Abstract
Dental caries in very young children may be severe, result in serious infection, and require general anesthesia for treatment. Dental caries results from a shift within the biofilm community specific to the tooth surface, and acidogenic species are responsible for caries. Streptococcus mutans, the most common acid producer in caries, is not always present and occurs as part of a complex microbial community. Understanding the degree to which multiple acidogenic species provide functional redundancy and resilience to caries-associated communities will be important for developing biologic interventions. In addition, microbial community interactions in health and caries pathogenesis are not well understood. The purpose of this study was to investigate bacterial community profiles associated with the onset of caries in the primary dentition. In a combination cross-sectional and longitudinal design, bacterial community profiles at progressive stages of caries and over time were examined and compared to those of health. 16S rRNA gene sequencing was used for bacterial community analysis. Streptococcus mutans was the dominant species in many, but not all, subjects with caries. Elevated levels of S. salivarius, S. sobrinus, and S. parasanguinis were also associated with caries, especially in subjects with no or low levels of S. mutans, suggesting these species are alternative pathogens, and that multiple species may need to be targeted for interventions. Veillonella, which metabolizes lactate, was associated with caries and was highly correlated with total acid producing species. Among children without previous history of caries, Veillonella, but not S. mutans or other acid-producing species, predicted future caries. Bacterial community diversity was reduced in caries as compared to health, as many species appeared to occur at lower levels or be lost as caries advanced, including the Streptococcus mitis group, Neisseria, and Streptococcus sanguinis. This may have implications for bacterial community resilience and the restoration of oral health.
Introduction
Dental caries is the most common chronic disease of childhood [1]. It can occur in very young children, shortly after the eruption of teeth, and may be severe. For many children, early childhood caries is a source of pain and impaired quality of life, and for some it results in serious infection, hospitalization, and even fatality [2]. In this young age cohort treatment must often be completed under general anesthesia, accounting for a disproportionate fraction of total dental expenditures [3].
It is of particular importance to understand the microbial etiology of the onset of caries, since preventive interventions such as probiotics or vaccines will be most effective if they interrupt the process before irreversible damage is done to teeth. Once lesions advance beyond the white spot stage and the enamel surface is damaged, they cannot be biologically reversed. Moreover, the disease process may be refractory to ordinary preventive measures that involve biofilm removal such as tooth brushing, since the biofilm becomes more protected from mechanical disruption. Also, early caries experience appears to predispose to greater caries experience later in life, affecting the permanent dentition [4]–[7].
Recent advances, including data from the Human Microbiome Project, have lead to a new paradigm for understanding chronic, bacterially mediated diseases. Diseases of the oral cavity occur in a complex host-bacterial community interaction that often does not fit a single microbe pathogenesis model. Dental caries occurs as the result of a shift in the composition of a biofilm community specific to the human tooth surface. Frequent carbohydrate intake can disrupt the ecology of this community by the selection of acidogenic and acid tolerant species, and these acidogenic communities are responsible for caries development [8], [9]. Streptococcus mutans appears to be the most common acid producer in caries initiation [10], but S. mutans is not present in all children with caries, and when found it is part of a complex microbial community [11]–[15]. Understanding the degree to which multiple acidogenic species provide functional redundancy and resilience to caries-associated communities is important for developing biologic interventions. Additionally the importance of microbial community interactions in caries pathogenesis is not well understood, including the contribution of bacterial community members in promoting health, such as alkali production [16] or colonization resistance.
Technical advances in 16S rRNA gene analysis have made it possible to comprehensively examine the composition of microbial communities and to study differences between health and disease. The purpose of this study was to investigate bacterial community profiles associated with the onset of early childhood caries in the young primary dentition, and to compare them to bacterial communities found on healthy teeth and in dentally healthy children. In a combination cross-sectional and longitudinal design, bacterial community profiles at progressive stages of caries and over time were examined. 16S rRNA gene cloning and sequencing were used for bacterial community analysis. Streptococcus mutans was found to be the dominant species in many, but not all, subjects with caries; elevated levels of Streptococcus vestibularis/Streptococcus salivarius, Streptococcus sobrinus, and Streptococcus parasanguinis were also associated with caries, especially in subjects with no or low levels of S. mutans, suggesting these species are alternative pathogens. Bacterial community diversity was reduced in caries as compared to health, as many species appeared to occur in reduced numbers or be lost as caries advanced. This may have implications for bacterial community resilience and restoration of oral health.
Results
Demographics and Clinical Outcomes
Thirty-six subjects with caries and 36 healthy controls were recruited for this study. Baseline samples were collected from all subjects. For subjects with dental caries plaque was collected separately from the surfaces of three progressive stages of caries. Longitudinal samples were collected as described below. There were no significant differences between the caries and control groups for gender, race or ethnicity based on Chi-squared analysis. The distribution of race in the study population was 40% white, 38% black, and 22% other. The study population was 83% not Hispanic and 17% Hispanic, and 54% female. Ages ranged from 12 to 36 months old at enrollment (mean age was 23.6 months) and were not significantly different between the caries and control groups. There was no difference between the groups based on smoke exposure or history of antibiotic use in the previous 30 days based on Chi-squared analysis. Sample sizes were too small to analyze the effect of fluoride, since the majority of subjects (89% of caries subjects and 92% of control subjects) reported exposure to fluoride through drinking water.
Cloning and Sequencing
An average of 54 clones (minimum 44, maximum 102) were identified per sample. The total number of clones for all samples was 9,396. A total of 6,688 clones were sequenced from baseline samples (caries and control subjects) and 2,708 clones were sequenced from longitudinal samples (caries subjects only). The shortest sequence length was 563 base pairs, and the average sequence length was 1060 base pairs.
Taxonomic Identification and Disease Association
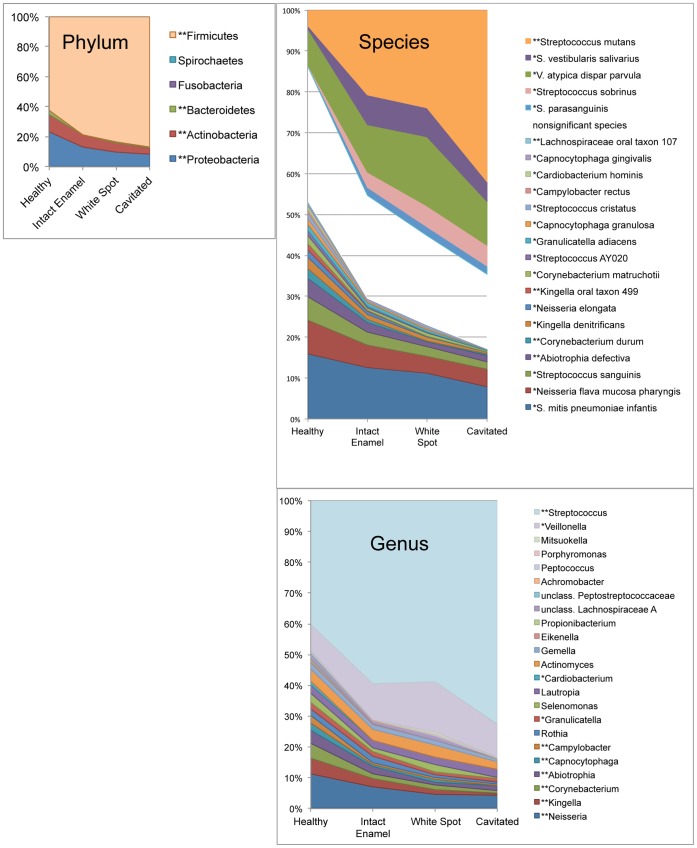
Overall 134 species were identified in this study, including two novel taxa. They could be assigned to 45 genera and six phyla. Only 9.65% of total clones represented uncultivated species. Baseline mean relative levels of bacterial taxa by advancing caries stage are plotted in Figure 1. At the level of phylum, Proteobacteria, Actinobacteria and Bacteroidetes decreased as caries stage increased, and Firmicutes increased. At the level of genus Veillonella and Streptococcus significantly increased with caries progression, and 8 other genera decreased as caries stage advanced (details in Figure 1). Five species-level taxa were significantly higher as caries stage increased, including Streptococcus mutans, the Streptococcus vestibularis/Streptococcus salivarius group, the Veillonella atypica/Veillonella dispar/Veillonella parvula group, Streptococcus sobrinus, and Streptococcus parasanguinis. Seventeen species significantly decreased as caries stage progressed (details in Figure 1).
