Abstract
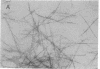
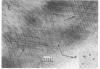
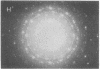
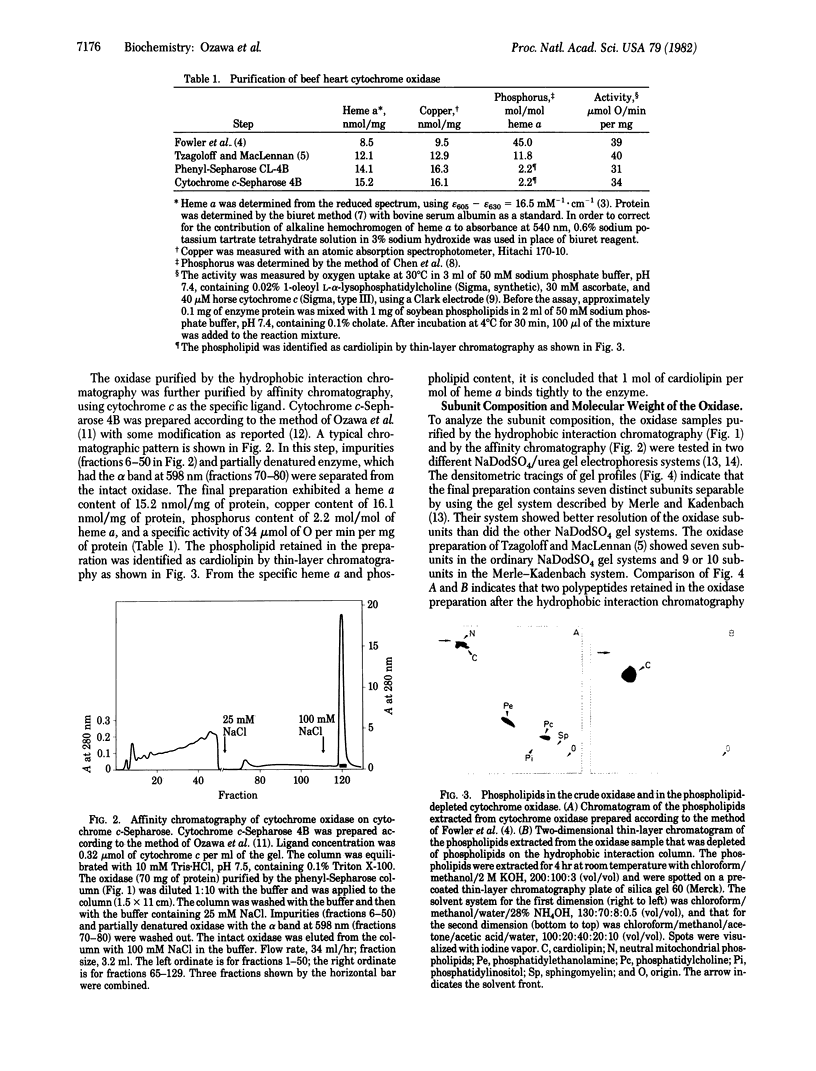
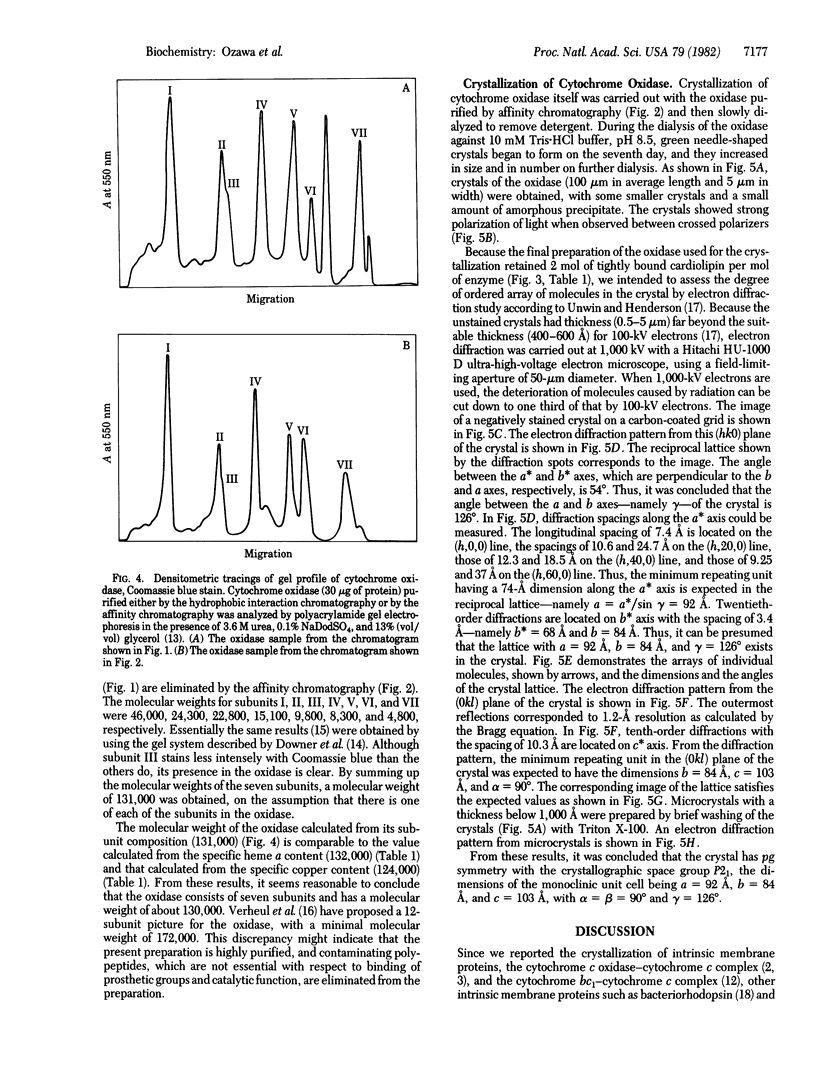
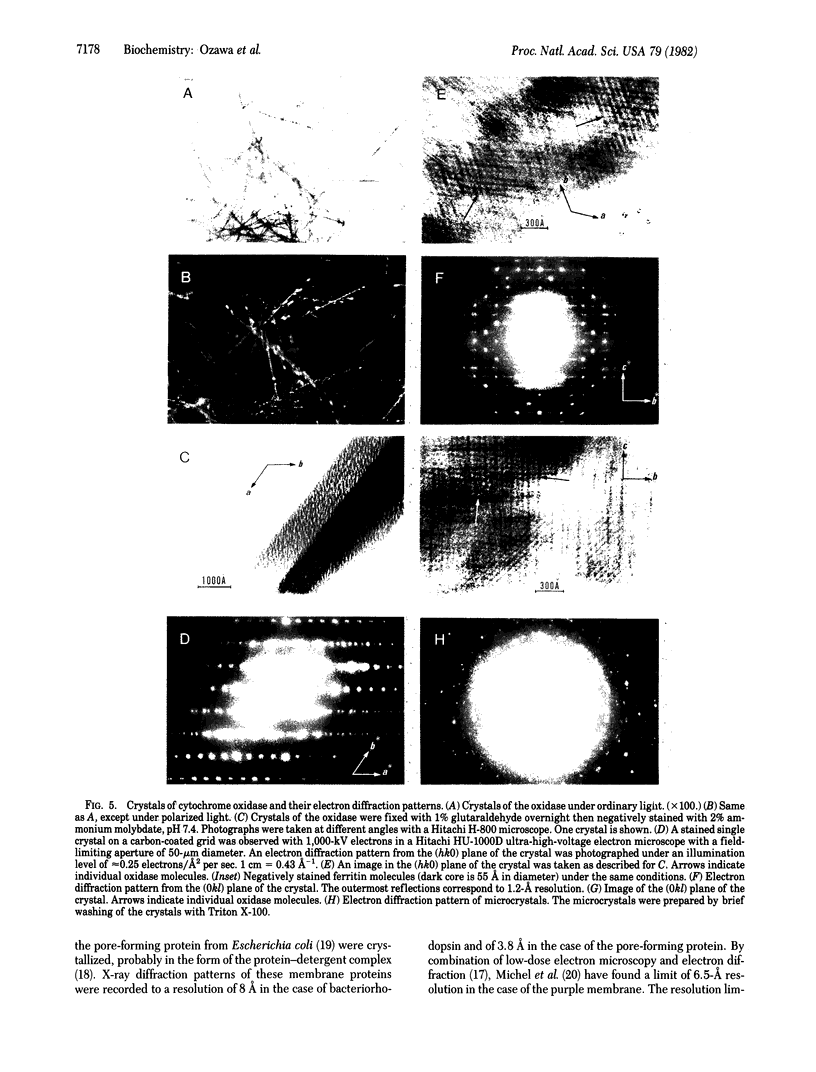
Cytochrome oxidase (ferrocytochrome c:oxygen oxidoreductase, EC 1.9.3.1) was purified from beef heart mitochondria. By washing the oxidase with detergent on a hydrophobic interaction column, phospholipids were depleted to the level of 1 mol of cardiolipin per mol of heme a. Hydrophobic impurities and partially denatured oxidase were separated from the intact oxidase on an affinity column with cytochrome c as the specific ligand. The final preparation of the oxidase contained seven distinct polypeptides. The molecular weight of the oxidase was estimated to be 130,000 from its specific heme a and copper content and from the subunit composition. Crystals of the oxidase were obtained by slow removal of the detergent from the buffer in which the oxidase was dissolved. The needle-shaped crystals were 100 microns in average length and 5 microns in width, and they strongly polarized visible light. Electron diffraction patterns were obtained with an unstained glutaraldehyde-fixed single crystal by electron microscopy using 1,000-kV electrons. From electron micrographs and the diffraction patterns of the crystal, it was concluded that the crystal is monoclinic in the space group P21, with unit cell dimensions a = 92 A, b = 84 A, and c = 103 A, and alpha = beta = 90 degrees, gamma = 126 degrees.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- FOWLER L. R., RICHARDSON S. H., HATEFI Y. A rapid method for the preparation of highly purified cytochrome oxidase. Biochim Biophys Acta. 1962 Oct 8;64:170–173. doi: 10.1016/0006-3002(62)90770-9. [DOI] [PubMed] [Google Scholar]
- Fry M., Blondin G. A., Green D. E. The localization of tightly bound cardiolipin in cytochrome oxidase. J Biol Chem. 1980 Oct 25;255(20):9967–9970. [PubMed] [Google Scholar]
