Abstract
We have constructed primed phi X174 DNA templates containing either acetylated or deacetylated aminofluorene adducts at the C-8 position of guanine. T4 DNA polymerase terminates synthesis one nucleotide before the acetylated adducts but incorporates an additional nucleotide opposite the deacetylated guanylaminofluorene. These observations can be explained by the known preferred conformations of the acetylated and deacetylated guanosinylaminofluorene nucleosides--the former favoring the syn conformation (so that in DNA the guanine is displaced from the helix by the fluorene ring) and the latter preferring the anti conformation (which allows normal base pairing of the guanine with cytosine). A similar differentiation between the two adducts was found with Escherichia coli DNA polymerase I. In contrast, avian myeloblastosis virus (AMV) reverse transcriptase, which terminated with a nucleotide inserted opposite the acetylated adducts, was less able to do so at the deacetylated adducts. The nucleoside incorporated by AMV reverse transcriptase opposite the acetylated adduct was exclusively cytidine, which suggests regular base pairing with the reacted guanosine nucleoside in the anti conformation; however, synthesis was completely blocked and unable to continue beyond this point. The differences between the termination patterns of the prokaryotic enzymes and AMV reverse transcriptase indicates that specific properties of a replicating polymerase can influence the conformation of a reacted nucleoside in the DNA, thus altering its recognition and possibly its mutagenic activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch H., Dworkin M., Miller J. A., Miller E. C. Electrophilic N-acetoxyaminoarenes derived from carcinogenic N-hydroxy-N-acetylaminoarenes by enzymatic deacetylation and transacetylation in liver. Biochim Biophys Acta. 1972 Dec 29;286(2):272–298. doi: 10.1016/0304-4165(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Battula N., Loeb L. A. On the fidelity of DNA replication. Lack of exodeoxyribonuclease activity and error-correcting function in avian myeloblastosis virus DNA polymerase. J Biol Chem. 1976 Feb 25;251(4):982–986. [PubMed] [Google Scholar]
- DeBaun J. R., Miller E. C., Miller J. A. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970 Mar;30(3):577–595. [PubMed] [Google Scholar]
- Evans F. E., Miller D. W., Beland F. A. Sensitivity of the conformation of deoxyguanosine to binding at the C-8 position by N-acetylated and unacetylated 2-aminofluorene. Carcinogenesis. 1980;1(11):955–959. doi: 10.1093/carcin/1.11.955. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Daune M. P. Dynamic structure of DNA modified with the carcinogen N-acetoxy-n-2-acetylaminofluorene. Biochemistry. 1974 Oct 8;13(21):4435–4440. doi: 10.1021/bi00718a028. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Hingerty B., Broyde S. Conformation of the deoxydinucleoside monophosphate dCpdG modified at carbon 8 of guanine with 2-(acetylamino)fluorene. Biochemistry. 1982 Jun 22;21(13):3243–3252. doi: 10.1021/bi00256a034. [DOI] [PubMed] [Google Scholar]
- King C. M. Mechanism of reaction, tissue distribution, and inhibition of arylhydroxamic acid acyltransferase. Cancer Res. 1974 Jun;34(6):1503–1515. [PubMed] [Google Scholar]
- King C. M., Phillips B. N-hydroxy-2-fluorenylacetamide. Reaction of the carcinogen with guanosine, ribonucleic acid, deoxyribonucleic acid, and protein following enzymatic deacetylation or esterification. J Biol Chem. 1969 Nov 25;244(22):6209–6216. [PubMed] [Google Scholar]