Figure 1. Relative levels of bacterial taxa by advancing stage of caries.
Graphs at the level of phylum, genus and species are shown. Taxa are sorted by magnitude of change with stage of caries (linear mixed effects model estimates), so that taxa associated with health sort at the bottom and taxa associated with caries are shown at the top. “*” indicates taxa with p<0.05 and “**” indicates taxa with p<0.01 after the false discovery rate correction was applied. Only genera found at greater than 0.1% of total clones and species found at greater than 0.2% of total clones are shown, and only those taxa significantly associated with caries or health are shown in the species-level graph.
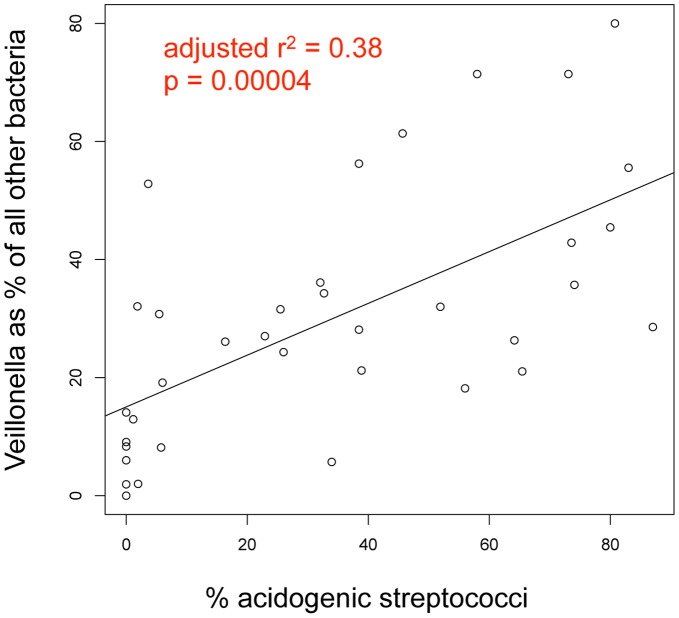
Figure 2 shows a significant correlation between the relative levels of Veillonella and the major acid-producing species.
Figure 2. Correlation between relative levels of Veillonella and acidogenic streptococci in white spot lesions.
The total % abundance of S. mutans, S. sobrinus, and S. vestibularis/salivarius combined is plotted against the abundance of the Veillonella atypica/dispar/parvula group expressed as a fraction of the remaining community. The result of a linear regression is shown as a line with the indicated parameters.
Cluster Analysis and Heterogeneity Among Subjects
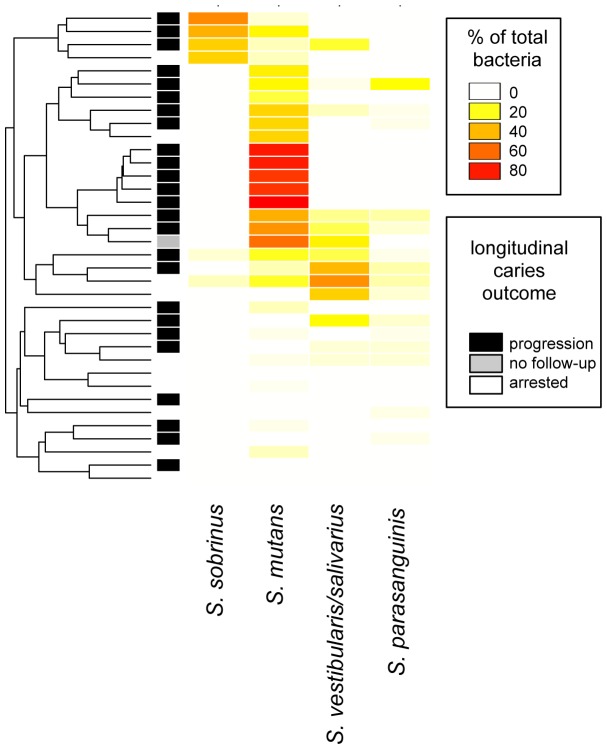
Figure 3 shows an abundance heatmap with sample clustering for the significant acid producing species in baseline samples from white spot lesions. This illustrates heterogeneity among subjects with respect to the different acid producers.
Figure 3. Heatmap and cluster analysis of baseline samples from white spot lesions.
Abundances of those bacterial species significantly associated with caries are shown, except for Veillonella which was ubiquitous and therefore omitted. The samples (one from each of 36 subjects) are arranged by hierarchical clustering using the average method and Bray-Curtis dissimilarity. Abundance as percentage of the total community is indicated by the color scale. The bar along the left side indicates longitudinal caries activity.
Community Diversity and Caries Stage
Overall more taxa decreased than increased as caries stage advanced within subjects, and this was true at all phylogenetic levels. The relationship between caries stage and bacterial community diversity for baseline samples is shown in Figure 4, upper panel. Overall, bacterial diversity decreased significantly (p<0.0001) as caries progressed from health to cavitated lesions.
Figure 4. Decreasing species diversity was observed with increasing caries severity both within and among subjects.
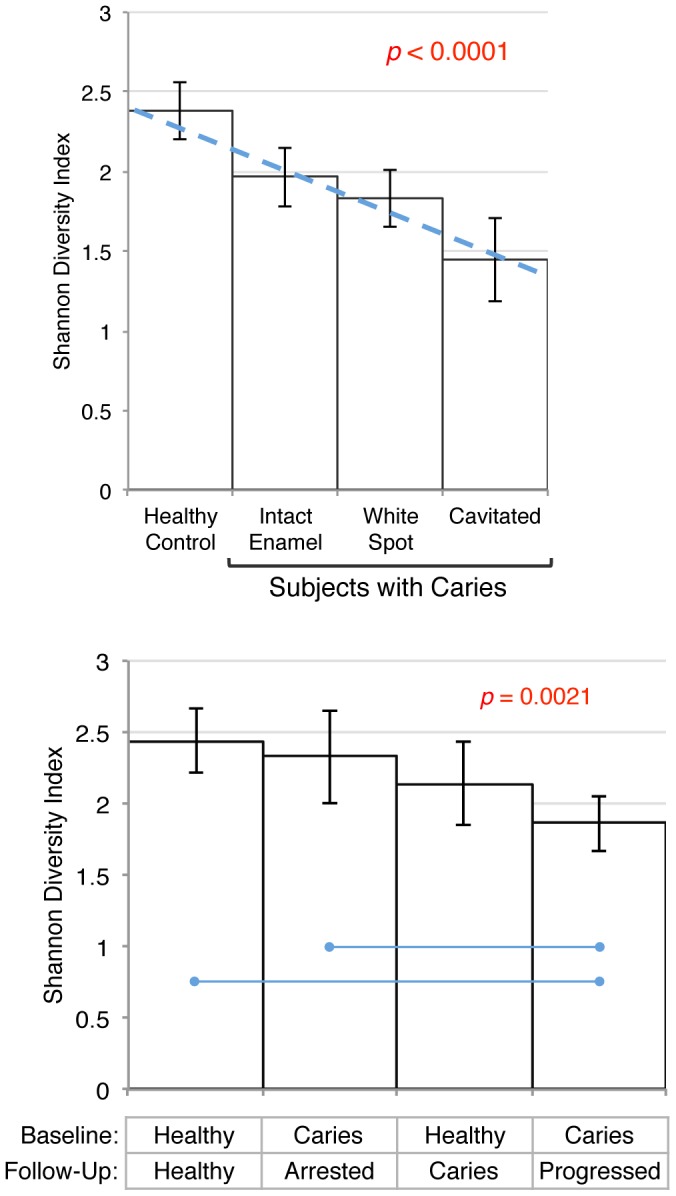
Mean Shannon Diversity Indices with 95% confidence intervals are shown. The upper panel shows diversity within subjects for stage of caries at baseline. Diversity was modeled using a linear mixed effects model (SAS PROC MIXED), and is shown as a dashed line (estimate = −0.26). Post hoc comparisons between sample types were significant, except between white spot and cavitated lesions. The lower panel shows species diversity comparisons among subjects by their baseline and longitudinal caries status for samples collected from noncarious enamel (the only type of sample available from all groups) using ANOVA. Significant post hoc comparisons are indicated by blue lines.