- Fry M., Green D. E. Cardiolipin requirement by cytochrome oxidase and the catalytic role of phospholipid. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1238–1246. doi: 10.1016/0006-291x(80)90622-1. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Capaldi R. A., Henderson R. Structure of cytochrome c oxidase in deoxycholate-drived two-dimensional crystals. J Mol Biol. 1979 Oct 25;134(2):305–327. doi: 10.1016/0022-2836(79)90037-8. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Rosenbusch J. P. Three-dimensional crystals of an integral membrane protein: an initial x-ray analysis. J Cell Biol. 1980 Jul;86(1):327–329. doi: 10.1083/jcb.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Capaldi R. A., Leigh J. S. Arrangement of cytochrome oxidase molecules in two-dimensional vesicle crystals. J Mol Biol. 1977 Jun 5;112(4):631–648. doi: 10.1016/s0022-2836(77)80167-8. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Merle P., Kadenbach B. The subunit composition of mammalian cytochrome c oxidase. Eur J Biochem. 1980 Apr;105(3):499–507. doi: 10.1111/j.1432-1033.1980.tb04525.x. [DOI] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D., Henderson R. Orthorhombic two-dimensional crystal form of purple membrane. Proc Natl Acad Sci U S A. 1980 Jan;77(1):338–342. doi: 10.1073/pnas.77.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D. Three-dimensional crystals of membrane proteins: bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1283–1285. doi: 10.1073/pnas.77.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Okumura M., Yagi K. Purification of cytochrome oxidase by using Sepharose-bound cytochrome c. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1102–1107. doi: 10.1016/s0006-291x(75)80499-2. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Suzuki H., Tanaka M. Crystallization of part of the mitochondrial electron transfer chain: cytochrome c oxidase--cytochrome c complex. Proc Natl Acad Sci U S A. 1980 Feb;77(2):928–930. doi: 10.1073/pnas.77.