- King C. M., Traub N. R., Cardona R. A., Howard R. B. Comparative adduct formation of 4-aminobiphenyl and 2-aminofluorene derivatives with macromolecules of isolated liver parenchymal cells. Cancer Res. 1976 Jul;36(7 Pt 1):2374–2381. [PubMed] [Google Scholar]
- Kriek E. Carcinogenesis by aromatic amines. Biochim Biophys Acta. 1974 Sep 9;355(2):177–203. doi: 10.1016/0304-419x(74)90003-1. [DOI] [PubMed] [Google Scholar]
- Kriek E. On the interaction of N-2-fluorenylhydroxylamine with nucleic acids in vitro. Biochem Biophys Res Commun. 1965 Sep 22;20(6):793–799. doi: 10.1016/0006-291x(65)90088-4. [DOI] [PubMed] [Google Scholar]
- Kriek E. On the mechanism of action of carcinogenic aromatic amines. I. Binding of 2-acetylaminofluorene and N-hydroxy-2-acetylaminofluorene to rat-liver nucleic acids in vivo. Chem Biol Interact. 1969 Oct;1(1):3–17. doi: 10.1016/0009-2797(69)90015-5. [DOI] [PubMed] [Google Scholar]
- Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972 Oct;32(10):2042–2048. [PubMed] [Google Scholar]
- Kriek E., Westra J. G. Structural identification of the pyrimidine derivatives formed from N-(deoxyguanosin-8-yl)-2-aminofluorene in aqueous solution at alkaline pH. Carcinogenesis. 1980 Jun;1(6):459–468. doi: 10.1093/carcin/1.6.459. [DOI] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
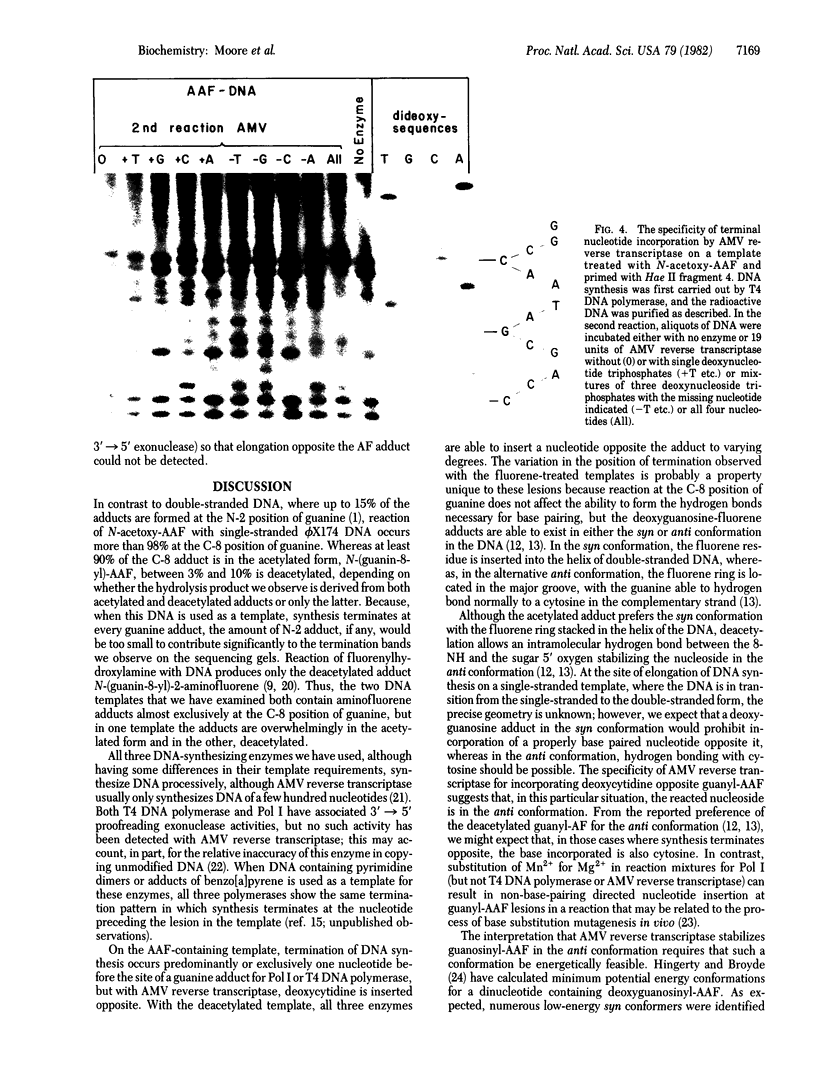
- Moore P. D., Rabkin S. D., Strauss B. S. Termination of vitro DNA synthesis at AAF adducts in the DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4473–4484. doi: 10.1093/nar/8.19.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Ross J., Metzger G., Werbin H. Conformation of 2-[(deoxyguanosin-8-ylacetyl)amino]fluorene differs in protein-free deoxyribonucleic acid and chromatin. Biochemistry. 1982 Mar 16;21(6):1369–1374. doi: 10.1021/bi00535a041. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformation of poly(dG-dC) . poly(dG-dC) modified by the carcinogens N-acetoxy-N-acetyl-2-aminofluorene and N-hydroxy-N-2-aminofluorene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4597–4601. doi: 10.1073/pnas.77.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Broyde S., Hingerty B. E. Z-DNA conformation of N-2-acetylaminofluorene modified poly(dG-dC).poly(dG-dC) determined by reactivity with anti cytidine antibodies and minimized potential energy calculations. Nucleic Acids Res. 1981 Oct 24;9(20):5459–5467. doi: 10.1093/nar/9.20.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Weinstein I. B., Rich A. Induction of the Z conformation in poly(dG-dC).poly(dG-dC) by binding of N-2-acetylaminofluorene to guanine residues. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1451–1455. doi: 10.1073/pnas.78.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Kriek E., Grunberger D. Circular dichroism and proton magnetic resonance studies of dApdG modified with 2-aminofluorene and 2-acetylaminofluorene. Carcinogenesis. 1980;1(11):897–902. doi: 10.1093/carcin/1.11.897. [DOI] [PubMed] [Google Scholar]
- Schut H. A., Wirth P. J., Thorgeirsson S. S. Mutagenic activation of N-hydroxy-2-acetylaminofluorene in the Salmonella test system: the role of deacetylation by liver and kidney fractions from mouse and rat. Mol Pharmacol. 1978 Jul;14(4):682–692. [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Visser A., Westra J. G. Partial persistency of 2-aminofluorene and N-acetyl-2-aminofluorene in rat liver DNA. Carcinogenesis. 1981;2(8):737–740. doi: 10.1093/carcin/2.8.737. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Pulkrabek P., Grunberger D., Weinstein I. B. Differential excision from DNA of the C-8 and N2 guanosine adducts of N-acetyl-2-aminofluorene by single strand-specific endonucleases. Cancer Res. 1977 Oct;37(10):3756–3760. [PubMed] [Google Scholar]