Bacterial community diversity was also significantly different among groups of subjects by ANOVA (p = 0.0021) as shown in Figure 4, lower panel. Post-hoc comparisons indicated that the samples taken from intact enamel at baseline from subjects with caries that progressed had significantly lower species diversity than that of subjects with caries that arrested and control subjects that remained healthy at follow-up.
Bacterial Community Shifts and Caries Stage within Subjects
Figure 5 shows a non-metric multidimensional scaling (NMDS) ordination based on Bray-Curtis dissimilarity for baseline samples from all stages of caries. A leftward shift with advancing caries stage can be observed in the plot. The ANOSIM test for all within-subject stages was significant (R = 0.232, p = 0.001) and pairwise comparisons revealed significant differences between healthy subjects and subjects with caries for all samples: intact enamel (R = 0.266, p = 0.002), white spot samples (R = 0.398, p = 0.002), and cavitated samples (R = 0.538, p = 0.002). The lower panels of Figure 4 illustrate the distribution of abundance within the samples for selected species.
Figure 5. Within-subject differences in plots of a non-metric multidimensional scaling (NMDS) ordination based on Bray-Curtis Dissimilarity.
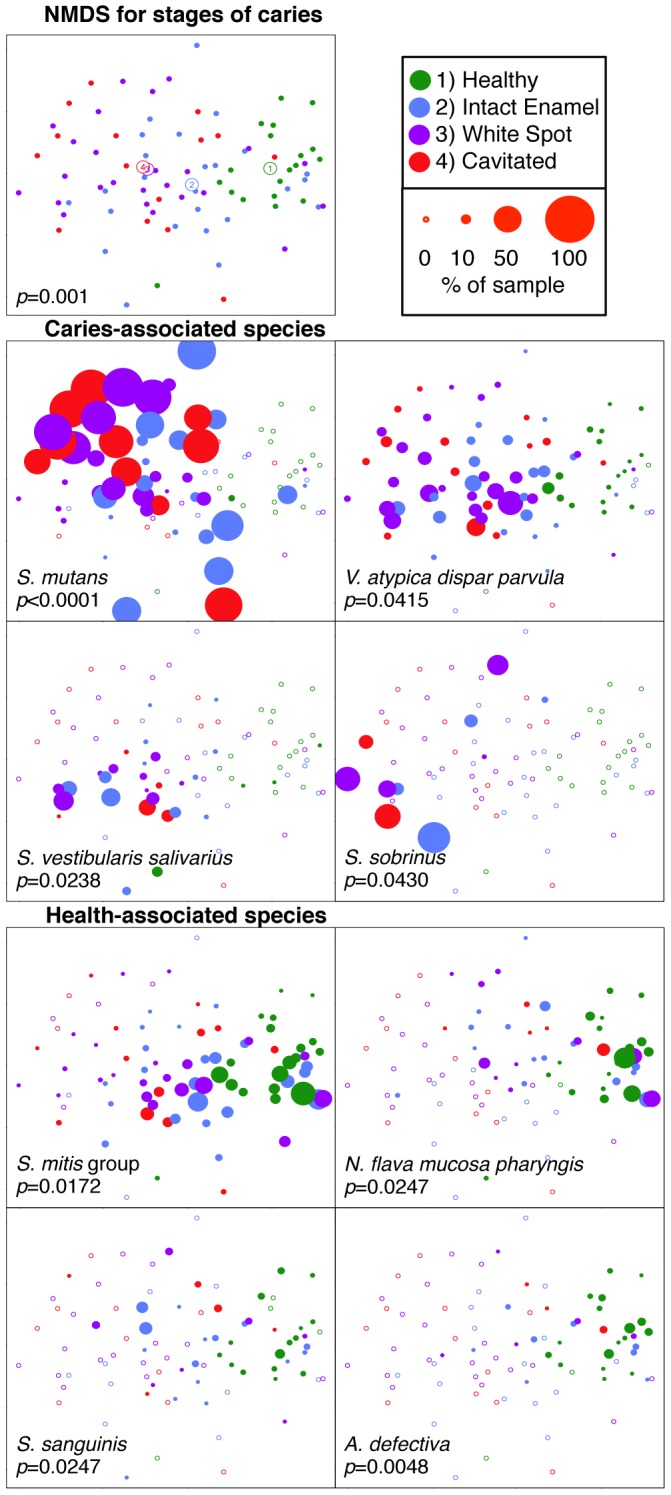
Baseline samples from only the subset of subjects whose caries status remained constant over time (caries subjects that continued to develop caries and healthy subjects that remained healthy) was included, and within-subject differences by stage of caries were observed. A single sample from each stage of caries is included for each subject, and each point represents a single sample. The top panel shows the NMDS plot, with the centroid for each stage of caries marked. The metaMDS algorithm used puts the largest dimension of change along the horizontal axis. The p-value is for the overall ANOSIM model. The points in lower panels are sized by abundance for the most common species significantly associated with caries and health, and p-values are for the linear mixed effects model estimates. Empty plot symbols represent samples where that species was not detected.
Longitudinal Analysis
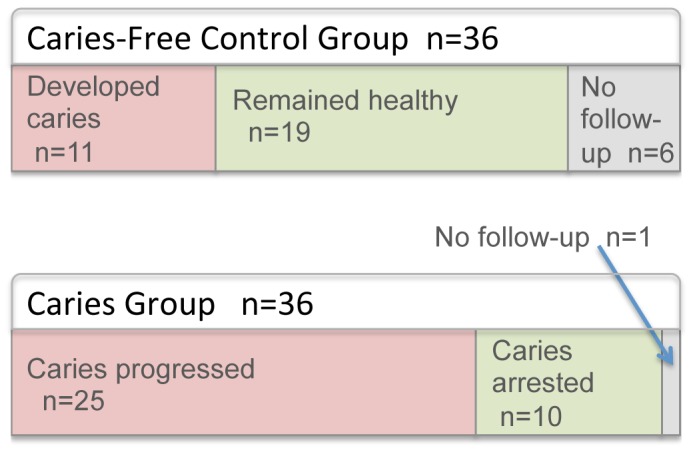
Subjects were followed longitudinally to assess their ongoing caries status. Twenty-one subjects with caries returned for a follow-up visit and sampling, and chart reviews were conducted for all remaining subjects. Sample sizes according to longitudinal caries status are shown in Figure 6. The relationship of baseline microbial community composition to longitudinal clinical outcomes was examined.
Figure 6. Sample sizes at baseline and outcomes at longitudinal follow-up.
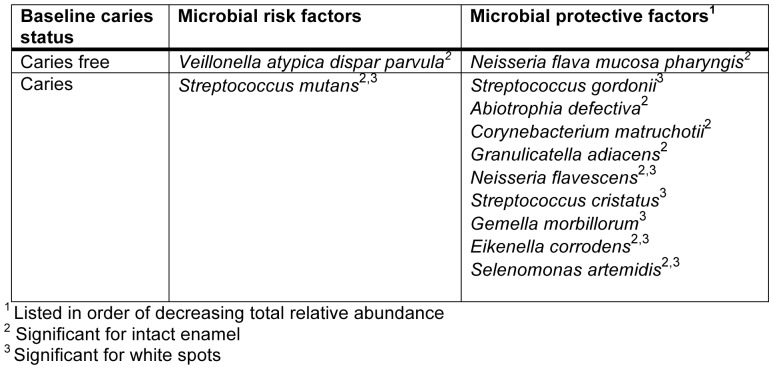
Levels of species occurring at greater than 0.2% of the total community at baseline were compared between progressed and arrested groups for intact enamel and white spot lesions by t-tests. Species that significantly predicted caries and health (candidate microbial risk and protective factors) are listed in Figure 7. Only Veillonella atypica/Veillonella dispar/Veillonella parvula remained significant after a false discovery rate correction was applied.
Figure 7. Candidate microbial risk and protective factors.
Candidate microbial risk and protective factors are listed for the onset of caries in subjects that were caries free at baseline and the progression of caries in subjects that had caries at baseline.