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Shimomura Y. Crystallization of the middle part of the mitochondrial electron transfer chain: cytochrome bc1-cytochrome c complex. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5084–5086. doi: 10.1073/pnas.77.9.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Hayashi H., Oda T. Studies on cytochrome oxidase. I. Fine structure of cytochrome oxidase-rich submitochondrial membrane. Arch Biochem Biophys. 1970 May;138(1):110–121. doi: 10.1016/0003-9861(70)90290-0. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Suzuki H., Ozawa T. The crystallization of mitochondrial cytochrome oxidase-cytochrome c complex. Biochim Biophys Acta. 1980 Mar 14;612(1):295–298. doi: 10.1016/0005-2744(80)90303-4. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., MacLennan D. H. Studies of the electron-transfer system. LXIV. Role of phospholipid in cytochrome oxidase. Biochim Biophys Acta. 1965 Jun 22;99(3):476–485. doi: 10.1016/s0926-6593(65)80201-6. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G., Senior A. E., Capaldi R. A., Hayashi H. Biological membrane structure. 3. The lattice structure of membranous cytochrome oxidase. Biochim Biophys Acta. 1972 Jul 3;274(1):38–48. doi: 10.1016/0005-2736(72)90278-7. [DOI] [PubMed] [Google Scholar]
- Verheul F. E., Draijer J. W., Dentener I. K., Muijsers A. O. Subunit stoichiometry of cytochrome c oxidase of bovine heart. Eur J Biochem. 1981 Oct;119(2):401–408. doi: 10.1111/j.1432-1033.1981.tb05622.x. [DOI] [PubMed] [Google Scholar]
- Vik S. B., Capaldi R. A. Conditions for optimal electron transfer activity of cytochrome c oxidase isolated from beef heart mitochondria. Biochem Biophys Res Commun. 1980 May 14;94(1):348–354. doi: 10.1016/s0006-291x(80)80227-0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T., Senior A. E., Hatase O., Hayashi H., Green D. E. Conformational changes in membranous preparations of cytochrome oxidase. J Bioenerg. 1972 Aug;3(5):339–344. doi: 10.1007/BF01516073. [DOI] [PubMed] [Google Scholar]