZE, Dobolyi A, Palkovits M. Activation of neurons in the hypothalamic dorsomedial nucleus via hypothalamic projections of the nucleus of the solitary tract following refeeding of fasted rats. Eur J Neurosci. 2010;31:302–314. doi: 10.1111/j.1460-9568.2009.07053.x. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Risold PY, Croizier S, Legagneux K, Brischoux F, Fellmann D, Griffond B. The development of the MCH system. Peptides. 2009;30:1969–1972. doi: 10.1016/j.peptides.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Sakamaki R, Uemoto M, Inui A, Asakawa A, Ueno N, Ishibashi C, Hirono S, Yukioka H, Kato A, Shinfuku N, Kasuga M, Katsuura G. Melanin-concentrating hormone enhances sucrose intake. Int J Mol Med. 2005;15:1033–1039. [PubMed] [Google Scholar]
- Schwarz J, Burguet J, Rampin O, Fromentin G, Andrey P, Tome D, Maurin Y, Darcel N. Three-dimensional macronutrient-associated Fos expression patterns in the mouse brainstem. PLoS One. 2010;5:e8974. doi: 10.1371/journal.pone.0008974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van der Ploeg LH, Howard AD. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol. 1997;272:R726–730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MM, Olszewski PK, Levine AS, Giraudo SQ. Effect of Agouti-related protein on development of conditioned taste aversion and oxytocin neuronal activation. Neuroreport. 2002;13:1355–1358. doi: 10.1097/00001756-200207190-00028. [DOI] [PubMed] [Google Scholar]