The NMDS plots shown in Figure 8 are keyed by longitudinal clinical status. ANOSIM analysis was significant for the overall model (R = 0.06819, p = 0.05), and pairwise comparisons revealed a significant difference between control subjects that remained healthy and those that developed caries (R = 0.1908, adjusted p = 0.015), as well as a difference between control subjects that remained healthy and caries subjects that progressed (R = 0.1571, adjusted p = 0.015).
Figure 8. Among-subject differences visualized by non-metric multidimensional scaling (NMDS) ordination based on Bray-Curtis Dissimilarity.
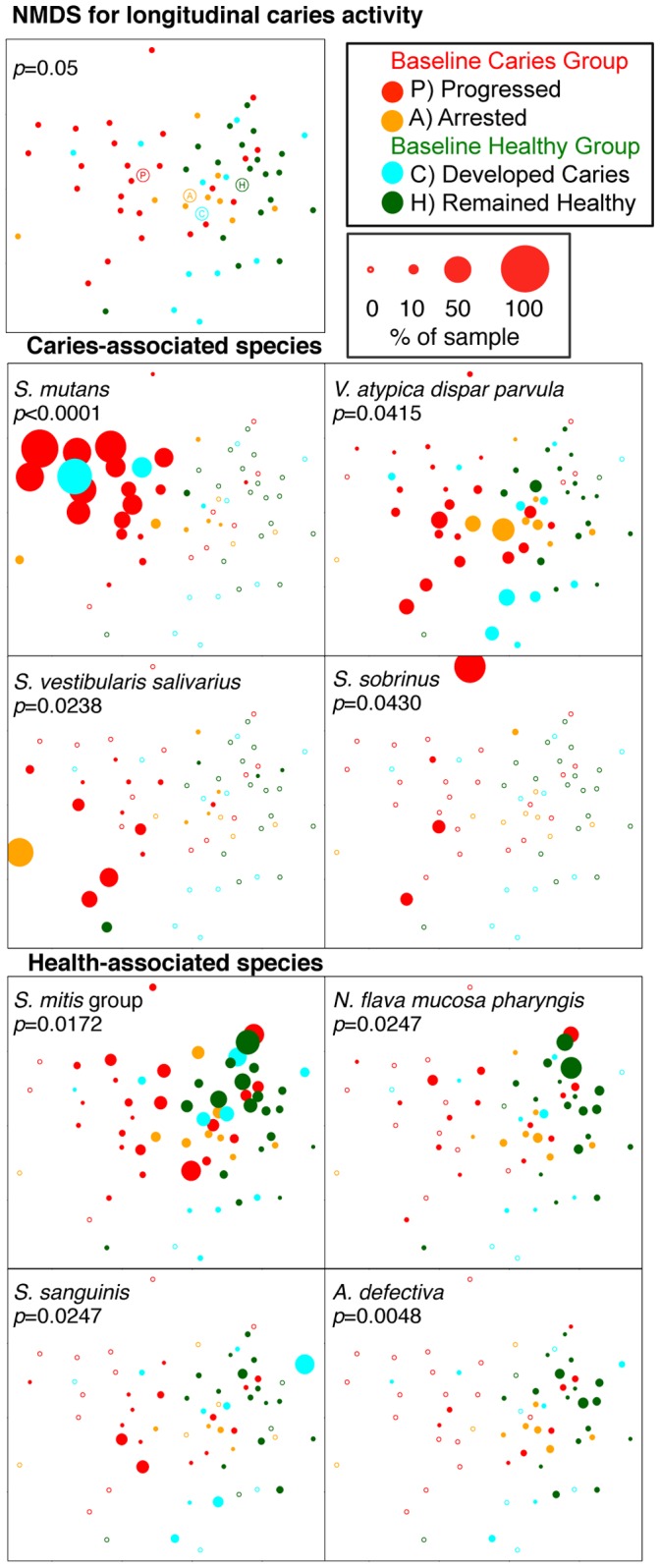
Among-subject differences by longitudinal caries activity were observed. The plots represent baseline community profiles for healthy subjects and subjects with caries. The baseline patient class and subsequent disease activity are color-coded. Samples from healthy enamel were the only stage available from all subjects and so were used here. The top panel shows the NMDS plot, with the centroid for each clinical group marked. The p-value is for the overall ANOSIM model. The points in lower panels are sized by abundance for the most common species significantly associated with caries and health, and p-values are for the linear mixed effects model estimates.
Repeat samples were obtained for 21 subjects with caries, and exploratory analyses were conducted to examine changes over time for major species. No differences in relative levels of species were observed over time for any stage of caries or for caries progressions for any of the species associated with advancing stage of caries within subjects (S. mutans, S. vestibularis/S. salivarius, V. atypica/V. dispar/V. parvula, S. mitis/S. pneumoniae/S. infantis/S. oralis, N. flava/N. mucosa/N. pharyngis, S. sobrinus, S. sanguinis, or A. defectiva). The comparison of Bray-Curtis dissimilarity over time within individuals revealed significantly greater similarity between samples from the same individual than between samples from different individuals, an indicator of long-term stability.
Discussion
For this study of the initiation of caries in young children, cross-sectional samples from dentally healthy children and from children with very early-stage caries were collected. Samples were collected from intact, noncarious tooth enamel from all subjects. Samples were also collected from two defined stages of caries from the subjects with the disease: from white spot lesions (the initial presentation of caries) and from cavitated lesions (more advanced and irreversible stage of caries). Subjects were followed longitudinally to determine progression of caries, and longitudinal samples were collected from those subjects who returned for follow-up. All subjects were given preventive counseling and treated with fluoride, yet a high proportion of subjects in both the caries group and the healthy group subsequently developed new lesions (Figure 6).
Bacterial community analysis was conducted using 16S rRNA gene cloning and sequencing, and bacteria were identified using an oral 16S rDNA database specifically curated for clinical data [17]. Bacterial taxa were measured as a percent of the total community. This open-ended approach allowed a comprehensive look at the bacterial communities associated with caries and dental health in children.
Caries-associated Species
Heterogeneity among samples
Figure 3 shows a cluster analysis for subjects with caries, and illustrates distinctive and heterogeneous profiles. As expected, the majority of subjects with caries exhibited high levels of S. mutans. However, perhaps the most interesting finding is that, as previously observed [11]–[13], [18], [19], not all subjects harbored high levels of S. mutans. In the present study two additional profiles, one dominated by S. sobrinus and one by S. vestibularis/S. salivarius, were observed. When present, one of these three species appeared to dominate the community, suggesting functional redundancy and competition rather than cooperative consortia. This has considerable clinical significance for diagnostic and therapeutic purposes. Interventions for caries have been focused almost entirely on S. mutans. It appears this target is appropriate for many, but not all, children. In addition, it seems possible that any of these three species could provide functional redundancy (lactate production and caries) if the others were eliminated by vaccination or other targeted therapy. In fact, one caries-active subject in the present study appeared to undergo a shift from an S. mutans-dominated profile to S. sobrinus, and another from an S. salivarius/S. vestibularis-dominated profile to S. mutans over time (data not shown). This potentially important observation will need to be investigated further in future studies. In other studies, additional species have been associated with disease when S. mutans was absent, including Lactobacillus species [11], [12], Bifidobacterium dentium [11], and Scardovia wiggsiae [15]. None were detected in significant levels in the current study, and this could be attributed to differences in study populations, severity of caries at the time of sampling, and methodologies, notably culture versus DNA-based methods.
For several of the samples taken from white spot lesions, a dominant, acid-producing species was not identified (Figure 3). About half of these subjects did not show progression of the white spot lesions observed at baseline (shown in dark bars in left column, Figure 3), suggesting that their lesions may have been developmental hypomineralizations rather than acid-induced demineralizations. These are difficult to distinguish clinically. In the remaining subjects, S. mutans, S. sobrinus or S. vestibularis/S. salivarius were all detected at low levels in other samples from the same subject. It was difficult to precisely sample these very young and often uncooperative subjects, and the target lesion may have been missed. It is also possible that genetically distinct strains of bacteria grouped with innocuous species by 16S rRNA analysis were the culprits, since the oral streptococci show high variability of their total genomes relative to their 16S rRNA genes [20].
S. mutans
S. mutans is highly acidogenic and aciduric, and considerable clinical and laboratory data implicates this species as the primary pathogen in human dental caries (reviewed in [10], [21], [22]). This role was corroborated in the present study. S. mutans was the most abundant species observed in samples from children with caries, and levels were highly significantly associated with lesion stage (Figure 1). It was also identified as a candidate risk factor for caries progression. Mean levels of S. mutans were higher in both intact enamel and white spot samples for subjects who developed new lesions as compared to subjects who did not (Figure 7). Although levels of S. mutans did not significantly predict the development of caries in previously healthy subjects, just two healthy control samples showed high levels of S. mutans at baseline (see S. mutans panel on Figure 8), and both of these subjects subsequently developed caries.
S. sobrinus
S. sobrinus is closely related to S. mutans, and these species are often referred to collectively as the mutans streptococci [20]. S. sobrinus was associated with caries in the current study (Figure 1), and appeared to be the primary pathogen in some subjects (Figure 3). Caries remained active over time in most of these subjects as shown in Figure 3, and S. sobrinus levels remained high in longitudinal samples (data not shown). In one subject with active caries S. mutans was replaced by S. sobrinus over time. In most samples dominated by S. sobrinus, low levels of S. mutans were present as well. Unlike S. mutans, it was not detected in any healthy control subjects, suggesting it may be a more specific predictor of disease than S. mutans.
The pathogenic potential of S. sobrinus has been established in a rat model [23], and it has been associated with childhood caries in several investigations from different geographic locales [13], [24]–[30]. It has often been found as a co-colonizer with S. mutans, and co-colonization has been consistently associated with greater caries risk and stronger association with caries [13], [24]–[30].
Salivarius group
S. vestibularis and S. salivarius are members of the salivarius group [31] and have been distinguished from each other based on phenotype. However, they cannot be reliably differentiated based on 16S rDNA sequence [17], and therefore the taxon designation here includes both species. Both species have been isolated from the human oral cavity, and in animal studies, isolates of S. salivarius have been shown to be strongly cariogenic, although less so than S. mutans [32]–[35]. In vitro studies suggest S. vestibularis is only mildly cariogenic [35], [36]. S. salivarius has been associated with caries in clinical studies using DNA-based methods [11], [37].
In the present study the salivarius group was significantly associated with caries, and appeared to be the primary pathogen in some subjects (Figure 3). The sample size is too small for analysis, but it appeared caries was less likely to remain active in these subjects than for those with an S. mutans or S. sobrinus-dominated profile as shown in Figure 3. However, S. salivarius levels remained high in most subjects with this profile who did progress (data not shown), providing support for a role in caries.
On the other hand, some strains of S. salivarius are known to produce urease, which hydrolyzes urea to ammonia, and so may be caries-protective [23], [38], [39]. A recent in vivo study found increased S. salivarius urease activity in plaque following a sucrose challenge [40], and S. salivarius has been associated with health in one study [41]. These conflicting findings might be explained by differences in detection methodology or genomic differences that are not detected using 16S rRNA methodologies. Future studies using higher resolution molecular techniques are needed to study this heterogeneous group of bacteria.
Streptococcus parasanguinis
In the present study S. parasanguinis was significantly associated with caries (Figure 1), but was not a dominant member of the community for any of the subjects, even those in whom no S. mutans was detected (Figure 3). S. parasanguinis has been significantly associated with caries in young children in two previous studies that utilized 16S rRNA methods [15], [37], and in another similar study it was found at high levels in an S. mutans-free subject with caries [11]. It ferments multiple carbohydrates to lactate and other organic acids [42], [43], and it appears to be moderately acid tolerant [44], consistent with a role in dental caries. However, it has also been associated with health in one study [41], and it has been reported to hydrolyze arginine to ammonia, and not to produce extracellular polysaccharide from sucrose [42]. So the contribution of S. parasanguinis to dental caries deserves further study.
Lactobacilli
Lactobacilli were rarely detected, and when present were at very low levels in this cohort of young children with the earliest stages of caries. Lactobacilli have shown a robust association with more advanced caries in many studies [11], [45]–[47] and in older children using a very similar experimental approach to the one used here [12], strongly suggesting that they are a later colonizer in microbial succession as caries-associated communities mature and shift to a lower pH.
Veillonella atypica/Veillonella dispar/Veillonella parvula
Three species of Veillonella have regularly been found in the oral cavity. These species have been distinguished from each other based on phenotype, but they cannot be reliably differentiated based on 16S rDNA sequence [17], [48], and therefore the taxon designation here includes all three Veillonella species. Veillonella rely solely on lactate and other organic acids as an energy source [49]. Veillonella was detected in most samples in the current study, and it was higher in samples from caries lesions (Figure 1, p = 0.0415, significant by linear mixed effect modeling). Veillonella was found to be significantly associated with caries in children in previous molecular studies as well [11], [15], [29], [37], [50]. Veillonella was highly correlated with the total of all known acid producing species, as shown in Figure 2. This is not surprising given its reliance on lactate as its nutrient source, and has potential clinical utility since Veillonella levels may serve as a sensitive biologic indicator and early warning of acid production. Among children without previous history of caries, Veillonella, but not S. mutans or other acid-producing species, predicted future caries (Figure 7). These findings need to be corroborated in a larger clinical study, and could lead to useful risk assessment methods for caries.
The contribution of Veillonella to caries in in vivo studies has been somewhat unclear, with laboratory studies showing effects on pH in both directions [51], [52]. Chemostat studies have shown levels of Veillonella species to increase as pH falls following glucose adminstration [53]–[56]. Mounting clinical data associating elevated levels of Veillonella with caries [11], [12], [15], [50], however, suggests that increasing levels of Veillonella do not halt caries by raising pH. Taken together with in vitro data [57] it appears Veillonella may even facilitate further acid production by S. mutans or other species by removing lactate from the environment and creating a higher pH microenvironment. A recent in vivo study showed that Veillonella mitigated the inhibitory effects of S. gordonii on S. mutans sugar metabolism [58], suggesting a specific interaction between S. mutans and Veillonella that may be more complex than pH.
Health-associated Species
A large number of species were associated with health in the current study. These species may be beneficial, and potential mechanisms are discussed below. Seventeen species were found at significantly higher relative levels in health (Figure 1) as compared to the more advanced stages of caries, with the major contributors being the Streptococcus mitis/Streptococcus pneumoniae/Streptococcus infantis/Streptococcus oralis group, the Neisseria flava/Neisseria mucosa/Neisseria pharyngis group, and Streptococcus sanguinis. At the level of phylum the Proteobacteria, Actinobaceria and Bacteroidetes were all health associated (Figure 1).
Several species were identified as candidate microbial protective factors against caries onset or progression from the longitudinal data as well (Figures 7 and 8). Baseline levels of the Neisseria flava/Neisseria mucosa/Neisseria pharyngis group were higher in control subjects that remained healthy when compared to control subjects that subsequently developed caries, suggesting a beneficial role in the caries process. Streptococcus gordonii, Abiotrophia defectiva, Corynebacterium matruchotii, Granulicatella adiacens, Neisseria flavescens, Streptococcus cristatus, Gemella morbillorum, Eikenella corrodens, and Selenomonas artemidis were all found at higher levels in subjects with caries that did not progress as compared to those whose caries progressed, suggesting a beneficial role for these species as well. Many of these candidates have been associated with health in previous clinical studies. Becker et al. found high levels of A. defectiva in a healthy subject and high levels of C. matruchotii in the intact enamel sample of a subject with caries. A. defectiva [29], [41], S. cristatus [11], [18], [29], [41], S. gordonii [59], and G. morbillorum [11], [41] were associated with health in previous investigations. N. flavescens was associated with health using next-generation sequencing and saliva samples [60]. N. flavescens is asaccharolytic [61]. S. cristatus and S. gordonii catabolize arginine to ammonia [62], [63] potentially raising pH. S. gordonii also inhibits biofilm formation [64] and bacteriocin production [64], [65] by S. mutans. C. matruchotii can utilize lactate [66], so it may also raise pH. S. artemidis produces propionic and acetic acids [67], which may be less destructive than lactic acid [68], [69]. Further laboratory study is required to determine the ability of these candidate species to protect against caries onset and progression.
Decreasing Species Diversity in Caries
In the present study diversity decreased with increasing caries stage within subjects, and among subjects was lowest in the group of subjects with caries that progressed (Figure 4). This confirms previous investigations that associated caries with a reduction in species diversity [12], [70]–[72] (Figure 4), and supports what has been called the “ecological plaque hypothesis” [57]. This explains caries as the result of an ecologic disruption of the normal, healthy bacterial community that occurs when carbohydrates are frequently available and acid-producing species lower pH through glycolytic activity. As a result acid-sensitive species are eliminated, and communities become dominated by just a few highly acid-tolerant species. This may accelerate further as species that normalize pH by the production of ammonia are lost [16], and this loss of diversity may have implications for bacterial community resilience and restoration of oral health.
Sequence Identification
Technical limitations of the current study include the small number of clones that were identified from each sample, and the “universal” primers used for 16S rRNA gene amplification that, although used with low stringency, may underrepresent some taxa. Degenerate universal primers have recently been developed and tested for multiple regions of the 16S rRNA gene that can be used in future studies to improve representation of community composition. Because oral streptococci show relatively low diversity in 16S rDNA sequence, longer reads are required to achieve resolution at the species level. Mean read length was 1060 bp, considerably longer than current next generation sequencing methodologies achieve. Future studies targeting the most highly variable regions of the 16S rRNA gene with optimized read lengths are needed to bring the power of deep sequencing approaches to understanding caries-associated bacterial communities.
Summary and Conclusions
Bacterial community profiles associated with the onset of early childhood caries in the young primary dentition were compared to bacterial communities found on healthy teeth and in dentally healthy children using a combination of a cross-sectional design representing the various stages of caries, and longitudinal clinical sampling. Differences between health and disease were observed at all taxonomic levels including phylum, genus and species. As expected, S. mutans was the dominant species in many, but not all, subjects with caries. Elevated levels of S. vestibularis/S. salivarius, S. sobrinus, and S. parasanguinis were significantly associated with caries, and were observed at especially high levels in subjects with little or no S. mutans, suggesting these species are alternative pathogens. Veillonella, which metabolizes lactate, was associated with caries and was highly correlated with total acid producing species. Among children without previous history of caries, Veillonella, but not S. mutans or other acid-producing species, predicted future caries. Bacterial community diversity was reduced in caries as compared to health, as many species appeared to occur at lower levels or be lost as caries advanced, including the Streptococcus mitis/Streptococcus pneumoniae/Streptococcus infantis/Streptococcus oralis group, the Neisseria flava/Neisseria mucosa/Neisseria pharyngis group, and Streptococcus sanguinis. This may have implications for bacterial community resilience and the restoration of oral health.
Materials and Methods
Ethics Statement
Approval from the Nationwide Children’s Hospital Institutional Review Board was obtained for this protocol, and written consent was obtained from the parents of all subjects.
Clinical Methods
Subject recruitment
Subjects with dental caries and a dentally healthy control group were recruited from the Nationwide Children’s Hospital Dental Clinic in Columbus, Ohio. General exclusionary criteria for either group included (i) age greater than 36 months, (ii) indications for infective endocarditis prophylaxis, and (iii) professional cleaning in the past 30 days. Only one child per family was included in each group. The inclusion requirement for the caries group was the presence of at least two maxillary incisors with white spot lesions, no cavitated lesion greater than 1 mm, and no existing restorations. Subjects with caries were asked to return every four to six weeks. Age-, race-, and gender-matched healthy control subjects that were caries-free and had no existing restorations were also recruited.
Sampling and clinical data collection
For the healthy subjects dental plaque was sampled from healthy enamel (stage 1). For the subjects with dental caries plaque was collected separately from the surfaces of each of three progressive stages: 2) intact enamel, 3) white spot lesions, and 4) cavitated lesions, if present. Therefore one sample was collected from each healthy subject, and either two or three samples were collected from each subject with caries, depending on whether cavitated lesions were present. For subjects with caries, all carious surfaces were scored. Dental plaque was collected by swiping the tooth or lesion surface with a coarse endodontic paper point. Each plaque sample was obtained by pooling from multiple teeth. Samples were placed in a sterile 1.5-ml microcentrifuge tube and frozen for storage.
A subset of caries subjects returned for a follow-up visit, during which the progression of any white spot or cavitated lesion was scored and the subject was re-sampled as above. Following the conclusion of the clinical study, longitudinal caries status for all healthy subjects and caries subjects who did not return for follow-up was determined by chart review.
During the first visit, a brief written survey regarding antibiotic history, fluoride status, and exposure to cigarette smoke was completed by the parent. At every visit, subjects received a toothbrush prophylaxis and fluoride varnish was applied. Parents received oral hygiene instructions and anticipatory guidance regarding the contribution of dietary factors to caries onset and progression.
Laboratory Methods
Sample preparation
Bacterial DNA was isolated using a bead beater as previously described [12].The bacterial DNA was purified using glass beads as previously described [73] and frozen until analysis.
PCR amplification, cloning, and sequencing
The 16S rRNA genes were amplified from the purified bacterial DNA, and the PCR products were cloned and sequenced as previously described [74]. Briefly, universal primers 5′-GTT TGA TCC TGG CTC AG-3′ (forward) and 5′-AAG GAG GTG ATC CAG CC-3′ (reverse) were used and the PCR products were examined by electrophoresis in 1% agarose and purified using the QIAquick PCR Purification Kit (QIAGEN, Valencia, CA). Amplicons were cloned into E. coli using a TOPO TA cloning kit (Invitrogen, San Diego, CA). Amplicons included the 16S rRNA gene hypervariable regions V5–V9.
Bacterial 16S rDNA Sequence Identification
Sequences from each clone were identified by comparing it to a local, curated oral microbiome database [17], found at http://microbiome.osu.edu, using BLAST [75]. Clinical sequences were required to be ≥98.0% similar to species-level taxonomic units in the database for identification. Sequences with similarity scores <98% were further analyzed by BLAST against the GenBank nr/nt database, the results manually examined, and chimeric sequences removed. Sequences that were observed only once and failed to match sequences in GenBank were not considered. Remaining novel sequences were added to the database.
Data Management and Statistical Analyses
Chi-squared analysis was used to compare the caries and control groups by gender, race, and ethnicity. A t-test was used to compare the two groups by age.
Levels of each species were calculated as a percent of total bacteria for each sample. Mean relative levels and 95% confidence intervals were determined for the most prevalent species using JMP (JMP, Version 7.0, SAS Institute Inc., Cary, NC). Repeated measures analysis was performed using PROC MIXED in SAS (SAS Institute Inc., SAS 9.2, Cary, NC) using the default structure. The sequential stages of health/caries from which samples were collected were assigned a numeric value and used in the PROC MIXED analysis. Using this scale, healthy control samples were assigned a value of 1, intact enamel samples from subjects with caries 2, white spot lesions 3, and cavitated lesions 4. A linear mixed effects model in PROC MIXED was used to calculate an estimate of the percent change in relative level for each taxon for caries stages 1 through 4, with α = 0.05, and the false discovery correction was applied.
Species abundance matrices were generated based on the BLAST classification of sequences. These matrices were then used to calculate the Bray-Curtis dissimilarity between each pair of samples. This statistic has been widely used in ecology to measure differences between communities and has a number of desirable properties, including giving sensible results for abundance matrices with many zeros and often showing good correlations with underlying environmental factors [76]. The calculation was performed with the vegdist function of the vegan package of the R programming language [76]. Direct comparisons were done on the Bray-Curtis matrix using the function t.test of the stats package of R [77], and ordinations were performed by non-metric multidimensional scaling (NMDS) using function metaMDS of the vegan package [76]. The centroids of points for the four different stages of caries/health were calculated. ANOSIM, a permutation-based test to measure differences between sample groups, was performed using the PRIMER 6 program (PRIMER-E, Plymouth, UK). Shannon diversity was calculated using a short R script that applied functions rrarefy and diversity from the vegan package [76], and was analyzed by SAS PROC MIXED for comparisons by severity and ANOVA for comparisons by subject group. Samples contained a varying number of sequences, with 44 being the smallest number for a sample that was included in the analysis. A random selection of 44 sequences was taken and the Shannon Diversity Index was calculated 10 times for each sample, and the average of these 10 indices was used for further analysis.
Longitudinal data was analyzed by calculating the Bray-Curtis dissimilarity matrix for subjects that were sampled at two time points, and the mean dissimilarities between samples from the same patient and samples from different subjects were compared by t-tests not assuming equal variance using the function t.test in stats package of R [77]. Longitudinal data was also explored at the species level. Mean abundance of the most abundant and significant species was compared for baseline and longitudinal samples using paired t-tests. Comparisons were made at each level of severity, and were tested for all caries samples, progressed samples only, and arrested samples only. t-tests were also used to compare progressed and arrested samples by the percent change in mean levels of these species between sampling times for enamel and white spot lesions.
t-tests and paired t-tests for post hoc comparisons were computed in JMP. The False Discovery Rate correction for multiple comparisons was determined using SAS.
Sample clustering and heatmap analysis
We generated heatmaps to graphically display species abundances for the most informative caries-associated species. Bray-Curtis dissimilarities between samples were determined using the species/sample abundance matrix for all white spot samples using function vegdist of vegan package in R [76], and hierarchical clustering of the samples was carried out with an average method using the function hclust of stats package in R [77]. The resulting dendrogram was reordered by assigning weights of 0 to the arrested samples and 1 to the progressed and using the mean as the agglomerative function. The reordered dendrogram and the abundance matrix for 4 caries-related species was given as input to the heatmap command of R.
Linear regression analysis of Veillonella atypica/Veillonella dispar/Veillonella parvula abundance
The combined abundance of the acidogenic species S. mutans, S. sobrinus, and S. vestibularis/S. salivarius was calculated for each white spot lesion sample. The abundance of V. atypica/V. dispar/V. parvula was calculated as fraction of the remaining organisms and the relationship between the two values was modeled by linear regression using command lm of R [77].
Funding Statement
This work was supported by grants R01DE016125, T32DE14320, and F30DE019339 from the National Institute of Dental and Craniofacial Research, National Institutes of Health (www.nidcr.nih.gov), and the OMNII Research Fellowship from the American Academy of Pediatric Dentistry (www.aapd.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.US Department of Health and Human Services (2000) Oral Health in America: A Report of the Surgeon General– Executive Summary National Institute of Dental and Craniofacial Research, National Institutes of Health.
- 2. Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E (2009) Beyond the dmft: the human and economic cost of early childhood caries. J Am Dent Assoc 140: 650–657. [DOI] [PubMed] [Google Scholar]
- 3. Kanellis MJ, Damiano PC, Momany ET (2000) Medicaid costs associated with the hospitalization of young children for restorative dental treatment under general anesthesia. J Public Health Dent 60: 28–32. [PubMed] [Google Scholar]
- 4. al-Shalan TA, Erickson PR, Hardie NA (1997) Primary incisor decay before age 4 as a risk factor for future dental caries. Pediatr Dent 19: 37–41. [PubMed] [Google Scholar]
- 5. Alm A, Wendt LK, Koch G, Birkhed D (2007) Prevalence of approximal caries in posterior teeth in 15-year-old Swedish teenagers in relation to their caries experience at 3 years of age. Caries research 41: 392–398. [DOI] [PubMed] [Google Scholar]
- 6. Mattila ML, Rautava P, Aromaa M, Ojanlatva A, Paunio P, et al. (2005) Behavioural and demographic factors during early childhood and poor dental health at 10 years of age. Caries research 39: 85–91. [DOI] [PubMed] [Google Scholar]
- 7. Peretz B, Ram D, Azo E, Efrat Y (2003) Preschool caries as an indicator of future caries: a longitudinal study. Pediatr Dent 25: 114–118. [PubMed] [Google Scholar]
- 8. Burne RA (1998) Oral streptococci… products of their environment. J Dent Res 77: 445–452. [DOI] [PubMed] [Google Scholar]
- 9. Marsh PD (2003) Are dental diseases examples of ecological catastrophes? Microbiology 149: 279–294. [DOI] [PubMed] [Google Scholar]
- 10. van Houte J (1994) Role of micro-organisms in caries etiology. J Dent Res 73: 672–681. [DOI] [PubMed] [Google Scholar]
- 11. Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, et al. (2008) Bacteria of dental caries in primary and permanent teeth in children and young adults. Journal of clinical microbiology 46: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, et al. (2010) Bacterial 16S sequence analysis of severe caries in young permanent teeth. Journal of clinical microbiology 48: 4121–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirose H, Hirose K, Isogai E, Miura H, Ueda I (1993) Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries research 27: 292–297. [DOI] [PubMed] [Google Scholar]
- 14. Kleinberg I (2002) A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 13: 108–125. [DOI] [PubMed] [Google Scholar]
- 15. Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, et al. (2011) Cultivable anaerobic microbiota of severe early childhood caries. Journal of clinical microbiology 49: 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burne RA, Marquis RE (2000) Alkali production by oral bacteria and protection against dental caries. FEMS microbiology letters 193: 1–6. [DOI] [PubMed] [Google Scholar]
- 17. Griffen AL, Beall CJ, Firestone ND, Gross EL, DiFranco JM, et al. (2011) CORE: A Phylogenetically-Curated 16S rDNA Database of the Core Oral Microbiome. PLoS One 6: e19051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Preza D, Olsen I, Aas JA, Willumsen T, Grinde B, et al. (2008) Bacterial profiles of root caries in elderly patients. Journal of clinical microbiology 46: 2015–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beighton D (2005) The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol 33: 248–255. [DOI] [PubMed] [Google Scholar]
- 20. Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T (1995) Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. International journal of systematic bacteriology 45: 406–408. [DOI] [PubMed] [Google Scholar]
- 21. Tanzer JM, Livingston J, Thompson AM (2001) The microbiology of primary dental caries in humans. J Dent Educ 65: 1028–1037. [PubMed] [Google Scholar]
- 22. Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiological Reviews 50: 353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanzer JM, Kurasz AB, Clive J (1985) Inhibition of ecological emergence of mutans streptococci naturally transmitted between rats and consequent caries inhibition by Streptococcus salivarius TOVE-R infection. Infect Immun 49: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hughes CV, Dahlan M, Papadopolou E, Loo CY, Pradhan NS, et al. (2012) Aciduric microbiota and mutans streptococci in severe and recurrent severe early childhood caries. Pediatr Dent 34: 16–23. [PMC free article] [PubMed] [Google Scholar]
- 25. Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, et al. (2005) Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. Journal of medical microbiology 54: 661–665. [DOI] [PubMed] [Google Scholar]
- 26. Seki M, Yamashita Y, Shibata Y, Torigoe H, Tsuda H, et al. (2006) Effect of mixed mutans streptococci colonization on caries development. Oral Microbiol Immunol 21: 47–52. [DOI] [PubMed] [Google Scholar]
- 27. Loyola-Rodriguez JP, Martinez-Martinez RE, Flores-Ferreyra BI, Patino-Marin N, Alpuche-Solis AG, et al. (2008) Distribution of Streptococcus mutans and Streptococcus sobrinus in saliva of Mexican preschool caries-free and caries-active children by microbial and molecular (PCR) assays. J Clin Pediatr Dent 32: 121–126. [PubMed] [Google Scholar]
- 28. Choi EJ, Lee SH, Kim YJ (2009) Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int J Paediatr Dent 19: 141–147. [DOI] [PubMed] [Google Scholar]
- 29. Kanasi E, Johansson I, Lu SC, Kressin NR, Nunn ME, et al. (2010) Microbial risk markers for childhood caries in pediatricians’ offices. J Dent Res 89: 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer CA, Kent R Jr, Loo CY, Hughes CV, Stutius E, et al. (2010) Diet and caries-associated bacteria in severe early childhood caries. J Dent Res 89: 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawamura Y, Hou XG, Todome Y, Sultana F, Hirose K, et al. (1998) Streptococcus peroris sp. nov. and Streptococcus infantis sp. nov., new members of the Streptococcus mitis group, isolated from human clinical specimens. International journal of systematic bacteriology 48 Pt 3: 921–927. [DOI] [PubMed] [Google Scholar]
- 32. Drucker DB, Shakespeare AP, Green RM (1984) The production of dental plaque and caries by the bacterium Streptococcus salivarius in gnotobiotic WAG/RIJ rats. Arch Oral Biol 29: 437–443. [DOI] [PubMed] [Google Scholar]
- 33. Horton WA, Jacob AE, Green RM, Hillier VF, Drucker DB (1985) The cariogenicity of sucrose, glucose and maize starch in gnotobiotic rats mono-infected with strains of the bacteria Streptococcus mutans, Streptococcus salivarius and Streptococcus milleri. Arch Oral Biol 30: 777–780. [DOI] [PubMed] [Google Scholar]
- 34. Willcox MD, Drucker DB, Hillier VF (1988) In-vitro adherence of oral streptococci in the presence of sucrose and its relationship to cariogenicity in the rat. Arch Oral Biol 33: 109–113. [DOI] [PubMed] [Google Scholar]
- 35. Willcox MD, Knox KW, Green RM, Drucker DB (1991) An examination of strains of the bacterium Streptococcus vestibularis for relative cariogenicity in gnotobiotic rats and adhesion in vitro. Arch Oral Biol 36: 327–333. [DOI] [PubMed] [Google Scholar]
- 36. Chestnutt IG, MacFarlane TW, Stephen KW (1994) An in vitro investigation of the cariogenic potential of oral streptococci. Arch Oral Biol 39: 589–593. [DOI] [PubMed] [Google Scholar]
- 37. Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, et al. (2002) Molecular analysis of bacterial species associated with childhood caries. Journal of clinical microbiology 40: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen YY, Burne RA (1996) Analysis of Streptococcus salivarius urease expression using continuous chemostat culture. FEMS microbiology letters 135: 223–229. [DOI] [PubMed] [Google Scholar]
- 39. Tanzer JM, Kurasz AB, Clive J (1985) Competitive displacement of mutans streptococci and inhibition of tooth decay by Streptococcus salivarius TOVE-R. Infect Immun 48: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toro E, Nascimento MM, Suarez-Perez E, Burne RA, Elias-Boneta A, et al. (2010) The effect of sucrose on plaque and saliva urease levels in vivo. Arch Oral Biol 55: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Corby PM, Bretz WA, Hart TC, Schork NJ, Wessel J, et al. (2007) Heritability of oral microbial species in caries-active and caries-free twins. Twin Res Hum Genet 10: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whiley RA, Fraser HY, Douglas CW, Hardie JM, Williams AM, et al. (1990) Streptococcus parasanguis sp. nov., an atypical viridans Streptococcus from human clinical specimens. FEMS microbiology letters 56: 115–121. [DOI] [PubMed] [Google Scholar]
- 43. Kikuchi K, Enari T, Totsuka K, Shimizu K (1995) Comparison of phenotypic characteristics, DNA-DNA hybridization results, and results with a commercial rapid biochemical and enzymatic reaction system for identification of viridans group streptococci. Journal of clinical microbiology 33: 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paddick JS, Brailsford SR, Kidd EA, Beighton D (2005) Phenotypic and genotypic selection of microbiota surviving under dental restorations. Applied and environmental microbiology 71: 2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, et al. (2004) Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. Journal of clinical microbiology 42: 3128–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chhour KL, Nadkarni MA, Byun R, Martin FE, Jacques NA, et al. (2005) Molecular analysis of microbial diversity in advanced caries. Journal of clinical microbiology 43: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loesche WJ, Syed SA (1973) The predominant cultivable flora of carious plaque and carious dentine. Caries research 7: 201–216. [DOI] [PubMed] [Google Scholar]
- 48. Marchandin H, Teyssier C, Simeon De Buochberg M, Jean-Pierre H, Carriere C, et al. (2003) Intra-chromosomal heterogeneity between the four 16S rRNA gene copies in the genus Veillonella: implications for phylogeny and taxonomy. Microbiology 149: 1493–1501. [DOI] [PubMed] [Google Scholar]
- 49. Rogosa M (1964) The Genus Veillonella. I. General Cultural, Ecological, and Biochemical Considerations. J Bacteriol 87: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lima KC, Coelho LT, Pinheiro IV, Rocas IN, Siqueira JF Jr (2011) Microbiota of dentinal caries as assessed by reverse-capture checkerboard analysis. Caries research 45: 21–30. [DOI] [PubMed] [Google Scholar]
- 51. Noorda WD, Purdell-Lewis DJ, van Montfort AM, Weerkamp AH (1988) Monobacterial and mixed bacterial plaques of Streptococcus mutans and Veillonella alcalescens in an artificial mouth: development, metabolism, and effect on human dental enamel. Caries research 22: 342–347. [DOI] [PubMed] [Google Scholar]
- 52. Mikx FH, Van der Hoeven JS (1975) Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch Oral Biol 20: 407–410. [DOI] [PubMed] [Google Scholar]
- 53. Bradshaw DJ, Marsh PD (1998) Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res 32: 456–462. [DOI] [PubMed] [Google Scholar]
- 54. Bradshaw DJ, Marsh PD, Schilling KM, Cummins D (1996) A modified chemostat system to study the ecology of oral biofilms. J Appl Bacteriol 80: 124–130. [DOI] [PubMed] [Google Scholar]
- 55. Bradshaw DJ, McKee AS, Marsh PD (1989) Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res 68: 1298–1302. [DOI] [PubMed] [Google Scholar]
- 56. McDermid AS, McKee AS, Ellwood DC, Marsh PD (1986) The effect of lowering the pH on the composition and metabolism of a community of nine oral bacteria grown in a chemostat. J Gen Microbiol 132: 1205–1214. [DOI] [PubMed] [Google Scholar]
- 57. Marsh PD (1994) Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8: 263–271. [DOI] [PubMed] [Google Scholar]
- 58. Liu J, Wu C, Huang IH, Merritt J, Qi F (2011) Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology 157: 2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D (2001) The predominant microflora of nursing caries lesions. Caries research 35: 397–406. [DOI] [PubMed] [Google Scholar]
- 60. Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, et al. (2011) Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boone DR, Castenholz RW, Garrity GM (2001) Bergey’s manual of systematic bacteriology/George M. Garrity, editor-in-chief. New York: Springer. v. <v. 1–2 in 4>p.
- 62. Lin X, Lamont RJ, Wu J, Xie H (2008) Role of differential expression of streptococcal arginine deiminase in inhibition of fimA expression in Porphyromonas gingivalis. J Bacteriol 190: 4367–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dong Y, Chen YY, Snyder JA, Burne RA (2002) Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Applied and environmental microbiology 68: 5549–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kuramitsu HK, Wang BY (2006) Virulence properties of cariogenic bacteria. BMC Oral Health 6 Suppl 1S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang BY, Kuramitsu HK (2005) Interactions between oral bacteria: inhibition of Streptococcus mutans bacteriocin production by Streptococcus gordonii. Applied and environmental microbiology 71: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Howell A Jr, Pine L (1961) The classification of organisms termed Leptotrichia (Leptothrix) buccalis. IV. Physiological and biochemical characteristics of Bacterionema matruchotii. Bacteriol Rev 25: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moore LVH, Johnson JL, Moore WEC (1987) Selenomonas noxia sp. nov., Selenomonas flueggei sp. nov., Selenomonas infelix sp. nov., Selenomonas dianae sp. nov., and Selenomonas artemidis sp. nov., from the Human Gingival Crevice. International journal of systematic bacteriology 37: 271–280. [Google Scholar]
- 68. Margolis HC, Moreno EC, Murphy BJ (1985) Importance of high pKA acids in cariogenic potential of plaque. J Dent Res 64: 786–792. [DOI] [PubMed] [Google Scholar]
- 69. Margolis HC, Zhang YP, Lee CY, Kent RL Jr, Moreno EC (1999) Kinetics of enamel demineralization in vitro. J Dent Res 78: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 70. Arif N, Sheehy EC, Do T, Beighton D (2008) Diversity of Veillonella spp. from sound and carious sites in children. J Dent Res 87: 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang W, Jiang Y, Li C, Liang J (2011) Investigation of supragingival plaque microbiota in different caries status of Chinese preschool children by denaturing gradient gel electrophoresis. Microbial ecology 61: 342–352. [DOI] [PubMed] [Google Scholar]
- 72. Li Y, Ge Y, Saxena D, Caufield PW (2007) Genetic profiling of the oral microbiota associated with severe early-childhood caries. Journal of clinical microbiology 45: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leys EJ, Griffen AL, Strong SJ, Fuerst PA (1994) Detection and strain identification of Actinobacillus actinomycetemcomitans by nested PCR. Journal of clinical microbiology 32: 1288–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kumar PS, Griffen AL, Moeschberger ML, Leys EJ (2005) Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiology 43: 3944–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 76.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, et al. (2011) vegan: Community Ecology Package.
- 77.R Development Core Team (2011) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.